Abstract
Prior studies demonstrated that immunization of macaques with simian immunodeficiency virus (SIV) Gag-Pol and Env recombinants of the attenuated poxvirus modified vaccinia virus Ankara (MVA) provided protection from high levels of viremia and AIDS following challenge with a pathogenic strain of SIV (V. M. Hirsch et al., J. Virol. 70:3741–3752, 1996). This MVA-SIV recombinant expressed relatively low levels of the Gag-Pol portion of the vaccine. To optimize protection, second-generation recombinant MVAs that expressed high levels of either Gag-Pol (MVA-gag-pol) or Env (MVA-env), alone or in combination (MVA-gag-pol-env), were generated. A cohort of 24 macaques was immunized with recombinant or nonrecombinant MVA (four groups of six animals) and was challenged with 50 times the dose at which 50% of macaques are infected with uncloned pathogenic SIVsmE660. Although all animals became infected postchallenge, plasma viremia was significantly reduced in animals that received the MVA-SIV recombinant vaccines as compared with animals that received nonrecombinant MVA (P = 0.0011 by repeated-measures analysis of variance). The differences in the degree of virus suppression achieved by the three MVA-SIV vaccines were not significant. Most importantly, the reduction in levels of viremia resulted in a significant increase in median (P < 0.05 by Student's t test) and cumulative (P = 0.010 by log rank test) survival. These results suggest that recombinant MVA has considerable potential as a vaccine vector for human AIDS.
Recent advances in understanding the pathogenesis of simian immunodeficiency virus (SIV) infection of macaques and the creation of SIV-human immunodeficiency virus (HIV) chimeras, have provided valid animal models for the evaluation of vaccines to prevent human AIDS (for review, see references 3, 44, and 53). Protection against experimental infection of nonhuman primates by SIV or SIV-HIV chimeras has been achieved with a variety of vaccine strategies (3, 43), including subunits or recombinant proteins, inactivated whole virus, live vector-based vaccines, and prime-boost combinations. However, the most potent level of protection is provided by vaccination with a genetically related, attenuated, live strain with deleted accessory genes such as nef (2, 18, 20, 37, 54, 65, 73), with the level of protection correlating with the replicative capacity of the vaccine virus (38, 55, 73). The safety of using attenuated live HIV vaccines in humans is a continuing concern and is an issue that cannot be readily addressed. Indeed, the attenuated live SIV vaccines can cause disease in neonatal macaques and in some adult macaques (7, 74, 75) without evidence of reversion. Nevertheless, the efficacy of attenuated live SIV vaccines suggests that an effective AIDS vaccine should mimic the processing, maturation, and presentation of lentiviral antigens during natural infection. Theoretically this could be achieved with recombinant eukaryotic expression vectors based on plasmid DNA, viral vectors, or bacteria (for a review, see references 3, 43, and 69).
AIDS vaccines that are based on recombinant viral vectors such as poxviruses (1, 5, 10, 11, 34, 42, 58, 62), alphaviruses (12, 15, 52), and adenoviruses (14, 64) appear to provide some protection in primate models. An obvious concern with the safety of live recombinant AIDS vaccines is that the viral vector itself should not induce life-threatening infections when administrated to immunocompromised individuals (63). Because of this concern, several highly attenuated poxvirus vector strains with limited pathogenic potential in humans have been developed (51, 57, 58). These include NYVAC (derived from the Copenhagen strain of vaccinia virus), ALVAC (derived from canarypox virus), fowlpox virus, and MVA (modified vaccinia virus Ankara). Studies with macaques immunized with recombinant vaccines based on NYVAC have demonstrated partial protection of macaques from intravenous and mucosal challenge with SIVmac251 (1, 10), and canarypox virus-based HIV-ALVAC recombinant vaccines are currently being evaluated in clinical trials in humans (22, 67).
Whereas NYVAC was derived by the deletion of specific virulence genes from the Copenhagen strain, MVA is an attenuated vaccinia that was derived by over 500 serial passages of the Ankara strain on primary chick embryo fibroblasts (CEF). This passage resulted in multiple genomic deletions totaling approximately 31 kb that altered the ability of MVA to replicate productively in mammalian cells (4, 6, 13, 16, 47, 49) but allowed the efficient expression of inserted recombinant genes (71, 72). MVA was avirulent even in immunosuppressed animals and has an excellent safety record after use in over 120,000 humans in the smallpox eradication campaign (46–48). We previously reported that immunization with a trivalent (Env and Gag-Pol) SIV-MVA recombinant resulted in significant modulation of viremia after subsequent intravenous challenge with highly pathogenic, uncloned SIVsmE660 (34). Rhesus macaques immunized with this MVA-SIV recombinant were better able to control viremia after SIV challenge than macaques immunized with the Wyeth-SIV recombinant (34). Two out of four MVA-SIV vaccinees have remained healthy for over 5 years after challenge. This initial study utilized a modified prime-boost regimen that consisted of multiple priming with MVA recombinant virus followed by a final boost with inactivated whole SIV administered without adjuvant (34). While the final antigen boost had no effect upon neutralizing antibody titers, its role in modulating viremia could not be dismissed. The first generation MVA-SIV recombinant utilized the weaker P7.5 vaccinia virus promoter to express the Gag-Pol antigens rather than the more active synthetic promoter used to express the Env glycoproteins. Thus the recombinant expressed considerably less Gag-Pol than Env antigen. The development of Gag-specific antibodies prior to challenge in macaques that remained healthy suggested that the Gag-specific immune response was a critical component of protective immunity. Since there is little reason to suspect that anti-Gag antibody responses might contribute to the effective control of SIV replication, this observation suggested that Gag-specific antibodies might be a surrogate marker for effective Gag-specific cytotoxic T lymphocytes (CTLs).
The purpose of the present study was dual. First, we wished to evaluate the protective effects of prior immunization with MVA-SIV recombinant vaccines as a sole immunogen without boosting with Env protein. A second goal was to optimize expression of Gag-Pol and to evaluate the relative roles of Gag-Pol and Env antigens in mediating protection from AIDS in the SIV-macaque model. To achieve these goals, we constructed a second generation of MVA recombinants that expressed Env or Gag-Pol precursors of SIV from the efficient early-late vaccinia virus promoter. Three recombinants were constructed that expressed Gag-Pol alone (MVA-gag-pol), Env alone (MVA-env), or Gag-Pol and Env (MVA-gag-pol-env). These second-generation MVA recombinants were then evaluated for expression in vitro, as well as for immunogenicity and protective efficacy in rhesus macaques.
MATERIALS AND METHODS
Vaccinia virus recombinants.
MVA was originally obtained from A. Mayr, Veterinary Faculty, University of Munich, Germany, and virus stocks were routinely propagated in CEF. The SIVsmH-4 sequences encoding Env or Gag-Pol protein precursors were inserted into the PmeI site of the MVA transfer plasmids under the control of the efficient synthetic early-late vaccinia virus promoter (17, 21) and are subsequently referred to as pLW22env and pMC03gag-pol, respectively. The expression cassette of pLW22env was flanked by the sequences for homologous recombination into the site of deletion II (49) in the MVA genome and contained the Escherichia coli β-galactosidase gene driven by the vaccinia virus early-late promoter (P7.5). The expression cassette of pMC03gag-pol was flanked by sequences homologous to deletion III in the MVA genome and contained the P7.5-GUS (E. coli β-glucuronidase) operon. To generate recombinant MVA virus, monolayers of nearly confluent CEF were infected with 0.05 PFU of MVA per cell in six-well plates and were transfected with 10 μg of plasmid DNA using the PerFect Transfection Kit (Invitrogen, San Diego, Calif.) at 90 min after infection, as recommended by the manufacturer. At 48 h after infection, the cells were harvested and processed as described previously (68). MVA recombinants expressing the SIVsmH-4 env (MVA-env) and gag-pol (MVA-gag-pol) genes were selected by β-galactosidase or GUS screening in the presence of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyronoside) or X-Glu (5-bromo-4-chloro-3-indolyl-β-d-glucuronide) (Gold BioTechnology, St. Louis, Mo.), respectively. Expression of SIV Env and Gag-Pol proteins was demonstrated by immunostaining of recombinant plaques with sera from SIV-infected macaques and Western blot analysis of CEF and BS-C-1 cells infected with selected recombinant clones of MVA. After five consecutive rounds of plaque purification, recombinant viruses were amplified in CEF and were purified by centrifugation through a 36% sucrose cushion. The titers of these stocks were determined by immunostaining of infected CEF with SIV-specific monkey sera. The double recombinant MVA-gag-pol-env was selected after transfection of transfer plasmid pMC03gag-pol into CEF infected with MVA-env recombinant.
