Abstract
Schisandra chinensis is a functional fruit with tonic effect and was widely used by traditional Chinese medicine for treatment and health care. The quality of Schisandra chinensis fruit may vary by different storage condition. In this study, the influence of ambient temperature, humidity, packaging materials and period of storage on the quality of Schisandra chinensis fruits were investigated. The contents of main active components lignans and organic acids were simultaneously determined by ultra-performance liquid chromatography coupled to quadrupole electrostatic field orbitrap high resolution mass spectrometry (UPLC Orbitrap HRMS). The antioxidant activity was determined using DPPH radical scavenging capacity, ABTS+ inhibition rate and FRAP value. The correlation of multicomponent and antioxidant activity was analyzed by grey relevance analysis. Taking the changes of multicomponent and antioxidant activity as investigation index, Schisandra chinensis fruits under different storage conditions was comprehensively evaluated. Schisandrol A, malic acid, sorbic acid, schizandrin A, schizandrin B, and schisandrol B were the main effective components of antioxidant activity. Ambient temperature at 5 °C and humidity at 40 % were more suitable for Schisandra chinensis fruits and kraft paper bag was better packaging material. Do not exceed 1 year was the effective storage period. For the safety evaluation, no aflatoxin was detected within the storage period of 2 years, demonstrated the storage was satisfactory. This study provided a reference for the high-quality storage and standardized operating procedures for storage of Schisandra chinensis fruits.
Keywords: Schisandra chinensis fruits, Storage conditions, Multicomponent, Antioxidant activity, Comprehensive evaluation
Highlights
-
•
The influence of storage on Schisandra chinensis fruits was first investigated.
-
•
The fruits quality was integrated evaluated by constituent and antioxidant activity.
-
•
The ambient temperature, humidity, packaging materials and period were studied.
-
•
It provided a reference for high-quality storage of Schisandra chinensis fruits.
1. Introduction
There are about 50 species of Schisandra plants in the world, mainly distributed in China, Korea, Japan, the Russian Far East and India. Schisandra chinensis (Turcz.) Baill. is the dry ripe fruit of the magnoliophyta group [1]. As a medicinal material in Northeast China, it is mainly produced in Heilongjiang, Jilin and Liaoning provinces. Schisandra chinensis fruit has pharmacological effects such as anti-aging, antioxidation, antibacterial, anti-convulsion, anti-depression, anti-tumor, liver protection and hypoglycemia [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11]], and presents high medicinal and health value. The lignans and organic acids are the main bioactive composition for those various effects [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]]. As people pay more and more attention to health care, the demand for health food is increasing. Therefore, Schisandra chinensis fruit was more and more widely used in traditional Chinese medicine for treatment and health care [23].
In the production of Chinese herbal medicines, storage is one of the most important factors that affects their internal quality after harvesting and processing. If the storage conditions were improper, the Chinese herbal materials would be mildew, moth or deterioration, which caused a serious adverse impact on the efficacy and quality. Therefore, suitable and effective storage conditions can avoid the above phenomena in the storage process of Chinese medicinal materials and play an essential role in ensuring the quality and efficacy [[24], [25], [26], [27], [28], [29], [30]]. With the continuous increase of market demand of Schisandra chinensis fruit materials, the standardization of storage technology and the comprehensive quality evaluation system will be the basis and trend for the high-quality development of Schisandra chinensis fruit in the future. According to the good manufacturing practice (GAP) for traditional Chinese medicine, environmental factors for storage generally include temperature, humidity, and period. In addition, packaging materials will also affect the quality during storage. According to the types of medicinal materials, plastic woven bags, gunny bags and kraft paper bags were usually applied in packaging in GAP. However, there is no uniform standard for the storage technology of Schisandra chinensis fruits. With the change of bioactive components of Schisandra chinensis fruits during storage, the influence on its antioxidant activity is still unclear.
Based on the above, this study investigated the influencing factors of storage conditions on the quality of Schisandra chinensis fruits. The different storage conditions, including packaging materials, ambient temperature, humidity and storage period were evaluated. The quality of Schisandra chinensis fruits during storage periods were determined by both of multicomponent’ content and antioxidant activity. Based on the grey correlation analysis, the relationship of multicomponents and antioxidant activity were established. The correlation degree could be used to explain the pharmacodynamics material basis of the antioxidant activity of Schisandra chinensis fruits. In order to ensure the standardization of storage conditions, the content of aflatoxin was detected within storage periods for safety evaluation. This study provided a reference for the high-quality storage of Schisandra chinensis fruits.
