Highlights
-
•
Succinyltransferase CPT1A was highly elevated in ENKTL-NT and was associated with a dismal prognosis.
-
•
CPT1A induces succinylation of 14–3–3theta at the K85 site, promoting ENKTL-NT proliferation.
-
•
Paclitaxel combined with knockdown of CPT1A significantly inhibited the proliferation of ENKTL-NT compared to paclitaxel alone.
Keywords: Extranodal natural killer/T-cell lymphoma, Carnitine palmitoyltrasferase 1A, 14–3–3theta, Succinylation, Proliferation, Apoptosis
Abstract
Background
The aggressive and refractory extranodal natural killer/T-cell lymphoma, nasal type (ENKTL-NT) is a subtype of non-Hodgkin's lymphoma. Succinylation promotes progression in a variety of tumors, but its mechanism in ENKTL-NT is unclear.
Methods
Bioinformatic analysis was performed to screen differentially expressed genes in the ENKTL dataset. Cell transfection techniques were used for knockdown and overexpression of genes. The mRNA and protein expression were detected using RT-qPCR and western blot, respectively. Immunohistochemical staining was used to assess protein expression in situ. For the detection of cell proliferation activity, CCK-8, clonal formation, and EDU staining assays were used. Flow cytometry was employed to detect apoptosis. Co-immunoprecipitation was utilized for the identification of protein interactions and succinylation modifications.
Results
Succinyltransferase CPT1A was highly elevated in ENKTL-NT and was associated with a dismal prognosis. CPT1A knockdown suppressed SNK-6 cells’ proliferation and induced apoptosis, while these effects were reversed by the overexpression of 14–3–3theta. Co-immunoprecipitation results showed that CPT1A caused succinylation of 14–3–3theta at site of K85, thereby enhancing the protein stability. Suppression of CPT1A-induced succinylation of 14–3–3theta by ST1326 resulted in the inhibition of SNK-6 cell proliferation and increased apoptosis. Paclitaxel combined with knockdown of CPT1A significantly inhibited the proliferation of ENKTL-NT compared to paclitaxel alone.
Conclusion
CPT1A induces succinylation of 14–3–3theta at the K85 site, promoting ENKTL-NT proliferation. The anti-ENKTL activity of paclitaxel was improved when combined with CPT1A knockdown.
Introduction
NK/T cell lymphoma (NK/T cell lymphoma), a subtype of non-Hodgkin lymphoma (NHL), is derived from abnormal transformation of natural killer (NK)-cells and cytotoxic T-cells. NKTL is relatively common in Asia, compared to other regions, such as Latin America, Europe and North America. NKTLs can be classified to be nodal (nNKTL) and extra-nodal (eNKTL). Most eNKTLs occur in the nasal cavity, thereby commonly termed nasal-type eNKTL (ENKTL-NT)[1,2]. ENKTL-NT is an aggressive malignancy with a dismal prognosis. In the development of this aggressive tumor, ENKTL-NT can escape immune surveillance based on the abnormally changed molecular characteristics of the tumor and disordered immune function of the patients[[2], [3], [4]]. Immunotherapy with anti-PD-1, anti-CD30 and anti-CD38 monoclonal antibodies holds great promise as a novel therapeutic option[[2], [3], [4]]. To date, radiation therapy, immunotherapy and chemotherapy based on asparaginase were commonly adopted to treat the cancer[2]. However, treatment of patients with stage III/IV NK/T-cell lymphoma or those who have relapsed or are refractory remains a major challenge. Further understanding the pathogenesis of this disease is necessary to find more effective target for the therapy.
Succinylation is a post-translational protein modification involving the attachment of a succinyl group from a succinyl donor to a lysine residue. Succinylation can alter the processes of enzymes and metabolic pathways such as theglycolysis, tricarboxylic acid cycle, ketone body formation, the electron transport chain,fatty acid oxidation, and the urea cycle. The succinyltransferases identified are CPT1A, p300, and KAT2A, and the desuccinylases SIRT5 and SIRT7. Overexpression of CPT1A, the rate-limiting enzyme in the fatty acid beta-oxidation pathway, may cause a switch from glycolysis to lipolysis in cells[5]. In contrast, p300 and KAT2A have important histone acetyltransferase activities and are involved in biological processes such as DNA repair, transcriptional regulation, proliferation, and differentiation in the nucleus.
CPT1A, which is found on the outer mitochondrial membrane, converts acyl-CoA to acyl-carnitine in order to transfer fatty acids into the mitochondria for further oxidation[6]. In addition to its function as an fatty acid oxidase enzyme in cancer cells, CPT1A has been implicated in various cellular processes, as interacting proteins that regulate lipid metabolism via fatty acid oxidase have been identified in nasopharyngeal carcinoma[7], breast cancer[8], and lymphocytic leukemia[9] in the context of tumorigenesis. CPT1A expression levels are markedly elevated in NK/T cell lymphomas, and this elevation correlates with tumor invasion, according to previous research[10]. The researchers additionally confirmed that inhibiting CPT1A caused NK/T cell lymphoma cells to undergo apoptosis and inhibited tumor migration. Further experiments suggest that this effect may be related to the metabolic reprogramming of tumor cells. In addition, studies have shown that several natural products and chemical agents have the ability to inhibit CPT1A and its associated signaling pathways and could be used as a potential treatment strategy for ENKTL[11].
The 14–3–3 family, a group of highly conserved regulatory molecular chaperones, was involved in cell cycle processes, cell signaling, and metabolic regulation[12]. 14–3–3 theta can regulate the function of these proteins and related biological processes by binding to other proteins[13]. 14–3–3 theta is involved in the proliferation and metastasis of HER2-positive breast cancer cells and has potential clinical applications in the prognostic assessment of tumors. However, studies on the role of 14–3–3 theta in extranodal natural killer/T-cell lymphoma, nasal type (ENKTL-NT) are currently unclear.
In this experiment, bioinformatic analysis of 20 ENKTL-NT and 19 intra-lymph node NK/T lymphomas (NKTL) revealed that ENKTL-NT showed substantial expression of the succinyltransferase CPT1A and that high expression of CPT1A in ENKTL-NT indicated a poor prognosis. CPT1A caused the succinylation of 14–3–3theta at the K85 site to promote proliferation of ENKTL-NT. Combining paclitaxel with CPT1A knockdown increased anti-ENKTL efficacy of paclitaxel.
Materials and methods
Bioinformatic analysis
The NK/T-cell lymphoma dataset GSE90597 was downloaded from the Gene Expression Omnibus database[14]. The dataset consists of 66 cases of NK/T cell lymphoma, including 19 cases of intranodal lymphomas and 47 cases of extranodal lymphomas (20 nasal type NK/T lymphomas, 15 non-nasopharyngeal, and 12 unknown types).Differential analysis of genes was performed using the Limma package of the R software (version 4.0) with a p-value< 0.05 and a log2 (fold change) >1.5. Heat maps, volcano maps, and principal component analysis of differentially expressed genes were performed using the pheatmap package, the ggplot2 package, and the ggcorrplot package, respectively. CPT1A expression was divided into high and low expression groups based on median gene expression, followed by a Kaplan-Meier survival curve analysis of CPT1A on overall survival (OS).
