Abstract
Background
A wide variety of materials are used for lumbar interbody fusion, but there is no unified consensus on the superiority of one material over another. The aim of this systematic review and network meta-analysis (NMA) is to compare and rank the various TLIF interbody materials based on fusion rates.
Methods
We queried PubMed, EMBASE and Scopus from inception until August 2023, in which 2135 studies were identified. Inclusion criteria were applied based on the PRISMA guidelines. The fusion assessment employed the Bridwell's criteria with a length of follow-up of at least 12 months. The NMA was conducted to compare multiple approaches from multiple studies using the frequentist framework with STATA16.
Results
In total, 13 TLIF studies involving 1919 patients with 1981 lumbar interbody levels fulfilled our eligibility criteria. Seven different cage materials were utilized: polyetheretherketone (PEEK, as the reference), allograft, autograft, PEEK with titanium coating (TiPEEK), titanium, carbon/carbon fiber reinforced polymer (CFRP) and 3D-printed titanium. The average patient age was 60.9 (SD = 7.5) years old. When compared to PEEK, the other six materials did not have a significantly different rate of lumbar fusion. However, the SUCRA number of the 3D-printed titanium, TiPEEK, Ti, allograft, autograft, CFRP, and PEEK were 0.8, 0.6, 0.5, 0.5, 0.4, 0.4, and 0.3 consecutively.
Conclusions
Based on a network meta-analysis within the confines of our clinical study, 3D-printed titanium interbody cage may promote the highest success rate of fusion while PEEK may be the material with the least success rate of fusion in TLIF.
Keywords: 3D printing, TLIF, Lumbar interbody fusion, PEEK, Titanium, Material, Fusion rate
1. Introduction
Transforaminal lumbar interbody fusion (TLIF) was first reported by Harms and Rolinger in 1982.1 It has become popular among spine surgeons because the procedure is safe and effective.2,3 Although the overall lumbar interbody fusion rates for TLIF are high (above 89 %),4,5 failed TLIF cage could generate pain and disability requiring revision surgery.6 The results of revision procedures have been relatively poor, with only 30–50 % rate of functional success.7 Careful surgical strategies including interbody material and optimal graft selection are important to achieve the highest fusion rate.
Various types of interbody material are available. Each has both advantages and disadvantages over the others. The most predominant used spacers are titanium and polyetheretherketone (PEEK) because they are biocompatible and able to provide the mechanical stability.8 The other options to PEEK are CFRP, titanium-coated PEEK, femoral strut allograft, morselized local autograft, as well as hydroxyapatite-polymer composites and coatings. Although spine interbody implants are commonly used, the optimal material selection remains controversial with few clinical studies comparing fusion rates between interbody materials.
To the best or our knowledge, there has been only one report of standard pairwise meta-analyses on role of titanium (Ti) and PEEK cages which have shown no significant difference in terms of lumbar fusion rates.9 Several studies have evaluated the role of interbody material in cervical spinal fusion, which cannot be used to interpret in our study.10, 11, 12 This study aimed to compare the fusion rates between different types of interbody materials used for TLIF. Using the network meta-analysis (NMA), we can identify and rank the interbody materials based on the lumbar interbody fusion rates when using TLIF procedures.
2. Methods
This study constitutes a systematic review with a NMA. We performed the method and reporting structure following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for NMA.