2. Materials and methods
2.1. Instruments and reagents
Ultra-performance liquid chromatography coupled with quadrupole electrostatic field orbitrap high resolution mass spectrometry (Q Exactive, Thermo Fisher Scientific Inc.); high performance liquid chromatography-triple quadrupole mass spectrometry (1290–6470, Agilent Scientific Inc.); Infinite M200 PRO multifunctional microplate reader (Beijing Longyue Biotechnology Development Co., Ltd).
DPPH free radical scavenging ability kit and total antioxidant capacity (T-AOC) test kit (including ABTS+ method and FRAP method) were purchased from Nanjing Jiancheng Bioengineering Institute. Methanol, acetonitrile and ammonium acetate (HPLC-grade, Fisher Scientific), formic acid (HPLC grade, Sigma-Aldrich).
2.2. Standards reference and Schisandra chinensis fruits
Standards reference (purity>99 %, Aladdin) used in this study included: Schisandrol A (Y30N10H104712), schisandrol B (P05J9S52197), schisantherin A (P13O8S45575), schisantherin B (P07M6S1), schizandrin A (R12O8F45508), schizandrin B (Y26F11Y17148), schisandrin C (P10A11F111646), gomisin D (M05J9S65019), gomisin J (M22O11S128159), schisanhenol (PJ0607SA13), quinic acid (A06N11L130218), malic acid (S08J12I36815), citric acid (SM0425GA14), sorbic acid (W05J10L89937), chlorogenic acid (Y20A11K111541). Mix references stock solution: Aflatoxin B1 (1.0 μg/mL), aflatoxin B2 (0.30 μg/mL), aflatoxin G1 (1.0 μg/mL) and aflatoxin G2 (0.3 μg/mL) (210,301-2106-D1, Shanghai ANPEL).
Fresh Schisandra chinensis fruits were harvested in early September and dried in the planting area in Jingyu County, Jilin Province. They were selected, numbered and stored under different conditions began in early October. According to the GAP, the packaging materials for Chinese medicine materials were plastic woven bags, gunny bags and kraft paper bags. For storage temperature, was not exceeded 25 °C in GAP. As Schisandra chinensis fruit is a type of fruit medicine and contains organic acids as main component, the storage temperature should be below room temperature and 5 °C and 15 °C were chosen in this study. For storage humidity, was required not exceed 60 % in GAP. As the color of dried Schisandra chinensis fruits will be darken under too dry environment and a relatively humid is easy to mildew, the humidity of 40 %, 50 % and 60 % were optimized in this experiment. Chinese medicinal materials are usually stored for one year and not exceed two years to ensure the efficacy. The storage periods for Schisandra chinensis fruits investigated were within two years.
2.3. Lignans and organic acids determination
2.3.1. Reference solution preparation
A total of 15 reference substances of lignans and organic acids were accurately weighed. Dissolved in 70 % methanol-water to 1 mg/mL and prepared a mixed reference stock solution. And then the stock solution were gradually diluted to different concentrations for quantitative analysis.
2.3.2. Sample solution preparation
The dried Schisandra chinensis fruits with different storage conditions were taken out at 3, 6, 9, 12 and 24 months, respectively. And the fruits with different storage periods were powdered and sieved. The extraction process was according to the extraction method of Schisandra chinensis fruits in Chinese Pharmacopoeia (2020 edition) [1]. Accurately weighed 0.25 g powder into Erlenmeyer flask with cap and added methanol 10 mL. Extracted by ultrasound for 20 min and filtrated. The extraction were repeated 3 times and the filtrates were mixed and shook well. After dilution and passing through a 0.22 μm microporous membrane, the sample solution were for analysis.
2.3.3. Instrument condition
Two kinds of main components lignans and organic acids in Schisandra chinensis fruit were detected using UPLC Orbitrap HRMS simultaneously. The parameters of UPLC and MS were optimized respectively to obtain the best separation, ionization efficiency and sensitivity [31].
Chromatography conditions: Thermo Scientific Syncronis C18 column (100 mm × 2.1 mm, 1.7 μm). Mobile phase: 0.1 % formic acid (A) - acetonitrile (B). Gradient elution: 0~5 min, 10 % B; 5~10 min, 10 %~70 % B; 10~30 min, 70 %~100 % B; 30~40 min, 100 % B. Column temperature: 30 °C. Flow rate: 0.2 mL/min. Injection volume: 5 μL.
