Abstract
Adenovirus (Ad) cell entry involves sequential interactions with host cell receptors that mediate attachment (CAR), internalization (αvβ3 and αvβ5), and penetration (αvβ5) of the endosomal membrane. These events allow the virus to deliver its genome to the nucleus. While integrins αvβ3 and αvβ5 both promote Ad internalization into cells, integrin αvβ5 selectively facilitates Ad-mediated membrane permeabilization and endosome rupture. In the experiments reported herein, we demonstrate that the intracellular domain of the integrin β5 subunit specifically regulates Ad-mediated membrane permeabilization and gene delivery. CS-1 melanoma cells expressing a truncated integrin β5 or a chimeric (β5-β3) cytoplasmic tail (CT) supported normal levels of Ad endocytosis but had reduced Ad-mediated gene delivery and membrane permeabilization relative to cells expressing a wild-type integrin β5. Thin-section electron microscopy revealed that virion particles were capable of being endocytosed into cells expressing a truncated β5CT, but they failed to escape cytoplasmic vesicles and translocate to the nucleus. Site-specific mutagenesis studies suggest that a C-terminal TVD motif in the β5CT plays a major role in Ad membrane penetration.
A unique feature of human adenovirus (Ad) is the efficiency with which it delivers its nucleic acid payload to the host cell nucleus. This is reflected in the low virus particle/infectious unit ratio observed with many host cell types (11, 27). Based on this property, as well as its broad tissue tropism, replication-defective Ad vectors are currently under evaluation for in vivo gene therapy (4, 37). Ad has also facilitated investigation of different gene products in vitro, as well as uncovered several important host cell functions including RNA processing (2, 9) and cell cycle regulation (8, 45).
Although there is relatively little information on how Ad penetrates the barrier of the host cell plasma membrane, viral entry involves a sequence of distinct virus-host cell interactions. High-affinity virus binding to cells is mediated by the fiber protein interaction with a 46-kDa cell receptor known as CAR (1, 39). Following attachment, Ad type 2 (Ad2) particles are rapidly internalized via the penton base capsid protein interaction with cell integrins αvβ3 and αvβ5 (43). Ad2 internalization also requires dynamin (40), a GTPase involved in the formation of clathrin-coated pits, as well as several signaling molecules including phosphoinositide-3-kinase (26) and the Rho family of small GTPases (25) which promote cortical actin polymerization.
In order to penetrate the barrier of the host cell membrane, Ad particles disrupt cell endosomes (20), allowing partially uncoated virions to be released into the cytoplasm where they transit to nuclear pore complexes (6). While the precise mechanism by which Ad penetrates the endosome has not been delineated, several features of this process have recently come to light. Ad induces the release of small molecules from cells at pH 6.0 (33, 34) and promotes the formation of channels in artificial lipid bilayers (3, 32). Ad-mediated membrane permeabilization requires the interaction of the penton base protein with αv integrins (33, 34), and interaction of the Ad3 penton base with cell surface αv integrins alone facilitates DNA delivery into cells (16). Activation of the 22-kDa Ad-encoded cysteine protease, a molecule which participates in virus penetration and uncoating (10), has been reported to require integrin interactions with the virus (20).
In a previous study, we demonstrated that integrin αvβ5 promotes Ad-mediated membrane permeabilization in a CS-1 melanoma cell model (42). CS-1 cells expressing αvβ5 also have increased susceptibility to Ad infection compared to cells expressing integrin αvβ3. In further experiments reported herein, we identify a region in the β5 integrin subunit that selectively mediates Ad cell entry.
MATERIALS AND METHODS
Ad, antibodies, and recombinant penton base.
Ad2 was obtained from the American Type Culture Collection, and a recombinant Ad encoding green fluorescent protein (GFP), Ad.RSV.GFP, was generated as previously described (23). Virions were isolated by sedimentation on CsCl density gradients (13). Ad2 was radiolabeled with Na125I to a specific activity of 107 cpm/μg (Iodogen; Pierce). The P1F6 function-blocking monoclonal antibody to αvβ5 has been previously described (7, 41). Recombinant penton base was produced in Tn5B insect cells and purified as previously described (44).
Construction of β5 integrin expression vectors and CS1/β5 transfected cell lines.
A plasmid (pCI/β5) encoding the entire β5 integrin subunit (30) was used as a template in PCRs to create altered forms of the β5 subunit. Wild-type β5 integrin (β5wt) cDNA for eucaryotic cell expression was generated by PCR (30) using a pair of oligonucleotide primers, primers 1 (5′-CTAGGATCCGGCGCCCCACCATGCCGCGG-3′) and 2 (5′-CTAGAATTCCTATCAGTCCACAGTGCCATTGTA-3′) using Taq polymerase. cDNA containing a chimeric integrin with a β5-β3 cytoplasmic domain was made by a two-step PCR. The first step was performed with primers 1 and 3 (5′-CGTGATATTGGTGAAGGTAGACGTGGCCTCTTTGTATAATGGATTTGAAGC-3′). The DNA fragment generated from this PCR was then used as the template in a second PCR using primers 1 and 4 (5′-CTAGAATTCTCATTAAGTGCCCCGGTACGTGATATTGGTGAAGGT-3′). To generate β5 deletion mutants, PCRs were performed with primer 1 and primer 5 (5′-CTAGAATTCCTATCAAGTGCCATTGTAGGATTTGTT-3′) to create β5Δ2 or with primer 6 (5′-CTAGAATTCCTATCAGTAGGATTTGTTGAACTTGTT-3′) to create β5Δ5, with primer 7 (5′-CTAGAATTCCTATCAGTCCACAGTGTGCGTGGAGAT-3′) to create β5Δ15, with primer 8 (5′-CTAGAATTCCTATCAAGTGTGCGTGGAGATAGGCTT-3′) for β5Δ17, and with primer 9 (5′-CTAGAATTCCTATCAGTATAATGGATTTGAAGCCAT-3′) for β5Δ25. Primers 10 (5′-CTAGAATTCCTATCAGGCCACAGTGCCATTGTA-3′) and 11 (5′-CTAGAATTCCTATCAGTCCACAGCGCCATTGTA-3′) were used to generate the point mutants D799A and T797A, respectively. The PCR-generated DNA fragments encoding each β5 subunit was inserted into the mammalian expression plasmid pcDNA3 (Invitrogen, Carlsbad, Calif.) between BamHI and EcoRI sites. Each construct was verified by automated DNA sequencing.