2.1. Search strategy and evidence selection
We queried PubMed, EMBASE, Scopus and Web of Science for relevant studies that matched our criteria from inception until August 2023. The search strategy consisted of keywords: “lumbar interbody fusion” AND “cage” with materials documentation “polyetheretherketone” OR “PEEK” OR “titanium” OR “carbon” OR “allograft” OR “autograft” OR “3D printing” OR “tantalum”. Only studies with full English-language text availability were considered. Two independent reviewers (SP1 and SP2) performed the two-stage screening for study relevance, first by title and abstract, and then, by the full text article review. A third senior author (SN) made a final judgment for any disagreement between the reviewers during the evidence selection process. The critical appraisal of our eligible articles was independently assessed and extracted by two authors (SP1 and SP2). using a standardized data extraction form including study design, sample size, patient characteristics i.e., age and sex, TLIF cage materials, types of bone graft, TLIF surgical levels, fusion assessment methods, clinical and radiographic outcomes and follow-up time. The risk of bias assessment for the nonrandomized trials was evaluated using the Newcastle-Ottawa Scale.13
2.2. Inclusion criteria
Eligibility criteria for our study included1: a diagnosis of degenerative lumbosacral spine disease2; a comparison between materials of Ti, PEEK, Ti-coated PEEK, allograft, autograft, CFRP, tantalum and 3D-printed Ti and3 a patient population of adult patients with more than 12 months of postoperative follow-up. Exclusion criteria included1: case reports, reviews, in vitro biomechanical studies, in vivo animal studies and computer modeling studies2; a diagnosis of idiopathic scoliosis, spinal infection, spinal oncology, autoimmune disease, and/or trauma3; surgical interventions of spondylectomy, corpectomy and corrective osteotomy procedures4; unspecified or uncommon interbody material5; expandable cage and6 use of bone morphogenic protein (BMP).
The rate of lumbar interbody fusion was the primary outcome. Lumbar fusion was evaluated using computed tomography (CT) scan or radiographic x-ray with a length of follow-up of at least 12 months. The criteria for fusion were defined as complete trabecular bridging of grafted bone material with or without a visual gap or grade I or II based on Bridwell's criteria.14,15 Grade I is defined as fusion with remodeling and trabeculae present; grade II is defined as an intact graft, but not fully remodeled and incorporated without lucencies. Some studies also defined nonunion as greater than 4° of motion on dynamic imaging.
2.3. Data synthesis and analysis