Q Exactive mass spectrometry conditions: Electrospray ion source (ESI). Lignans presented higher signal in positive ion mode, while negative ion mode was more sensitive for organic acids determination. Scanning range m/z 50–1000 in full scan mode. Spray voltage: 3400 V. Sheath gas flow rate: 35 arb and auxiliary gas flow rate: 10 arb. Ion transfer tube temperature: 310 °C and vaporizer temperature: 310 °C.
2.4. Aflatoxins determination
The aflatoxins were prepared and detected by LC-MS according to the general rule 2351-aflatoxin determination method of the Chinese Pharmacopoeia (2020 edition) [32].
2.4.1. Reference solution preparation
The stock solution was diluted with 70 % methanol into a series of reference solutions of aflatoxins B1, G1, B2 and G2.
2.4.2. Sample solution preparation
The fruits powder was accurately weighted, added 3 g sodium chloride and 75 mL 70 % methanol solution, stirred with high speed and centrifuged. Taken 15 mL supernatant and diluted it with water to 50 mL, shaken well and centrifuged. Taken 20 mL supernatant, passed through the immunoaffinity column, and eluted with 20 mL water. Discard the water wash, let the air enter the column and squeezed the water out of the column. Then eluted with 1.5 mL methanol and collected the eluent. Diluted the eluent with water to 2 mL and shook well. Then filtered with 0.22 μm microporous filter membrane and taken the filtrate for analysis.
2.4.3. Instrument condition
Chromatography conditions: Agilent ZORBAX Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm). Mobile phase: 2 mmol/L ammonium acetate in water (A) - methanol (B). Gradient elution: 0~4.5 min, 25 % B; 4.5~5 min, 25 %~90 % B; 5~6 min, 90 % B; 6~6.5 min, 90 %~25 % B; 6.5~10 min, 25 % B. Column temperature: 25 °C. Flow rate: 0.3 mL/min. Injection volume: 2 μL.
Agilent 1290–6470 mass spectrometry conditions: Electrospray ion source (ESI). Spray voltage: 3300 V. Sheath gas flow rate: 38 arb and auxiliary gas flow rate: 10 arb. Ion transfer tube temperature: 320 °C and vaporizer temperature: 300 °C. Positive ionization in MRM scan mode with scanning range m/z 150–500. Collision energy: 20–38 eV. Residence time: 50 ms. Transmission voltage: 160 V. Accelerating voltage: 3 V.
2.5. Antioxidant activity determination
The determination of DPPH free radical scavenging ability, and ABTS+ and FRAP total antioxidant capacity were according to the test procedure of kit instructions.
2.5.1. DPPH assay
For the DPPH assay, 400 μL extracts or blank solvent (methanol) was mixed with 400 μL prepared DPPH solution in a centrifuge tube. The mixture was incubated in the dark for 30 min at room temperature. Then 200 μL was placed into 96-well plate. The absorbance value was measured at 517 nm. The free radical scavenging rate was calculated according to the following formula:
| DPPH scavenging rate (%) = (Ablank - Atest) / Ablank × 100% |
2.5.2. ABTS + assay
The ABTS+ assay was determined as the procedure. 10 μL extract or blank solvent (methanol) was mixed with 20 μL prepared enzyme solution and 170 μL ABTS+ solution to the 96-well plate. The mixture was incubated for 6 min at room temperature. The absorbance of the mixture was recorded at 734 nm. Calculated the ABTS+ inhibition rate according to the formula below:
| ABTS+ inhibition rate (%) = (Ablank - Atest) / Ablank × 100% |
2.5.3. FRAP assay
For FRAP assay, 5 μL of sample extract or blank solvent (methanol) mixed with 180 μL FRAP working solution in a 96-well plate, incubated at 37 °C for 5 min, and recorded the absorbance at 593 nm. FeSO4·7H2O was prepared as a standard solution, and diluted to several concentrations. The series solution was treated as the procedure mentioned above, established a calibration curve (y = 3.5051x - 0.2586, R = 0.9997), afterwards calculated FRAP value of the samples.
2.6. Statistical analysis
The t-test for assess the significant difference and correlation analysis using SPSS19.0 statistical analysis software. And the Figures were generated using Graphpad Prism 8.4.3.