Expression of SIV proteins by MVA recombinant viruses.
To assess in vitro expression of SIV proteins by MVA-SIV recombinant viruses, CEF or BS-C-1 cell monolayers were infected with 10 PFU of nonrecombinant or recombinant MVA viruses (in triplicate) per cell in six-well tissue culture plates. After 24 or 48 h, the medium and infected cells were harvested separately. The medium was clarified by low-speed centrifugation and filtration through a 0.45-μm-pore-size Millex-HV filter unite (Millipore, Bedford, Mass.). Cells were harvested in phosphate-buffered saline, were pelleted by centrifugation, and were solubilized on ice for 10 min in a 10 mM Tris-HCl buffer (pH 7.5) that was supplemented with 1% Triton X-100 (Fluka Chemical Corp., Ronkonkoma, N.Y.), 1% sodium deoxycholate (Sigma, St. Louis, Mo.), and 150 mM NaCl. These samples were tested for reverse transcriptase (RT) activity. SIV p27 capsid (CA) protein concentrations were measured by SIV Core Antigen Capture Assay (Coulter Corporation, Miami, Fla.). To prepare samples for radioimmunoprecipitation assay, CEF or BS-C-1 cells were infected with MVA or MVA-SIV recombinant viruses and were pulsed with 35S-labeled cysteine and methionine (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) for 24 h, beginning 2 h after infection. Infected cells and media were collected as described above and were immunoprecipitated with sera from an SIV-infected macaque or with anti-gp130 recombinant immunoglobulin G (IgG) antibody IgG1-201 (23) by using GammaBind G Sepharose beads (Amersham Pharmacia Biotech, Inc.) as previously described (21).
Cells and viruses.
Monkey BS-C-1 and CEF were grown in minimal essential medium with NEAA supplemented with 10% fetal calf serum (FCS). CEMx174 cells used for SIV rescue were grown in RPMI 1640 supplemented with glutamine and 10% FCS. Peripheral blood mononuclear cells (PBMC) were separated by centrifugation through Lymphocyte Separation Medium (ICN Biomedicals, Inc., Aurora, Ohio) and were maintained for 4 days in RPMI 1640 supplemented with 10% interleukin-2 (IL-2) and 5 μg of phytohemaglutinin (PHA) per ml, and the PBMC were subsequently maintained in a similar medium lacking PHA.
The challenge virus was a cell-free virus stock of uncloned SIVsmE660 (25) which had been passaged in pig-tailed macaque PBMC and titrated for infectivity by intravenous inoculation of 10-fold serial dilutions into rhesus macaques to determine the dose at which 50% of macaques are infected (MID50). This virus was highly pathogenic and highly related, but not identical, to the molecular clone SIVsmH-4 used to construct the recombinant vaccinia viruses.
Animals and immunization schedule.
Twenty-four juvenile, simian type D retrovirus- and simian T-lymphotropic virus type 1-seronegative rhesus macaques (Macaca mulatta) were immunized intramuscularly with 108 PFU of recombinant MVA-gag-pol (group A, n = 6), MVA-env (group B, n = 6), MVA-gag-pol-env (group C, n = 6), or nonrecombinant MVA (group D, n = 6) at 0, 4, 16, and 28 weeks. The animals were bled periodically throughout the immunization protocol, and plasma samples were assayed for SIV-specific antibody by enzyme-linked immunosorbent assay (ELISA) and neutralizing antibody. The animals were then challenged intravenously 4 weeks later with 50 MID50 of the SIVsmE660 virus stock described earlier (25, 32). Blood and plasma samples were collected biweekly before challenge, on the day of challenge, and subsequently at 3, 7, 10, and 14 days; 3, 4, 6, and 8 weeks; and monthly thereafter.
Quantitative RT-PCR of plasma SIV RNA.
A plasma SIV RNA viral load real-time quantification assay based on the Applied Biosystems Prism 7700 Sequence Detection System (70) was adapted for use with SIVsmE660. Plasma samples for analysis were collected using EDTA as an anticoagulant and were stored at −70°C until analysis. Viral RNA was isolated from macaque plasma samples by using the QIAamp Viral RNA Kit (Qiagen, Inc., Santa Clarita, Calif.) and were treated with amplification-grade DNase I (Life Technologies, Gaithersberg, Md.) as recommended by the manufacturer. Replicate aliquots of the test RNA were subjected to RT-PCR using a two-step, two-enzyme protocol with SIV-Gag consensus primers S-GAG03 and S-GAG04 and SIV-Gag consensus TaqMan probe P-SUS-05 (70). The TaqI-XbaI DNA fragment (2,453 bp) from the gag coding region derived from the SIVsmH-4 sequence was cloned between HindIII and XbaI sites of the plasmid pTRI-19(polyA), and RNA transcribed with T7 RNA-polymerase from this template was used as standard control template. pTRI-19(polyA) plasmid containing a polyA30 insert in the multiple cloning site was constructed on the base of triple tandem promoter cloning vector TRIPLEscript (Ambion, Austin, Tex.) and was kindly provided by K. Suryanarayana and J. D. Lifson (Laboratory of Retroviral Pathogenesis, National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, Md.). The polyA-tailed full-length RNA control template was purified on oligo(T) agarose and was quantified by measurements of A260 based on the calculated extinction coefficient for the transcript sequence. A serial fivefold dilution series of the standard RNA template was assayed in duplicate to generate a standard curve for each assay. RT-PCR was performed for each plasma sample in triplicate, including one control reaction for potential DNA contamination processed without the addition of RT. The assay results were normalized to the volume of plasma extracted and were expressed as SIV RNA copy equivalents per ml of plasma, as described for HIV type 1 (59, 60). Interassay variation had a coefficient of less than 25%.
Evaluation of parameters of SIV infection by flow cytometry and SIV isolation.
Following virus challenge, a comprehensive virological analysis was performed on sequential plasma, PBMC, and lymph node biopsy specimens. Lymphocyte subsets (CD4+, CD8+, CD2+, and CD20+) were evaluated by fluorescence-activated cell sorting on whole heparinized blood samples by using methods previously described (33). Virus rescue was conducted by stimulation of 5 × 106 PBMC with 10% IL-2 and PHA (5 μg/ml) in RPMI 1640 media supplemented with glutamine, penicillin-streptomycin, and 10% FCS for 4 days, followed by cocultivation with an equal number of CEMx174 cells (25, 36). Virus rescue from lymph nodes was accomplished by disruption of fresh lymph node tissue into a single cell suspension by gentle rubbing through a cell strainer (Falcon) and by stimulation of 5 × 106 lymph node cells with PHA and IL-2 as was done with PBMC, followed by cocultivation with CEMx174 cells. The analyses included lymph node histopathology with in situ hybridization analysis for expression of SIV RNA as previously described (33, 35).
Electronmicroscopy of BS-C-1 cells infected with recombinant viruses.
Monolayers of BS-C-1 cells in Lab-Tech chamber slides (Nunc, Inc., Naperville, Ill.) were inoculated with 10 PFU of MVA-SIV recombinant viruses per cell. After 24 h, the cells were fixed in 2% glutaraldehyde-Milloning's phosphate buffer (pH 7.35) (Tousimis Research Corp., Rockville, Md.) and then were fixed in 1% osmium tetroxide, dehydrated in a graded alcohol series, and embedded in epoxy resin. Thin sections were cut and stained with uranyl acetate and lead citrate. Samples were examined on a LEO transmission electron microscope at 80 kV (Courtesy of Jackie Muller, Food and Drug Administration, Rockville, Md.).