The direct evidence (head-to-head trials) were analyzed using a random-effect model and reported coefficient, standard error, p-value, and 95 % confidence interval (CI). The odds ratio (OR) with 95 % confidence intervals (CIs) were reported in the forest plot. The positive coefficient means OR greater than one. On the other hand, a negative coefficient means OR less than one. The statistical analyses in this study were performed by the biostatistician (SP2) using Stata (release 16, Stata- Corp LLC, TX, USA). The ranking probability of each TLIF cage material was calculated. This was achieved by calculating the log odds ratio for each type of interbody materials compared with the reference, namely, PEEK. The surface under the cumulative ranking (SUCRA) was also created to represent the cumulative probability for each interbody material to demonstrate the most desirable material option to gain the highest success fusion rate. The rank is presented both graphically and numerically for comparison in which the graph presents the area under the curve to indicate the probability of each material option. The numeric presentation offers overall ranking ranging from 0 to 100 %; a ranking closer to 100 % indicates a higher likelihood of that material option to be in the top rank, while a ranking closer to 0 provides a material option of lower likelihood of the bottom rank, than other options. We further analyzed the quality of the included studies for discrepancies using the NewCastle-Ottawa Scale (NOS).
3. Results
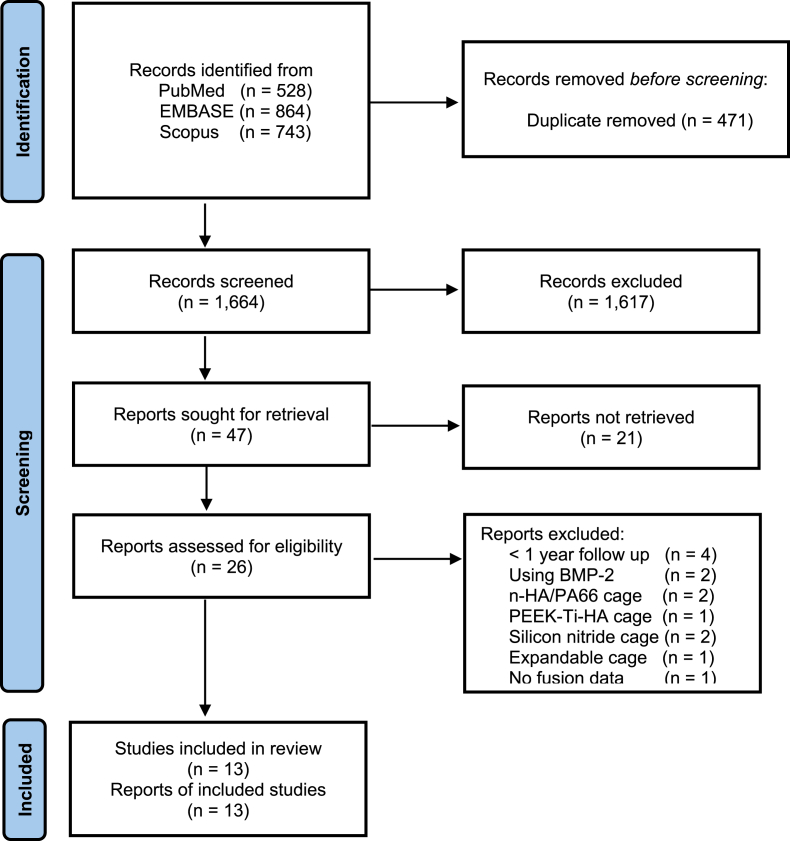
We identified 2135 articles: 528 from PubMed, 864 from EMBASE and 743 from Scopus (Fig. 1). In all, 471 were duplicated references; and the remaining 1664 articles underwent title and abstract review, of which 26 proved potentially eligible for our study and underwent full text review. Of these 26 TLIF studies with head-to-head comparisons of interbody materials, 13 studies were excluded (five studies using uncommon materials (n-HA/PA66, Silicon nitride, PEEK-Ti-HA), four studies with follow-up time less than one year, two studies using BMP, one study using expandable cage and one study without report of fusion rate).
Fig. 1.
The PRISMA flow diagram of the include studies.
A total of 13 TLIF studies were included in the NMA (Fig. 1). These studies included eight retrospective studies, three prospective studies and two randomized controlled trial with 1919 patients performed on 1981 lumbar interbody levels (Table 1). The study included 845 males and 1074 females with the average age of 60.9 years (SD = 7.5). The systematic review of the comparison of the different interbody materials is shown in Table 2. The fusion rate of each interbody material was PEEK (90.2 %), Ti (89.2 %), CFRP (100 %), allograft (96.5 %), TiPEEK (94.4 %), autograft (94.8 %) and 3D-printed Ti (90.9 %).
Table 1.
Characteristics of the included studies.
| Authors | Design | Average age | Male/Female | Materials | Autograft | Synthetic graft | L1-2 | L2-3 | L3-4 | L4-5 | L5-S1 |
| Cutler 2006(47) | R | 45.8 | 9/12 | Allograft | 21 | 21 (DBM) | |||||
| 50.2 | 8/10 | PEEK | 18 | 18 (DBM) | |||||||
| Lv 2015(48) | R | 51 | 32/52 | PEEK | 84 | 61 | 23 | ||||
| 53 | 37/59 | Autograft | 67 | 29 | |||||||
| Mura 2011(49) | R | 54.2 | M = 62 | PEEK | 56 | ||||||