3. Results and discussion
3.1. Determination of lignans and organic acids in Schisandra chinensis fruits by UPLC orbitrap HRMS
10 lignans and 5 organic acids were identified by comparing retention time and accurate mass with individual standards (Supplementary material, Figs. S1–S2). The validation of UPLC Orbitrap HRMS method was performed by linear regression, limits of detection (LOD) and quantification (LOQ), precision, repeatability and recovery. The results are shown in Supplementary material, Table S1. The established UPLC Orbitrap HRMS method was accurate and reliable, which can be used for the simultaneous determination of lignans and organic acids in Schisandra chinensis fruits.
3.2. Effects of different storage conditions on lignan and organic acid components of Schisandrachinensis fruits
The dried Schisandra chinensis fruits were packed with plastic woven bags, gunny bags or kraft paper bags and were stored under 5 °C or 15 °C with humidity at 40 %, 50 % or 60 %. The same amount of fruits was taken for determination of lignan and organic acid components on 0, 3, 6, 9, 12 and 24 months, respectively. The contents of lignans and organic acids in Schisandra chinensis fruits under different storage conditions were obtained and shown in Supplementary material, Table S2.
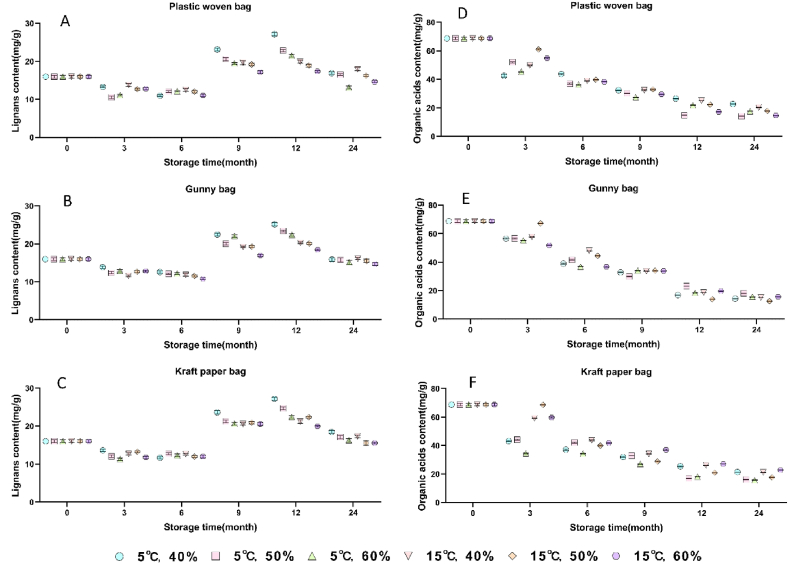
For lignans, under the same temperature, humidity and packaging materials, the contents of most lignans showed a trend of increasing first and then decreasing with the extension of storage period during the first one year. Among the lignans detected, the contents of schisantherin A and schisantherin B reached a peak when stored for 6 months. The contents of schisanhenol, schizandrin A and schisandrin C reached maximum at 9 months. The contents of gomisin J, schisandrol B and schizandrin B showed an increasing trend during the storage period, while the contents of schisandrol A and gomisin D showed a downward trend. As the storage period from one to two years, the contents of 10 lignans showed a downward trend and reached a relatively stable value. The Chinese Pharmacopoeia (2020 edition) prescribes the content of schisandrol A should not be lower than 0.40 %. According to this limit, two-year period Schisandra chinensis fruits of all storage conditions were not up to this standard, along with some samples at 15 °C and 50 % or 60 % humidity.
The total content changes of 10 lignans was also compared, as shown in Fig. 1(A-C). It can be seen that the total contents of 10 lignans under each packaging material generally showed a trend of first increasing and then decreasing during the storage period. At 12 months of storage, the total lignans content was generally higher. As the temperature and/or humidity increased, the total lignans contents were in decreased trends.
Fig. 1.
Effects of different storage conditions on the content of lignans (A,B,C) and organic acids (D,E,F) in Schisandra chinensis fruits.
From the results of the content of 5 organic acids, it can be seen that when ambient temperature, humidity and packaging materials are same, and the contents of quinic acid and citric acid reached maximum at 6 months and the content of chlorogenic acid reached a peak at 9 months during the storage period. The contents of malic acid and sorbic acid showed a downward trend.
For the packaging materials, the total content of 5 organic acids measured in each storage conditions were presented in Fig. 1(D-F). The results showed that under the same ambient temperature and humidity conditions, the total organic acidcontent in Schisandra chinensis fruits were decreased during the 2-years storage period. With the increasing of temperature and/or humidity, the total organic acids contents were generally decreased.