Drug sensitivity prediction
We used all ENKTL-NT samples from the GSE90597 dataset to predict the sensitivity of the drug. Drug IC50 values were predicted using the oncoPredict R program and the Genomics of Drug Sensitivity in Cancer (GDSC) database.
Magnetic bead sorting of NK cells
Blood from healthy adults was mixed with tissue diluent (TBDscience, China, cat: 2010C1119) in a 1:1 ratio. The mixture was added to an equal volume of cell separation solution and centrifuged for 15 min at a speed of 1500 rpm. The second layer of cells was then transferred to a centrifuge tube containing 5 ml of cell wash (TBDscience, China, cat: 2010 × 1118), mixed thoroughly, and centrifuged for 5 min at 1800 rpm. NK cells were sorted by Magnetic beads using the NK Cell Isolation Kit, human (Miltenyi, Germany, cat: 130–092–657). Subsequently, the cell concentration was adjusted to 1 × 10^7 cells/100μL. The cells were resuspended in 200 μl PBS containing anti-human CD3-FITC and anti-human CD56-PE and incubated for 15 min at 4 °C in the dark. The cell isolation refer to previous methods[15]. The CD3-negative and CD56- positive cells were identified as NK cells
Cell culture and pharmaceutical interventions
All cells were cultured in medium containing 10% fetal bovine serum (Gibco, USA) in a cell culture incubator at 37 °C, 5% CO2, and saturated humidity. YTS, Jurkat and SNK-6 cells were cultured in RPMI-1640 medium (Gibco, USA), RPMI-1640 medium (Gibco, USA) and RPMI-1640 medium (Gibco, USA) with 1000 U/ml interleukin-2 (Sigma-Aldrich, USA), respectively. NK cells were cultured in X-VIVO 10 medium (Lonza, Switzerland) with 10 ng/ml IL-15 (Peprotech, USA). NK-92 cells were cultured in α-MEM medium (Life Technologies, Germany) with 150 U/ml interleukin-2 (Sigma-Aldrich, USA). With reference to the concentration and stimulation time of ST1326 in previous literature, 2 μM, 4 μM and 8 μM of ST1326 was used to culture cells for 48 h[9].
4D label-free succinylation modification quantitative proteome
This experiment was performed to detect succinylation in SNK-6 cells using the 4D Label-Free Succinylation Modification Quantitative Proteome from Hangzhou Jingjie Biotechnology Co.,Ltd..SNK-6 cells from CPT1A SiRNA knockdown (n = 3) and SiRNA-normal control (NC) (n = 3) groups were collected. Under sonication, cells were lysed by adding a lysate buffer containing 8 M urea, 1% protease inhibitor, 3 M TSA, and 50 mM NAM. Enzymatic digestion was performed with trypsin. The addition of dithiothreitol resulted in a final concentration of 5 mM. Then, Lodoacetamide was added. IP buffer (100 mM NaCl, 1 mM EDTA, 50 mM Tris–HCl, 0.5% NP-40, pH 8.0) was used to dissolve the peptides. The supernatant was transferred to succinylated resin (PTM Bio, China, cat: PTM-402) and incubated overnight at 4 °C with a rotary agitator. The resin was rinsed four times with IP buffer solution and twice with deionized water following incubation. The peptides bound to the resin were eluted three times with an eluent containing 0.1% trifluoroacetic acid, and the eluate was collected, vacuum chilled, and desiccated. The eluate was vacuum freeze-dried and analyzed by liquid chromatography-mass spectrometry (LC-MS) following evacuation and desalting in accordance with the C18 ZipTips instructions.
Transfection
Vector-NC, CPT1A-siRNA1, CPT1A-siRNA2, 14–3–3θ-siRNA, CPT1A-overexpression plasmid, 14–3–3 theta -overexpression plasmid, wild-type 14–3–3 theta overexpression plasmid with HA tag, K11→R, K85→R site-mutated 14–3–3 theta overexpression plasmid (HA-14–3–3 theta-WT, HA-14–3–3 theta-K11R, HA-PLOD2-K85R), and the corresponding empty vector control plasmid were designed and supplied by Shanghai Genechem Co.,Ltd..SNK-6 cells were inoculated into six-well plates at a density of 5.0 × 10^4 cells/well, and transfection was performed after the cells were fully adhered to the wall. The transfection procedure was carried out according to the manufacturer's instructions, and the six-well plates were incubated in an incubator for 12hours after transfection and then changed. The proportion of cells carrying fluorescence was greater than 80%. After 1 week of continuous screening, western blot was used to verify the efficiency of transfection.
Establishment of a subcutaneous tumor-bearing mouse model
A total of 24 specific pathogen-free (SPF) female nude mice (18–22 g, 6–8 weeks old) were purchased from the Southern Medical University Laboratory Animal Center. The mice were housed at 16∼26 °C, 40%∼70% relative humidity and 12 h of light in an SPF-class facility. Wild-type (WT), CPT1A knockdown (KD), CPT1A overexpression (OE), CPT1A (OE) + 14–3–3 theta (KD) SNK-6 cells were then collected, and the cell density was adjusted to 2 × 10^6 cells/ml using phosphate buffer. 100 ul of SNK-6 cell suspension was injected into the axillary fat pad. Mice were divided into SNK-6-WT, CPT1A (OE), CPT1A (OE) + 14–3–3 theta (KD), normal control, paclitaxel and paclitaxel + CPT1A (KD) according to the experimental design protocol, with 4 mice in each group. Both the largest and smallest diameters of the tumor were measured every three days. The tumor volume(V) = (a × b2)/2 (mm3).The animal study was approved by the animal care and use ethics committee of the First Hospital of China Medical University (Approved number: 2023PS1045K).
Apoptosis detection by flow cytometry
AnnexinV-PE+7-AAD Apoptosis Assay Kit (Abnova, China, cat: KA3809) protocols were followed to detect apoptosis through flow cytometry (BD). The cells were digested with trypsin and centrifuged to extract them. The cells were then suspended in 100 uL of phosphate buffer after being rinsed with PBS. The cell suspension was added to 5 uL of Annexin V-PE and 5 uL of 7-AAD and incubated for 15 min at ambient temperature. 400 uL of buffer was added to the tubes and then assayed using a Cytomics FC500 flow cytometer(BD), as previous reference[16].
CCK-8 assay
SNK-6 cells were grouped according to the purpose of the experiment.Logarithmic growth phase SNK-6 cells were plated. CCK-8 was added at 20 μl/well and incubated in an incubator at 37 °C 5% CO2 for 3 h. Optical density (OD) values at 450 nm were measured using an enzyme marker. Cell proliferation capacity (%) = [(treated group - blank group)/(control group - blank group)] × 100%.
Colony formation assay
The logarithmic growth phase of groups of treated SNK-6 cells was obtained.Each culture dish was inoculated uniformly with 500 cells, and the solution was changed every two days. The cells were cultured until they formed colonies visible to the naked eye. After two rinses with PBS, the cells were fixated for 15 min in methanol. Following this, the cells were stained with crystal violet. Then,we calculated the number of colonies that formed.