CS-1 hamster melanoma cells (14), which produce endogenous integrin αv but not β subunits (38), were generously provided by Caroline Damsky (University of California at San Francisco). CS-1 cells stably transfected with either a β5wt integrin subunit or a chimeric β subunit consisting of a β5 ectodomain, a β5 transmembrane domain, and a β3 cytoplasmic domain were described previously (18). CS-1 cell lines expressing deletion or site-directed β5 integrin subunits were generated by transfection with Lipofectamine (Gibco BRL, Gaithersburg, Md.). Stably transformed cells were selected by growth in medium containing 500 μg of G418 per ml and by adhesion in plastic tissue culture wells.
Flow cytometry, Ad-mediated gene delivery, and cell adhesion assays.
The P1F6 function-blocking monoclonal antibody to αvβ5 was used to measure integrin expression on transfected CS-1 cells by flow cytometry (22). Control cell samples were incubated in phosphate-buffered saline (PBS) lacking P1F6. A fluorescein-labeled goat anti-mouse immunoglobulin G (heavy plus light chain) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was used as the secondary antibody. To measure Ad-mediated gene delivery, cells were gently detached from tissue culture dishes with PBS containing 5 mM EDTA, washed with PBS, and then resuspended in complete Dulbecco modified Eagle medium (DMEM). One-milliliter aliquots of 2 × 106 cells were chilled on ice to 4°C, various amounts of Ad.RSV.GFP were then added to the cell samples, and incubation was continued for 60 min at 4°C with frequent mixing. The cells were then washed once with PBS, resuspended in 10 ml of complete DMEM, and transferred to 10-cm-diameter tissue culture dishes. After the cells were cultured for 48 h at 37°C in a 5% CO2 incubator, the cells were detached with 5 mM EDTA in PBS, washed with PBS, and resuspended in 3% formaldehyde in PBS. GFP expression was measured by flow cytometry as previously described (40).
Cell adhesion assays were performed in a manner similar to that previously described (36). The wells in 48-well tissue culture plates were coated with various amounts of purified Ad2 penton base protein in PBS overnight at 4°C. The plates were then washed with PBS, and nonspecific binding sites were blocked by incubating the plate with PBS containing 2% bovine serum albumin (BSA) at room temperature for 1 h. After the wells were rinsed briefly with PBS, 105 CS-1 cells in 200 μl of serum-free medium were added to each well and incubated at 37°C for 1 h. Unattached cells were removed by washing the wells three times with PBS. The adherent cells were then stained with crystal violet for 30 min at room temperature. The plates were washed gently with water and drained to dry. The cell-associated stain was solubilized with 200 μl of 10% acetic acid and then quantified in a SpectraMax 250 spectrophotometer (Molecular Devices) at A600. The reaction (A600) was linear from 0 to 1.5 × 105 cells per well.
Ad2 binding, internalization, and membrane permeabilization assays.
Ad2 binding and internalization were performed as previously described (40). 125I-labeled Ad2, approximately 2 × 105cpm/sample, was incubated with 2 × 106 cells at 4°C in the presence or absence of a 200-fold excess of unlabeled Ad2 for 30 min. After the cells were washed with ice-cold PBS, cell-associated radioactivity was counted by pelleting the cells. Specific Ad2 binding was determined by subtracting the amount of cell-associated radioactivity obtained in the presence of a 200-fold-excess unlabeled virions. For Ad2 internalization, the cells were incubated at 37°C for various times. Uninternalized virus particles were removed by incubating the samples with 10× trypsin-EDTA (Gibco BRL) at 37°C for 7 min, followed by washing with PBS at 4°C. The remaining radioactivity associated with the cell pellets, attributed to internalized virions, was then counted.
Ad-mediated cell membrane permeabilization assays were performed as previously described (42) with some modifications. Cells were incubated with [3H]choline chloride (2 μCi/ml; NEN Life Science Products, Inc., Boston, Mass.) in complete medium at 37°C for 1.5 h. The cells were then chilled on ice and washed three times with ice-cold virus binding buffer (10 mM HEPES-buffered saline [pH 7.0], 0.2% BSA, 1 mM CaCl2, 1 mM MgCl2, 50 mM NaN3). Twenty micrograms of purified Ad2 (approximately 8 × 1010 particles) was added to 106 cells, and the samples were incubated at 4°C for 1 h. Then the cells were washed once with ice-cold membrane permeabilization buffer (50 mM morpholineethanesulfonic acid [MES]-buffered saline [pH 6.0], 0.2% BSA, 1 mM CaCl2, 1 mM MgCl2, 50 mM NaN3), resuspended to 400 μl in the same buffer, and then incubated at 37°C for 1 h. After the cells were pelleted by centrifugation, the radioactivity in the supernatants was counted. Cells incubated in the absence of Ad2 were used as a control for nonspecific release of [3H]choline. The percentage of [3H]choline release specifically mediated by Ad particles was determined by the following formula: [(release with Ad − release without Ad)/release without Ad] × 100.
Subcellular localization of Ad particles by thin-sectioning electron microscopy.