It was found that the contents of lignans in Schisandra chinensis fruits was not always reduced during the whole storage. This may be due to the transformation between the lignan components in the Schisandra chinensis fruits, so that the content of some lignans first increased rather than showed a trend of decreasing. Consider the instability of organic acids, the contents continued to decrease during storage. In summary, the ambient temperature of 5 °C and humidity of 40 % were the more suitable conditions for Schisandra chinensis fruits of long-period storage. The kraft paper bags was more suitable than the other two packaging materials. Since in the Chinese Pharmacopoeia (2020 edition), the content of schisandrol A should be higher than 0.40 % and the effective storage period of Schisandra chinensis fruits should be within 1 year.
3.3. Effects of different storage conditions on the antioxidant activity of Schisandra chinensis fruits extracts
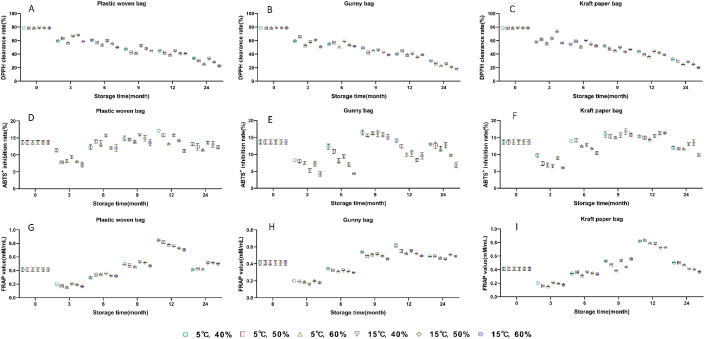
The DPPH clearance rate (%), ABTS+ inhibition rate (%) and FRAP value (mM/mL) of Schisandra chinensis fruits with different packaging materials under the ambient temperature of 5–15 °C and humidity of 40–60 % during 2-years storage periods were calculated according to the kit instructions. The results were shown in Fig. 2. For DPPH and ABTS+ detection, the decrease in absorbance value indicated that the antioxidant capacity of the sample was enhanced. The FRAP value was higher, the reducing ability of the antioxidant was stronger.
Fig. 2.
DPPH clearance rate (A,B,C), ABTS+ inhibition rate (D,E,F), FRAP value (G,H,I) of Schisandra chinensis fruits under different storage conditions.
As shown in Fig. 2(A-C), it can be seen that the DPPH clearance rate of fruits samples showed a downward trend with the extension of storage period. It was found that the change trend of DPPH clearance rate was consistent with that of the total contents of organic acids during the storage period. It indicated that organic acid components may have a greater influence on the antioxidant activity of DPPH clearance.
In the case of ABTS+ inhibition rate, it showed a trend of first increasing and then decreasing within the storage time (Fig. 2(D-F)). Between 9 and 12 months of storage, the ABTS+ inhibition rate reached higher. The samples stored for 12 months at 5 °C temperature and 40 % humidity presented the highest ABTS+ inhibition rate.
It can be seen from Fig. 2 (G-I), the FRAP value of Schisandra chinensis fruits was increased during the storage period of the first year and reached a maximum at 12 months. Then the FRAP value decreased at 24 months of storage.
The results showed that the change trend of ABTS+ inhibition rate and FRAP value was almost the same as the change of total lignans. It indicated that lignans presented a greater influence on the antioxidant activity of ABTS+ inhibition and FRAP value.
3.4. Correlation of multicomponent and antioxidant activity of Schisandra chinensis fruits with different storage conditions
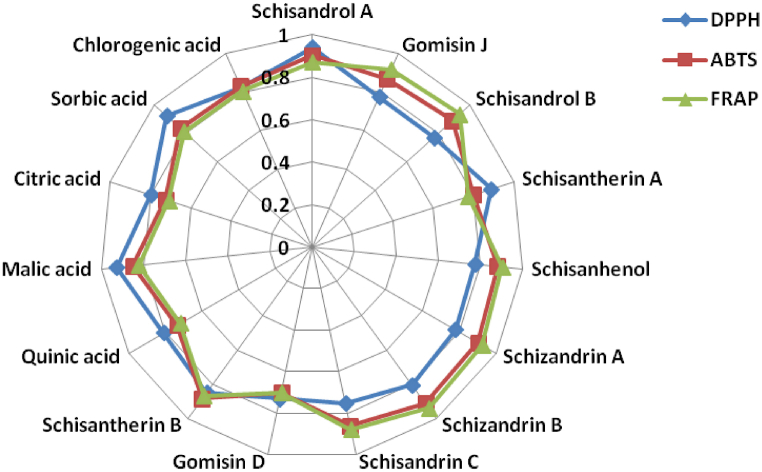
The DPPH clearance rate (%), ABTS+ inhibition rate (%) and FRAP value (mM/mL) of the Schisandra chinensis fruits were defined as the reference sequence, and the contents of lignans and organic acids in the samples were defined as the comparison sequence. Grey correlation analysis was carried out to evaluate the contribution of each component to the antioxidant activity. The correlation coefficient between antioxidant assays and multicomponent were shown in Table S3. The results of correlation coefficients were visualized and presented in Fig. 3.