EDU staining
SNK-6 cells were divided into vector-NC, CPT1A-siRNA1 and CPT1A-siRNA2 group and treated accordingly. Cells were inoculated into confocal dishes at 1 × 10^[5] cells/dish. After overnight incubation, cells were added to EdU working solution (10μmol/L, Beyotime Biotechnology, Shanghai) and incubated for 2hours. The cells were washed three times in phosphate buffer. Paraformaldehyde at a concentration of 4% was used to fix the cells. Add 100 L/well of 0.3% Triton X-100 and incubate at ambient temperature for 15 min. Add 100 uL of DAPI solution as well as incubate for 35 min at ambient temperature. Finally, the samples were photographed by fluorescence microscopy.
Western blot
Tumor tissue and cells were placed in RIPA lysis buffer (Beyotime Biotechnology, China), protease inhibitor and phosphatase inhibitors were added, and the slurry was homogenised. The supernatant was separated by centrifugation at 4 °C for 15 min at 12,000 rpm.Utilizing a BCA reagent (AbbVie, China), the protein concentration of the supernatant was measured. Electrophoresis on a 10% SDS-PAGE gel was used to separate proteins, which were then transferred to a PVDF membrane (Millipore).The PVDF membrane was meticulously removed and occluded for 30 min with 5% bovine serum albumin.The primary antibody anti-Succinyllysine (PTM Biolabs, China, cat:PTM-401), CPT1A (Abcam, Cambridge, UK, cat: ab220789), 14–3–3 theta (Abcam, Cambridge, UK, cat: ab264319), caspase3 (Abcam, Cambridge, UK, cat: ab208161), BAX (Proteintech, USA, cat: 50599-2-Ig), HA (Proteintech, USA, cat: 51064-2-AP), FLAG (Abcam, Cambridge, UK, cat: ab205606), β-actin (Proteintech,USA, cat: 66009-1-Ig) antibody solution was incubated overnight at 4 °C with the membranes. The membranes were rinsed with TBST three times. At room temperature, membranes were incubated with goat anti-rabbit IgG/HRP or goat anti-rabbit IgG/HRP for one hour.Membranes were washed three times with TBST. Protein levels were quantified using ImageJ 1.48 software after imaging on a gel imaging system according to the instructions for the electrochemiluminescence (ECL) kit, with reference to the previous literature[17].
RT-qPCR
Total RNA was extracted from tissues and cells using the RNA Extraction Kit. The absorbance of the extracted RNA at 260 nm was measured and quantified using a NanoDrop ND-100 spectrophotometer (MIULAB, Hangzhou, China), and the ratio of absorbance at 260/280 nm was used to determine the purity of the extracted total RNA. CDNA was synthesized by reverse transcription using the PrimeScriptRT kit (Takara, Japan), and the expression of each gene was determined using RT-PCR with the CPT1A, 14–3–3 theta, and β-actin primer sequences.The primer sequences were CPT1A forward (5′–3′):ATGCGCTACTCCCTGAAAGT; CPT1A reverse (5′–3′):GTGGCACGACTCATCTTGC; 14–3–3 theta forward:CCTGCACGCTGGCTAAAAC, and 14–3–3 theta reverse:CACTGTCTGATGTCCAAAGTGTT; β-actin forward:CATTAAGGAGAAGCTGTGCT; β-actin reverse:GTTGAAGGTAGTTTCGTGGA.
Immunohistochemical staining
Tissue samples were obtained from Patients in the First Hospital of China Medical University and the xenograft tumors from the animal study. Written informed consent for publication was obtained from all patients. This research was approved by the Ethics Committee of the First Hospital of China Medical University (approved number: 2023PS1004K). Paraffin-embedded tumors were serially sectioned at 3um. Tissue sections were deparaffinized in xylene and gradient ethanol after baking at 70 °C for 4 h. The sections were boiled for 8 min in 1x LBP buffer and then allowed to cool to room temperature. Sections were treated overnight at 4 °C with 200ul of anti-CPT1A (Abcam) and anti-Ki67 antibody (Proteintech, USA). After PBS cleansing, the sections were put to C solution. PBS washed the sections after 10 min. After 10 min, PBS washed the pieces after adding D solution. DAB was poured over tissue sections, and immersion in distilled water stopped the reaction after color development. Hematoxylin-stained sections were dehydrated, rendered transparent, and sealed. All sections were viewed on an inverted microscope (Olympus CKX53, Japan). Immunohistochemistry results were positive (+) if cells had yellow or brownish-yellow granules with a clear background and a positive cell count of more than 10%. The rest were negative (-). Senior pathologists independently interpreted the results and the average gray value of Ki-67 staining was caculated, according to the previous literature[18].
Co-immunoprecipitation (Co-IP)
Logarithmic 293T cells were inoculated into 6-well plates at 1 × 10^[5] cells per well. Plasmids or siRNAs were transfected after plating. Cells were collected and lysed 48 h after transfection. On ice for 1.5 h, collected cells were lysed in NP-40 buffer (50 mmol/L Tris–HCl (pH 7.4), 150 mmol/L NaCl, 0.1% NP-40, and protease inhibitor). The supernatant was centrifuged at 15,000 rpm for 25 min at 4 °C and treated with anti-Flag or anti-HA beads for 2 h. Anti-succinylated, anti-CPT1A, and anti-14–3–3 theta antibodies were treated for 1 h with the supernatant. The supernatant was incubated with ProteinA/G beads for 2 h at 4 °C and rinsed 5 times with pre-cooled NP-40 buffer. Western blot analysis was standard.
Statistical analysis
The statistical analysis of the study was performed using GraphPad Prism 8. Comparisons between two groups were analyzed using the unpaired Student's t-test. Comparisons between three or more groups were analyzed using one-way ANOVA. The p values less than 0.05 were considered significantly different. *: p < 0.05; **: p < 0.01, ***: p < 0.001,****: p < 0.0001; #: p < 0.05, ##: p < 0.01, ###: p < 0.001.
Results
-
1.