Assessment of humoral immune responses.
Humoral responses to SIV antigens were measured by ELISA by using Costar 3690 polystyrene plates (Corning Inc., Corning, N.Y.) coated by SIVsmH-4 gp130 or Gag-Pol proteins. The selective interaction of a lectin GNA (from Galanthus nivalis) with glycoproteins of SIV (45) was used to coat plates with SIVsmH-4 gp130. Serum-free medium from the CHO-SIVsmH-4 gp130 (clone AD5) cell line (26, 36, 61) was used as a source of recombinant gp130 glycoprotein (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health). To prepare plates for ELISA, 50 μl of GNA lectin (Sigma) solution in phosphate-buffered saline (10 μg/ml) was added to each well and was incubated at 4°C overnight. ELISA plates were then blocked with Superblock in Tris-buffered saline (Pierce, Rockford, Ill.) as recommended by the manufacturer, and an optimized dilution of recombinant SIVsmH-4 gp130 was added for 3 h at 37°C. Unbound proteins were removed by washing three times with Tris-buffered saline containing 0.05% Tween 20, and dilutions of macaque plasma in 10% Superblock were added for 2 h at 37°C. The bound macaque antibodies were detected with an alkaline-phosphatase-conjugated goat antibody specific for the Fc fragment of human IgG (Pierce) and developed with the p-nitrophenyl phosphate substrate (Sigma) as previously described (23). Endpoint titers were determined as the reciprocal of the highest serum dilution that gave an optical absorbance value of two standard deviations above the average values obtained with negative control sera. For quantitative measurement of Gag-Pol-specific antibodies, the plates were coated directly with gradient-purified, disrupted SIV-like particles produced in BS-C-1 cells infected with MVA-gag-pol recombinant virus. Medium from the BS-C-1 cells infected with MVA was used as diluent for macaque plasmas to prevent the binding of vaccinia-virus-specific antibodies to MVA proteins from contaminating the viruslike particle preparation. Vaccinia-virus-specific ELISA antibody titers were determined by assays described earlier (34).
Statistical methods.
Repeated measures analysis of variance (ANOVA) were used to test for differences in all the outcomes between groups receiving different immunization regimens. Where appropriate, analyses were performed on log-transformed data. Analyses of residuals showed distributions consistent with normality. Viral load data below the level of detection of the assay were assigned values of 300 copy eq/ml for repeated measures ANOVA. These data were also analyzed by a method for left-censored log-normally distributed data at individual times, which yielded slightly higher P values than ANOVA at those times but did not affect any conclusions about statistical significance. Statistical calculations were carried out using the SAS System for Windows (Release 6.12; SAS Institute Inc., Cary, N.C.). Cumulative survival rates for groups of vaccinated and control macaques were analyzed with Kaplan-Meier survival curves (39).
RESULTS
Evaluation of expression of viral antigens by new MVA recombinants.
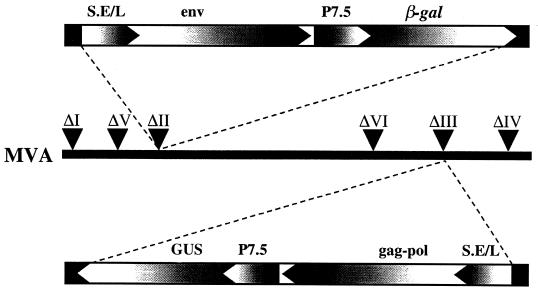
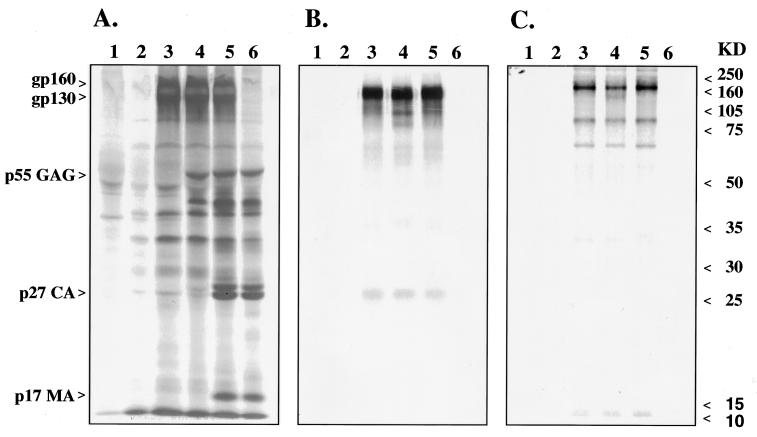
Three MVA recombinants (MVA-gag-pol, MVA-env, and MVA-gag-pol-env) were constructed as depicted in Fig. 1. These recombinants differed from the previous MVA-SIV recombinant (34) in that both Env and Gag-Pol precursors were expressed under control of the highly effective, synthetic, early-late vaccinia virus promoter. In addition, gene cassettes for the present recombinants were inserted into two separate sites within the MVA genome (deletions II and III). This contrasted with the sole use of deletion III in the original MVA-SIV recombinant. Selected clones of recombinant viruses were propagated and characterized by immunoprecipitation of expressed SIV proteins from BS-C-1 cells infected with MVA-SIV recombinants. As shown in Fig. 2A, BS-C-1 cells infected with MVA-env, the first generation MVA-SIV, and MVA-gag-pol-env recombinant viruses produced similar amounts of Env proteins (Fig. 2A, lanes 3, 4, and 5). Immunoprecipitation with an SIV Env-specific macaque monoclonal antibody confirmed the identities of the Env precursor gp160 (Fig. 2C), and cleaved gp120 envelope glycoprotein (Fig. 2B). As expected from the use of the stronger promoter for Gag-Pol expression, the level of expression of Gag proteins in MVA-gag-pol and MVA-gag-pol-env recombinant viruses was more robust than that observed with the first-generation MVA-SIV recombinant (Fig. 2A, lanes 6, 5, and 4, respectively). The processing of the Gag-Pol precursors also differed from that of the original recombinant. Culture supernatants of cells infected with the first-generation recombinant contained mainly processing intermediates of Gag-Pol and Gag precursors (Fig. 2A, lane 4). In contrast, significant amounts of processed SIV Gag proteins (capsid CA p27 and matrix MA p17) were observed in cells infected with MVA-gag-pol (lane 6) or MVA-gag-pol-env (lane 5), consistent with efficient maturation of SIV pseudovirions.
FIG. 1.
Representation of recombinant MVA virus genomes. Insertion of the expression cassette consisting of the sequences coding the SIVsmH-4 env precursor regulated by the synthetic early-late promoter (S.E/L) and the β-gal gene regulated by the vaccinia virus early-late P7.5 promoter is indicated by dashed lines to the site of deletion II within the MVA genome. Insertion of a cassette containing the sequences coding the SIVsmH-4 gag-pol precursor regulated by the S.E/L promoter and the GUS gene regulated by the P7.5 promoter is indicated by dashed lines to the site of deletion III. The directions of promoters and open reading frames are indicated.
FIG. 2.
Expression of SIV proteins in monkey cell line BS-C-1 infected with recombinant MVA viruses. Radioimmunoprecipitation of viral proteins from culture supernatants (panels A and B) and cell extracts (panel C) of BS-C-1 monkey cells infected with different MVA-SIV recombinant viruses. Extracts shown in panel A were immunoprecipitated with plasma from an SIV-infected macaque, and extracts shown in panels B and C were immunoprecipitated with a macaque SIVsm-gp120-specific monoclonal antibody, IgG-201 (23). Lanes 1, immunoprecipitation from mock-infected cells; lanes 2, cells infected with nonrecombinant MVA; lanes 3, recombinant MVA-env; lanes 4, original MVA-SIV recombinant virus; lanes 5, MVA-gag-pol-env; lanes 6, MVA-gag-pol. Molecular mass markers are listed at the right in kilodaltons.