Fig. 3.
Radar chart of correlation between multicomponents and antioxidant activities of Schisandra chinensis fruits.
The higher grey correlation coefficient of component, the stronger correlation between the component and the antioxidant activity, and the greater contribution of component to antioxidant activity. The results of grey correlation analysis showed that schisandrol A, malic acid and sorbic acid were the top three with higher correlation coefficient in DPPH clearance rate. For ABTS+ inhibition rate, the grey correlation coefficients of schizandrin B, schizandrin A and schisandrol A were in the top three, were all greater than 0.9. While for the FRAP value, schizandrin B, schisandrol B and schizandrin A were in the top three, all of which were greater than 0.9. According to the correlation results, it can be seen that in the activity of DPPH clearance, the contribution rate of organic acid components was larger. While in the activity of ABTS+ inhibition and FRAP value, the contribution rate of lignan components was larger.
3.5. Determination of aflatoxins in Schisandra chinensis fruits with different storage conditions
For the safety evaluation, the aflatoxins B1, B2, G1 and G2 were determined by UPLC QQQ MS in MRM mode and the monitoring ion pairs were shown in Supplementary material, Fig. S3 and Table S4. The UPLC QQQ MS method was validated and the regression equations, related coefficients, linear range, LOD, LOQ, precision, repeatability and recovery of aflatoxins B1, B2, G1 and G2 were tested. The established UPLC QQQ MS method was accurate and reliable for the determination of aflatoxins B1, B2, G1 and G2. The Schisandra chinensis fruits with storage periods of 24 months under all storage conditions were investigated and no aflatoxin was detected. It showed that during the two years storage period, no mildew occurred in the Schisandra chinensis fruits.
4. Conclusions
In this study, the influence of different storage conditions on the quality of Schisandra chinensis fruits was investigated. The quality evaluation of Schisandra chinensis fruits was carried out using multicomponent content and antioxidant activity, and the correlation of them. The effects of humidity and period of storage on the quality of Schisandra chinensis fruits were more significant. Therefore, the strict control of humidity and period of storage in the operating procedure was the key step during storage. The comprehensive analysis showed that the temperature of 5 °C and humidity of 40 % were more suitable for storing Schisandra chinensis fruits packed with kraft paper bag. And the effective storage period should not exceed 1 year. The antioxidant activity of Schisandra chinensis fruits was highly related to its lignans and organic acids components. And schisandrol A, malic acid, sorbic acid, schizandrin A, schizandrin B, and schisandrol B were the main effective components of antioxidant activity. For the safety evaluation by aflatoxins determination, the storage conditions were appropriate during the two years period. This study provided a scientific reference for the effective and safe storage of Schisandra chinensis fruits.
Data availability statement
The data included in article and supplementary material in article.
Funding statement
The authors appreciate the support of the National Key R&D Program of the Ministry of Science and Technology (2019YFC1710704).
