Abstract
To investigate the optimal processing of maize porridge, the volatile compounds and texture under different cooking methods and time have been studied. A total of 51 volatile compounds were identified in maize porridge. Notably, the major volatiles, aldehydes and esters exhibited a relatively high content in electric pressure cooker (EPC), and esters tend to significantly increase after cooking. Among aldehydes, nonanal and hexanal played a great role in flavor due to their relatively high content. Volatile compounds of maize porridge in different cooking methods could be clearly distinguished by multiple chemometrics. Furthermore, texture analysis revealed that almost all the indicators in the EPC can reach the lowest value at 60 min. To summarize, different cooking methods had a more significant influence on the volatile compounds and texture compared to time. This study helps to improve the sensory attributes of maize porridge, and thus contributes to healthier and more sustainable production.
Keywords: GC–MS, Multiple chemometrics, Texture analysis, Porridge processing
Highlights
-
•
Flavor, texture and sensory analysis were used to evaluate maize porridge quality.
-
•
Multiple chemometrics methods were performed to analyze the results.
-
•
Aldehydes and esters as key volatile compounds have a high content in electric pressure cooker.
-
•
EPC-60 min attained the highest sensory comprehensive score.
-
•
Different cooking methods had more effect on the volatile compounds and texture than cooking time.
1. Introduction
Maize, widely cultivated in China, tropical and temperate regions, was the most productive cereal worldwide. Maize porridge, a common form of maize products in the human diet, served as a substantial source of maize consumption. Recently, the contribution of maize to human nutrition and health has been revisited (Poole, Donovan, & Erenstein, 2021). However, there was limited information on the effect of cooking methods on the volatile compounds and texture of maize porridge.
Flavor significantly impacts human consumption. The flavor and texture of cereal foods were mostly formed during processing due to process-induced changes in the grain biopolymers and flavor-active compounds (Wang et al., 2023). Some grain biopolymers can be degraded as precursors during processing to produce different volatile compounds. Currently, existing studies have given us insight into the volatile compounds of maize porridge (Ekpa, Fogliano, & Linnemann, 2021). However, the finding was based on maize varieties from Nigeria and Benin. The volatile compounds of Chinese maize porridge largely remained unknown. During actual production, various factors affect the sensory quality and volatile compounds of cereal products. Cooking methods and varieties can significantly vary rice sensory quality and flavor components (Hu, Lu, Guo, & Zhu, 2020). The thermal degradation of odor and taste compounds may lead to a decrease in the level of certain key substances or the formation of new substances, which ultimately had effects on the sensory characteristics of the cereal. Furthermore, subjecting brown rice to high-pressure cooking conditions altered its volatile compounds and subsequently affected its flavor and acceptability (Yu et al., 2021). Pre-soaking brown rice prior to cooking resulted in a softer and stickier texture of cooked brown rice (Zhu, Yu, Yin, Wu, & Zhang, 2022). The hardness of cooked brown rice decreased by increasing the soaking temperature and time, and stickiness after soaking for 60 min was higher than that after 30 min. Moreover, employing high-temperature air fluidization techniques has been shown to enhance the cooking quality, palatability, and storage stability of brown rice (Li et al., 2021). Previous studies mainly focused on the factors that influence sensory quality and flavor in cereal products, especially in rice products. While research on the effect of different cooking methods on the volatile compounds and texture of maize porridge is limited.
This study investigated the effects of different cooking methods on the volatile compounds and texture properties of Chinese maize porridge. The Songyuan maize, renowned for its sweet, smooth flavor, has high popularity in China, was used in this research. The electromagnetic oven (EC) employed the electromagnetic induction principle to generate heat at the bottom of the pot, instead of using open flame. The electric rice cooker (IC) was a modern type of cooker that can be used for steaming, boiling, stewing, simmering, and other processing. The electric pressure cooker (EPC) represented an advancement over the traditional pressure cooker and electric rice cooker, which combined the advantages of both pressure cooker and electric rice cooker. The three most commonly used cookers in Chinese households were employed in this study. The volatile compounds of maize porridge were characterized using headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography–mass spectrometry (GC–MS). Previous studies on food texture have demonstrated the importance of texture testing (Wang et al., 2023; Zhu et al., 2024). Texture instruments could simulate the hardness, adhesiveness, cohesiveness, and elasticity of porridge during human chewing. In this research, texture instruments were used to analyze the texture of maize porridge. Additionally, sensory evaluation was conducted to better understand and meet consumer preferences. This study aimed to investigate the effect of cooking methods on the volatile compounds and texture of maize porridge in a scientific way. The findings will enhance our understanding of the volatile compounds of maize porridge. This is a great option to enhance the sensory qualities of maize porridge and improve the acceptance.
2. Materials and methods
2.1. Materials
Songyuan maize (Sweet maize varieties in Jilin province, Non-gmo maize, protein content 6.5 g/100 g, fat content 2.0 g/100 g, carbohydrate 75.1 g/100 g) was processed in Liaoyang City, Liaoning Province, China.
2.2. Porridge cooking and sample preparation
100 g of unground maize were respectively placed in an electric pressure cooker (EPC) (Supor Inc., Zhejiang, China), electromagnetic oven (EC) (Supor Inc., Zhejiang, China), electric rice cooker (IC), (Triangle Inc., Guangdong, China). With 900 W power supply, 2000 g of water will be added to keep the maize: water = 1:20 and cook for different time (30 min, 40 min, 50 min, 60 min). To avoid any effect of retrogradation, the texture and volatile compounds analysis were started immediately after cooking.
2.3. Volatile compounds analysis
The volatile compounds of the maize porridge samples were determined by headspace solid-phase microextraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC–MS). The standardized operation was as follows:
HS-SPME: all samples weighed (8 ± 0.02 g), placed into 20 mL headspace bottles, added 10 μL internal standard (1,2-dichlorobenzene, 5.155 μg/mL) and heated in a water bath at 60 °C for 30 min under agitation at 200 rpm. Then, the fiber (50/30 μm PDMS/CAR/DVB 2 cm) was immediately desorbed into the GC–MS injection port at 280 °C for 5 min.
GC–MS analysis: Clarus690-SQ8T (PerkinElmer, USA) was used according to the description of Xu et al. (2022) to analyze the volatile compounds. The Elite-5MS column (30 m × 0.25 mm × 0.5 μm) was used, with high purity helium as the carrier (1.0 mL /min). The initial column temperature was maintained at 40 °C for 3 min and then increased to 100 °C at 6 °C/min. Finally, it raised to 230 °C at 10 °C/min with a 3 min hold time. The MS electronic energy was EI 70 eV, and the mass scanning range was 30 m/z to 500 m/z. The volatile components were identified by matching retention index (RI, determined by n-alkane C5-C22) of mass spectra to reference standards of the National Institute of Standards and Technology (NIST). Relative quantification was carried out according to the ratio of the ion flow peak area of each compound detected in the total ion flow chromatogram to the internal standard (10 μL, 1,2-dichlorobenzene, 5.155 μg/mL) peak area (Song, Porter, Whitaker, Lee, & Wang, 2023).
2.4. Texture profile analysis (TPA)
A texture analyzer (TA-XT plus, Stable Micro Systems Ltd., UK) was employed for the textural analysis of maize porridge samples. The porridge samples were arrayed on the platform and tested. A two-cycle compression program (TPA) was employed for textural analysis, and TPA parameters were as follows: 60% strain, the pre-test speed, test speed, and post-test speed were set at 1.0, 30, and 1 mm/s respectively, and the model of cylindrical probe is P/36. Each sample was repeated 5 times.
2.5. Sensory evaluation
The establishment of the reference method were obtained and modified from those reported by Mestres, Briffaz, and Valentin (2019). The evaluation team consisted of 20 sensory evaluators (10 males and 10 females, ages ranging from 23 to 30) from the Qingdao University laboratory. The team members were trained for two weeks to clearly evaluate the maize porridge samples. The samples (30 mL) were placed in 50 mL plastic cups, encoded with three-digit numbers, presented to the team members randomly, and then evaluated in separate compartments at a controlled temperature (35 °C). Each sample was analyzed in triplicate. Finally, 5 descriptors were generated to describe and differentiate the different porridge samples. They described the appearance, texture, aroma, and taste of the porridges. The intensity of the 5 descriptors was scored on a 0–9 scale (from 0, weak, to 9, strong). Since our aim was to study the effect of cooking methods and time on the volatile compounds and texture of maize porridge, comparing the sensory scores of maize porridge in different cooking methods and time, we could know the flavor change of maize porridge and the effect of different cooking methods on the flavor.
2.6. Data analysis
All data in the experiment were obtained by three or more parallel tests and were expressed as mean ± standard deviation. SPSS 26.0 (SPSS Inc., Chicago, IL, USA) was implemented to analyze significant differences (P < 0.05) in the data generated from the samples. It was mainly based on the one way analysis of one way (ANOVA) test and Duncan's multiple comparisons test. SIMCA-P (14.1, Umea, Sweden) and TBtools (1.120, China) were separately performed for the principal component analysis (PCA) and clustering heat map analysis. OriginPro 2021 (OriginLab Corporation, Northampton, MA, USA) was used to draw the remaining graphs.