CPT1A may be involved in the regulation of ENKTL-NT
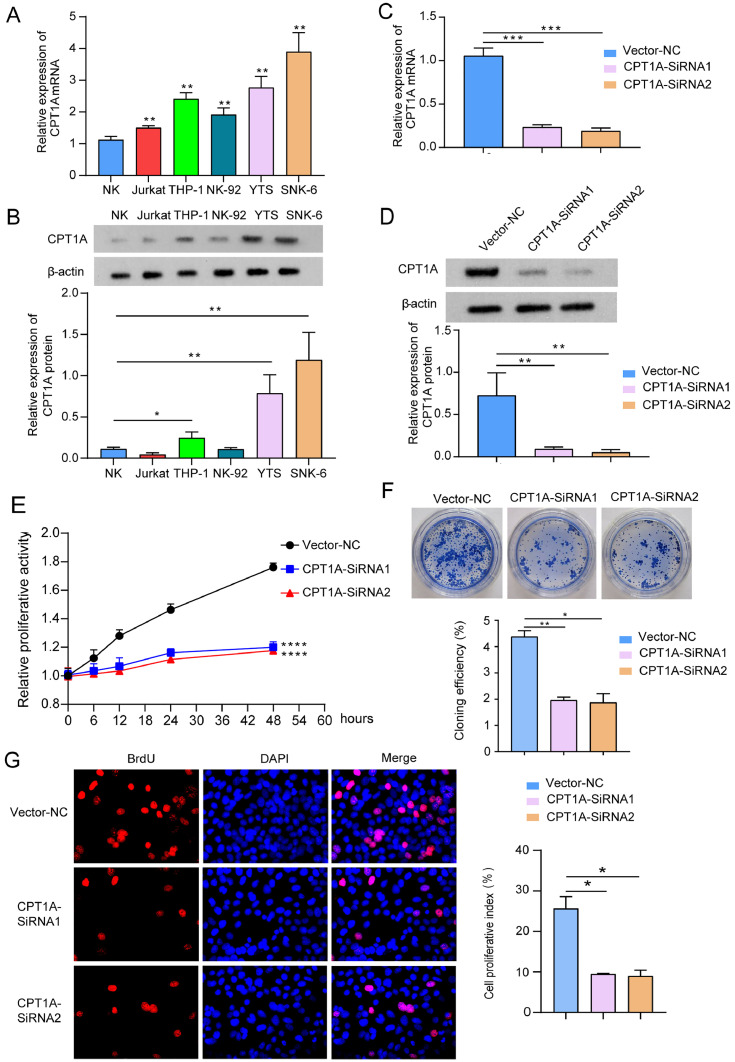
Considering the sample composition characteristics of the GSE90597 dataset, we selected 20 cases of ENKTL-NT for comparative analysis with 19 cases of intra-lymph node NK/T lymphoma. The screening threshold for differentially expressed genes was p-value < 0.05 and log2(fold change) > 1.5. A total of 1642 differentially expressed genes were identified, and we show the top 1000 differentially expressed genes as a heat map (Fig. 1. A). In ENKTL-NT, 538 differentially expressed genes were upregulated and 1104 differentially expressed genes were downregulated (Fig. 1. B). The results of principal component analysis showed that the NKTL group could be significantly distinguished from the ENKTL-NT group (Fig. 1. C). Succinylation transferases that have been identified include CPT1A and EP300, which regulate succinylation modifications of target proteins. Based on the above analysis of the GSE90597 database, we found that the succinyltransferase CPT1A was highly expressed in ENKTL-NT (Fig. 1. D), while EP300 was lowly expressed (data not shown). Further analysis of the GSE90597 dataset showed that the high expression of CPT1A in ENKTL-NT was associated with a poor prognosis (Fig. 1. E). Results from our clinical sample collection showed that the protein expression level of CPT1A was significantly upregulated in ENKTL-NT tissue compared to normal controls (Fig. 1. F and G). This suggests that CPT1A may be involved in the regulation of ENKTL-NT.
-
2.
Knockdown of CPT1A significantly inhibits the proliferation of SNK-6 cells
In this study, the mRNA and protein expression levels of CPT1A were detected in different cell lines using RT-qPCR and western blot, respectively. Compared with normal NK cells, the mRNA and protein expression of CPT1A were most significantly upregulated in SNK-6 cells (Fig. 2. A and B). Therefore, SNK-6 cells were selected for the follow-up study. Knockdown of CPT1A in SNK-6 cells was performed by transfection of CPT1A-siRNA. Both CPT1A-siRNA1 and CPT1A-siRNA2 significantly down-regulated the mRNA and protein expression of CPT1A compared to the vector-NC group (Fig. 2. C and D). Inhibition of CPT1A expression significantly reduced the proliferative activity (Fig. 2. E) and cell clone formation (Fig. 2. F) of SNK-6 cells, which was further confirmed by EdU staining (Fig. 2. G).
-
3.
CPT1A-induced succinylation of 14–3–3 theta promotes ENKTL-NT proliferation
Co-IP results showed that CPT1A knockdown significantly reduced CPT1A binding to 14–3–3 theta while downregulating 14–3–3 theta succinylation levels, and input results showed that both CPT1A and total 14–3–3 theta protein were reduced (Fig. 3. A). Subsequently, we successfully constructed SNK-6 cell models that overexpressed 14–3–3 theta and knocked down 14–3–3 theta (Fig. 3. B and C). In addition, knockdown of CPT1A significantly promoted degradation of 14–3–3 theta protein (Fig. 3. D). Overexpression of 14–3–3theta rescue the downregulation of 14–3–3 theta and proliferation induced by CPT1A knockdown (Fig. 3. E∼G). CPT1A overexpression strongly enhanced CPT1A expression and promoted tumor development in tumor, which was mitigated by 14–3–3 theta knockdown in an in vivo mouse model of SNK-6 cell tumor (Fig. 3. H and I). Immunohistochemical staining also showed that the expression of the cell proliferation antigen Ki67 was significantly reduced in the CPT1A(OE) with 14–3–3 theta (KD) tumor tissue compared to the CPT1A(OE) group (Fig. 3. J).
-
4.
The K85 site of 14–3–3 theta is a modification site for CPT1A
CPT1A's quantitative proteomic effect on succinylation in SNK-6 cells was examined using 4D label-free succinylation modification. Further study revealed two succinylated sites of 14–3–3 theta, K11 and K85. We created human influenza virus haemagglutinin (HA)-tagged 14–3–3 theta wild type and K11R, K85R mutant. The K85R mutant degraded faster (Fig. 4. A). The group transfected with the HA-14–3–3 theta (K85R) plasmid expressed less HA-tagged protein after endogenous 14–3–3 theta knockdown (Fig. 4. B). This shows that 14–3–3 theta's K85 mutation reduces succinylation, accelerating degradation. CO-IP demonstrated a substantial reduction in binding of the HA-14–3–3 theta (K85R) plasmid to FLAG-CPT1A, showing that the K85 site is a succinylation modification site (Fig. 4. C). CCK-8 and clone formation assays demonstrated that 14–3–3 theta knockdown significantly inhibited SNK-6 cell growth compared to HA-Control. Compared to the 14–3–3 theta-KD+HA-control group, transfection with HA-14–3–3 theta (WT) and (K11R) dramatically increased SNK-6 cell growth and reversed the inhibitory effect of low expression. HA-14–3–3 theta (K85R) did not affect SNK-6 cell proliferation compared to the 14–3–3 theta-KD+HA-control group.Transfection with exogenous 14–3–3 theta (WT) restored cell growth, while transfection with K11R did not. The ability of 14–3–3 theta (K85R) to promote cell proliferation was diminished because 14–3–3 theta (K85R) attenuated succinylation (Fig. 4. D and E).
-
5.