CRediT authorship contribution statement
Yanhua Sheng: Writing – original draft, Methodology, Investigation, Formal analysis. Jinxu Dong: Validation, Software. Rui Wang: Formal analysis. Yikai Wang: Visualization, Validation. Xin Huang: Writing – review & editing, Supervision, Resources, Project administration. Changbao Chen: Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32194.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.National Pharmacopoeia Committee . 2020. ChP 2020 Edition; p. 68. [Google Scholar]
- 2.Liu Y.Y., Huang S.Q., Li Y.Z., Fan H., Zhang H.W., Deng C., Song X.M., Zhang D.D., Wang W. Research progress on lignans and pharmacological activities in plants of Schisandra. Chin. Tradit. Herb. Drugs. 2022;6:1903–1918. [Google Scholar]
- 3.Xu M.J., Yan T.X., Gong G.W., Wu B., He B.S., Du Y.Y., Xiao F., Jia Y. Purification, structural characterization, and cognitive improvement activity of a polysaccharides from Schisandra chinensis. Int. J. Biol. Macromol. 2020;163:497–507. doi: 10.1016/j.ijbiomac.2020.06.275. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q.Q., Wei W.J., Li Y., Gao K. Triterpenoids and lignans from Schisandra chinensis and their inhibition activities of Cdc25A/B phosphatases. Nat. Prod. Res. 2020:1–6. doi: 10.1080/14786419.2020.1802268. [DOI] [PubMed] [Google Scholar]
- 5.Liu X.K., Guo Y.L., Cai G.Z., Gong J.Y., Wang Y., Liu S.Y. Chemical composition analysis of Schisandra chinensis fructus and its three processed products using UHPLC-Q-Orbitrap/MS-based metabolomics approach. Nat. Prod. Res. 2020:1–4. doi: 10.1080/14786419.2020.1858416. [DOI] [PubMed] [Google Scholar]
- 6.Li J.H., Li W., Luo S., Ma C.H., Liu S.X. Alternate ultrasound/microwave digestion for deep eutectic hydrodistillation extraction of essential oil and polysaccharide from Schisandra chinensis (Turcz.) Baill. Molecules. 2019;7:1288–1310. doi: 10.3390/molecules24071288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai W.Y., Wang H.E., Wang B.Y., Yu H.S., Li Z. Research progress on chemical constituents and pharmacological activities of Schisandra chinensis. Chin. Tradit. Pat. Med. 2019;9:2177–2183. [Google Scholar]
- 8.Wang X.R., Liu Y., Niu Y.Y., Wang N.X., Gu W. The Chemical composition and functional properties of essential oils from four species of Schisandra growing wild in the Qinling Mountains, China. Molecules. 2018;23:1645. doi: 10.3390/molecules23071645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X.Y., Wang J.Q., Xin Y., Li F. Optimization of the extraction process of polysaccharide from Schisandra Chinensis (Turcz.) Baill. J. Phys.: Conf. Ser. 2019;1176:62032–62041. [Google Scholar]
- 10.Li B. Study on chemical constituents of Schisandra chinensis. Guide Chin. Med. 2018;21:20–21. [Google Scholar]
- 11.Yin F.Z., Dai H., Li L., Lu T.L., Li W.D., Cai B.C., Yin W. Study of organic acids in Schisandrae Chinensis Fructus after vinegar processing. J. Separ. Sci. 2017;20:4012–4021. doi: 10.1002/jssc.201601447. [DOI] [PubMed] [Google Scholar]
- 12.Xing N.N., Qu H.D., Ren W.C., Ma W. Main chemical constituents and modern pharmacological action of Schisandrae Chinensis Fructus: a review. Chin. J. Exp. Tradit. Med. Form. 2021;15:210–218. [Google Scholar]
- 13.Ma Y.C., Feng T.T., Han Y.B., Fan C.C., Liu Y.F., Liang S., Wang X.Q., Dai L.J. Research progress on chemical constituents and pharmacology of Schisandra chinensis. Acta Chin. Med. Pharm. 2020;11:67–71. [Google Scholar]
- 14.Li Q.S., Deng Y.H., Wang Y.L., Ye L., Wu L.L., Zheng J.C., Luo X.Y., Yi S.L., Deng Q., Sun L.J. Antioxidant and anti-Fusarium activities of Schisandra chinensis extract and its effect on basic characteristics of dried Lutjanus sanguinaus. Food Ferment. Ind. 2020;7:130–135. [Google Scholar]
- 15.Liu J., Xu J., Guo J.T. Review of active constituents and pharmacological activities of Schisandrae Chinensis Fructus. J. Exp. Tradit. Med. Form. 2019;11:206–215. [Google Scholar]
- 16.Huang Y., Liu X., Tao W., Pan G.T., Mei Z.G. Advances in chemical constituents of Schisandra chinensis and activities of anti-type 2 diabetes. Chin. Tradit. Herb. Drugs. 2019;7:1739–1744. [Google Scholar]