3. Results and discussion
3.1. Influence of cooking methods on volatile compounds of maize porridge
3.1.1. PCA and Heatmap, hierarchical cluster analysis
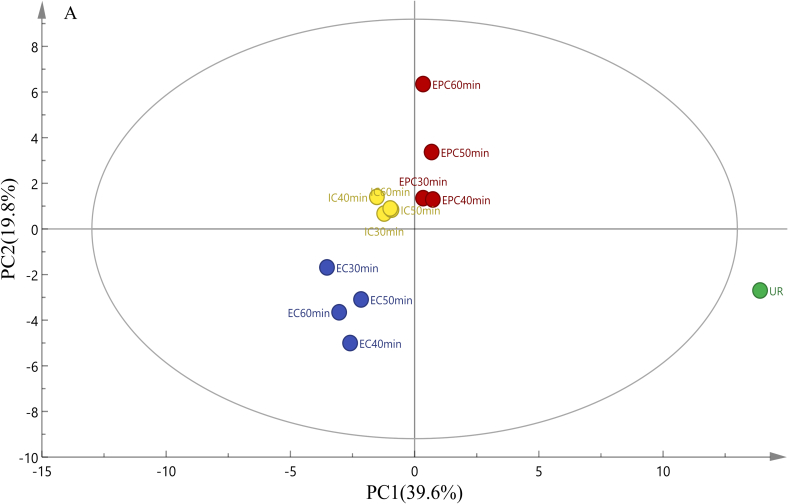
The principal component analysis (PCA) was a statistical technique that used orthogonal transformation to convert a set of observations of possibly correlated variables into a set of values of linearly uncorrelated variables (Greenacre et al., 2022). Typically, the PCA model was chosen as the separation model when the contribution ratio closed to 60% (Chen et al., 2020). A PCA chart (Fig. 1A) was established based on the concentration of volatile compounds to better understand the changes in volatile compounds under different cooking methods. The PCA showed that PC1 and PC2 accounted for 59.4% of the total variance (39.6% and 19.8%, respectively), which was closer to 60%, indicating that the PC1 and PC2 can explain most of the volatile compound information. Overall, the maize porridge samples of different cooking methods were scattered in different parts of the PCA chart, demonstrating that cooking methods had a significant effect on the volatile compounds of maize porridge.
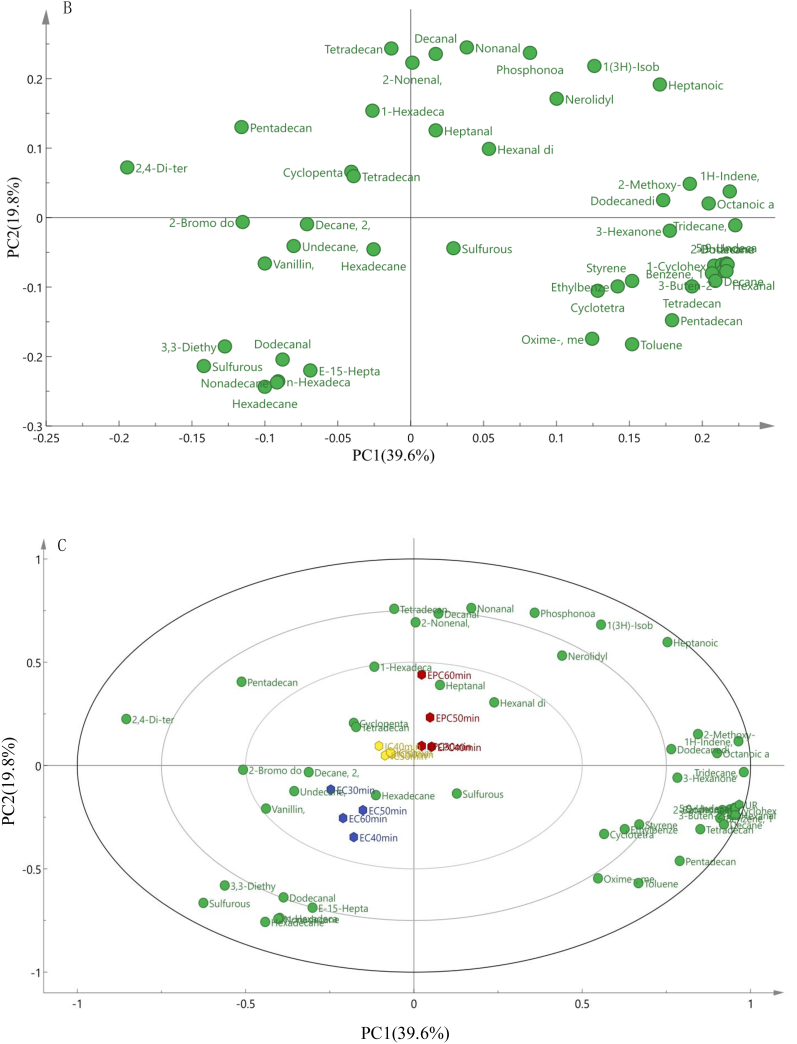
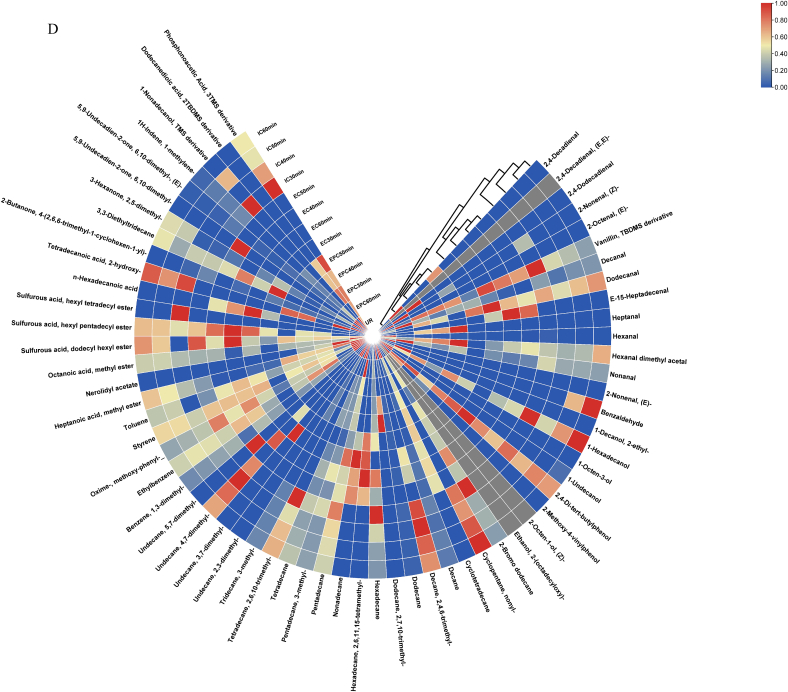
Fig. 1.
PCA analysis (A), the loading plot (B), the bi-plot results (C) and heatmap analysis (D) of aroma components in maize porridge prepared in 3 kinds of cookers with different cooking time, EPC: Maize porridge prepared in the electric pressure cooker; EC: Maize porridge prepared in the electromagnetic oven; IC: Maize porridge prepared in the electric rice cooker; UR: Uncooked maize.
According to PCA results, the thirteen kinds of maize porridge samples were divided into 4 groups based on the volatile compounds. As shown in Fig. 1B and Fig. 1C, the loading plot and bi-plot graph can explain the cluster results of PCA. The first group contained IC30-min, IC40-min, IC50-min, and IC60-min, scattered throughout the PCA score plot in the upper left quadrant. This may be due to tetradecane 2, 6, 10-trimethyl- and cyclopentane nonyl-. The second group had EC30-min, EC40-min, EC50-min, and EC60-min, which were clustered in the lower left quadrant. This may be related to several aromatic compounds. The third group included EPC30-min, EPC40-min, EPC50-min, and EPC60-min, gathered in the upper right quadrant. This may be caused by the high content of aldehydes, such as heptanal and hexanal. The fourth group consisted of Uncooked maize (UR) and was distributed in the lower right quadrant.
A heatmap was constructed based on the concentration of volatile compounds in maize porridge samples (Fig. 1D). The volatile compounds containing aldehydes, alcohols, hydrocarbons, aromatic compounds, esters, ketones, and acids, were identified and displayed in the heatmap. Each average concentration of volatile compound in maize porridge samples was shown (from blue to red) in Fig. 1(D) by colored boxes on the heatmap. The first group contained the cooking method of IC 30, 40, 50, and 60 min, owing to a generally high content of hydrocarbons. The second group contained the cooking method of EC for 30, 40, 50, and 60 min, which might be due to a relatively high content of aromatic compounds and a low content of acids. The third group included the cooking method of EPC at 30, 40, 50, and 60 min. This result may be explained by the fact that they had a relatively high content of aldehydes and esters. The fourth group comprised UR sample. Except for acids, UR samples contained higher levels of all other substances than the other groups.