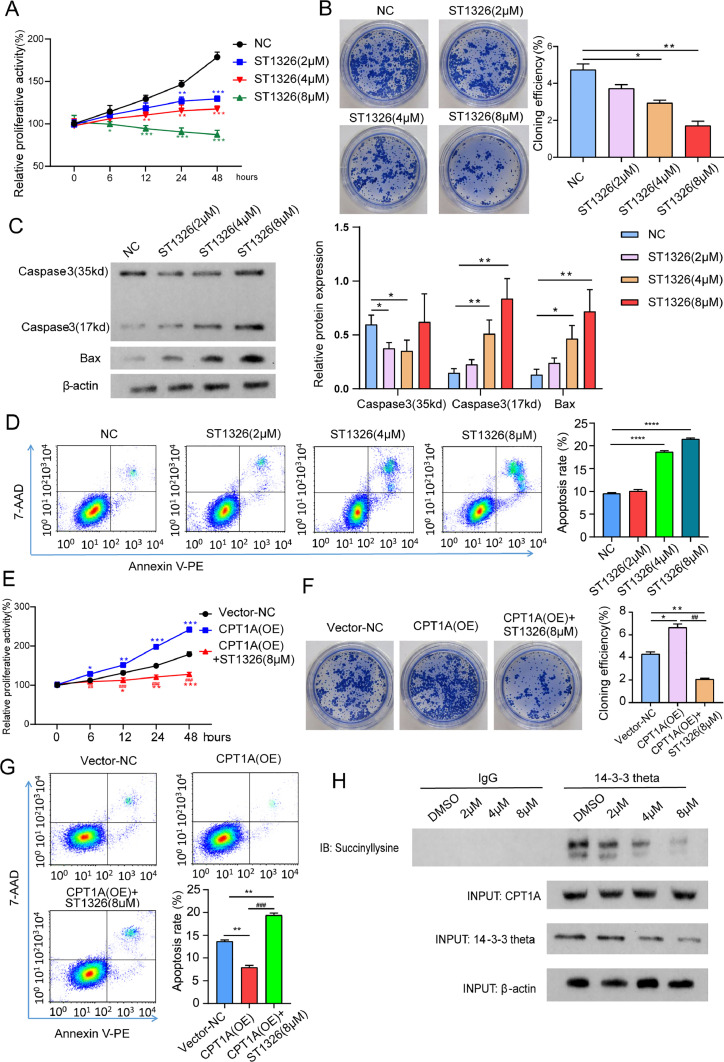
ST1326 inhibits SNK-6 proliferation by reducing CPT1A-mediated 14–3–3 theta succinylation
Teglicar (ST1326) is a selective and reversible inhibitor of the liver isoform of carnitine palmitoyltransferase 1 (L-CPT1) with the ability to inhibit the function of CPT1A. First, we found that 8 μM ST1326 most significantly inhibited the proliferation of SNK-6 cells (Fig. 5. A and B). Notably, ST1326 promoted apoptosis in SNK-6 cells as evidenced by increased expression of pro-apoptotic proteins (caspase-3 and Bax) (Fig. 5. C and D).Despite the overexpression of CPT1A, 8 µM ST1326 significantly inhibited the proliferation and apoptosis of SNK-6 cells (Fig. 5. E∼G). Finally, we demonstrated that ST1326 reduced 14–3–3 theta succinylation in a concentration-dependent manner and also attenuated total 14–3–3θ protein by CO-IP assay (Fig. 5. F).
-
6.
CPT1A knockdown combined with paclitaxel shows enhanced antitumor efficacy
Drug sensitivity prediction using the GSE90597 dataset revealed that high CPT1A expression may lead to reduced sensitivity of ENKTL to paclitaxel and docetaxel. The prediction results showed that drug IC50 values for paclitaxel and docetaxel showed typical significance in the CPT1A-high group. The significant upregulation of drug IC50 suggests that CPT1A-high expression may lead to reduced sensitivity of ENKTL to paclitaxel and docetaxel. We found that the IC20 and IC50 of paclitaxel in SNK-6 cells were 5.87 mM and 15.9 mM, respectively (Fig. 6. A). Knockdown of CPT1A further promoted the inhibitory effect of paclitaxel on proliferative activity and clone formation of SNK-6 cells compared to the paclitaxel group (Fig. 6. B and C) and promoted apoptosis of SNK-6 cells (Fig. 6. D). In vivo experiments also showed that knockdown of CPT1A in combination with paclitaxel inhibited tumor growth and reduced Ki67 expression in tumor tissue compared to paclitaxel alone (Fig. 6. E and F).
Fig. 1.
CPT1A may be involved in the regulation of ENKTL-NT.
A. The top 1000 differentially expressed genes between NKTL and ENKTL-NT in the GSE90597 dataset, shown as a heat map. B. Volcano plot of the differentially expressed genes. C. Principal component analysis between the NKTL group and the ENKTL-NT group. D. mRNA expression of CPT1A and EP300 in GSE90597 in NKTL and ENKTL-NT. E. The effect of CPT1A on ENKTL-NT survival in the GSE90597 dataset was analyzed using the Kaplan-Meier survival curve method. F and G. Western blot and immunohistochemical staining were performed to detect protein expression and in situ expression of CPT1A in ENKTL-NT tissue and normal nasal mucosa tissue, respectively. 8 samples were included in each of the ENKTL-NT and normal nasal mucosa groups. *: p < 0.05; **: p < 0.01.
Fig. 2.
Knockdown of CPT1A significantly inhibits the proliferation of SNK-6 cells.
A. mRNA expression of CPT1A in normal NK cells, Jurkat, THP-1, NK-92, YTS and SNK-6 cell lines. Comparison with normal NK cells. B. Protein expression of CPT1A in normal NK cells, Jurkat, THP-1, NK-92, YTS, and SNK-6 cell lines. Comparison with normal NK cells. C and D. RT-qPCR and western blot were used to detect the mRNA and protein expression of CPT1A in SNK-6 cell lines, respectively. E. CCK-8 assay to detect the proliferative activity of SNK-6 cells. F. Effect of knockdown of CPT1A on the rate of clone formation in SNK-6 cells. G. EDU was applied to assay the effect of knockdown of CPT1A on the proliferation of SNK-6 cells. *: p < 0.05; **: p < 0.01, ***: p < 0.001,****: p < 0.0001.
Fig. 3.
14–3–3 Theta succinylated by CPT1A promotes ENKTL proliferation.
A. Co-IP assay to detect succinylation modifications under CPT1A-SiRNA knockdown conditions. B and C. Expression of mRNA and proteins was analyzed using RT-qPCR and western blotting, after 14–3–3 theta was knocked down and overexpressed, respectively.D. Western blot detection of 14–3–3 theta protein expression after knockdown of CPT1A.E and F. The effect of 14–3–3 theta overexpression on CPT1A and 14–3–3 theta protein expression was examined using western blot.G. SNK-6 cell proliferation activity was assayed by CCK-8.H. Clonogenesis assay to detect the proliferative ability of SNK-6 cells.I. In order to determine how the CPT1A (OE) and 14–3–3 theta (KD) plasmids affected the expression of CTP1A and 14–3–3 theta proteins, western blot analysis was performed.J. The photographs as well as volume measurements of mouse models with subcutaneous tumors. K. Immunohistochemical detection of cell proliferation antigen Ki67 expression in tumor tissues. *: p < 0.05; **: p < 0.01, ***: p < 0.001; ##: p < 0.01, ###: p < 0.001.