CS-1 cells (4 × 106) were incubated with Ad2 particles at a multiplicity of infection (MOI) of 5,000 in DMEM containing 10 mM HEPES (pH 7.5) and 0.5% BSA for 60 min at 4°C and then warmed to 37°C for 2 to 15 min. The cells were then washed with ice-cold PBS and then collected by low-speed centrifugation. The samples were then fixed with 2% glutaraldehyde in PBS for 30 min at 22°C, washed three times for 10 to 15 min each, and then washed once with PBS and then with H2O. The cells were then postfixed with 1% osmium tetroxide for 1 h at 22°C, washed with H2O, and then stained with 1% uranyl acetate overnight at 4°C. The cells were dehydrated with serial increasing concentrations of ethanol and proxypropane and then embedded with Epona (Ted Pella, Inc.). Thin sections were stained with 2% uranyl acetate for 1 min and examined with a Philips EM-208 transmission electron microscope. A minimum of 100 virus particles in approximately 20 randomly selected sections were quantitated, and their location, either free in the cytoplasm or inside vesicles, was noted.
RESULTS
Ad entry and infection of CS-1 cells expressing wild-type or mutated αvβ5 integrins.
To identify the mechanism of αvβ5-dependent Ad cell entry, we investigated the role of the β5 integrin cytoplasmic tail (CT). CS-1 cells express endogenous αv integrin subunits but do not produce β3 or β5 integrin mRNA (38). We therefore expressed αvβ5 integrins in CS-1 cells by transfecting them with a cDNA plasmid encoding a wild-type β5 integrin subunit consisting of its ectodomain, transmembrane (TM) anchor, and a full-length β5CT. Alternatively, cells were transfected with cDNA encoding the β5 ectodomain-TM, a β3CT, or a β5-β3CT or a truncated β5CT (Fig. 1). Cells expressing the different β5 integrins were selected by growth in G418 and also by adhesion in tissue culture plates. Each of the transfected cell types expressed similar levels of αvβ5 integrin as determined by flow cytometric analysis (Fig. 2).
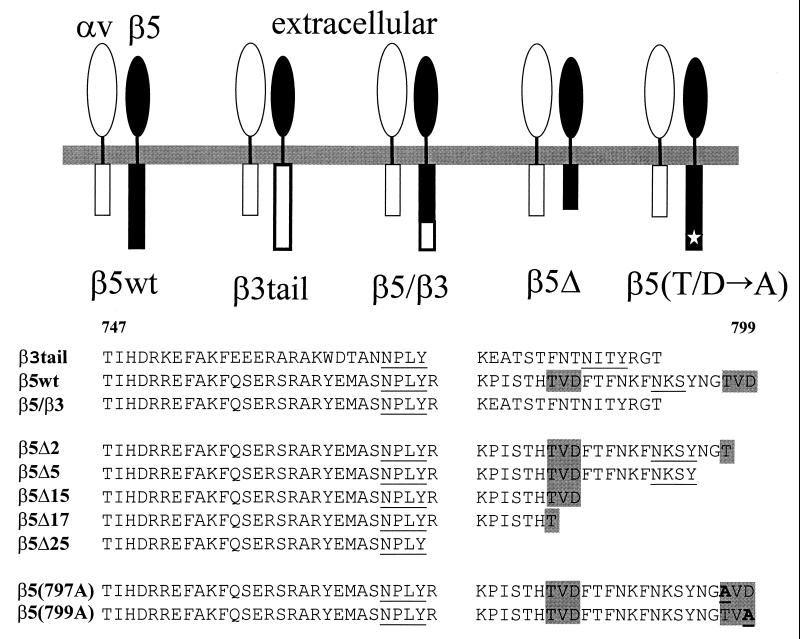
FIG. 1.
Schematic diagram and alignment of the amino acid sequences of the different αvβ5 integrins analyzed in these studies. (Top) αv (open symbols), β5 (black symbols), and β3 (open symbols) represent the individual subunits of an integrin heterodimer. (Bottom) The sequences of the predicted cytoplasmic domains are shown for each individual β5 or β3 integrin (residues 747 to 799). NPXY and NXXY motifs, previously identified as cell migration (17) or internalization sequences (24) are indicated by the underlined sequences. TVD motifs in the β5 integrin are highlighted by the shaded boxes.
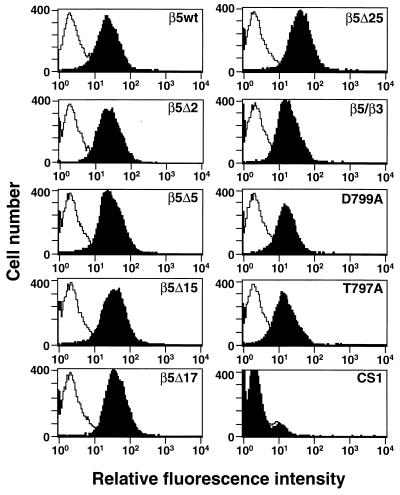
FIG. 2.
Analysis of αvβ5 integrin expression on CS-1 cells by flow cytometry. CS-1 cells were transfected with cDNA constructs encoding the indicated β5 subunits and following drug selection were analyzed for αvβ5 integrin expression using the P1F6 function-blocking monoclonal antibody (black profiles). Control cell samples were incubated with fluorescein isothiocyanate-labeled secondary antibody alone (white profiles).
