Introduction
B-cell lymphomas are subtypes of non-Hodgkin lymphoma that may originate in lymph nodes or other organs trafficked by B-cells, including the skin, digestive tract, and central nervous system.1,2
Primary central nervous system lymphomas (PCNSL) of the B-cell origin begin in the central nervous system or eyes and comprise 4% of all intracranial malignancies.1 Over 90% of PCNSLs are of the diffuse large B-cell lymphoma (DLBCL) type.1 Conversely, primary cutaneous B-cell lymphomas originate in the skin and represent 25% of all cutaneous lymphomas.3 Primary cutaneous follicle center lymphoma (PCFCL) is one of the 3 subtypes of primary cutaneous B-cell lymphomas with an indolent course.
When B-cell lymphomas arise in 2 different organs, it may suggest either metastasis from one primary site to the other, or the manifestation of a systemic B-cell lymphoma metastasizing to both sites. This presentation is rarely caused by 2 distinct primary B-cell lymphomas originating from separate organs. We report an extremely rare case of a patient with PCNSL, DLBCL, who subsequently developed PCFCL, and we highlight the utility of immunosequencing in differentiating between 2 B-cell lymphomas.
Case report
A 61-year-old man was referred to our cutaneous lymphoma clinic with asymptomatic facial lesions that developed over the preceding 6 to 7 weeks. Notably, he had a history of PCNSL, DLBCL, diagnosed and treated 1 year before this visit, which remained in remission for the previous 6 months.
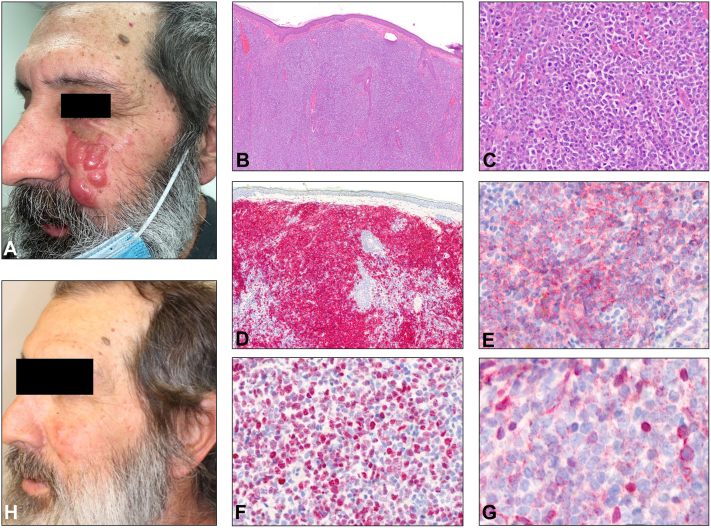
Physical exam revealed a cluster of well-circumscribed, firm, shiny, salmon-pink nodules and tumors on the left cheek (Fig 1, A). The lesions appeared clinically concerning for cutaneous metastasis of his PCNSL, DLBCL.
Fig 1.
Clinical and histopathological features of skin lesions. A, Cluster of firm, pink nodules on the left cheek. B and C, Dense dermal infiltrate of large, atypical monomorphous lymphocytes (hematoxylin & eosin: [B] 20×, [C] 40×). These cells stain positive for (D) CD20 (20×), (E) CD10 (100×), (F) BCL-6 (100×), and a variable staining pattern highlighting nonneoplastic reactive cells for (G) BCL-2 (100×). H, Resolution of nodules on the left cheek posttreatment.
Punch biopsy of the left cheek revealed a dense dermal infiltrate of large atypical monomorphous lymphocytes in the dermis separated by a grenz zone from the overlying epidermis (Fig 1, B and C). Poorly organized germinal centers were highlighted by CD20, CD10, and B-cell lymphoma 6 (BCL-6) positivity on immunostaining (Fig 1, D–F). The neoplastic B cells were negative for multiple myeloma oncogene 1 (MUM1), CD21, cyclin D1, and CD138. BCL-2 had a variable staining pattern highlighting nonneoplastic reactive cells (Fig 1, G). High-throughput sequencing (HTS) of the B-cell receptor revealed a dominant immunoglobulin (Ig)H-sequence, corresponding to a monoclonal B-cell proliferation (Table I) (Adaptive Biotechnologies). Overall, histopathological, immunohistochemical, and molecular features favored a diagnosis of follicle center B-cell lymphoma with diffuse large B-cell morphology, prompting the need to distinguish a primary process from a metastatic one.
Table I.