Fig. 4.
The K85 site of 14–3–3θ is a modification site for CPT1A.
A. Construction of HA-14–3–3 theta (WT), HA-14–3–3 theta (K11R), HA-14–3–3 theta (K85R) plasmids. The three plasmids were transfected into SNK-6 cells and the protein synthesis was inhibited by the addition of the protein synthesis inhibitor 10 μg/ml actinomycin cycloheximide (CHX) at 0, 2, 4, 6 and 10 h before cell collection. The assay times were set at 0, 2, 4, 6, and 10 h. The expression levels of HA-tagged proteins at different time points after transfection with HA-14–3–3 theta (WT), HA-14–3–3 theta (K11R), and HA-14–3–3 theta (K85R) were detected by western blot.B. Western blot was utilized to detect the effect of different HA-14–3–3 theta plasmids on the expression of the 14–3–3 theta protein.C. Co-IP assay for exogenous transfection of 14–3–3 theta.D and E. The effect of 14–3–3 theta modification succinylation site mutations on cell proliferation was assayed by CCK-8 and clone formation assays. *: p < 0.05; **: p < 0.01, ***: p < 0.001; ##: p < 0.01, ###: p < 0.001.
Fig. 5.
ST1326 inhibits SNK-6 proliferation by reducing CPT1A-mediated 14–3–3 theta succinylation.
A and B. The CCK-8 test and clone creation assay were used to determine whether or not ST1326 caused SNK-6 cells to proliferate at various doses.C. Western blot to detect the expression of Caspase-3 and Bax proteins.D. Flow cytometry to detect apoptosis.E and F. The impact of ST1326 on SNK-6 cell proliferation in the context of CPT1A overexpression was determined using CCK-8 and clonogenic assays.G. Apoptosis detection by flow cytometry.F. In SNK-6 cells that were stimulated by varying doses of ST1326, a Co-IP experiment was carried out in order to determine the succinylation and expression of CPT1A and 14–3–3 theta. *: p < 0.05; **: p < 0.01, ***: p < 0.001; ##: p < 0.01, ###: p < 0.001.
Fig. 6.
CPT1A knockdown combined with Paclitaxel shows enhanced antitumor efficacy.
A. SNK-6 cells were stimulated with 0 nM, 1 nM, 5 nM, 10 nM, 25 nM, 50 nM and 100 nM paclitaxel for 48 h. CCK-8 assayed the IC20 and IC50 values of different concentrations of paclitaxel on SNK-6 cells. B and C. CCK-8 and clone formation assays were applied to detect the effect of knockdown of CPT1A on paclitaxel drug toxicity. D. Apoptosis was detected by flow cytometry. E. Measurement of tumor tissue volume. F. Immunohistochemical staining was used to detect Ki67 expression in tumor tissue in situ. *: p < 0.05; **: p < 0.01, ***: p < 0.001; ##: p < 0.01, ###: p < 0.001.
Discussion
Succinylation, also known as succination, is a common type of protein modification that involves the addition of succinic anhydride to proteins. The catalytic efficiency of fatty acid β-oxidation is limited by CPT1A, and its absence or abnormal regulation can lead to metabolic disorders. CPT1A also exhibits lysine succinyl transferase (LSTase) activity, which can regulate enzyme activity and the metabolism of substrate proteins. As the main mediator of mitochondrial membrane transferase, CPT1A is essential for the regulation of metabolic homeostasis and the proliferation of tumor cells[11]. Our study found that CPT1A induces succinylation of 14–3–3 theta at the K85 site, promoting ENKTL-NT proliferation. The anti-ENKTL effectiveness of paclitaxel significantly improved when it was combined with CPT1A knockdown.
The mechanisms by which CPT1A plays a role in promoting tumor progression are diverse, but largely dependent on fatty acid oxidation[19], in pancreatic adenocarcinoma[5] and clear cell renal carcinoma[20]. Excessive fatty acid oxidation is induced by the overexpression of CPT1A to provide more energy for tumor cells[11], and also aggravates the radioresistance of tumor cells[21]. The microenvironment underlying a tumor is characterized by an inflammatory response and oxidative stress. In particular, under hypoxic conditions, HIF1α is hyperactivated and its expression of CPT1A is promoted[11].
There have been many studies on CPT1A as a regulatory protein of fatty acid oxidation metabolism, but its role as a modifier protein is less. Recent studies have shown that succinylation is also associated with protein stability[22]. Succinyl groups introduce hydrophobic and charge changes to the protein surface, increasing the water solubility, stability, and immunogenicity of the protein. CPT1A, for instance, induces the succinylation of S100 calcium-binding protein A1 at the K47 site, resulting in the accumulation of S100 calcium-binding protein A1 in gastric cancer cells and fostering gastric carcinogenesis[23]. The upregulation of leucine-rich pentapeptide structure and eukaryotic initiation factor 3b, respectively, by CPT1A and SIRT5 promotes the pathogenesis of clear cell renal cell carcinoma[24]. This is consistent with our finding that CPT1A increases the stability of the protein in late ENKTL-NT, modified by succinylation.
Previous studies have found that fatty acid metabolism is involved in apoptosis in cancer cells[25]. Moreover, hypoxia induces hypoxia-inducible factors and apoptosis. CPT1A's reprogramming of fatty acid metabolism enables cells to obtain the energy required for proliferation and survival in hypoxic conditions[26]. In contrast, it has been shown that CPT1A is lowly expressed in clear cell renal cell carcinoma and promotes the proliferation of clear cell renal cell carcinoma[20]. Furthermore, hypoxia-inducible factors mechanistically inhibit CPT1A expression, thereby reducing fatty acid transport and increasing the formation of lipid droplets in the cytoplasm[27]. This may be related to tissue heterogeneity. Here, we did not observe fatty acid oxidation in SNK-6 cells for the time being. However, similar to previous studies, CPT1A is clearly involved in the regulation of apoptosis in ENKTL-NT. Interestingly, CPT1A regulated apoptosis indirectly by modifying the protein 14–3–3 theta.
14–3–3 theta proteins with functions in cell cycle regulation, apoptosis, and protein transport have been identified in prior research.14–3–3 theta inhibits apoptosis by binding pro-apoptotic factors, such as Bcl-2, Bad, and Bax[28]. 14–3–3 theta proteins are generally regarded as pro-tumorigenic in cancer, with the exception of 14–3–3 sigma, which is regarded as a tumor suppressor[29]. By preventing their translocation to the nucleus, 14–3–3 theta also inhibits the activity of pro-apoptotic transcription factors (including Foxo3a and c-Abl). Previous studies involving post-transcriptional modification of the 14–3–3 theta protein are lacking, especially in lymphoma[29]. We demonstrate for the first time that 14–3–3 theta is succinylated in lymphoma and that its inhibitory effect on apoptosis is enhanced. This provides a new perspective for us to explore the 14–3–3 theta promoting the progression of lymphoma in the future.