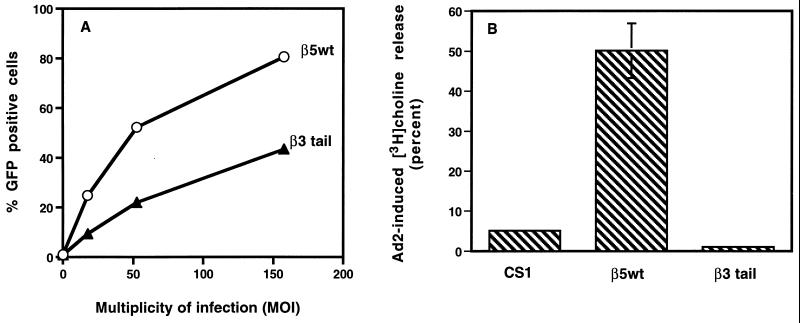
Next, we compared Ad2 interactions with CS-1 cells expressing the wild-type β5 integrin or the chimeric integrin containing a β5 ectodomain and a β3CT (β3 tail). Each cell type showed very similar Ad binding and internalization (data not shown). However, CS-1 cells expressing the wild-type β5 integrin supported two- to fourfold-higher levels of Ad-mediated gene delivery than cells expressing the β3 tail (Fig. 3A). The β5wt-expressing cells also supported 10-fold-higher levels of Ad-induced membrane permeabilization (Fig. 3B). Together, the results of these initial experiments suggest that the CT of the β5 integrin subunit specifically regulates Ad cell entry.
FIG. 3.
Analysis of Ad-mediated gene delivery and membrane permeabilization in CS-1 cells expressing a wild-type integrin αvβ5 or αvβ5-β3CT (β3tail). (A) CS-1 cells expressing β5wt or a β5 integrin with a β3CT were infected at different MOIs with Ad encoding GFP and then analyzed for expression 48 h postinfection by flow cytometry. (B) CS-1 cells and CS-1 expressing β5wt or a β5-β3CT were analyzed for Ad-mediated membrane permeabilization by [3H]choline release as described in Materials and Methods.
Identification of a region in the β5CT that regulates Ad-mediated gene delivery.
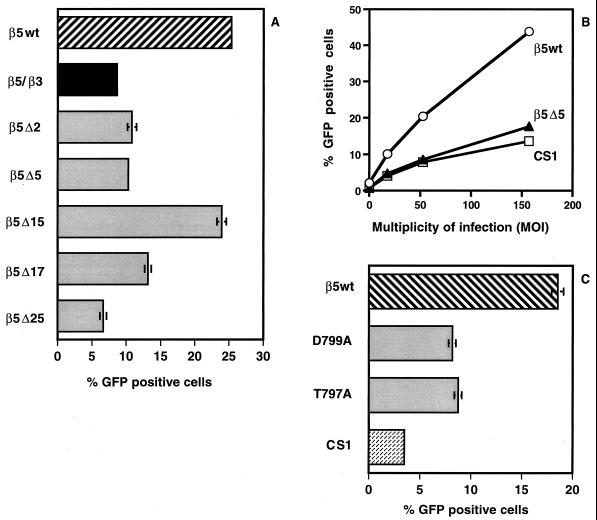
To identify the amino acid residues in the β5CT that promote Ad cell entry, we expressed a number of truncated β5 and chimeric β5-β3 integrins in CS-1 cells and assessed their ability to support Ad-mediated gene delivery. We focused on the C-terminal 25 amino acids of the β5 CT, as this region displays the greatest divergence among the different β integrin subunits (17). Cells expressing a β5-β3 chimeric integrin in which the C-terminal 24 amino acid residues of β5CT were replaced by the corresponding residues in β3CT supported smaller amounts of Ad-gene delivery relative to cells expressing the wild-type β5 integrin (Fig. 4A). These findings suggested that the C terminus of the β5 integrin CT regulates Ad-mediated gene delivery in CS-1 cells. To more precisely identify the amino acids responsible, we examined Ad-mediated gene delivery in cells expressing integrin β5 truncation or site-specific mutants. Removal of as few as two or five amino acid residues from the C terminus of the β5CT significantly decreased Ad-mediated gene delivery, similar to the level seen in CS-1 cells lacking αv integrins (Fig. 4A and B). Interestingly, deletion of an additional 10 residues from the C terminus of the β5CT (β5Δ15) which results in a new C-terminal TVD sequence (residues 782 to 784) identical to the one present on the native C-terminal β5CT (residues 797 to 799) (Fig. 1), restored Ad-mediated gene delivery nearly to the level seen in cells bearing the wild-type β5 integrin (Fig. 4A). Deletion of two additional residues, β5Δ17, significantly decreased Ad-mediated gene delivery. These findings suggested that the C-terminal TVD motif (residues 782 to 784) regulates Ad-mediated gene delivery and that exposure of the “internal” TVD sequence (residues 782 to 784) can partially compensate for the C-terminal TVD deletion.
FIG. 4.
Ad-mediated gene delivery in cells expressing different αvβ5 integrins. Ad-mediated gene delivery to CS-1 cells expressing deletion or chimeric forms of αvβ5 integrins (A and B) or alanine substitutions (C) was measured 48 h postinfection. The MOI for the experiments shown in panels A and C is 50.
To explore further the potential role of the C-terminal TVD motif, we expressed mutants of β5CT with two different alanine substitution (T797A or D799A) in CS-1 cells. As shown in Fig. 4C, the D799A and T979A mutations caused a significant decrease in Ad-mediated gene delivery. Together, these results suggest that the C-terminal TVD motif specifically regulates Ad-mediated gene delivery.
Mutations in β5CT selectively impair Ad-mediated membrane permeabilization.
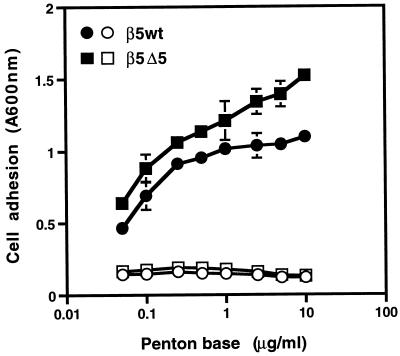
Efficient Ad-mediated gene delivery is mediated in part by the interaction of the virus penton base protein with αv integrins. However, αv integrin-mediated gene delivery regulates both virus internalization and efficient endosome disruption. To examine whether cells expressing mutated β5 integrins were still capable of interacting with the Ad penton base protein, we performed cell adhesion assays. Cells expressing the β5Δ5 integrin exhibited dose-dependent binding to the Ad2 penton base, similar to cells expressing the β5wt integrin, and adhesion required the presence of divalent metal cations (Fig. 5). These results indicated that truncations of β5CT did not impair receptor functional interactions with the Ad penton base protein.