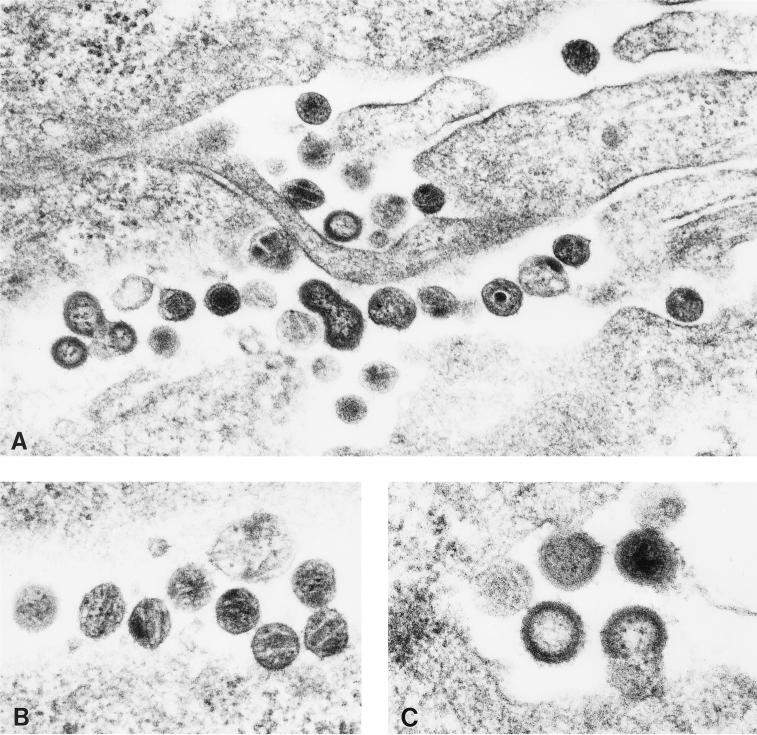
To compare the levels of SIV-gag-pol proteins expressed in mammalian cells, culture supernatants and cytoplasmic extracts were assayed for RT activity and SIV p27 protein. As shown in Table 1, higher levels of RT activity and antigen were detectable in BS-C-1 cells infected with the MVA-gag-pol and MVA-gag-pol-env recombinant viruses than in the cells infected with the original recombinant. The amount of CA protein produced by cells infected with both newer recombinant viruses expressing Gag-Pol was comparable to that produced by permissive cells infected with SIV. As expected, RT activity and CA protein were not detectable in supernatants of cells infected with MVA or MVA-env recombinant virus. To confirm the production of SIV-like particles, electron microscopy was performed on BS-C-1 cells infected for 24 h with the MVA-gag-pol-env recombinant virus. As shown in Fig. 3, numerous SIV-like particles were observed at all stages of virion maturation. Such virus-like particles were not observed in cells infected with the original MVA-SIV recombinant virus (data not shown), consistent with lower levels of expression and processing of Gag-Pol in the original recombinant.
TABLE 1.
Production of SIV RT and CA protein in culture media of BS-C-1 cells infected with MVA-SIV recombinant virusesa
| MVA recombinant | RT activity (103 cpm)b | CA antigen (ng/ml)b |
|---|---|---|
| MVA-SIV (Original) | 54 ± 6 | 16 ± 1 |
| MVA-gag-pol-env | 1,683 ± 27 | 870 ± 12 |
| MVA-gag-pol | 1,706 ± 92 | 870 ± 12 |
BS-C-1 cells were infected with 10 PFU of indicated MVA-SIV recombinant per cell, and samples were collected as described in Materials and Methods.
Results are normalized to 106 infected cells and are presented as average values of three independent experiments ± standard derivations.
FIG. 3.
Production of SIV-like particles from BSC-1 cells 24 h after infection with the MVA-gag-pol-env recombinant virus. Electron micrograph of infected cells with immature and mature SIV-like particles (panel A; magnification, ×54,000) and detailed pictures of mature VLPs (panel B; magnification, ×85,000) and stages of VLPs budding from the cell surface and maturing (panel C; magnification, ×68,000).
Immunogenicity of MVA-SIV recombinant viruses in rhesus macaques.
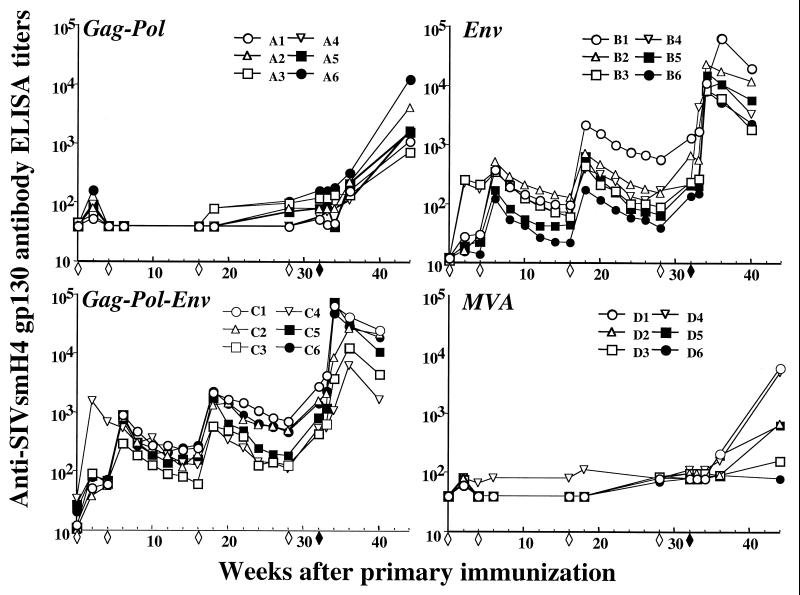
A cohort of 24 macaques was immunized intramuscularly with 108 PFU of MVA-gag-pol (group A), MVA-env (group B), MVA-gag-pol-env (group C), or nonrecombinant MVA (group D) at 0, 1, 4, and 7 months and were challenged 4 weeks later with uncloned SIVsmE660. Antibody responses to vaccinia virus and SIV antigens were monitored in sequential plasma samples by ELISA (Fig. 4) and neutralization assays (shown in more detail in reference 56). As shown in Fig. 4, immunization with the Env antigen (groups B and C) resulted in Env-specific antibody production after the second immunization. Levels of antibody peaked 2 weeks after the immunization and subsequently declined. Each subsequent immunization resulted in a boost in the antibody titer and a moderate incremental increase in the peak titer. As expected, macaques immunized with MVA-gag-pol or MVA did not develop Env-specific antibody. A similar pattern was observed with respect to the Gag-specific responses in macaques immunized with MVA-gag-pol and MVA-gag-pol-env (data not shown). Vaccinia-virus-specific ELISA antibody titers of all animals (data not shown) peaked by the second immunization. As shown in Table 2, neutralizing antibodies generated to the vaccine strain SIVsmH-4 were observed in plasma from macaques immunized with either MVA-env or MVA-gag-pol-env. However, there was no detectable neutralization of the challenge virus SIVsmE660 on the day of challenge. As expected, none of the macaques immunized with MVA-gag-pol or MVA exhibited any neutralizing activity to either the vaccine or challenge virus (data not shown). CTL responses were not monitored in this cohort since none of these macaques expressed major histocompatibility complex (MHC) class I alleles with known associated peptide epitopes that could be used in CTL monitoring, and prior immunization with recombinant MVA prevented the use of target cells infected with recombinant vaccinia viruses for functional killing assays.
FIG. 4.
Anti-SIVsmH-4 gp130 ELISA antibody titers in sera of immunized and control macaques. Six macaques per group were inoculated four times (open diamonds) with recombinant or nonrecombinant MVA and challenged 4 weeks later with SIVsmE660 (filled diamonds). Serial dilutions of plasma were incubated with recombinant SIVsmH-4 gp130 bound to microtiter plates treated by lectin from G. nivalis. End-point titers were defined as the reciprocal of the highest sera dilution that gave an optical absorbance at least two standard deviations greater in value than average values obtained with negative control sera.