Overactivation of CPT1A allows cells to achieve redox homeostasis and inhibit cellular anoikis[30]. Previous studies have shown that non-Hodgkin's lymphoma patients undergoing anthracycline therapy, such as doxorubicin-containing chemotherapy, promote the activation of carnitine acyltransferases[31]. It has been shown that high CPT1A expression is linked to antineoplastic drug resistance. Mantle cell lymphoma is an uncommon type of non-Hodgkin's lymphoma that affects lymphoid B cells[32]. High expression of CPT1A in mantle cell lymphoma is positively associated with poor response to standard doses of chemotherapy. Overexpression of CPT1A is also associated with platinum resistance in epithelial ovarian cancer[33]. Knockdown of CPT1A reduces histone acetylation and improves castration resistance in prostate cancer[34]. Notably, fatty acid oxidation activated by the PGC1α/CEBPB/CPT1A axis exacerbates radioresistance[35]. This suggests to us that CPT1A inhibition may alleviate chemotherapy tolerance of advanced ENKTL-NT.
Chemotherapy is one of the main treatments for advanced ENKTL and commonly used drugs include cyclophosphamide, vincristine and adriamycin. Paclitaxel is an anti-tumor drug widely used in cancer chemotherapy. In previous chemotherapy regimens, combining paclitaxel with other chemotherapeutic agents or radiotherapy as a first-line treatment option has improved survival and relieved symptoms in patients with ENKTL. Previous studies have found that CPT1A promotes antiandrogen resistance in prostate cancer[34] and immune checkpoint blockade related immunotherapy tolerance in lung cancer[36]. As expected, by combining CPT1A knockdown and paclitaxel, we significantly increased the antitumor effect of paclitaxel. Our study provides an important basis for the treatment of ENKTL-NT with paclitaxel. We further elucidated the regulatory mechanism of CPT1A in ENKTL-NT, which provided the basis for clinical study of Etomoxir, an inhibitor of CPT1A.
In addition, CPT1A has been used as a target for the treatment of liver fibrosis[37] and CPT1A-induced ferroptosis in lung cancer cells[36], suggesting that there may be multiple regulatory mechanisms of CPT1A in advanced ENKTL. Overall, current studies are insufficient to understand the complex relationship between CPT1A and tumor resistance, particularly in non-Hodgkin's lymphoma. Further large-scale studies are needed in the future to explore the role of CPT1A in anti-tumor drug resistance and its potential therapeutic applications. Given that CPT1A is a key enzyme in lipid metabolism and energy production, we should pay more attention to the side effects, toxicity and safety of inhibitors when inhibiting CPT1A.
There are still shortcomings in our study.When CPT1A was knocked down in combination with paclitaxel, both proliferation viability and clone formation of SNK-6 cells were inhibited, whether this was a superimposed effect or improved paclitaxel resistance is not well understood. Furthermore, the mechanisms leading to CPT1A overexpression in ENKTL-NT have not been investigated. Although the role of CPT1A in ENKTL-NT has been partially elucidated, additional research is required to comprehend how it is influenced by multiple factors, such as microenvironmental and genetic factors. For example, in the tumor microenvironment, T lymphocytes and their immunoreactive cells, vascular endothelial cells and surrounding tissue cells may influence the expression of CPT1A, thereby regulating pathways such as exogenous nutrient uptake, metabolism and lipid synthesis in ENKTL-NT. Similarly, IL-17 signaling can mediate CPT1A expression[38].
In conclusion, studies on paclitaxel and CPT1A will help to deepen our understanding of the treatment of ENKTL-NT, identify new therapeutic strategies and alleviate drug resistance problems.
Funding
This work was supported by the 345 Talent Project in Shengjing Hospital of China Medical University.
Ethical statement
The animal study was approved by the animal care and use ethics committee of the First Hospital of China Medical University (Approved number: 2023PS1045K).
Tissue samples were obtained from Patients in the First Hospital of China Medical University. Written informed consent for publication was obtained from all patients. This research was approved by the Ethics Committee of the First Hospital of China Medical University (approved number: 2023PS1004K)
CRediT authorship contribution statement
Xiao Cui: Writing – original draft, Validation, Supervision, Software, Resources, Methodology, Investigation, Conceptualization. Chengcheng Cao: Visualization, Resources, Methodology, Formal analysis. Xinyang Li: Software, Project administration, Methodology, Formal analysis, Data curation. Biyan Lin: Visualization, Validation, Project administration, Methodology, Investigation. Aihui Yan: Project administration, Methodology, Formal analysis, Conceptualization. Ying Yang: Writing – review & editing, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
There are no conflicts of interest.
Contributor Information
Aihui Yan, Email: yanmenxueshu@163.com.
Ying Yang, Email: yangy5@sj-hospital.org.
Data availability
The data in this study can be obtained from the corresponding author.
References
- 1.Wang H., Fu B.B., Gale R.P., Liang Y. NK-/T-cell lymphomas. Leukemia. 2021;35(9):2460–2468. doi: 10.1038/s41375-021-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tse E., Zhao W.L., Xiong J., Kwong Y.L. How we treat NK/T-cell lymphomas. J. Hematol. Oncol. 2022;15(1):74. doi: 10.1186/s13045-022-01293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konjević G., Jurisić V., Banićevic B., Spuzić I. The difference in NK-cell activity between patients with non-Hodgkin's lymphomas and Hodgkin's disease. Br. J. Haematol. 1999;104(1):144–151. doi: 10.1046/j.1365-2141.1999.01129.x. [DOI] [PubMed] [Google Scholar]
- 4.Jurisic V., Colovic N., Konjevic G., Minic I., Colovic M. An aggressive extramedullary cutaneous plasmacytoma associated with extreme alterations in the innate immune system. Onkologie. 2010;33(3):113–115. doi: 10.1159/000278713. [DOI] [PubMed] [Google Scholar]
- 5.Xu R., Liu Y., Ma L., et al. NQO1/CPT1A promotes the progression of pancreatic adenocarcinoma via fatty acid oxidation. Acta Biochim. Biophys. Sin. (Shanghai) 2023;55(5):758–768. doi: 10.3724/abbs.2023066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mørkholt A.S., Wiborg O., Nieland J.G.K., Nielsen S., Nieland J.D. Blocking of carnitine palmitoyl transferase 1 potently reduces stress-induced depression in rat highlighting a pivotal role of lipid metabolism. Sci. Rep. 2017;7(1):2158. doi: 10.1038/s41598-017-02343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Q., Tan Z., Shi F., et al. PGC1α/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation. Cancer Sci. 2019;110(6):2050–2062. doi: 10.1111/cas.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaban S., Shearer R.F., Lee L.S., et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciardi M.R., Mirabilii S., Allegretti M., et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood. 2015;126(16):1925–1929. doi: 10.1182/blood-2014-12-617498. [DOI] [PubMed] [Google Scholar]