FIG. 5.
Analysis of integrin-mediated cell adhesion to the Ad penton base. Adhesion of CS-1 cells expressing wild-type αvβ5 or a truncated β5CT (β5Δ5) to immobilized Ad2 penton base was measured in the absence (filled symbols) or presence (open symbols) of 10 mM EDTA as described in Materials and Methods.
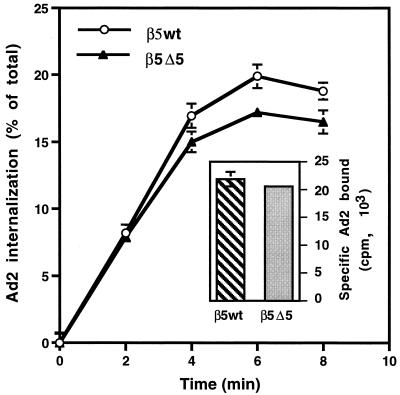
We next asked whether cells expressing β5Δ5CT were capable of supporting Ad internalization. 125I-labeled Ad2 showed very similar levels of binding to cells bearing β5wt or β5Δ5CT. Virions were also capable of being internalized into both cell types (Fig. 6). These findings indicate that deletions of β5CT which reduced Ad-mediated gene delivery did not alter either virus binding or endocytosis.
FIG. 6.
Comparison of Ad binding and internalization in CS-1 cells expressing wild-type or a truncated β5CT. The specific binding of radiolabeled Ad particles to each cell type is shown in the inset. Virus internalization was measured by resistance to trypsin digestion following warming of infected cells to 37°C for various lengths of time.
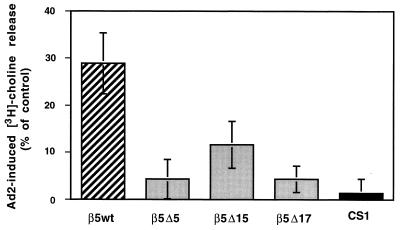
Following internalization, viral particles must escape the early endosome in order to transit to the cell nucleus (21). Ad-mediated cell membrane permeabilization has been used as a surrogate assay for virus penetration of the early endosome. Membrane permeabilization requires reduced pH and is not affected by the presence of 50 mM sodium azide, which inhibits ligand internalization. We therefore tested whether Ad2 particles were capable of permeabilizing CS-1 cells expressing wild-type or mutated β5 integrins. Cells expressing β5Δ5CT had a markedly reduced ability to support Ad-mediated membrane permeabilization compared to cells bearing the wild-type β5 integrin (Fig. 7). Cells expressing the β5Δ15 integrin with a C-terminal TVD sequence also showed reduced Ad-mediated membrane permeabilization activity, although they supported higher permeabilization activity than cells expressing the β5Δ5 or β5Δ17 integrin. Together, these findings indicate that C-terminal β5CT mediates Ad-mediated membrane permeabilization.
FIG. 7.
Ad-mediated membrane permeabilization of CS-1 cells. CS-1 cells expressing wild-type β5 or a truncated β5CT or cells lacking these integrins (CS-1) were loaded with [3H]choline, and Ad-mediated membrane permeabilization was measured at pH 6.0 for 1 h at 37°C in the presence of 50 mM sodium azide to prevent viral internalization. Nonspecific [3H]choline release was determined for each cell type in the absence of Ad.
Electron microscopic visualization of Ad entry into CS-1 cells expressing wild-type β5 integrins or mutant β5CT.
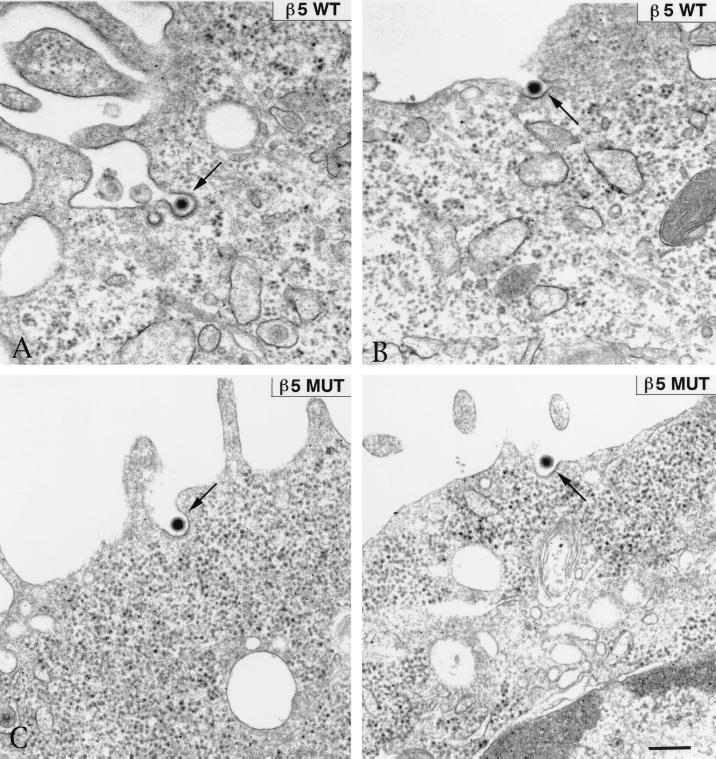
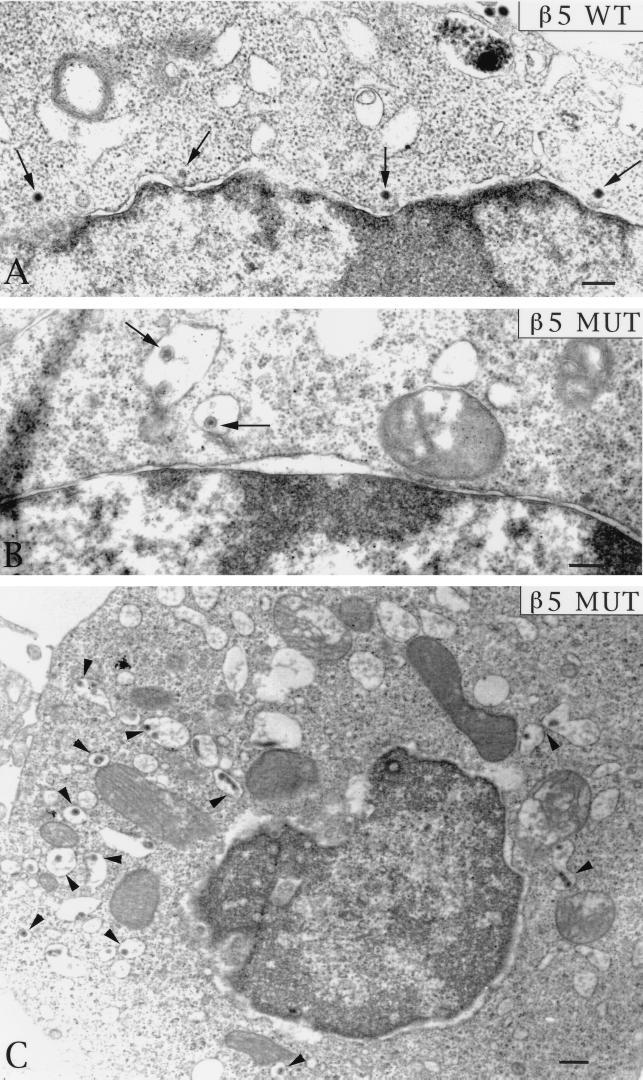
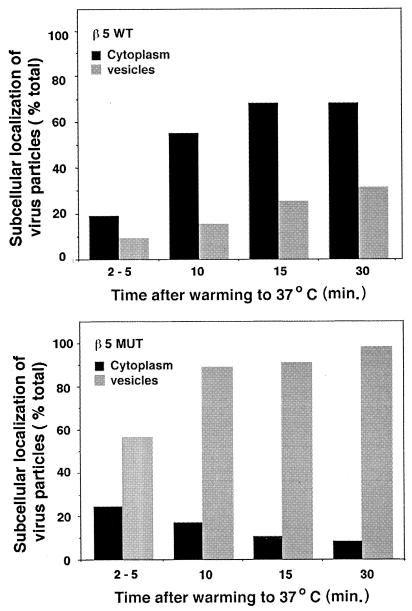