- 17.Bian Z.H., Jin S., Zhou C.G., Hu M.M. Screening and UPLC-QTOF-MS/MS analysis of active fractions of Schisandra chinensis against methicillin-resistant Staphylococcus aureus in vitro. Chin. J. Hosp. Pharm. 2018;19:2008–2012. [Google Scholar]
- 18.Yu H.R., Tian Z.K., Gao X., Chen X.Y., Wu L. Research advances in pharmacological effects of polysaccharide from Schisandra Chinensis. Chem. Eng. 2018;7:64–67. [Google Scholar]
- 19.Zhang M.X., Huang G.Y., Bai Y.Q., Li H., Yang B. Research advances in chemical constituents and hepatoprotective effect of schisandrae sphenantherae fructus and schisandrae chinensis fructus. China J. Chin. Mater. Med. 2021;5:1017–1025. doi: 10.19540/j.cnki.cjcmm.20201127.601. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Yu J.H., Wang X.T., Wang C.M., Li H., Chen J.G., Sun J.H. Immunomodulatory effect of Schisandra chinensis lignans in cyclophosphamide- induced immunodeficiency mice. J. Jilin Univ. - Med. Ed. 2019;2 294-299, 472. [Google Scholar]
- 21.Hong S.H., Li M., Jeung E.B., Lee G.S., Hong E.J., Choi Y.W., An B.S. Therapeutic effects of Schisandra chinensis on the hyperprolactinemia in rat. Int. J. Oncol. 2017;4:1448–1454. doi: 10.3892/ijo.2017.3881. [DOI] [PubMed] [Google Scholar]
- 22.Xu L.J., Grandi N., Del V.C., Mandas D., Corona A., Piano D., Esposito F., Parolin C., Tramontano Enzo. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J. Microbiol. 2015;4:288–293. doi: 10.1007/s12275-015-4652-0. [DOI] [PubMed] [Google Scholar]
- 23.Nowak A., Szyda M.Z., Błasiak J., Nowak A., Zhang Z., Zhang B.L. Potential of Schisandra chinensis(Turcz.) Baill. in human health and nutrition: a review of current knowledge and therapeutic perspectives. Nutrients. 2019;11:333. doi: 10.3390/nu11020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng L.Z., Xiong C.H., Pei Y.P., Zhu Z.Q., Zheng X., Zhang Y., Yang X.H., Liu Z.L., Xiao H.W. Effects of various storage conditions on total phenolic, carotenoids, antioxidant capacity, and color of dried apricots. Food Control. 2022;136:108846–108856. [Google Scholar]
- 25.Liang S.J., Wen Z.J., Tang T.X., Liu Y.F., Dang F.L., Xie T.X., Wu H. Study on flavonoid and bioactivity features of the pericarp of Citri Reticulatae ‘chachi’ during storage. Arab. J. Chem. 2022;3:103653–103667. [Google Scholar]
- 26.Yang M., Jiang Z.D., Wen M.C., Wu Z.F., Zha M.Y., Xu W., Zhang L. Chemical variation of Chenpi (Citrus Peels) and corresponding correlated bioactive compounds by LC-MS metabolomics and multibioassay analysis. Front. Nutr. 2022;9:825381–825398. doi: 10.3389/fnut.2022.825381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y., Guo W.B., Liu Y.X., Sang Y.Y., Yang W.T., Guo M.R., Cheng S.B., Chen G.G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao) Sci. Hortic. 2021;288:110372–110381. [Google Scholar]
- 28.Fu X.J., Xing S.P., Xiong H.W., Min H., Zhu X.J., He J.L., Feng J.X., Mu H.L. Effects of packaging materials on storage quality of peanut kernels. PLoS One. 2018;3:e0190377–e0190387. doi: 10.1371/journal.pone.0190377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X.F., Luo C.Q., Xing J.Y., Han Z.Z., Li T., Wu W.W., Xu H., Zhan R.T., Chen W.W. Optimization of storage conditions of the medicinal herb Ilex asprella against the sterigmatocystin producer Aspergillus versicolor using response surface methodology. Toxins. 2018;10:499–510. doi: 10.3390/toxins10120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q.T., Kong W.J., Yang M.H., Guo W.Y. Review of scientific preservation techniques for traditional Chinese medicine becoming mouldy during storage. China J. Chin. Mater. Med. 2015;7:1223–1229. [PubMed] [Google Scholar]
- 31.Sheng Y.H. Master Thesis; 2023. Study on Quality Evaluation of Schisandra Chinensis (Turcz.) Baill. Fruits Medicinal Material. [Google Scholar]
- 32.National Pharmacopoeia Committee . 2020. ChP 2020 Edition. general rule 2351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data included in article and supplementary material in article.