TABLE 2.
Neutralizing antibody titers to the vaccine (SIVsmH-4) and challenge (SIVsmE660) viruses on the day of challenge
| Vaccine | Macaque no. | Reciprocal neutralizing dilution titer for:
|
|
|---|---|---|---|
| SIVsmH-4 | SIVsmE660 | ||
| MVA-env | B1 | <30 | <30 |
| B2 | 286 | <30 | |
| B3 | <30 | <30 | |
| B4 | <30 | <30 | |
| B5 | <30 | <30 | |
| B6 | 86 | <30 | |
| MVA-gag-pol-env | C1 | 274 | <30 |
| C2 | <30 | <30 | |
| C3 | <30 | <30 | |
| C4 | 107 | <30 | |
| C5 | 85 | <30 | |
| C6 | 88 | <30 | |
MVA-SIV immunization did not prevent infection with SIV.
Following challenge with SIVsmE660, an increase in the titer of SIV-specific antibody titers was observed with anamnestic Env responses observed in macaques immunized with MVA-env and MVA-gag-pol-env (Fig. 4). These antibodies were capable of neutralizing the vaccine virus but not the challenge virus, as is described in detail elsewhere (56). A similar type of anamnestic Gag-specific antibody response was observed in macaques immunized with MVA-gag-pol or MVA-gag-pol-env (data not shown). These anamnestic immune responses indicated that each of the macaques became infected following SIV challenge. Indeed, as shown in Table 3, SIV was isolated from PBMC of all 24 macaques. However, virus isolation was transient from PBMC samples of four macaques (A1, A3, B1, and C4). Isolation of virus was not successful on samples collected from these four animals after 12 weeks postinoculation, consistent with extremely low viral loads in these animals. Single cell suspensions of lymph node mononuclear cells were also evaluated for infectious virus at 1, 2, and 4 weeks after challenge. SIV was recovered from each of these cultures (data not shown).
TABLE 3.
Summary of virus isolation results from PBMC of MVA-immunized macaques
| Immunogen | Macaque | Week post-SIV Inoculation
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 20 | 28 | 36 | 44 | ||
| MVA-gag-pol | A1 | + | + | + | + | − | − | − | − | − | − | − | − |
| A2 | + | + | + | + | + | + | + | + | + | + | + | + | |
| A3 | + | + | + | − | − | + | + | − | − | − | − | − | |
| A4 | + | + | + | + | + | + | + | + | + | + | + | + | |
| A5 | + | + | + | + | + | + | + | + | + | + | + | + | |
| A6 | + | + | + | + | + | + | + | + | + | + | + | + | |
| MVA-env | B1 | + | + | + | + | − | + | + | − | − | − | − | − |
| B2 | + | + | + | + | + | + | + | + | + | + | + | + | |
| B3 | + | + | + | + | + | + | + | + | + | + | + | + | |
| B4 | + | + | + | + | + | + | + | + | + | + | + | + | |
| B5 | + | + | + | + | + | + | + | + | + | + | + | + | |
| B6 | + | + | + | + | + | + | + | + | + | + | + | + | |
| MVA-gag-pol-env | C1 | + | + | + | + | + | + | + | + | + | + | + | + |
| C2 | + | + | + | + | + | + | + | + | + | + | + | + | |
| C3 | + | + | + | + | + | + | + | + | + | + | + | + | |
| C4 | + | + | + | + | + | + | − | − | − | − | − | − | |
| C5 | + | + | + | + | + | + | + | + | + | + | + | + | |
| C6 | + | + | + | + | + | + | + | + | + | + | + | + | |
| MVA | D1 | + | + | + | + | + | + | + | + | + | + | + | + |
| D2 | + | + | + | + | + | + | + | + | + | + | Dead | ||
| D3 | + | + | + | + | + | + | + | + | + | Dead | |||
| D4 | + | + | + | + | + | + | + | + | + | + | + | Dead | |
| D5 | + | + | + | + | + | + | + | + | Dead | ||||
| D6 | + | + | + | + | + | + | + | + | + | + | + | + | |
Immunization significantly modifies viral load following SIV challenge.
The levels of plasma viral RNA were assessed sequentially throughout the course of infection. Figure 5 depicts the plasma viremia for each animal of the four immunization groups. Two of the MVA control macaques (D6 and D3) had high plasma viral loads persistently until they were euthanized at 16 and 20 weeks, respectively. Neither of these animals developed SIV-specific antibody. One animal in this group was lost to the study due to an anesthetic death at 16 weeks postchallenge. The other three macaques in the control group demonstrated high levels of persistent viremia; two animals were euthanized due to AIDS at 32 weeks (D2) and 60 weeks (D5) post-SIV challenge, and one animal is still alive. Although the levels of plasma viremia overlapped the levels in MVA-SIV-immunized macaques, the plasma viral RNA levels were generally higher in the MVA control macaques than in macaques immunized with MVA-SIV recombinants.
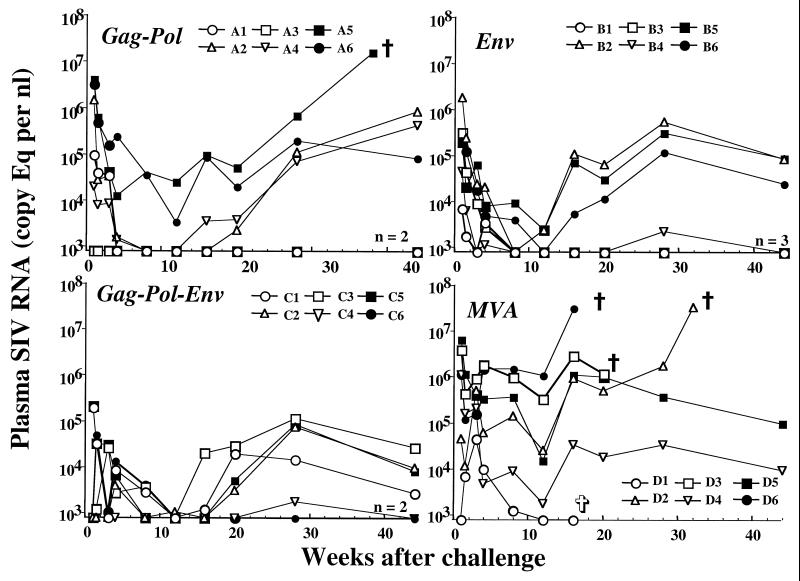
FIG. 5.
Plasma viral load in immunized and control macaques after challenge with uncloned SIVsmE660. Sequential levels of plasma viral RNA over the first 45 weeks after SIV challenge are shown for animals immunized prior to challenge with MVA-gag-pol, MVA-env, MVA-gag-pol-env, or MVA. Plasma viral load was determined by real-time RT-PCR as described in Materials and Methods. Results are expressed as number of copies of SIV genomic RNA equivalent per milliliter of plasma. Plasma samples having values under assay threshold sensitivity were given a value of 800 copy eq/ml. Animals sacrificed because of clinical manifestations of AIDS (ἀ) and the animal that died of causes unrelated to AIDS (✞) are labeled.
Considerable variability in plasma viremia was observed in the SIV-immunized animal groups. A proportion of animals in each MVA-SIV-immunized group (MVA-gag-pol, n = 2; MVA-env, n = 3; and MVA-gag-pol-env, n = 2) exhibited sustained control of plasma viremia to below the limits of detection of the assay. These same macaques became virus culture negative by 12 weeks postchallenge (Table 3). Two macaques immunized with the MVA-gag-pol vaccine demonstrated sustained control of viremia (A1 and A3), two others controlled viremia transiently (A2 and A4), and two exhibited sustained high levels of viremia (A5 and A6). A similar pattern was observed in macaques immunized with the MVA-env vaccine. Three MVA-env-immunized macaques demonstrated sustained control of viremia (B1, B3, and B4), and three animals exhibited persistent moderate viremia (B2, B5, and B6). As in the other two groups, two animals immunized with MVA-gag-pol-env controlled viremia well (C4 and C6), and the four animals exhibited persistent but moderate viremia. In each immunization group, there were a number of animals with transient control of viremia and subsequent increasing levels.