On the basis of these findings, we considered the possibility that cells bearing altered forms of β5CT would internalize virus particles but would not allow Ad penetration of the cell endosome. To examine this possibility, we analyzed Ad entry into CS-1 cells expressing wild-type β5 or β5Δ5 integrin at various times by thin-section transmission electron microscopy. Each cell type was incubated with Ad particles at 4°C for 60 min to allow virus attachment and then warmed to 37°C for various times. The samples were then fixed, processed, and examined by transmission electron microscopy. At approximately 2 to 5 min after cells were heated to 37°C, Ad particles were seen in the process of being internalized into clathrin-coated invaginations in CS-1 cells expressing either wild-type β5 or β5Δ5CT mutant integrin (Fig. 8). These findings were consistent with the similar rates of internalization of 125I-labeled Ad for each cell type (Fig. 7). At later times after warming, Ad capsids were frequently seen in the cytoplasm in close proximity to the nuclear membrane in CS-1 cells expressing β5wt integrin, indicating that Ad particles had successfully achieved endosome rupture (Fig. 9A). In striking contrast, virions inside CS-1 cells expressing β5Δ5CT were rarely seen free in the cytoplasm but instead were found inside large vacuoles (Fig. 9B and C). Quantitative analysis of the electron micrographs substantiated these findings (Fig. 10). A progressive increase of virions located free in the cytoplasm was observed in β5wt-expressing cells. In contrast, thin sections of cells expressing the β5 mutant integrin showed an accumulation of virions inside large vesicles with very few (<10% of total) particles observed free in the cytoplasm (Fig. 10). Thus, the mutations of β5CT which impair membrane permeabilization also inhibit virion escape from cytoplasmic vesicles. These defects likely account for the decreased gene delivery seen in cells expressing altered β5CT integrins.
FIG. 8.
Transmission electron micrographs of Ad internalization into CS-1 cells. Cells expressing a wild-type (WT) or mutant (MUT) β5 integrin with a deleted cytoplasmic tail (β5Δ5). Following attachment of Ad2 particles to cells at 4°C for 60 min, the cells were warmed to 37°C for 5 min and then fixed, embedded, and processed further for thin-sectioning transmission electron microscopy. Bar, 200 nm.
FIG. 9.
Transmission electron micrographs of the late stages of Ad entry in CS-1 cells. (A) Ad particles (indicated by the arrows) were observed free in the cytoplasm in close proximity to the nucleus in β5wt integrin-expressing cells at 10 to 15 min after warming to 37°C. In contrast, Ad particles were located inside large vacuoles in cells expressing a mutant (MUT) β5 integrin (β5Δ5) at 10 to 15 min (B) or at 30 min (C) after warming to 37°C.
FIG. 10.
Morphometric analysis of Ad entry into cells expressing a wild-type β5 integrin or an integrin with a mutated β5 CT. The location of Ad particles, at least 100 per time point in randomly selected cell sections, was determined directly from electron micrographs. Virus particles were identified as being free in the cytoplasm or inside large uncoated vesicles in cells expressing the wild-type β5 (upper panel) or the Δ5β5 integrin (lower panel). Differences in the amount of internalized Ad particles observed at the earliest time point (2 to 5 min) for each cell type are likely due to small variations in the warming times (1 to 2 min). Note that viruses accumulate in vesicles in cells expressing the mutant integrin relative to cells expressing the wild-type receptor. MUT, mutant.