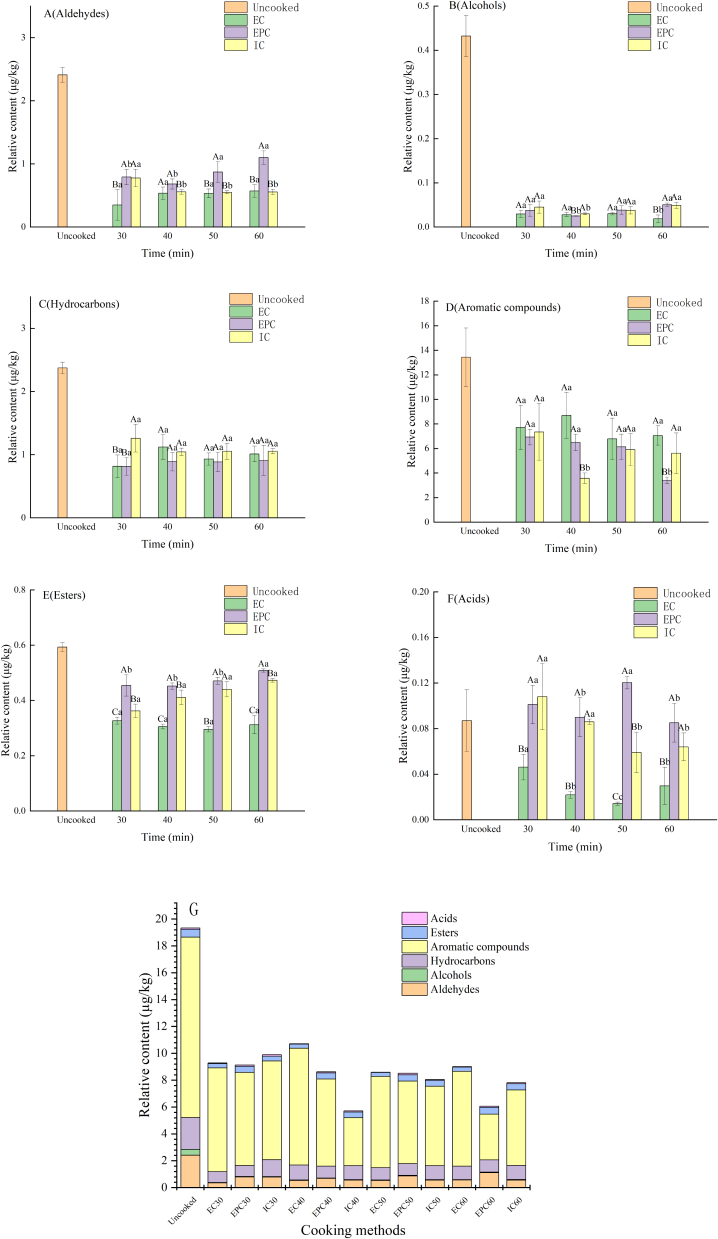
3.1.2. Change of key volatile compounds in maize porridge during cooking
As shown in Fig. 2, a total of 51 volatile compounds in maize porridge samples were classified and presented in the form of bar charts. The content of volatile compounds tended to increase or decrease largely after cooking. Fig. 2(A) showed the variation in aldehyde content with different cooking time and methods. Previous research has identified aldehydes as having green, fruity, and malty aromas, which exerted a significant effect on the flavor of cereals (Zheng, Wang, Xiong, & Zhang, 2023). Meanwhile, aldehydes were regarded as crucial volatile compounds in rice porridge recently (Wang et al., 2023). In this study, the content of aldehydes in EPC tended to increase when the heating time changed from 30 min to 60 min, potentially due to the generation of aldehydes by lipolytic enzymes and heating can speed up this reaction (Guo et al., 2023). Conversely, the increase in EC and IC was negligible. This phenomenon might be explained by varying degrees of Maillard reaction in different cooking methods or that the produced aldehydes were partially evaporated in EC and IC (Zhang et al., 2018a). Higher heating rates in EC and IC produced significant amounts of water vapor, which carried away the partial volatile aldehydes.
Fig. 2.
The content of different kinds of volatile compounds relative to 1,2-dichlorobenzene in maize porridge samples, Aldehydes (A); Alcohols (B); Hydrocarbons (C); Aromatic compounds (D); Esters (E); Acids (F); The relative content of all volatile compounds in different cooking methods (G); EPC: Maize porridge prepared in the electric pressure cooker; EC: Maize porridge prepared in the electromagnetic oven; IC: Maize porridge prepared in the electric rice cooker; Uncooked: Uncooked maize.
As depicted in Fig. 2(B), alcohols had the lowest content among all volatile compounds. In this research, alcohols content exhibited a significant declining trend after cooking. Additionally, there was no clear regularity in alcohols variation between the three cooking methods.
Hydrocarbons content can be observed in Fig. 2(C). The n-hydrocarbons identified ranged from C8 to C19 and may arise from decarboxylation and breakdown of carbon chains of longer fatty acids. Most of them had no flavor characteristics (Zhang et al., 2019). In EPC and IC, the content changed more steadily. Only in EC, the content of hydrocarbon substances increased first and then decreased at 40 min.
Fig. 2(D) displayed that the aromatic compounds had the largest content among all the volatile compounds released from maize porridge. Previous research found that aromatic compounds with more than six carbon atoms were derived from high-temperature rearrangement or polymerisation reactions (Zhang et al., 2022). In this study, the aromatic compounds in EPC exhibited a declining trend. The content in IC decreased first and then increased in 40 min, with the total concentration peaked at 30 min. It worth noting that EC had the highest aromatic compounds content. The content of aromatic compounds in EC tended to increase first and then decrease, reaching the peak at 40 min.
In Fig. 2(E), the change of ester content in different cooking methods can be noticed. Previous studies have shown that ester aromas were essential for the fruity character alcoholic beverages and sorghum wine (Holt, Miks, de Carvalho, Foulquié-Moreno, & Thevelein, 2019; Tanwar, Panghal, Chaudhary, Kumari, & Chhikara, 2023). Some esters can be blended with other volatile components to make the overall flavor softer and contribute significantly to food flavor. It was evident that uncooked maize had the highest ester content. EPC had a higher ester content compared to IC and EC. A possible explanation may be that the compact structure of maize starch was destroyed by the high pressure in EPC, resulting in a higher esters content released from maize in EPC compared to EC and IC. More specifically, the total content of esters increased with time in all three cooking methods.
As presented in Fig. 2(F), the acids content was quite low as similar to the results of alcohols. EC held a remarkably lower acid content compared to EPC and IC. At EPC-50 min, it reached the highest acids content.
Different volatile components had their specific changes over time in different cooking methods. In general, most of the volatile compounds were stable with cooking time. Key volatile components such as aldehydes and esters were relatively high in EPC. According to ordinate data, the volatile compounds in maize porridge were mainly aromatic compounds and aldehydes, with minimal levels of alcohols and acids. Although the content of alcohols was relatively low, it played a synergistic role in the overall aroma. The content of all volatile compounds in different cooking methods was presented in Fig. 2(G).
3.1.3. Analysis of volatile compounds in maize porridge
According to GC–MS analysis, a total of 51 volatiles were presented, including 6 aromatic compounds, 4 alcohols, 19 hydrocarbons, 5 esters, 12 aldehydes, 4 acids, and 1 ketone (Table 1). The Maillard reaction, lipoxygenase and fatty acid metabolism all contributed to the formation of heat-induced volatile compounds (Krause et al., 2022). Although there were differences in the volatile substances of maize in different cooking methods compared to those found in the maize porridge, there were still similarities overall (Zhang, Gao, Zhang, Feng, & Zhuang, 2022). Pico, Tapia, Bernal, and Gómez (2018) found higher levels of aromatic compounds and esters in maize starch, which was consistent with our results and indirectly supported the reliability of our study. The aldehydes, alcohols, and esters contained in maize porridge after cooking showed significant changes in quantity and content compared to the uncooked maize. Among the detected volatile compounds, (E)-2-octenal and 2, 3-dimethyl-undecane were only found in uncooked maize, while 20 volatile compounds were presented after cooking. Aromatic compounds, aldehydes and ketones were the most abundant volatile compounds in raw maize. Except for acids, most volatile compounds maintained relatively constant contents during cooking.
Table 1.
Changes of key aroma compounds of maize porridges prepared in 3 kinds of cookers with different cooking time.