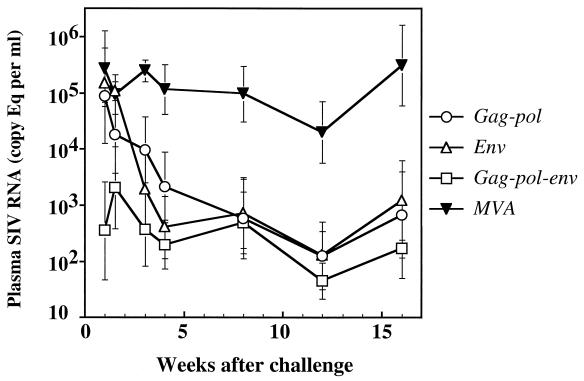
To determine whether there was a significant reduction in plasma viremia in MVA-SIV vaccinees, geometric mean plasma viral RNA levels were compared over the first 16 weeks as shown in Fig. 6. After this time point, two animals in the control group were lost to follow-up, making statistical analyses less meaningful. Macaques in each of the MVA-SIV groups had lower virus loads than the nonrecombinant MVA group. Statistical analyses of plasma virus load from weeks 1 to 16 in the four groups demonstrated that the difference between the control macaques (group D) and the macaques immunized with SIV antigens was highly significant (P = 0.0011 by ANOVA). However, significant differences in SIV plasma viral load were not observed between the three SIV-immunized groups (P = 0.30).
FIG. 6.
Geometric means of plasma viral load values for groups of immunized and control macaques after challenge with uncloned SIVsmE660. Significant reductions in geometric mean plasma viral load were observed in macaques immunized prior to SIV challenge with recombinant MVA expressing SIVsmH-4 proteins Gag-Pol, Env, or Gag-Pol-Env as compared to those immunized with nonrecombinant MVA (repeated measures ANOVA, P = 0.0011). The error bars represent the standard errors of the means of duplicated measurements for six macaques in each group.
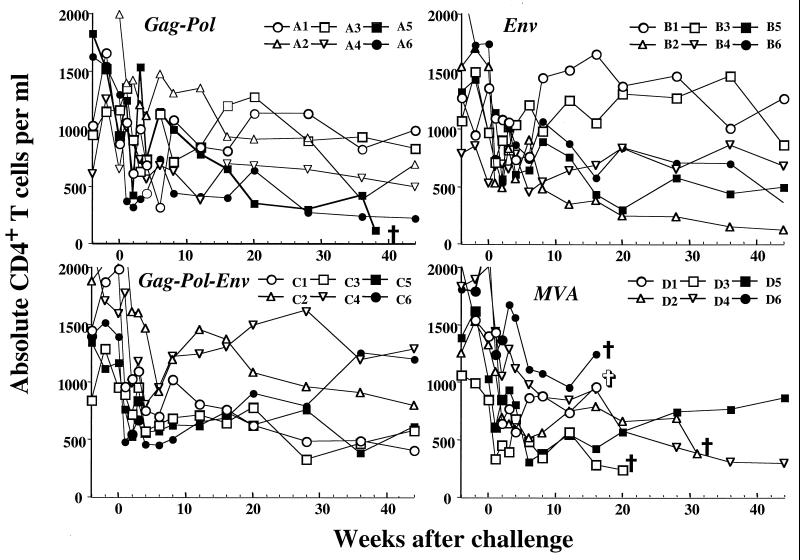
Control of viremia correlates with stability of CD4+ lymphocytes.
The absolute numbers of circulating CD4+ lymphocytes were followed sequentially in each of the macaques as depicted in Fig. 7. As observed in previous studies, loss of CD4+ lymphocytes was not an accurate predictor of rapid disease progression. Only one of the macaques that progressed rapidly to AIDS exhibited a significant decline in the absolute number of circulating CD4+ lymphocytes (D3). Peripheral CD4+ T-cell numbers tended to decrease over the course of infection in the majority of the macaques. However, long-term maintenance of CD4+ lymphocyte numbers was observed in some of the animals in each of the groups immunized with MVA-SIV recombinant viruses. A total of seven macaques have maintained CD4+ lymphocyte numbers within normal limits, exhibit low plasma viremia, and have no other clinical evidence of AIDS-related disease (lymphadenopathy, splenomegaly, or thrombocytopenia). Two of these were immunized with MVA-gag-pol, three were immunized with MVA-env, and two were immunized with MVA-gag-pol-env. Macaques which have maintained relatively normal levels of peripheral CD4+ T cells also exhibited low plasma viremia (A1, A3, B1, B3, B4, C4, and C6) (Fig. 7). Notably, none of the macaques immunized with the MVA nonrecombinant have remained healthy. One of these macaques has survived but exhibits moderate plasma viremia, moderate CD4 depletion, and lymphadenopathy.
FIG. 7.
Peripheral blood CD4+ T-lymphocyte levels in immunized and control macaques infected with uncloned SIVsmE660. Sequential levels of peripheral CD4+ T cells over the first 45 weeks after SIV challenge are shown for animals immunized prior to challenge with MVA-gag-pol, MVA-env, MVA-gag-pol-env, or MVA. Animals sacrificed because of clinical manifestations of AIDS (ἀ) and the animal that died of causes unrelated to AIDS (✞) are labeled. CD4+ T-lymphocyte levels were evaluated by fluorescence-activated cell sorting on whole heparinized blood samples using methods previously described (33).
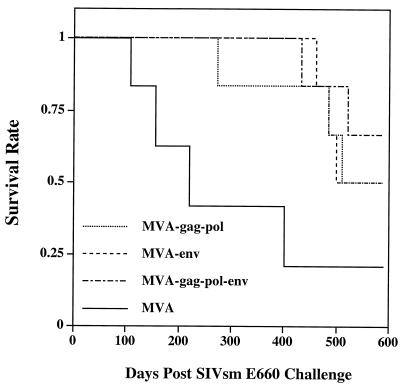
Significant increase in survival of MVA-SIV-immunized macaques.
Animals were monitored for evidence of immunosuppression and AIDS-related symptoms and were euthanized at the first definitive signs of AIDS. At 19 months after SIV challenge, one control macaque had survived, whereas the ten survivors in the MVA-SIV-vaccinees included three in the MVA-gag-pol group, three in the MVA-env group, and four in the MVA-gag-pol-env group. Cumulative survival rates of the four groups of MVA-immunized macaques are shown in a Kaplan-Meier plot in Fig. 8. Vaccination with any of the MVA-SIV recombinant viruses significantly prolongs the survival of immunized macaques compared to the survival of control animals (P = 0.010 by the log rank test). Cumulative survival of MVA-gag-pol- (P = 0.115), MVA-env- (P = 0.025), and MVA-gag-pol-env- (P = 0.041) immunized macaques were superior to the cumulative survival for the control group. By 590 days after challenge with SIVsmE660, the median length of survival of members of the MVA-immunized control group was 221 days, which was significantly shorter than the 551-day median of members of the MVA-gag-pol group (P = 0.049), the 546-day median of members of the MVA-env group (P = 0.015), and the >590-day median of members of the MVA-gag-pol-env group (P = 0.012) when compared by the Student's t test. However, the three SIV-immunized groups were indistinguishable in terms of survival.
FIG. 8.
Survival rates for immunized and control macaques. Kaplan-Meier plot of cumulative survival rates in the first 590 days after challenge indicates significant differences between MVA-immunized- and MVA-SIV-immunized macaques.