DISCUSSION
Ad entry into susceptible host cells is a multistep process requiring interaction of multiple outer virus capsid proteins with distinct cell receptors. Ad penton base interactions with integrins αvβ3 and αvβ5 facilitate virus internalization via a clathrin-coated pit pathway. Somewhat surprisingly, integrin αvβ5 was previously shown to selectively facilitate Ad-mediated membrane permeabilization and Ad-mediated gene delivery. In light of these findings, it is interesting that αvβ5 rather than αvβ3 integrins predominate in the same tissue infected by Ad in vivo, including the upper respiratory tract (19) and eye (31).
In the current study, we sought to obtain further clues to the mechanism(s) involved in Ad membrane penetration by comparing Ad infection in CS-1 cells expressing altered β5 integrins with those bearing the wild-type receptor. Initial experiments showed that replacement of the C-terminal 25 amino acids of β5CT with those of a related integrin subunit, β3, caused a significant reduction in Ad-mediated gene delivery, thus suggesting that β5CT plays a major role in regulating virus infection. This finding was confirmed by analyzing a number of deletion and substitution mutants of β5CT. Such mutants had significantly impaired Ad-mediated gene delivery. Interestingly, a TVD motif which is present in two separate regions of β5CT, but not in any other β integrin subunit, plays a significant role in virus infection. Deletions or substitutions of the C-terminal TVD sequence negatively impacted Ad infection. While the more internal TVD sequence β5CT could not compensate for small deletions or the alanine substitutions in the C-terminal TVD motif, removal of all of the C-terminal flanking residues adjacent to it restored Ad-mediated gene delivery and partially reconstituted Ad-mediated membrane permeabilization. This finding suggests that exposure of this internal TVD sequence reconstitutes viral function. We cannot rule out the possibility, however, that other amino acid residues flanking the TVD motif or located further upstream of this sequence could influence β5 integrin function. The addition of a VD sequence to the C terminus of β3CT by site-directed mutagenesis (thus creating a TVD motif) did not confer on this integrin the ability to support efficient Ad-mediated gene delivery (data not shown). Thus, further experimentation will therefore be necessary to more precisely define the sequences in β5CT that regulate Ad-mediated gene delivery.
A major finding of the current study was that β5CT is selectively involved in mediating membrane penetration rather than other steps of viral entry. Thus deletions or substitutions of the C-terminal β5CT which reduced Ad-mediated gene delivery did not impair cell adhesion to the penton base or alter virus attachment or internalization into cells. This result is consistent with those of previous mutagenesis studies showing that an NPXY motif, present in β5 and β3 integrin subunits (and in all forms of β5CT used for our studies), regulates cell migration (17) as well as β1-mediated bacterial invasion (24). Instead, alterations of the C-terminal β5CT inhibited Ad-mediated membrane penetration as measured by release of [3H]choline at low pH. Ad-mediated membrane permeabilization is thought to correspond to the ability of Ad to disrupt cell endosomes at low pH. Consistent with this notion, CS-1 cells bearing mutant β5 integrins had numerous virions inside large cytoplasmic vacuoles, whereas cells expressing wild-type β5 integrins contained virions free in the cytoplasm and translocated to the nuclear pore complex (Fig. 9 and 10). These results confirm that the reduced Ad-mediated gene delivery in cells expressing mutant β5CT occurs as a consequence of a defect in virus penetration.
An obvious question arising from these experiments is how does β5CT orchestrate Ad-mediated membrane penetration? One possibility is that β5CT interacts with another host cell factor, perhaps via the C-terminal TVD motif, and this interaction is required for membrane penetration. Although a cofactor for the β5 integrin subunit has not yet been reported, host cell molecules which interact with other integrin subunits have been identified. For example, the NXXY motif mediates the association of β1CT with ICAP-1 protein (5) and between β3CT and endonexin (12). β1 integrin interaction with CD98 (15), an early marker of T-cell activation, has also been shown to enhance cell fusion by Newcastle disease virus (29) and human immunodeficiency virus (28). Moreover, membrane fusion during fertilization involves integrin interactions (35). While the precise mechanism(s) involved in β5-mediated Ad entry requires further investigation, the unanticipated findings reported herein may help to unravel the complex events associated with virus penetration of host cells.
ACKNOWLEDGMENTS
This work was supported by NIH grants EY11431 and HL54342.
We express our gratitude to Shuang Huang for helpful discussions and Shonna Fleck and Pat Mathias for technical assistance.
Footnotes
Manuscript 12502-IMM of The Scripps Research Institute.