| Volatile compounds(μg/kg) |
Cooking time/min |
||||||
|---|---|---|---|---|---|---|---|
| RI | Cooker | Uncooked maize | 30 | 40 | 50 | 60 | |
| Aldehydes | |||||||
| 2,4-Decadienal | 1413 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | 0.014 ± 0.004a | 0.019 ± 0.004a | |||
| IC | ND | ND | ND | ND | |||
| 2,4-Dodecadienal | 1315 | EC | ND | ND | ND | ND | ND |
| EPC | 0.01 ± 0.001 | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 2-Nonenal, (Z)- | 1158 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | 0.009 ± 0.001b | 0.017 ± 0.004a | |||
| IC | 0.012 ± 0.003 | ND | ND | ND | |||
| 2-Octenal, (E)- | 1056 | EC | 0.278 ± 0.023 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Decanal | 1200 | EC | 0.056 ± 0.008 | 0.066 ± 0.001Aa | 0.041 ± 0.001Cc | 0.038 ± 0.001Cd | 0.052 ± 0.006Bb |
| EPC | 0.067 ± 0.010Ab | 0.050 ± 0.001Ac | 0.065 ± 0.004Ab | 0.129 ± 0.040Aa | |||
| IC | 0.060 ± 0.008Aa | 0.047 ± 0.002Bb | 0.056 ± 0.004Ba | 0.057 ± 0.001Ba | |||
| Dodecanal | 1591 | EC | 0.016 ± 0.002 | 0.017 ± 0.001Aa | 0.013 ± 0.002Ab | 0.010 ± 0.001Bb | 0.021 ± 0.011Aab |
| EPC | 0.014 ± 0.001Ba | 0.009 ± 0.002Ab | ND | ND | |||
| IC | 0.014 ± 0.003ABab | 0.010 ± 0.001Ab | 0.013 ± 0.001Aa | 0.015 ± 0.001Aa | |||
| E-15-Heptadecenal | 1601 | EC | ND | ND | 0.022 ± 0.001a | 0.018 ± 0.002b | 0.017 ± 0.001b |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Heptanal | 897 | EC | ND | ND | ND | ND | ND |
| EPC | 0.073 ± 0.027a | 0.087 ± 0.049a | 0.127 ± 0.038a | ND | |||
| IC | ND | ND | ND | ND | |||
| Hexanal | 787 | EC | 1.622 ± 0.050 | 0.013 ± 0.001Bc | 0.032 ± 0.011Aab | 0.017 ± 0.007Bb | 0.039 ± 0.003Ba |
| EPC | 0.050 ± 0.013Aa | 0.029 ± 0.011Aa | 0.049 ± 0.001Aa | 0.060 ± 0.033Aa | |||
| IC | 0.062 ± 0.035Aa | 0.024 ± 0.002Ab | 0.012 ± 0.003Bc | 0.019 ± 0.004Cbc | |||
| Hexanal dimethyl acetal | 975 | EC | 0.050 ± 0.001 | 0.014 ± 0.001Bb | 0.045 ± 0.014Ba | 0.052 ± 0.018Ba | 0.032 ± 0.005Ba |
| EPC | 0.065 ± 0.011Ab | 0.074 ± 0.003Ab | 0.092 ± 0.012Aa | 0.036 ± 0.001Bc | |||
| IC | 0.043 ± 0.012Ab | 0.038 ± 0.009Bb | 0.041 ± 0.012Bb | 0.066 ± 0.002Aa | |||
| Nonanal | 1099 | EC | 0.301 ± 0.027 | 0.128 ± 0.016Bb | 0.206 ± 0.016Ba | 0.224 ± 0.005Ba | 0.240 ± 0.012Ba |
| EPC | 0.328 ± 0.048Ab | 0.247 ± 0.017Bc | 0.346 ± 0.050Ab | 0.732 ± 0.019Aa | |||
| IC | 0.387 ± 0.030Aa | 0.295 ± 0.025Ab | 0.294 ± 0.004Ab | 0.275 ± 0.017Bb | |||
| Vanillin | 952 | EC | 0.096 ± 0.001 | 0.222 ± 0.012Aa | 0.184 ± 0.038Aa | 0.173 ± 0.028Aa | 0.198 ± 0.037Aa |
| EPC | 0.186 ± 0.013Ba | 0.183 ± 0.001Aa | 0.175 ± 0.049Aa | 0.109 ± 0.005Bb | |||
| IC | 0.205 ± 0.044ABa | 0.143 ± 0.002Bb | 0.134 ± 0.001Bc | 0.123 ± 0.018Bc | |||
| Alcohols | |||||||
| 1-Decanol, 2-ethyl- | 1169 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | 0.018 ± 0.003 | |||
| IC | ND | ND | ND | ND | |||
| 1-Hexadecanol | 1842 | EC | 0.026 ± 0.003 | 0.029 ± 0.008Aa | 0.028 ± 0.005ABa | 0.030 ± 0.003Aa | 0.019 ± 0.009Ca |
| EPC | 0.038 ± 0.013Aa | 0.025 ± 0.001Bb | 0.038 ± 0.010Aa | 0.033 ± 0.001Ba | |||
| IC | 0.045 ± 0.014Aa | 0.030 ± 0.002Ab | 0.038 ± 0.008Aa | 0.045 ± 0.003Aa | |||
| 1-Octen-3-ol | 978 | EC | 0.357 ± 0.039 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| 1-Undecanol | 1666 | EC | 0.049 ± 0.005 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | 0.008 ± 0.001 | |||
| Hydrocarbons | |||||||
| 2-Bromo dodecane | 1679 | EC | 0.021 ± 0.003 | 0.028 ± 0.001Bb | 0.034 ± 0.004Aa | 0.027 ± 0.002Ab | 0.028 ± 0.001Ab |
| EPC | 0.029 ± 0.009ABa | 0.023 ± 0.003Ba | 0.029 ± 0.008Aa | 0.028 ± 0.007ABa | |||
| IC | 0.038 ± 0.007Aa | 0.026 ± 0.001Bb | 0.026 ± 0.005Aab | 0.025 ± 0.001Bb | |||
| Cyclopentane, nonyl- | 1626 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.034 ± 0.001b | 0.030 ± 0.001c | 0.037 ± 0.006ab | 0.042 ± 0.001a | |||
| Cyclotetradecane | 1447 | EC | 0.060 ± 0.001 | 0.035 ± 0.001Aa | 0.032 ± 0.011Aab | 0.028 ± 0.001Ab | 0.028 ± 0.002Ab |
| EPC | 0.026 ± 0.004Ba | 0.030 ± 0.003Aa | 0.034 ± 0.009Aa | 0.026 ± 0.003Aa | |||
| IC | ND | ND | ND | ND | |||
| Decane | 998 | EC | 0.881 ± 0.020 | 0.318 ± 0.012Bc | 0.366 ± 0.001Aa | 0.347 ± 0.020ABab | 0.354 ± 0.004Bb |
| EPC | 0.310 ± 0.052ABab | 0.343 ± 0.024Aab | 0.307 ± 0.021Bb | 0.351 ± 0.008Ba | |||
| IC | 0.391 ± 0.038Aa | 0.358 ± 0.012Ab | 0.386 ± 0.028Aa | 0.377 ± 0.014Aa | |||
| Decane, 2,4,6-trimethyl- | 1260 | EC | ND | ND | 0.056 ± 0.007Aa | ND | 0.074 ± 0.012Aa |
| EPC | ND | ND | ND | ND | |||
| IC | 0.065 ± 0.006ab | 0.071 ± 0.013Aa | 0.061 ± 0.001b | 0.052 ± 0.002Bc | |||
| Dodecane | 1197 | EC | 0.618 ± 0.043 | 0.090 ± 0.001Aa | 0.089 ± 0.007Aa | 0.071 ± 0.006Bb | 0.078 ± 0.012Aab |
| EPC | 0.078 ± 0.010Ba | 0.099 ± 0.009Aa | 0.094 ± 0.016Aa | 0.117 ± 0.031Aa | |||
| IC | 0.101 ± 0.013Aa | 0.087 ± 0.003Aa | 0.090 ± 0.005Aa | 0.092 ± 0.005Aa | |||
| Dodecane, 2,7,10-trimethyl- | 1273 | EC | ND | ND | ND | ND | ND |
| EPC | ND | 0.040 ± 0.002b | 0.058 ± 0.004a | ND | |||
| IC | ND | ND | ND | ND | |||
| Hexadecane | 1715 | EC | 0.121 ± 0.006 | 0.139 ± 0.003ABa | 0.171 ± 0.044Aa | 0.117 ± 0.014Aa | 0.086 ± 0.005Bb |
| EPC | 0.108 ± 0.022Ba | 0.110 ± 0.031Aa | 0.120 ± 0.016Aa | 0.118 ± 0.007Aa | |||
| IC | 0.193 ± 0.063Aa | 0.131 ± 0.008Aa | 0.112 ± 0.027Aa | 0.108 ± 0.006Ab | |||
| Hexadecane, 2,6,11,15-tetramethyl- | 1736 | EC | ND | 0.040 ± 0.002a | 0.050 ± 0.015 A | 0.032 ± 0.004b | 0.045 ± 0.009a |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Nonadecane | 1691 | EC | ND | 0.034 ± 0.004b | 0.031 ± 0.007b | 0.034 ± 0.001b | 0.045 ± 0.001a |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Pentadecane | 1689 | EC | ND | 0.070 ± 0.003Bb | 0.063 ± 0.002Bc | 0.069 ± 0.001Ab | 0.123 ± 0.018Aa |
| EPC | 0.074 ± 0.004Ba | 0.061 ± 0.011Ba | 0.057 ± 0.012Aa | 0.149 ± 0.082Aa | |||
| IC | 0.116 ± 0.009Aa | 0.082 ± 0.007Ab | 0.067 ± 0.010Abc | 0.061 ± 0.001Bc | |||
| Pentadecane, 3-methyl- | 1748 | EC | 0.054 ± 0.002 | 0.024 ± 0.004ABab | 0.031 ± 0.005Aa | 0.019 ± 0.002Ab | 0.020 ± 0.002Ab |