High-throughput sequencing data demonstrating dominant (malignant) B-cell clones and their frequencies within all B-cells for each indicated biopsy site
| Biopsy site | Frequency of dominant Ig sequence∗ |
||
|---|---|---|---|
| IgH—sequence A | IgH—sequence B | IgΚ—sequence C | |
| Left cheek | 5.97% | Not detected | Not detected |
| Brain | Not detected | 15.38% | 17.17% |
Sequence A:ATCTCCAGAGACAATTCAAAGAACATCCTATATCTTCACATGACTTCTCTGAGAGTCGAAGACACGGCCATTTATTATTGTGCGAAAGATAAAAATTATGTTGATCATCGAGCGTCGATTCTTGAATATTGGGGCCAGGGAGCC.
Sequence B:CAGAGACAATTCTAAGAACACCCTATATTTACACATGAGCAGCCTGAGAGCCGAAGACACGGCCATATATTACTGT.
Sequence C:GCTGAAGACGTGGCATTTTATTATTGTCAGCAATATTATATTACTCCCTCTCTTTTTGGCCAGGGG.
The frequency of dominant Ig sequences within all B-cells of the same Ig (H or K) lineage are displayed in the table. IgH sequence A was only detected in the left cheek, while IgH sequence B and IgK sequence C were only identified in the brain.
A bone marrow biopsy, peripheral blood flow cytometry, and a positron emission tomography-computed tomography scan were performed to complete systemic workup. The results were unremarkable for systemic involvement of B-cell lymphoma.
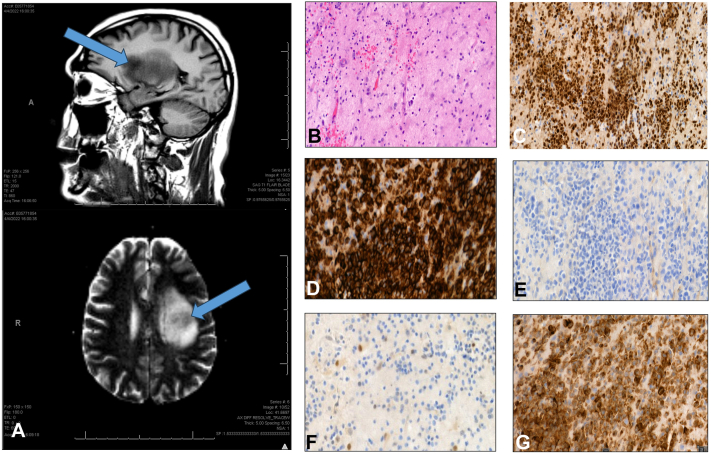
A detailed review of the patient’s PCNSL, DLBCL history was performed. Initial brain magnetic resonance imaging 1 year prior revealed a large, heterogenous enhancing mass in the left insula (Fig 2, A). Stereotactic brain biopsy showed angiocentric lymphoid cells staining positive for CD20 and BCL-2 and negative for CD10 and BCL-6 (Fig 2, B–G). The neoplastic B-cells also tested positive for c-MYC and MUM1. The patient achieved remission after 8 rounds of bi-weekly methotrexate over a 4-month period at 3500 mg/m2 per dose and whole brain radiation therapy (24 Gy in 2 Gy fractions), completed 7 and 5 months before the onset of facial lesions, respectively.
Fig 2.
Radiologic and histopathological features of brain lesion. A, Enhancing space occupying lesion measuring 2.6 cm × 2.4 cm × 3.5 cm with local mass effect. B, Angiocentric lymphoid cells (hematoxylin & eosin: [B] 40×) that are (C) MUM1-positive (100×), (D) CD20-positive (100×), (E) CD10-negative (100×), (F) BCL-6-negative (100×), and (G) BCL-2-positive (100×).
We conducted retrospective HTS of the B-cell receptor in the PCNSL, DLBCL biopsy to identify the clonal signature of the malignant B-cells. HTS revealed distinct dominant B-cell clones in both brain and skin biopsies, confirming a diagnosis of PCFCL (Table I).
The patient subsequently received intravenous rituximab, 800 mg weekly for 4 weeks, leading to the resolution of facial lesions (Fig 1, H). He tolerated the infusions without side effects and remains in remission 6 months after completion of the treatment.
Discussion
We present an exceptionally rare case of a patient with 2 distinct extranodal B-cell lymphomas that independently developed in the skin and brain. We considered the possibility of the cutaneous lesions originating from the brain or other systemic source. However, histopathological findings, lack of systemic involvement, and HTS revealing distinct clones between the 2 tumor sites confirmed the diagnosis of PCFCL. Typically, PCFCL stains positive for CD20 and BCL-6 and negative for BCL-2, MUM1, and c-MYC, with variable positivity results for CD10.3 PCNSL, DLBCL, though, is typically positive for CD20, MUM1, and BCL-2 and is almost always negative for CD10.3 In our case, the cutaneous lesions were positive for CD20, CD10, and BCL-6, while the central nervous system (CNS) lesion was positive for CD20, MUM1, c-MYC, and BCL-2. The significant differences in MUM-1, BCL-2, c-MYC, CD10, and BCL-6 staining between the brain and skin biopsies strongly indicate 2 independent processes. This was further supported by HTS, showing 2 distinct dominant B-cell clones between the 2 tumor sites.