| 54.2 | F = 38 | CFRP | 58 | ||||||||
| Nemoto 2014(50) | R | 40.7 | 22/1 | Titanium | 23 | 7 | 16 | ||||
| 42.9 | 23/2 | PEEK | 25 | 10 | 15 | ||||||
| Rickert 2017(51) | RCT | 67.7 | 6/14 | Ti PEEK | 22 | 22 (HA + B-TCP) | 1 | 9 | 14 | ||
| 68.3 | 6/14 | PEEK | 20 | 20 (HA + B-TCP) | 1 | 10 | 15 | ||||
| Vazifehdan 2019(52) | R | 71 | M = 167 | PEEK | |||||||
| 71 | F = 252 | Titanium | |||||||||
| Canseco 2021(53) | R | 63.0 | 46/62 | PEEK | 108 | 1 | 9 | 73 | 26 | ||
| 59.0 | 15/14 | Titanium | 29 | 2 | 24 | 3 | |||||
| Khan 2022(46) | P | 63.5 | 42/72 | PEEK | N/A | ||||||
| 63.5 | 51/63 | 3D-Printed Ti | N/A | ||||||||
| Kim 2022(45) | R | 58.53 | 21/22 | PEEK | 43 | 43 (DBM) | 6 | 30 | 7 | ||
| 59.17 | 21/19 | 3D-Printed Ti | 40 | 40 (DBM) | 4 | 28 | 8 | ||||
| Li 2020(54) | P | 66.3 | 15/19 | PEEK | 34 | 1 | 1 | 1 | 21 | 10 | |
| 67.6 | 15/18 | Allograft | 33 | 1 | 1 | 21 | 10 | ||||
| Singhatanadgige 2022(55) | RCT | 64.1 | 15/26 | PEEK | 50 | 50 (DBM) | 7 | 25 | 18 | ||
| 62.7 | 13/28 | TiPEEK | 49 | 49 (DBM) | 7 | 37 | 5 | ||||
| Tanida 2016(56) | R | 65 | 15/25 | PEEK | 51 | 2 | 4 | 30 | 15 | ||
| 62.5 | 36/41 | Titanium | 93 | 1 | 7 | 14 | 54 | 17 | |||
| Wu 2019(57) | P | 55.3 | 76/97 | PEEK | 173 | 2 | 4 | 8 | 74 | 85 | |
| 54.2 | 92/114 | Allograft | 206 | 2 | 3 | 97 | 104 |
| Authors | F/U length (mo.) | fusion assessment method | Fusion rate | VAS back Pre-op | VAS back Post-op | VAS leg Pre-op | VAS leg Post-op | ODI score Pre-op | ODI score Post-op |
|---|---|---|---|---|---|---|---|---|---|
| Cutler 2006 | 12 | Xray | 20/21 | 63 | 20.7 | ||||
| 12 | Xray | 18/18 | 63 | 22.8 | |||||
| Lv 2015 | 35 | CT | 79/84 | 42 | 21 | ||||
| 35 | CT | 91/96 | 45 | 19 | |||||
| Mura 2011 | 12 | CT, dynamic Xray | 56/56 | ||||||
| 12 | CT, dynamic Xray | 58/58 | |||||||
| Nemoto 2014 | 24 | CT | 22/23 | 6 | 1 | 5.6 | 0.6 | ||
| 24 | CT | 16/25 | 6.1 | 1.28 | 5.4 | 0.7 | |||
| Rickert 2017 | 12 | CT, dynamic x-ray | 20/22 | 7.2 | 3.6 | 6 | 3.7 | 42 | 27 |
| 12 | CT, dynamic x-ray | 20/22 | 5.2 | 2.4 | 5.8 | 1.6 | 39 | 16 | |
| Vazifehdan 2019 | 50 | x-ray, CT | 296/323 | ||||||
| 50 | x-ray, CT | 91/96 | |||||||
| Canseco 2021 | 12 | x-ray | 100/108 | 7.2 | 2.9 | 7.1 | 1.8 | 43.2 | 22.0 |
| 12 | x-ray | 25/29 | 7.5 | 4.2 | 7.8 | 2.0 | 40.0 | 18.0 | |
| Khan 2022 | 12 | dynamic x-ray |
83/114 | 45.2 | 29.5 | ||||
| 12 | dynamic x-ray |
102/114 | 43.4 | 24.9 | |||||
| Kim 2022 | 12 | CT | 42/43 | 2.0 | 2.3 | 6.0 | 3.0 | 45.6 | 36.9 |
| 12 | CT | 38/40 | 4.4 | 2.0 | 6.3 | 2.9 | 50.8 | 38.4 | |
| Li 2020 | 24 | dynamic x-ray |
30/34 | 50.9 | 29.0 | ||||
| 24 | dynamic x-ray |
30/33 | 48.4 | 25.9 | |||||
| Singhatanadgige 2022 | 12 | CT | 45/50 | 6.3 | 1.4 | 7.3 | 1.7 | 48.2 | 12.7 |
| 12 | CT | 47/49 | 6.6 | 1.5 | 6.6 | 0.9 | 53.5 | 12.4 | |
| Tanida 2016 | 24 | CT, dynamic xray | 41/51 | ||||||
| 24 | CT, dynamic xray | 77/93 | |||||||
| Wu 2019 | 24 | dynamic x-ray |
167/173 | 50.1 | 28.0 | ||||
| 24 | dynamic x-ray |
201/206 | 49.8 | 26.8 |
Table 2.