| EPC | 0.018 ± 0.005Ba | 0.019 ± 0.003Ba | 0.020 ± 0.003Aa | 0.021 ± 0.002Aa | |||
| IC | 0.029 ± 0.005Aa | 0.020 ± 0.001Bb | 0.020 ± 0.004Aab | 0.021 ± 0.001Ab | |||
| Tetradecane | 1271 | EC | 0.431 ± 0.008 | 0.192 ± 0.040Aa | 0.193 ± 0.040Aa | 0.150 ± 0.011Aa | 0.143 ± 0.012Ca |
| EPC | 0.155 ± 0.035Aa | 0.162 ± 0.037Aa | 0.173 ± 0.033Aa | 0.194 ± 0.009Ba | |||
| IC | 0.221 ± 0.060Aab | 0.172 ± 0.009Ab | 0.188 ± 0.034Aab | 0.212 ± 0.005Aa | |||
| Tetradecane, 2,6,10-trimethyl- | 1708 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.025 ± 0.002a | 0.012 ± 0.001c | 0.016 ± 0.003b | 0.017 ± 0.002b | |||
| Tridecane, 3-methyl- | 1368 | EC | 0.142 ± 0.007 | ND | ND | ND | ND |
| EPC | 0.013 ± 0.001Ab | 0.020 ± 0.003Aa | 0.021 ± 0.005Aa | 0.023 ± 0.002Aa | |||
| IC | 0.020 ± 0.007Aa | 0.020 ± 0.002Aa | 0.020 ± 0.002Aa | 0.023 ± 0.001Aa | |||
| Undecane, 2,3-dimethyl- | 1169 | EC | 0.046 ± 0.003 | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Undecane, 3,7-dimethyl- | 1337 | EC | ND | ND | ND | ND | 0.048 ± 0.006 |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Undecane, 4,7-dimethyl- | 1329 | EC | ND | ND | 0.061 ± 0.015 A | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | 0.026 ± 0.008ab | 0.034 ± 0.004Ba | 0.029 ± 0.002a | 0.024 ± 0.001b | |||
| Undecane, 5,7-dimethyl- | 1337 | EC | ND | ND | ND | 0.072 ± 0.008 | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Aromatic compounds | |||||||
| 1H-Indene, 1-methylene- | 1182 | EC | 0.084 ± 0.009 | ND | ND | ND | ND |
| EPC | 0.011 ± 0.001Ba | 0.012 ± 0.001Aa | 0.016 ± 0.002Aa | 0.033 ± 0.021Aa | |||
| IC | 0.015 ± 0.001Aa | 0.011 ± 0.001Ab | 0.010 ± 0.001Bb | 0.009 ± 0.001Bb | |||
| Benzene, 1,3-dimethyl- | 859 | EC | 1.999 ± 0.086 | 0.392 ± 0.003Cb | 0.939 ± 0.041Aa | 0.528 ± 0.381Aab | 0.783 ± 0.034Aa |
| EPC | 0.750 ± 0.007Bb | 0.823 ± 0.006Aa | 0.723 ± 0.030Abc | 0.668 ± 0.038Bc | |||
| IC | 0.883 ± 0.008Aa | 0.809 ± 0.022Ab | 0.730 ± 0.040Ac | 0.668 ± 0.117ABc | |||
| Ethylbenzene | 854 | EC | 0.237 ± 0.114 | 0.025 ± 0.001Cc | 0.150 ± 0.011Aa | 0.158 ± 0.096Aab | 0.085 ± 0.025Ab |
| EPC | 0.196 ± 0.018Aa | 0.180 ± 0.033Aa | 0.105 ± 0.001Ab | 0.041 ± 0.022Bc | |||
| IC | 0.112 ± 0.063Ba | 0.122 ± 0.027Aa | 0.126 ± 0.071Aa | 0.112 ± 0.008Aa | |||
| Oxime-, methoxy-phenyl-_ | 910 | EC | 3.392 ± 1.984 | 3.122 ± 1.071Aa | 2.729 ± 0.956Aa | 2.015 ± 0.473Aa | 1.870 ± 0.332Aa |
| EPC | 2.152 ± 0.599Aa | 1.784 ± 0.456ABa | 1.803 ± 0.593Aa | 1.520 ± 0.179Aa | |||
| IC | 2.822 ± 0.993Aa | 1.738 ± 0.061Bb | 1.567 ± 0.002Bc | 1.455 ± 0.452Aabc | |||
| Styrene | 888 | EC | 1.866 ± 0.010 | 1.254 ± 0.048Aa | 1.235 ± 0.130Aa | 1.244 ± 0.053Aa | 1.298 ± 0.001Aa |
| EPC | 1.091 ± 0.012Bb | 1.218 ± 0.005Aa | 1.063 ± 0.025Bb | 0.917 ± 0.101Cab | |||
| IC | 1.224 ± 0.035Aa | 0.900 ± 0.316Ab | 1.122 ± 0.042ABb | 1.144 ± 0.019Bb | |||
| Toluene | 758 | EC | 5.854 ± 0.182 | 2.922 ± 0.672Aa | 3.641 ± 0.728Aa | 2.838 ± 0.692Aa | 3.025 ± 0.392Aa |
| EPC | 2.732 ± 0.001Ba | 2.477 ± 0.165Bb | 2.421 ± 0.383Bab | 0.988 ± 0.002Bc | |||
| IC | 2.292 ± 1.146ABa | ND | 2.355 ± 1.133Ba | 2.186 ± 1.060Aa | |||
| Esters | |||||||
| Heptanoic acid, methyl ester | 1020 | EC | 0.529 ± 0.014 | 0.253 ± 0.007Ca | 0.235 ± 0.002Ca | 0.238 ± 0.007Ca | 0.254 ± 0.030Ca |
| EPC | 0.402 ± 0.028Ac | 0.402 ± 0.007Ac | 0.430 ± 0.008Ab | 0.468 ± 0.004Aa | |||
| IC | 0.302 ± 0.010Bd | 0.337 ± 0.018Bc | 0.382 ± 0.019Bb | 0.412 ± 0.002Ba | |||
| Octanoic acid, methyl ester | 1122 | EC | 0.043 ± 0.002 | 0.014 ± 0.001Bb | 0.016 ± 0.002Aab | 0.018 ± 0.001Ba | 0.019 ± 0.001Aa |
| EPC | 0.019 ± 0.001Aa | 0.019 ± 0.002Aa | 0.019 ± 0.002ABa | 0.019 ± 0.001Aa | |||
| IC | 0.018 ± 0.001Ab | 0.019 ± 0.001Ab | 0.022 ± 0.001Aa | 0.022 ± 0.001Aa | |||
| Sulfurous acid, dodecyl hexyl ester | 1548 | EC | 0.022 ± 0.001 | 0.030 ± 0.004Aa | 0.029 ± 0.005Aa | 0.019 ± 0.002Ab | 0.018 ± 0.002ABb |
| EPC | 0.021 ± 0.008Aa | 0.019 ± 0.004Ba | 0.022 ± 0.003Aa | 0.021 ± 0.001ABa | |||
| IC | 0.027 ± 0.009Aa | 0.021 ± 0.002Ba | 0.022 ± 0.005Aa | 0.024 ± 0.003Aa | |||
| Sulfurous acid, hexyl pentadecyl ester | 1657 | EC | ND | 0.024 ± 0.001Aa | 0.025 ± 0.001Aa | 0.021 ± 0.001Ab | 0.021 ± 0.001Ab |
| EPC | 0.011 ± 0.002Ba | 0.012 ± 0.001Ba | ND | ND | |||
| IC | 0.015 ± 0.004Ba | 0.011 ± 0.002Ba | 0.015 ± 0.003Ba | 0.015 ± 0.002Ba | |||
| Sulfurous acid, hexyl tetradecyl ester | 1549 | EC | ND | ND | ND | ND | ND |
| EPC | ND | ND | ND | ND | |||
| IC | ND | 0.022 ± 0.004 | ND | ND | |||
| Acids | |||||||
| Dodecanedioic acid,2TBDMS derivative | 1473 | EC | 0.020 ± 0.005 | ND | ND | ND | ND |
| EPC | 0.016 ± 0.007a | 0.012 ± 0.001a | 0.012 ± 0.004a | ND | |||
| IC | ND | ND | ND | ND | |||
| n-Hexadecanoic acid | 1940 | EC | ND | 1.016 ± 0.031a | 0.022 ± 0.003b | 0.014 ± 0.001c | 0.030 ± 0.016bc |
| EPC | ND | ND | ND | ND | |||
| IC | ND | ND | ND | ND | |||
| Phosphonoacetic Acid,3TMS derivative | 1230 | EC | 0.067 ± 0.022 | ND | ND | ND | ND |
| EPC | 0.085 ± 0.010Ab | 0.069 ± 0.015Ab | 0.100 ± 0.001Aa | 0.074 ± 0.014Ab | |||
| IC | 0.108 ± 0.029Aa | 0.076 ± 0.001Ab | 0.051 ± 0.017Bc | 0.055 ± 0.012Ac | |||
| Tetradecanoic acid, 2-hydroxy- | 1204 | EC | ND | ND | ND | ND | ND |
| EPC | ND | 0.009 ± 0.001Aa | 0.009 ± 0.002Aa | 0.011 ± 0.003Aa | |||
| IC | ND | 0.010 ± 0.001Aa | 0.008 ± 0.001Aa | 0.009 ± 0.001Aa | |||
| Ketones | |||||||
| 3-Hexanone, 2,5-dimethyl- | 933 | EC | 1.482 ± 0.032 | 1.248 ± 0.005Aa | 1.120 ± 0.011Ac | 1.158 ± 0.032Bbc | 1.176 ± 0.035Cb |
| EPC | 1.178 ± 0.094ABab | 1.129 ± 0.062Ab | 1.171 ± 0.025ABb | 1.239 ± 0.020Ba | |||
| IC | 1.134 ± 0.012Bb | 1.128 ± 0.038Ab | 1.262 ± 0.068Aa | 1.283 ± 0.010Aa | |||
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. RI: retention indices; EPC: Maize porridge prepared in the electric pressure cooker; EC: Maize porridge prepared in the electromagnetic oven; IC: Maize porridge prepared in the electric rice cooker.