- 10.Khurana P., Burudpakdee C., Grupp S.A., Beier U.H., Barrett D.M., Bassiri H. Distinct bioenergetic features of human invariant natural killer T cells enable retained functions in nutrient-deprived states. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.700374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang M., Dong X., Xiao L., et al. CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma. Cell Death. Dis. 2022;13(4):331. doi: 10.1038/s41419-022-04730-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obsilová V., Silhan J., Boura E., Teisinger J., Obsil T. 14-3-3 proteins: a family of versatile molecular regulators. Physiol. Res. 2008;57(Suppl 3):S11–S21. doi: 10.33549/physiolres.931598. [DOI] [PubMed] [Google Scholar]
- 13.Jin J.P., Zhang Z., Bautista J.A. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit. Rev. Eukaryot. Gene Expr. 2008;18(2):93–124. doi: 10.1615/critreveukargeneexpr.v18.i2.10. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J., Guo C., Ma Z., Liu H., Yang C., Li S. Identification of a novel gene expression signature associated with overall survival in patients with lung adenocarcinoma: a comprehensive analysis based on TCGA and GEO databases. Lung Cancer. 2020;149:90–96. doi: 10.1016/j.lungcan.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Vuletic A., Konjevic G., Milanovic D., Ruzdijic S., Jurisic V. Antiproliferative effect of 13-cis-retinoic acid is associated with granulocyte differentiation and decrease in cyclin B1 and Bcl-2 protein levels in G0/G1 arrested HL-60 cells. Pathol. Oncol. Res. 2010;16(3):393–401. doi: 10.1007/s12253-009-9241-2. [DOI] [PubMed] [Google Scholar]
- 16.Jurisic V., Srdic-Rajic T., Konjevic G., Bogdanovic G., Colic M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J. Membr. Biol. 2011;239(3):115–122. doi: 10.1007/s00232-010-9309-7. [DOI] [PubMed] [Google Scholar]
- 17.Scherbakov A.M., Vorontsova S.K., Khamidullina A.I., et al. Novel pentacyclic derivatives and benzylidenes of the progesterone series cause anti-estrogenic and antiproliferative effects and induce apoptosis in breast cancer cells. Invest. New Drugs. 2023;41(1):142–152. doi: 10.1007/s10637-023-01332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marković M., Jurišić V., Petrović M., Dagović A., Stanković V., Mitrović S. Appearance of ductal breast and colon carcinoma with gastrointestinal stromal tumor (GIST) in a female patient: an extremely rare case. Rom. J. Morphol. Embryol. 2018;59(2):613–617. [PubMed] [Google Scholar]
- 19.Li R., Li X., Zhao J., et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics. 2022;12(2):976–998. doi: 10.7150/thno.63751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H., Zhao H., Ren Z., et al. Overexpression CPT1A reduces lipid accumulation via PPARα/CD36 axis to suppress the cell proliferation in ccRCC. Acta Biochim. Biophys. Sin. (Shanghai) 2022;54(2):220–231. doi: 10.3724/abbs.2021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang N., Xie B., Xiao W., et al. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat. Commun. 2022;13(1):1511. doi: 10.1038/s41467-022-29137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papanicolaou K.N., O'Rourke B., Foster D.B. Metabolism leaves its mark on the powerhouse: recent progress in post-translational modifications of lysine in mitochondria. Front. Physiol. 2014;5:301. doi: 10.3389/fphys.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C., Zhang C., Li X., et al. CPT1A-mediated succinylation of S100A10 increases human gastric cancer invasion. J. Cell Mol. Med. 2019;23(1):293–305. doi: 10.1111/jcmm.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W., Che X., Qu X., et al. Succinylation regulators promote clear cell renal cell carcinoma by immune regulation and RNA N6-methyladenosine methylation. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.622198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Guo W., Guo Y., et al. Apoptosis induction in human prostate cancer cells related to the fatty acid metabolism by wogonin-mediated regulation of the AKT-SREBP1-FASN signaling network. Food Chem. Toxicol. 2022;169 doi: 10.1016/j.fct.2022.113450. [DOI] [PubMed] [Google Scholar]
- 26.Rios-Colon L., Kumar P., Kim S., et al. Carnitine Palmitoyltransferase 1 Regulates Prostate Cancer Growth under Hypoxia. Cancers. (Basel) 2021;13(24):6302. doi: 10.3390/cancers13246302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du W., Zhang L., Brett-Morris A., et al. HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism. Nat. Commun. 2017;8(1):1769. doi: 10.1038/s41467-017-01965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masters S.C., Fu H. 14-3-3 proteins mediate an essential anti-apoptotic signal. J. Biol. Chem. 2001;276(48):45193–45200. doi: 10.1074/jbc.M105971200. [DOI] [PubMed] [Google Scholar]
- 29.Hermeking H. The 14-3-3 cancer connection. Nat. Rev. Cancer. 2003;3(12):931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 30.Tian T., Lu Y., Lin J., et al. CPT1A promotes anoikis resistance in esophageal squamous cell carcinoma via redox homeostasis. Redox. Biol. 2022;58 doi: 10.1016/j.redox.2022.102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldner R., Laschan C., Lohninger A., et al. Effects of doxorubicin-containing chemotherapy and a combination with L-carnitine on oxidative metabolism in patients with non-Hodgkin lymphoma. J. Cancer Res. Clin. Oncol. 2006;132(2):121–128. doi: 10.1007/s00432-005-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerdtsson A.S., Rodrigues J de Matos, Eskelund C.W., et al. Overexpression of the key metabolic protein CPT1A defines mantle cell lymphoma patients with poor response to standard high-dose chemotherapy independent of MIPI and complement established highrisk factors. Haematologica. 2023;108(4):1092–1104. doi: 10.3324/haematol.2022.281420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J., Wu G., Song L., et al. NKX2-8 deletion-induced reprogramming of fatty acid metabolism confers chemoresistance in epithelial ovarian cancer. EBioMedicine. 2019;43:238–252. doi: 10.1016/j.ebiom.2019.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi M., Stoykova G.E., Salzmann-Sullivan M., et al. CPT1A supports castration-resistant prostate cancer in androgen-deprived conditions. Cells. 2019;8(10):1115. doi: 10.3390/cells8101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han S., Wei R., Zhang X., et al. CPT1A/2-mediated FAO enhancement-a metabolic target in radioresistant breast cancer. Front. Oncol. 2019;9:1201. doi: 10.3389/fonc.2019.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L., Chen C., Zhao C., et al. Targeting carnitine palmitoyl transferase 1A (CPT1A) induces ferroptosis and synergizes with immunotherapy in lung cancer. Signal. Transduct. Target. Ther. 2024;9(1):64. doi: 10.1038/s41392-024-01772-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fondevila M.F., Fernandez U., Heras V., et al. Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis. J. Hepatol. 2022;77(1):15–28. doi: 10.1016/j.jhep.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Majumder S., Amatya N., Revu S., et al. IL-17 metabolically reprograms activated fibroblastic reticular cells for proliferation and survival. Nat. Immunol. 2019;20(5):534–545. doi: 10.1038/s41590-019-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study can be obtained from the corresponding author.