DISCUSSION
Our initial studies with MVA-SIV recombinants in rhesus macaques suggested that prior immunization provided protection against high levels of viremia and AIDS (34). However, due to small group sizes (n = 4) and heterogeneity in virus load, the differences between the control and MVA-SIV-immunized groups did not reach statistical significance. Nonetheless, two of the MVA-SIV immunized macaques remained healthy 4 years postchallenge. The goal of the present study was to determine definitively whether MVA-SIV recombinant vaccines provide benefit in terms of reduced levels of viremia and prolonged survival. Indeed, immunization with any of the three MVA-SIV recombinants resulted in significant control of plasma viremia and partial protection from AIDS following intravenous challenge with pathogenic SIV. Protection from high levels of viremia and rapid disease progression was observed in all three groups immunized with MVA-SIV recombinants. None of the macaques immunized with MVA-SIV recombinants developed rapidly progressive disease, whereas two such animals were observed in the control group. Most importantly, the reduction in plasma viremia in MVA-SIV-immunized macaques was associated with a significant increase in median length of survival (almost 1 year).
Although immunization with MVA-SIV recombinants improved survival following SIV challenge, progression to AIDS was still evident in the majority of animals, albeit with considerably delayed onset. Therefore, as of 19 months postchallenge, 10 of the MVA-SIV-immunized macaques (56%) survived (MVA-gag-pol, n = 3; MVA-env, n = 3; MVA-gag-pol-env, n = 4). This contrasts with the survival of only one of the MVA control macaques. While the majority of the MVA-SIV-immunized surviving macaques show some evidence of disease progression (declining CD4 T cells, lymphadenopathy), four (25%) have the characteristics of long-term nonprogressors of SIV or HIV infection (MVA-gag-pol, n = 2; MVA-env, n = 1; and MVA-gag-pol-env, n = 1). These characteristics include stable peripheral CD4+ T cells, the inability to consistently culture infectious virus from PBMC, normal lymph node morphology, and plasma viral RNA levels below the limits of detection of the assay. Historical data on other SIV-infected macaques suggest that animals with such virologic and clinical characteristics can remain clinically stable indefinitely. Cumulatively, these data demonstrate that immunization with MVA-SIV recombinant viruses protects macaques from high levels of viremia, significantly prolongs survival, and may prevent the development of AIDS in a small subset of these animals.
The present study did not directly address the immune mechanisms responsible for protection. However, it is evident that the protection from high levels of viremia observed in this study was not mediated by neutralizing antibody. First, protection was observed in macaques immunized with a Gag-Pol recombinant of MVA where neutralizing antibody could play no role in protection. Second, since macaques were not boosted with purified Env protein prior to challenge, neutralizing antibody titers for the vaccine strain were extremely low at the time of challenge (56). Third and most importantly, the neutralizing antibody response was highly type specific, since it neutralized only the vaccine strain (SIVsmH-4) and not the challenge strain (SIVsmE660). The type specificity of the neutralizing antibody response was even more evident following challenge (56). Sequential analysis of plasma samples from these macaques revealed that neutralization of the challenge strain by plasma was not evident until 12 weeks postchallenge. In addition, this neutralizing response did not appear more rapidly in the SIV-vaccinated groups than in the control group (56). Other antibody functions such as antibody-dependent cell-mediated cytolysis (8) or formation of immune complexes that enhance immunogenicity of SIV antigens may play a role in modulating SIV infection (19, 27, 28, 50) but were not assessed in the present study.
Consistent with growing evidence of the important role of CTLs in protection from HIV and SIV infections (24, 40, 41, 66), we believe it probable that the control of viremia observed in this study was mediated by cellular immune responses. Indeed, studies in this laboratory and others have demonstrated potent CTL responses in animals immunized with MVA vectors (9, 29–31, 68, 69). In particular, we evaluated Gag-specific CTL in rhesus macaques expressing the MHC class I allele Mamu-A*01 following immunization with the same MVA-gag-pol recombinant virus used in the present study. During immunization, these macaques developed robust Gag-specific CTL responses as assayed by both functional killing and tetrameric MHC class I/peptide-binding assays (68). Following SIV challenge, we observed a reduction in plasma viremia in MVA-gag-pol vaccinees as compared with macaques immunized with nonrecombinant MVA. The strength of the CTL response to immunization correlated inversely with level of plasma viremia following challenge, suggesting a role for memory CTL in protection from high levels of viremia (69).
In summary, the present study confirms our previous observation that prior immunization with MVA-SIV recombinant viruses results in significant control of viremia and prolonged survival following challenge with pathogenic SIV. The protective effects appeared to be mediated directly by the MVA recombinant without SIV protein boosting. Regardless of whether the macaques were immunized with an MVA recombinant expressing SIV Env or Gag-Pol, a similar degree of protection was achieved, suggesting that each antigen may contribute to protection equally. While the level of protection from AIDS was clearly less than optimal, it should be noted that the challenge (intravenous, pathogenic, and neutralization resistant) used in this study was a highly stringent test of the protective effect of this vaccination regimen. This challenge is likely to be more rigorous than the type of exposure involved in human infection with HIV-1, estimated to be generally less than 1% per exposure episode. This factor, in combination with the observation that there has been greater success in protecting against mucosal challenge (10, 62), suggests that such a vaccine approach may be highly effective in humans.
ACKNOWLEDGMENTS
We thank R. Byrum (Bioqual, Rockville, Md.) for assistance in collecting animal specimens, N. Cooper for help with tissue culture and MVA vaccine stock purification, K. Suryanarayana and J. D. Lifson (Laboratory of Retroviral Pathogenesis, National Cancer Institute-FCRDC) for providing pTRI-19 (polyA) plasmid, and S. Whitted and R. Goeken for technical assistance. We also thank D. C. Montefiori (Duke University Medical Center, Durham, N.C.) for critical review of the manuscript.