There have been 4 previous cases of PCNSL metastasizing to the skin, but the CNS and skin samples were clonally and histologically identical.4 Additionally, in these cases, patients were in remission for an average of 23 months, while our patient was in remission for 6 months.4 There was also concern that the patient’s cutaneous lymphoma was the primary cancer and was not clinically apparent by the time it metastasized to the CNS. This order of events is a rare occurrence, happening in less than 4% of all non-Hodgkin lymphoma cases; almost all cases have skin manifestations prior to CNS involvement.5,6 Again, the histology and sequencing in our study eliminate this hypothesis.
There have been well-documented cases of methotrexate-associated B-cell lymphoproliferative disorders, prompting consideration that our patient’s PCFCL might have been related to the methotrexate administered for his preceding PCNSL. However, most reported cases involved patients using methotrexate chronically for management of rheumatologic conditions. Approximately 40% of cases demonstrated spontaneous partial or complete resolution of methotrexate-associated B-cell lymphoproliferative disorders upon discontinuation of methotrexate.7 It appears unlikely that development of PCFCL in our patient was directly caused by methotrexate, though it cannot be ruled out entirely.
Our patient represents an unusually rare case of a cutaneous B-cell lymphoma developing after a CNS B-cell lymphoma. Previous PCNSL studies showed no increased risk of secondary cutaneous lymphomas, and no cases have been reported to date.8,9 This is also the first case to demonstrate HTS utility in establishing 2 genetically distinct B-cell clones in 2 different B-cell lymphomas.4 It may be beneficial to utilize HTS in differentiating second primary hematologic malignancies from metastases of existing hematologic cancers.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of interest
Dr Nikbakht is an advisory board member for Helsinn and Kyowa Kirin. Authors Joffe, Rohan, Mandel, Suriano, Banner, Drs Valiga, Porcu, Gong, Lee, and Alpdogan have no conflicts of interest to declare.
Footnotes
Authors Joffe and Rohan contributed equally to this work.
Funding sources: Dr Nikbakht is supported via a grant from the National Cancer InstituteR03CA252818.
Patient consent: The authors obtained written consent from patients for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available. Patient consent forms were not provided to the journal but are retained by the authors.
IRB approval status: Not applicable.
References
- 1.Grommes C., DeAngelis L.M. Primary CNS lymphoma. J Clin Oncol. 2017;35:2410–2418. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sapkota S., Shaikh H. StatPearls; 2023. Non-Hodgkin Lymphoma. [PubMed] [Google Scholar]
- 3.Vitiello P., Sica A., Ronchi A., Caccavale S., Franco R., Argenziano G. Primary cutaneous B-cell lymphomas: an update. Front Oncol. 2020;10:651. doi: 10.3389/fonc.2020.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel B.M., Moesch J., Karunamurthy A., et al. Cutaneous metastasis of primary diffuse large B-cell lymphoma of the central nervous system developing 4 years after complete remission: diagnosis confirmed by comparison of clones. J Cutan Pathol. 2022;49:90–94. doi: 10.1111/cup.14129. [DOI] [PubMed] [Google Scholar]
- 5.Giannini C., Dogan A., Salomao D.R. CNS lymphoma: a practical diagnostic approach. J Neuropathol Exp Neurol. 2014;73:478–494. doi: 10.1097/NEN.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Wickless H. Thinking about CNS metastasis in cutaneous lymphoma: analysis of existing data. Leuk Res Rep. 2017;8:14–18. doi: 10.1016/j.lrr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koens L., Senff N.J., Vermeer M.H., Willemze R., Jansen P.M. Methotrexate-associated B-cell lymphoproliferative disorders presenting in the skin: a clinicopathologic and immunophenotypical study of 10 cases. Am J Surg Pathol. 2014;38:999–1006. doi: 10.1097/PAS.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 8.Brown M.T., McClendon R.E., Gockerman J.P. Primary central nervous system lymphoma with systemic metastasis: case report and review. J Neuro Oncol. 1995;23:207–221. doi: 10.1007/BF01059952. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Pulido J.S., O'Neill B.P., Johnston P.B. Second malignancies in patients with primary central nervous system lymphoma. Neuro Oncol. 2015;17:129–135. doi: 10.1093/neuonc/nou105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.