REFERENCES
- 1.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Berget S M, Moore C, Sharp P A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumenthal R, Seth P, Willingham M C, Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986;25:2231–2237. doi: 10.1021/bi00356a057. [DOI] [PubMed] [Google Scholar]
- 4.Burcin M M, Schiedner G, Kochanek S, Tsai S Y, O'Malley B W. Adenovirus-mediated regulable target gene expression in vivo. Proc Natl Acad Sci USA. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D D, Wong C, Smith H, Liu J. ICAP-1, a novel β1 integrin cytoplasmic domain-associated protein, binds to a conserved and functionally important NPXY sequence motif of β1 integrin. J Cell Biol. 1997;138:1149–1157. doi: 10.1083/jcb.138.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 7.Cheresh D A, Spiro R C. Biosynthetic and functional properties of an arg-gly-asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 8.Chinnadurai G. Adenovirus 2Ip+locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983;33:759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- 9.Chow L T, Gelinas R E, Broker T R, Roberts R J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 10.Cotten M, Weber J M. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213:494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- 11.Couffinhal T, Kearney M, Sullivan A, Silver M, Tsurumi Y, Isner J M. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum Gene Ther. 1997;8:929–934. doi: 10.1089/hum.1997.8.8-929. [DOI] [PubMed] [Google Scholar]
- 12.Eigenthaler M, Hofferer L, Shattil S J, Ginsberg M H. A conserved sequence motif in the integrin β3 cytoplasmic domain is required for its specific interaction with β3-endonexin. J Biol Chem. 1997;272:7693–7698. doi: 10.1074/jbc.272.12.7693. [DOI] [PubMed] [Google Scholar]
- 13.Everitt E, Meador S A, Levine A S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977;21:199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farishian R A, Whittaker J R. Tyrosine utilization by cultured melanoma cells: analysis of melanin biosynthesis using [14C] thiouracil. Arch Biochem Biophys. 1979;198:449–461. doi: 10.1016/0003-9861(79)90519-8. [DOI] [PubMed] [Google Scholar]
- 15.Fenczik C A, Sethi T, Ramos J W, Hughes P E, Ginsberg M H. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 16.Fender P, Ruigrok R W H, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nature Biotechnol. 1997;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- 17.Filardo E J, Brooks P C, Deming S L, Cheresh D A. Requirement of the NPXY motif in the integrin β3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo. J Cell Biol. 1995;130:441–450. doi: 10.1083/jcb.130.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filardo E J, Deming S L, Cheresh D A. Regulation of cell migration by the integrin β subunit ectodomain. J Cell Sci. 1996;109:1615–1622. doi: 10.1242/jcs.109.6.1615. [DOI] [PubMed] [Google Scholar]
- 19.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greber U F, Webster P, Helenius A, Weber J. The role of the adenovirus protease in virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 21.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 22.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isberg R R, Tran Van Nhieu G. The mechanism of phagocytic uptake promoted by invasion-integrin interaction. Trends Cell Biol. 1995;120:120–124. doi: 10.1016/s0962-8924(00)88962-x. [DOI] [PubMed] [Google Scholar]
- 25.Li E, Stupack D, Bokoch G, Nemerow G R. Adenovirus endocytosis requires reorganization of the actin cytoskeleton mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyberg-Hoffman C, Shabram P, Li W, Giroux D, Aguilar-Cordova E. Sensitivity and reproducibility in adenoviral infectious titer determination. Nat Med. 1997;3:808–811. doi: 10.1038/nm0797-808. [DOI] [PubMed] [Google Scholar]
- 28.Ohgimoto S, Tabata N, Suga S, Nishio M, Ohta H, Tsurudome M, Komada H, Kawano M, Watanabe N, Ito Y. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. J Immunol. 1998;155:3585–3592. [PubMed] [Google Scholar]
- 29.Okamoto K, Tsurudome M, Ohgimoto S, Kawano M, Nishio M, Komada H, Ito M, Sakakura Y, Ito Y. An anti-fusion regulatory protein-1 monoclonal antibody suppresses human parainfluenza virus type 2-induced cell fusion. J Gen Virol. 1997;78:83–89. doi: 10.1099/0022-1317-78-1-83. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy H, Hemler M E. Cloning, primary structure and properties of a novel human integrin β subunit. EMBO J. 1990;9:1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rayner S A, Gallop J L, George A J T, Larkin D F P. Distribution of integrins αvβ5, αvβ3 and αv in normal human cornea: possible implications in clinical and therapeutic adenoviral infection. Eye. 1998;12:273–277. doi: 10.1038/eye.1998.63. [DOI] [PubMed] [Google Scholar]
- 32.Rosenkranz A A, Antonenko Y N, Smirnova O A, Yurov G K, Naroditsky B S, Sobolev A S. Avian adenovirus induces ion channels in model bilayer lipid membranes. Biochem Biophys Res Commun. 1997;236:750–753. doi: 10.1006/bbrc.1997.7040. [DOI] [PubMed] [Google Scholar]
- 33.Seth P, Pastan I, Willingham M C. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985;260:9598–9602. [PubMed] [Google Scholar]
- 34.Seth P, Willingham M C, Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984;259:14350–14353. [PubMed] [Google Scholar]
- 35.Snell W J, White J M. The molecules of mammalian fertilization. Cell. 1996;85:629–637. doi: 10.1016/s0092-8674(00)81230-1. [DOI] [PubMed] [Google Scholar]
- 36.Stupack D G, Li E, Kehler J A, Geahlen R L, Hahn K, Nemerow G R, Cheresh D A. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk-kinase. J Cell Biol. 1999;144:777–787. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teoh G, Chen L, Urashima M, Tai Y-T, Celi L A, Chen D, Chauhan D, Ogata A, Finberg R W, Webb I J, Kufe D W, Anderson K C. Adenovirus vector-based purging of multiple myeloma cells. Blood. 1998;92:4591–4601. [PubMed] [Google Scholar]
- 38.Thomas L, Chan P W, Chang S, Damsky C. 5-Bromo-2-deoxyuridine regulates invasiveness and expression of integrins and matrix-degrading proteinases in a differentiated hamster melanoma cell. J Cell Sci. 1993;105:191–201. doi: 10.1242/jcs.105.1.191. [DOI] [PubMed] [Google Scholar]
- 39.Tomko R P, Xu R, Philipson L. HCAR and MAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Huang S, Kapoor-Munshi A, Nemerow G R. Adenovirus internalization and infection required dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wayner E A, Orlando R A, Cheresh D A. Integrins αvβ3 and αvβ5 contribute to cell attachment to vitronectin but differently distribute on the cell surface. J Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 44.Wickham T J, Nemerow G R. Optimization of growth methods and recombinant protein production in BTI-Tn-5B1-4 insect cells using the baculovirus expression system. Biotechnol Prog. 1993;9:25–30. doi: 10.1021/bp00019a004. [DOI] [PubMed] [Google Scholar]
- 45.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]