REFERENCES
- 1.Abimuki A G, Robert-Guroff M, Benson J, Tartaglia J, Paoletti E, Gallo R C, Markham P D, Franchini G. Long-term survival of SIVmac251-infected macaques previously immunized with NYVAC-SIV vaccines. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:S78–S85. [Google Scholar]
- 2.Almond N, Kent K, Cranage M, Rud E, Clarke B, Stott E J. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet. 1995;345:1342–1344. doi: 10.1016/s0140-6736(95)92540-6. [DOI] [PubMed] [Google Scholar]
- 3.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12(Suppl. A):S133–S140. [PubMed] [Google Scholar]
- 4.Altenburger W, Suter C P, Altenburger J. Partial deletion of the human host range gene in attenuated vaccinia virus MVA. Arch Virol. 1989;105:15–27. doi: 10.1007/BF01311113. [DOI] [PubMed] [Google Scholar]
- 5.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 6.Antoine G, Scheiflinger F, Dorner F, Falkner F G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 7.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenecity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 8.Baum L L, Cassutt K J, Knigge K, Khattri R, Margolick J, Rinaldo C, Kleeberger C A, Nishanian P, Henrard D R, Phair J. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–2173. [PubMed] [Google Scholar]
- 9.Belyakov I M, Wyatt L S, Ahlers J D, Earl P, Pendleton C D, Kelsall B L, Strober W, Moss B, Berzofsky J A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benveniste R E, Morton W R, Clarck E A, Tsai C C, Ochs H D, Ward J M, Kuller L, Knott W B, Hill R W, Gale M J, Thouless M E. Inoculations of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988;62:2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retrovir. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard T J, Alcami A, Panayota A, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lack several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 14.Buge S L, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus simian immunodeficiency virus Env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caley I J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Frelinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type I immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propargation and generation of recombinant viruses in nonhuman mammalian cell line. Virology. 1997;244:365–396. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 17.Chackrabarti S, Sisler J R, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;21:1904–1907. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 18.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 19.Denisova G, Stern B, Raviv D, Zwickel J, Smorodinsky N I, Gershoni J M. Humoral immune response to immunocomplexed HIV envelope glycoprotein 120. AIDS Res Hum Retrovir. 1996;12:901–909. doi: 10.1089/aid.1996.12.901. [DOI] [PubMed] [Google Scholar]
- 20.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y M, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earl P L, Cooper N, Wyatt L S, Carroll M W, Elroy-Stein O, Moss B. Expression of proteins in mammalian cells using vaccinia viral vectors. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1998. pp. 16.15.1–16.19.11. [Google Scholar]
- 22.Excler J L, Plotkin S. The prime-boost concept applied to HIV preventive vaccines. AIDS. 1997;11(Suppl. A):S127–S137. [PubMed] [Google Scholar]
- 23.Glamann J, Burton D R, Parren P W H I, Ditzel H J, Kent K A, Arnold C, Montefiori D, Hirsch V M. Simian immunodeficiency virus (SIV) envelope-specific Fabs with high-level homologous neutralizing activity: recovery from a long-term-nonprogressor SIV-infected macaque. J Virol. 1998;72:585–592. doi: 10.1128/jvi.72.1.585-592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh W C, Markee J, Akridge R E, Meldorf M, Musey L, Karchmer T, Krone M, Collier A, Corey L, Emerman M, McElrath M J. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–557. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein S, Elkins W R, London W T, Hahn A, Goeken R, Martin J E, Hirsch V M. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J Med Primatol. 1994;23:75–82. doi: 10.1111/j.1600-0684.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 26.Haigwood N L, Misher L E, Chin S M, Blair M, Planelles V, Scandella C J, Steimer K S, Gardner M B, Yilma T, Hirsch V M, Johnson P R. Characterization of group specific antibodies in primates: studies with SIV envelope in macaques. J Med Primatol. 1992;21:82–90. [PubMed] [Google Scholar]
- 27.Haigwood N L, Watson A, Sutton W F, McCure J, Lewis A, Ranchis J, Travis B, Voss G, Letvin N, Hu S-L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 28.Haigwood N L, Zolla-Pazner S. Humoral immunity to HIV, SIV, and SHIV. AIDS. 1998;12(Suppl. A):S121–S132. [PubMed] [Google Scholar]
- 29.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Immunogenicity of intravenous and intramuscular administrations of modified vaccinia virus Ankara-based multi-CTL epitope vaccine for human immunodeficiency virus type 1 in mice. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 30.Hanke T, Neumann V C, Blanchard T J, Sweeney P, Hill A V S, Smith G L, McMichael A. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17:589–596. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- 31.Hanke T, Samuel R V, Blanchard T J, Neumann V C, Allen T M, Boyson J E, Sharpe S A, Cook N, Smith G L, Watkins D I, Cranage M P, McMichael A J. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J Virol. 1999;73:7524–7532. doi: 10.1128/jvi.73.9.7524-7532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsch V M, Johnson P R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from and African green monkey: species-specific variation in pathogenicity correlates with extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch V M, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins W R, Montefiori D. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson P R, Montefiori D C, Goldstein S, Hamm T E, Zhou J, Kitov S, Haigwood N L, Misher L, London W T, Gerin J L, Allison A, Purcell R H, Chanock R M, Hirsch V M. Inactivated whole-virus vaccine derived from a proviral molecular clone of simian immunodeficiency virus induces high levels of neutralizing antibodies and confers protection against heterologous challenge. Proc Natl Acad Sci USA. 1992;89:2175–2179. doi: 10.1073/pnas.89.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson R P, Desrosiers R C. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Biol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 38.Johnson R P, Lifson J D, Czajak S C, Cole K S, Manson K H, Glickman R, Yang J, Montefiori D C, Montelaro R, Wyand M S, Desrosiers R C. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73:4952–4961. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan E L. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–466. [Google Scholar]
- 40.Koup R A, Safrit J T, Cao Y Z, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type-1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 42.Leno M, Carter L, Venzon D J, Romano J, Markham P D, Limbach K, Tartaglia J, Paoletti E, Benson J, Franchini G, Robert-Guroff M. CD8(+) lymphocyte antiviral activity in monkeys immunized with SIV recombinant poxvirus vaccines: potential role in vaccine efficacy. AIDS Res Hum Retrovir. 1999;15:461–470. doi: 10.1089/088922299311213. [DOI] [PubMed] [Google Scholar]
- 43.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 44.Levy J A. The value of primate models for studying human immunodeficiency virus pathogenesis. J Med Primatol. 1996;25:163–174. doi: 10.1111/j.1600-0684.1996.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 45.Mahmood N, Hay A J. An ELISA utilizing immobilized snowdrop lectin GNA for the detection of envelope glycoproteins of HIV and SIV. J Immunol Methods. 1992;151:9–13. doi: 10.1016/0022-1759(92)90101-x. [DOI] [PubMed] [Google Scholar]
- 46.Mahnel H, Mayr A. Experiences with immunization against orthopox viruses of humans and animals using vaccine strain MVA. Berl Muench Tieraerztl Wochenschr. 1994;107:253–256. [PubMed] [Google Scholar]
- 47.Mayr A, Hochstein-Mintzel V, Stickl H. Abstammung, eigenschaften und verwendung des attenuierten vaccinia-stammmes MVA. Infection. 1975;3:6–14. [Google Scholar]
- 48.Mayr A, Stickl H, Muller H K, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defense mechanism. Zentralbl Bakteriol Hyg B. 1978;167:375–390. [PubMed] [Google Scholar]
- 49.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1992;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 50.Montefiori D C, Evans T G. Toward an HIV type 1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retrovir. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 51.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V M, Goldstein S, Elkins W R, Lifson J D, Piatak M, Restifo N P, Owerwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mossman S P, Bex F, Berglund P, Arthos J, O'Neil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljestrom P, Hoover E A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathanson N, Hirsch V M, Mathieson B J. The role of nonhuman primate models in the development of an AIDS vaccine. AIDS. 1999;13(Suppl. A):S113–S120. [PubMed] [Google Scholar]
- 54.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a Nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 55.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y Z, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMickael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 56.Ourmanov I, Bilska M, Hirsch V M, Montefiori D C. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci USA. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perkus M E, Tartaglia J, Paoletti E. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukocyte Biol. 1995;58:1–13. doi: 10.1002/jlb.58.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Piatak M, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K-C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 60.Piatak M, Luk K-C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques. 1993;14:70–77. [PubMed] [Google Scholar]
- 61.Planelles V, Haigwood N L, Mathas M L, Mann K A, Scandella C J, Lidster W D, Schuster J R, van Kuyk R, Marx P A, Gardner M B, Luciw P A. Functional and immunological characterization of SIV envelope glycoprotein produced in genetically engineered mammalian cells. AIDS Res Hum Retrovir. 1991;7:889–896. doi: 10.1089/aid.1991.7.889. [DOI] [PubMed] [Google Scholar]
- 62.Polacino P, Stallard V, Montefiori D C, Brown C R, Richardson B A, Morton W R, Benveniste R E, Hu S L. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J Virol. 1999;73:3134–3146. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 64.Robert-Guroff M, Kaur H, Patterson L J, Leno M, Conley A J, McKenna P M, Markham P D, Richardson E, Aldrich K, Arora K, Murty L, Carter L, Zolla-Pazner S, Sinangil F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J Virol. 1998;72:10275–10280. doi: 10.1128/jvi.72.12.10275-10280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rud E W, Cranage M, Yon J. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8(+) lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 67.Schulz A. Using recombinant vectors as HIV vaccine. IAVI Report. 1998;3:1–4. [Google Scholar]
- 68.Seth A, Ourmanov I, Kuroda M J, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suryanarayana K, Wiltrout T A, Vasques G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chane reaction. AIDS Res Hum Retrovir. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 71.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. A recombinant vector derived from the host-range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 73.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wyand M S, Manson K H, Lackner A A, Desrosiers R C. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat Med. 1997;3:32–36. doi: 10.1038/nm0197-32. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J-Y, Martin L N, Watson E A, Monteralo R C, West M, Epstein L, Murphey-Corb M. Simian immunodeficiency virus/Delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1998;158:1277–1286. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]