In addition to undecanal, four saturated aldehydes (vanillin, nonanal, decanal, and hexanal) that were considered to be the major flavor contributors to grains, were found in maize porridge. The four key volatile compounds maintain stability during cooking, particularly in EPC. Notably, the content in EPC was higher compared to EC and IC. Hexanal, the main product of linoleic acid oxidation, was reported to be abundant in wheat starch, maize and potato (Pico, Martínez, Bernal, & Gómez, 2017), with the green, grassy flavor (Nedele, Gross, Rigling, & Zhang, 2021). The substance was detected in all stages of the three cooking methods, with the highest level of 1.622 μg/kg in uncooked maize. The content in EPC was higher than that in the other cooking methods. The level of enzymatic reaction and autoxidation in various cooking methods could potentially be a contributing factor. Additionally, the high tightness in EPC might be another factor. 2,4-decadienal was a volatile compound produced by lipid oxidation that can be continuously consumed by more chemical reactions such as Maillard reaction, oxidation and degradation (do N. Batista et al., 2015). 2,4-decadienal, 2,4-dodecadienal and heptanal were only found in EPC. Nonanal, which had a volatile flavor of beef fat and green grass, was produced by oxidation (Huang et al., 2022). Compared with EC and IC, nonanal was highly expressed in EPC and peaked at EPC-60 min (0.732 μg/kg). There was an increasing trend in EC and EPC, but a decreasing trend in IC. Substances like (E)-2-octenal (0.278 μg/kg) were found only in raw maize. Vanillin was reported as a kind of important volatile compound and flavor in natural herbs (Martău, Călinoiu, & Vodnar, 2021). It was detected in this study by gas chromatography. Maize porridge samples in three different cooking methods all had high vanillin contents. The contents in three cooking methods all peaked at 30 min.
Alcohol volatiles are formed by the oxidative decomposition of unsaturated fatty acids (Zhang et al., 2018b). 1-octen-3-ol had a low odor threshold with the scent of mushrooms and herbs, and it plays an important role in the maize porridge (Gao et al., 2023). However, in this study, 1-octen-3-ol (0.357 μg/kg) was only identified in uncooked maize. The reasons for this were threefold: first, the water vapor took away the small molecular alcohols with lower boiling points; second, the inactivation of some alcohol reductase during heating might block the production of alcohols; third, alcohols might undergo esterification, condensation, cracking, and other reactions at higher temperatures (Zhang, Gao, et al., 2022). 1-Hexadecanol was the only alcohol that was detected in all three cooking methods. The content peaked at IC60-min (0.045 μg/kg). 1-Undecanol, was presented in uncooked maize but almost undetectable after cooking. This could also be due to the destructibility of starch by heat treatment, resulting in the evaporation of alcohol. (Li et al., 2021).
Hydrocarbons were one of the largest types of volatile compounds in this study, however, their contribution to the overall flavor is not significant due to their high aroma threshold (Ekpa et al., 2021). Ketones are products of fatty acid oxidative degradation (Shi et al., 2018) and most ketones possess a relatively high odor threshold when compared to other lipid-derived aroma compounds such as aldehydes or esters (Lomelí-Martín, Martínez, Welti-Chanes, & Escobedo-Avellaneda, 2021). 2,5-dimethyl-3-hexanone maintained a high content and peaked at a maximum of 1.482 μg/kg in uncooked maize. The concentration of 2,5-dimethyl-3-hexanone was recorded at its highest level at 30 min in EC, while it reached the maximum value at 60 min in EPC and IC.
Esters were synthesized through the dehydration condensation of fatty acids and alcohols, serving as a significant source of flavor in various flowers and fruits (Yan et al., 2018). Due to their low odor threshold, esters played a crucial role in maize porridge. Octanoic acid methyl ester, known for its sweet orange and wine flavor, exhibited an increasing concentration with longer cooking time in all three cooking methods. The highest concentration was found in uncooked maize (0.043 μg/kg). Heptanoic acid methyl ester, commonly employed in spice synthesis, was detected in all three cooking methods. Nonetheless, its concentration was consistently higher in EPC compared to the other methods. This may be related to the fact that a certain degree of pressure enhances esterification reactions, as previously discussed.
3.2. Influence of cooking methods on texture properties of maize porridge
The texture of maize porridge was examined using a double-cycle compression program. Seven indices were employed to assess its texture characteristics: hardness, adhesiveness, cohesiveness, elasticity, gumminess, chewiness, and resilience. Table 2 showed the results of a one-way analysis of variance (ANOVA) conducted for the cooking method and time variables, as well as a repeated measurements analysis employing a double-factor variance. The analysis was conducted across seven different indicators. In SPSS, P value was a commonly used statistical indicator to determine a parameter of the result of hypothesis test, the probability of sample observation or more extreme results when the null hypothesis is true (Leech, Barrett, & Morgan, 2014). A lower P value indicated a more significant difference in maize porridge texture properties. Therefore, we can use P value to measure whether cooking method and time have a significant effect on the texture of maize porridge. Table 2 showed that both cooking methods and time significantly affect the texture of maize porridge, with a P value <0.05 in at least one case in one-factor and two-factor conditions. Among them, the effect on hardness and elasticity was apparent. On the whole, the impact of cooking methods was more significant than that of cooking time on the textural properties.
Table 2.
Effect of time and cooker on texture properties of maize porridge samples.
| Cooking time/min |
P |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Units | Cooker | 30 | 40 | 50 | 60 | M | T | M × T | |
| Hardness | N | EC | 33.10 ± 5.75Aa | 24.40 ± 3.80Ab | 22.23 ± 1.75Ab | 19.64 ± 2.82Ab | *** | *** | NS |
| EPC | 15.28 ± 1.69Ca | 14.51 ± 1.61Ca | 10.52 ± 2.03Cb | 9.25 ± 0.45Cb | |||||
| IC | 22.41 ± 4.62Ba | 19.40 ± 2.25Ba | 14.59 ± 2.06Bb | 13.62 ± 1.05Bb | |||||
| Adhesiveness | N.mm | EC | 2.13 ± 0.54Ab | 2.60 ± 0.21Aab | 2.73 ± 0.19Aab | 3.52 ± 1.51Aa | * | NS | NS |
| EPC | 2.37 ± 0.33Aa | 2.17 ± 0.22Aa | 2.40 ± 0.33Aa | 1.53 ± 0.28Bb | |||||
| IC | 2.46 ± 0.67Aa | 3.30 ± 1.70Aa | 3.24 ± 1.03Aa | 3.02 ± 0.86ABa | |||||
| Cohesiveness | Ratio | EC | 0.09 ± 0.03Aa | 0.10 ± 0.01Aa | 0.11 ± 0.01Ca | 0.11 ± 0.02Aa | NS | *** | ** |
| EPC | 0.10 ± 0.01Ac | 0.11 ± 0.01Ac | 0.15 ± 0.01Aa | 0.13 ± 0.01Ab | |||||
| IC | 0.09 ± 0.01Abc | 0.07 ± 0.01Bc | 0.13 ± 0.02Bab | 0.17 ± 0.06Aa | |||||
| Elasticity | mm | EC | 4.90 ± 2.60Bb | 7.14 ± 1.04Aa | 7.54 ± 0.75Aa | 7.69 ± 0.59Aa | *** | *** | *** |
| EPC | 7.34 ± 1.04Aa | 6.14 ± 1.34Aab | 4.25 ± 0.77Bc | 5.02 ± 0.81Bbc | |||||
| IC | 4.94 ± 1.25Bb | 6.47 ± 1.82Aa | 8.33 ± 0.88Aa | 8.00 ± 1.18Aa | |||||
| Gumminess | N | EC | 3.00 ± 1.41Aa | 2.49 ± 0.49Aa | 2.42 ± 0.19Aa | 2.23 ± 0.32Aa | *** | NS | NS |
| EPC | 1.46 ± 0.19Bab | 1.57 ± 0.05Ba | 1.59 ± 0.37Ba | 1.19 ± 0.08Bb | |||||
| IC | 1.92 ± 0.26ABab | 1.40 ± 0.19Bb | 1.90 ± 0.30Bab | 2.26 ± 0.69Aa | |||||
| Chewiness | mj | EC | 12.59 ± 2.64Ab | 17.60 ± 2.89Aa | 18.17 ± 1.63Aa | 17.09 ± 1.84Aa | *** | * | *** |
| EPC | 10.74 ± 2.13ABa | 9.67 ± 2.23Ba | 6.97 ± 2.76Bab | 6.02 ± 1.15Bb | |||||
| IC | 9.66 ± 3.36Bb | 9.24 ± 3.48Bb | 15.73 ± 1.57Aa | 17.48 ± 2.51Aa | |||||
| Resilience | EC | 0.04 ± 0.02Aa | 0.03 ± 0.00Aa | 0.02 ± 0.00Aa | 0.02 ± 0.01Aa | *** | NS | NS | |
| EPC | 0.01 ± 0.00Bb | 0.02 ± 0.00Bab | 0.02 ± 0.00Aa | 0.01 ± 0.00Aab | |||||
| IC | 0.02 ± 0.00ABa | 0.02 ± 0.00Ba | 0.02 ± 0.01Aa | 0.02 ± 0.01Aa | |||||
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. NS, P > 0.05; *, 0.01 < P < 0.05, **, 0.001 < P < 0.01, ***, P < 0.001. Abbreviations are: M, cooking method; T, time; EPC: Maize porridge prepared in the electric pressure cooker; EC: Maize porridge prepared in the electromagnetic oven; IC: Maize porridge prepared in the electric rice cooker.
Hardness was the main physical parameter applied in the food industry to select maize for food use (Beswa, Siwela, Amonsou, & Kolanisi, 2020). As presented in Table 2, the hardness of three cooking methods all peaked at 30 min and decreased with time. The hardness of maize porridge was largely determined by the starch particles. When heated, water diffusion into the maize was sufficient and the starch located in the central part of the maize became fully gelatinized, which may explain the observed decrease in hardness (Li, Wen, Wang, & Sun, 2017). It was notable that the initial 50 min of the cooking process showed a more pronounced hardness alteration compared to later durations. More particularly, the maize porridge prepared in EPC consistently exhibited lower hardness values than that cooked in EC and IC. This difference can be attributed to the high-pressure environment induced by the EPC, causing the starch particles in the maize to change different shapes and rearrange. The rearrangement of particles may lead to the formation of micropores, which can absorb and retain larger amounts of water through capillarity (Cappa, Lucisano, Barbosa-Cánovas, & Mariotti, 2016). The increase in moisture content made the grain gelatinized more fully. Ultimately, it resulted in a decrease in both hardness and elasticity (Wu et al., 2019).
Elasticity refered to the ability of food to deform when exposed to force and then recover its original shape when the force is removed. Table 2 showed that the elasticity of maize porridge in EPC tend to decrease, while it increased in IC and EC, which could be explained by the statement made by Wu et al. (2019). Meanwhile, the maximum elasticity was observed at IC-50 min (8.33 mm).
3.3. Sensory assessment of different maize porridge samples
Sensory evaluation provided a more intuitive response to consumer preferences. The study evaluated the sensory characteristics of maize porridge using five indicators, as shown in Table 3. The primary factor affecting the overall score was identified as the hardness of the porridge. The texture score decreased with cooking time, corresponding to the observed decrease in porridge texture hardness during the texture analysis. Moreover, the EPC consistently maintained a lower hardness score, which was causally linked to its high pressure. This finding aligns with the results of the texture analyses conducted above. Among the three cooking methods, the IC had the highest cereal flavor score during the first 30 min. It was speculated that the rapid heating rate of the IC allowed the volatile compounds to be emitted in a shorter time. On the contrary, in the final 60 min, EPC achieved the highest score of cereal aroma due to its better airtightness compared with EC and IC. EPC-60 min had the highest comprehensive sensory score. At 40 and 50 min, the comprehensive sensory scores of IC were the highest. Nevertheless, there was no significant difference in scores between EPC and IC at this time. In addition, the highest comprehensive sensory scores at 30 and 60 min were in EPC. Color, aroma, and sweetness almostly showed the highest scores under the EPC condition. For consumers who prefer soft food, EPC was a good choice.
Table 3.
Effect of time and cooker on sensory evaluation of maize porridge samples.
| Cooking time/min |
|||||
|---|---|---|---|---|---|
| Cooker | 30 | 40 | 50 | 60 | |
| Appearance | |||||
| Color | EC | 4.70 ± 0.98Bc | 6.20 ± 0.85Bb | 7.15 ± 1.12Ba | 7.30 ± 1.42Aa |
| EPC | 6.45 ± 0.65Ad | 7.70 ± 0.54Ac | 8.25 ± 0.52Ab | 8.65 ± 0.47Aa | |
| IC | 5.15 ± 0.77Bc | 7.30 ± 0.76Ab | 7.60 ± 0.72Bab | 7.85 ± 0.83Aa | |
| Texture | |||||
| Hardness | EC | 8.85 ± 0.35Aa | 6.95 ± 0.79Ab | 6.80 ± 1.00Ab | 4.95 ± 0.72Ac |
| EPC | 4.75 ± 0.97Ca | 4.70 ± 0.76Ca | 3.05 ± 0.58Cb | 2.25 ± 0.68Bc | |
| IC | 7.15 ± 0.77Ba | 6.09 ± 0.76Ba | 4.85 ± 0.89Bb | 4.05 ± 1.00Ac | |
| Aroma | |||||
| Cereal | EC | 4.95 ± 0.84Cc | 5.85 ± 0.71Cb | 5.95 ± 0.84Cb | 6.80 ± 0.85Ba |
| EPC | 7.00 ± 0.62Bc | 8.15 ± 0.77Ab | 8.05 ± 0.72Ab | 8.85 ± 0.35Aa | |
| IC | 8.15 ± 0.83Aa | 7.05 ± 0.84Bb | 6.90 ± 0.75Bb | 6.85 ± 0.64Bb | |
| Taste | |||||
| Sweet | EC | 5.65 ± 0.83Bc | 6.90 ± 1.11Ab | 7.90 ± 0.75Aa | 8.17 ± 0.58Aa |
| EPC | 7.15 ± 0.71Ab | 7.55 ± 1.29Ab | 8.20 ± 0.66Aa | 8.75 ± 0.42Aa | |
| IC | 6.05 ± 0.79Bc | 7.50 ± 0.79Ab | 8.05 ± 0.72Aa | 8.50 ± 0.79Aa | |
| Comprehensive score | EC | 6.55 ± 0.65Ac | 7.00 ± 0.44Bb | 7.35 ± 0.56Bb | 8.15 ± 0.56Ba |
| EPC | 6.85 ± 0.47Ad | 7.65 ± 0.71Ac | 8.25 ± 0.68Ab | 8.65 ± 0.47Aa | |
| IC | 4.60 ± 1.09Bc | 7.80 ± 0.96Aab | 8.35 ± 0.64Aa | 7.25 ± 0.92Cb | |
Values represent the means ± SD; Different superscript lowercase letters indicate significant differences (P < 0.05) within a row; different superscript uppercase letters indicate significant differences (P < 0.05) within a column. EPC: Maize porridge prepared in the electric pressure cooker; EC: Maize porridge prepared in the electromagnetic oven; IC: Maize porridge prepared in the electric rice cooker.
4. Conclusions
This study explored the effect of three different cooking methods on volatile compounds and texture of maize porridge by a multifaceted method, including HS-SPME combined with GC–MS, multiple chemometrics analysis, and texture analysis. A total of 51 volatile compounds were identified, including 6 aromatic compounds, 4 alcohols, 19 hydrocarbons, 5 esters, 12 aldehydes, 4 acids, and 1 ketone. PCA results showed that cooking methods had a significant effect on volatile compounds. The considerable amounts of aldehydes and aromatic compounds indicated their importance in maize porridge. Moreover, the results from texture analysis revealed that both cooking time and method played a crucial role, with different cooking methods showing a more pronounced effect than cooking time. The comprehensive score peaked at EPC-60 min, and sensory evaluation of maize porridge in EPC was positive at different cooking time. Thereby, the EPC cooking method was strongly recommended. In summary, the study results may offer new insights into the sensory improvement of maize porridge and provide theoretical and technical references for industrial production.
Ethical statement
In this project, the rights and interests of the subjects are fully protected and meet the ethical requirements, and was approved by the Qingdao University Ethics committee (No. QDU-HEC-2023159).
CRediT authorship contribution statement
Shihao Wang: Writing – review & editing, Investigation. Kaixuan Chen: Writing – original draft. Ailing Tian: Data curation. Meifan Pan: Formal analysis. Xinyang Liu: Software. Lingyun Qu: Formal analysis. Jin Jin: Validation. Sijie Lv: Validation. Yanqiu Xu: Formal analysis. Wenzhe Yang: Funding acquisition. Xinfang Zhang: Software. Lili Zheng: Writing – original draft. Yani Zhang: Supervision. Xueliang Yang: Writing – review & editing. Feng Zhong: Writing – review & editing. Lirong Xu: Writing – review & editing, Conceptualization. Aiguo Ma: Writing – review & editing, Conceptualization.
Declaration of competing interest
This is a manuscript prepared by Kaixuan Chen et al. entitled “Effect of Cooking Methods on Volatile Compounds and Texture Properties in Maize Porridge” It is submitted to be considered for publication as an “article” in “Food Chemistry: X". Neither the full paper nor any part of its content has been published or has been submitted to any other journal for publication consideration. All the authors listed have approved the manuscript that is enclosed. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was financed by the National Natural Science Foundation of China (Project No.32202083), China Postdoctoral Science Foundation (Project No.2023 M731849), Qingdao Postdoctoral Innovation Project (Project No. QDBSH20220202216).
Data availability
Data will be made available on request.
References
- Beswa D., Siwela M., Amonsou E.O., Kolanisi U. Grain quality, provitamin a carotenoid profiles, and sensory quality of provitamin a-biofortified maize stiff porridges. Foods. 2020;9(12):1909. doi: 10.3390/foods9121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappa C., Lucisano M., Barbosa-Cánovas G.V., Mariotti M. Physical and structural changes induced by high pressure on corn starch, rice flour and waxy rice flour. Food Research International. 2016;85:95–103. doi: 10.1016/j.foodres.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Chen X., Chen H., Xiao J., Liu J., Tang N., Zhou A. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Research International. 2020;138 doi: 10.1016/j.foodres.2020.109717. [DOI] [PubMed] [Google Scholar]
- Ekpa O., Fogliano V., Linnemann A. Identification of the volatile profiles of 22 traditional and newly bred maize varieties and their porridges by PTR-QiTOF-MS and HS-SPME GC-MS. Journal of the Science of Food and Agriculture. 2021;101(4):1618–1628. doi: 10.1002/jsfa.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Liu M., Zheng L., Zhang T., Chang X., Liu H.…Sun J. Comparative analysis of key odorants and aroma characteristics in hot-pressed yellow horn (Xanthoceras sorbifolia bunge) seed oil via gas chromatography–ion mobility spectrometry and gas chromatography–olfactory-mass spectrometry. Foods. 2023;12(17):3174. doi: 10.3390/foods12173174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre M., Groenen P.J., Hastie T., d’Enza A.I., Markos A., Tuzhilina E. Principal component analysis. Nature Reviews Methods Primers. 2022;2(1):100. [Google Scholar]
- Guo K., Zhang Y., Zhang H., Wang L., Song H., Li Z. Odor changes in breast milk during different storage temperatures and times using GC× GC-O-MS. Food Research International. 2023;168 doi: 10.1016/j.foodres.2023.112792. [DOI] [PubMed] [Google Scholar]
- Holt S., Miks M.H., de Carvalho B.T., Foulquié-Moreno M.R., Thevelein J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiology Reviews. 2019;43(3):193–222. doi: 10.1093/femsre/fuy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Lu L., Guo Z., Zhu Z. Volatile compounds, affecting factors and evaluation methods for rice aroma: a review. Trends in Food Science & Technology. 2020;97:136–146. [Google Scholar]
- Huang Q., Dong K., Wang Q., Huang X., Wang G., An F.…Luo P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131103. [DOI] [PubMed] [Google Scholar]
- Krause S., Keller S., Hashemi A., Descharles N., Bonazzi C., Rega B. From flours to cakes: reactivity potential of pulse ingredients to generate volatile compounds impacting the quality of processed foods. Food Chemistry. 2022;371 doi: 10.1016/j.foodchem.2021.131379. [DOI] [PubMed] [Google Scholar]
- Leech N.L., Barrett K.C., Morgan G.A. Routledge; 2014. IBM SPSS for intermediate statistics: Use and interpretation. [Google Scholar]
- Li H., Wen Y., Wang J., Sun B. The molecular structures of leached starch during rice cooking are controlled by thermodynamic effects, rather than kinetic effects. Food Hydrocolloids. 2017;73:295–299. [Google Scholar]
- Li Y., Li Y., Chen Z., Bu L., Shi F., Huang J. High-temperature air fluidization improves cooking and eating quality and storage stability of brown rice. Innovative Food Science & Emerging Technologies. 2021;67 [Google Scholar]
- Lomelí-Martín A., Martínez L.M., Welti-Chanes J., Escobedo-Avellaneda Z. Induced changes in aroma compounds of foods treated with high hydrostatic pressure: a review. Foods. 2021;10(4):878. doi: 10.3390/foods10040878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martău G.A., Călinoiu L.F., Vodnar D.C. Bio-vanillin: towards a sustainable industrial production. Trends in Food Science & Technology. 2021;109:579–592. [Google Scholar]
- Mestres C., Briffaz A., Valentin D. Rice. AACC International Press; 2019. Rice cooking and sensory quality; pp. 385–426. [Google Scholar]
- do N. Batista L., Da Silva V.F., Pissurno É.C., da Conceição Soares T., de Jesus M.R., Kunigami C.N.…da Fonseca M.G. Formation of toxic hexanal, 2-heptenal and 2, 4-decadienal during biodiesel storage and oxidation. Environmental Chemistry Letters. 2015;13:353–358. [Google Scholar]
- Nedele A.K., Gross S., Rigling M., Zhang Y. Reduction of green off-flavor compounds: comparison of key odorants during fermentation of soy drink with lycoperdon pyriforme. Food Chemistry. 2021;334 doi: 10.1016/j.foodchem.2020.127591. [DOI] [PubMed] [Google Scholar]
- Pico J., Martínez M.M., Bernal J., Gómez M. Evolution of volatile compounds in gluten-free bread: from dough to crumb. Food Chemistry. 2017;227:179–186. doi: 10.1016/j.foodchem.2017.01.098. [DOI] [PubMed] [Google Scholar]
- Pico J., Tapia J., Bernal J., Gómez M. Comparison of different extraction methodologies for the analysis of volatile compounds in gluten-free flours and corn starch by GC/QTOF. Food Chemistry. 2018;267:303–312. doi: 10.1016/j.foodchem.2017.06.157. [DOI] [PubMed] [Google Scholar]
- Poole N., Donovan J., Erenstein O. Agri-nutrition research: revisiting the contribution of maize and wheat to human nutrition and health. Food Policy. 2021;100 doi: 10.1016/j.foodpol.2020.101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang L., Fang Y., Wang H., Tao H., Pei F.…Hu Q. A comprehensive analysis of aroma compounds and microstructure changes in brown rice during roasting process. LWT. 2018;98:613–621. [Google Scholar]
- Song X., Porter M.E., Whitaker V.M., Lee S., Wang Y. Identification of ethyl vanillin in strawberry (Fragaria× ananassa) using a targeted metabolomics strategy: From artificial to natural. Food Chemistry: X. 2023;20:100944. doi: 10.1016/j.fochx.2023.100944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar R., Panghal A., Chaudhary G., Kumari A., Chhikara N. A Review. Food Chemistry Advances; Phytochemical and Functional Potential of Sorghum: 2023. Nutritional; p. 100501. [Google Scholar]
- Wang S., Tian A., Zhao K., Zhang R., Lei Z., Qin X.…Ma A. Effect of cooking methods on volatile compounds and texture properties in rice porridge. LWT. 2023;115111 [Google Scholar]
- Wu T., Wang L., Li Y., Qian H., Liu L., Tong L.…Zhou S. Effect of milling methods on the properties of rice flour and gluten-free rice bread. LWT. 2019;108:137–144. [Google Scholar]
- Xu L., Mei X., Wu G., Karrar E., Jin Q., Wang X. Inhibitory effect of antioxidants on key off-odors in French fries and oils and prolong the optimum frying stage. LWT. 2022;162 [Google Scholar]
- Yan J.W., Ban Z.J., Lu H.Y., Li D., Poverenov E., Luo Z.S., Li L. The aroma volatile repertoire in strawberry fruit: a review. Journal of the Science of Food and Agriculture. 2018;98(12):4395–4402. doi: 10.1002/jsfa.9039. [DOI] [PubMed] [Google Scholar]
- Yu C., Zhu L., Zhang H., Bi S., Wu G., Qi X.…Zhou L. Effect of cooking pressure on phenolic compounds, gamma-aminobutyric acid, antioxidant activity and volatile compounds of brown rice. Journal of Cereal Science. 2021;97 [Google Scholar]
- Zhang K., Gao L., Zhang C., Feng T., Zhuang H. Analysis of volatile flavor compounds of corn under different treatments by GC-MS and GC-IMS. Frontiers in Chemistry. 2022;10 doi: 10.3389/fchem.2022.725208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wan C., Wang C., Chen H., Liu Y., Li S.…Qin W. Evaluation of the non-aldehyde volatile compounds formed during deep-fat frying process. Food Chemistry. 2018;243:151–161. doi: 10.1016/j.foodchem.2017.09.121. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lv H., Yang B., Zheng P., Zhang H., Wang X., Granvogl M., Jin Q. Characterization of thermally induced flavor compounds from the Glucosinolate Progoitrin in different matrices via GC-TOF-MS. Journal of Agricultural and Food Chemistry. 2022;70(4):1232–1240. doi: 10.1021/acs.jafc.1c04415. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang N., Fray R.G., Fisk I., Liu C., Li H., Han Y. Characterization of volatile aroma compounds after in-vial cooking of foxtail millet porridge with gas chromatography-mass spectrometry. Journal of Cereal Science. 2018;82:8–15. [Google Scholar]
- Zhang Y., Zhang S., Fan W., Duan M., Han Y., Li H. Identification of volatile compounds and odour activity values in quinoa porridge by gas chromatography–mass spectrometry. Journal of the Science of Food and Agriculture. 2019;99(8):3957–3966. doi: 10.1002/jsfa.9621. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Wang Z., Xiong F., Zhang G. Enzyme inactivation induced by thermal stabilization in highland barley and impact on lipid oxidation and aroma profiles. Frontiers in Nutrition. 2023;10:1097775. doi: 10.3389/fnut.2023.1097775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Yu C., Yin X., Wu G., Zhang H. Effects of soaking on the volatile compounds, textural property, phytochemical contents, and antioxidant capacity of brown rice. Foods. 2022;11(22):3699. doi: 10.3390/foods11223699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Chen S., Yin H., Han X., Xu M., Wang W.…Liu Y. Classification of oolong tea varieties based on computer vision and convolutional neural networks. Journal of the Science of Food and Agriculture. 2024;104(3):1630–1637. doi: 10.1002/jsfa.13049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.