Comparison of the different interbody materials.
| Material | Male (n) | Female (n) | Local bone (n) | DBM (n) | HA + B-TCP | L1-2 (n) | L2-3 (n) | L3-4 (n) | L4-5 (n) | L5-S1 (n) | Fusion Segment (n) | Segment Level (n) | Fusion rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEEK | 299 | 401 | 662 | 111 | 20 | 3 | 9 | 45 | 339 | 138 | 993 | 1101 | 90.2 % |
| Ti | 73 | 56 | 145 | N/A | N/A | 1 | 7 | 16 | 85 | 36 | 215 | 241 | 89.2 % |
| CFRP | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 58 | 58 | 100 % |
| Allograft | 116 | 144 | 260 | 21 | N/A | N/A | 3 | 4 | 118 | 114 | 251 | 260 | 96.5 % |
| TiPEEK | 19 | 42 | 71 | 49 | 22 | N/A | 1 | 16 | 51 | 5 | 67 | 71 | 94.4 % |
| Autograft | 37 | 59 | N/A | N/A | N/A | N/A | N/A | N/A | 67 | 29 | 91 | 96 | 94.8 % |
| 3D-Printed Ti | 72 | 82 | 40 | 40 | N/A | N/A | N/A | 4 | 28 | 8 | 140 | 154 | 90.9 % |
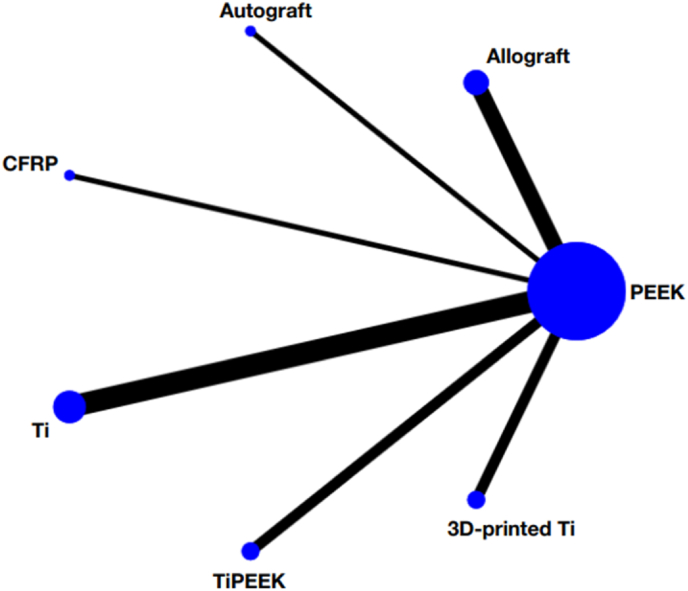
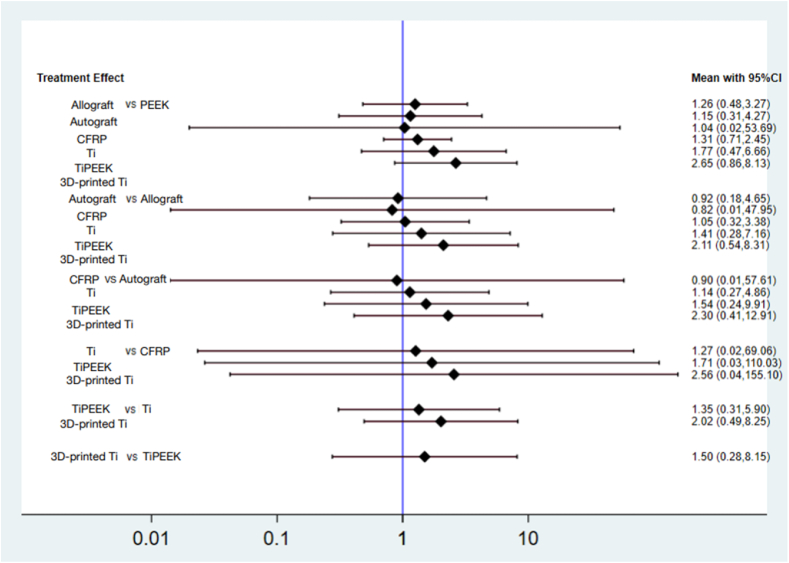
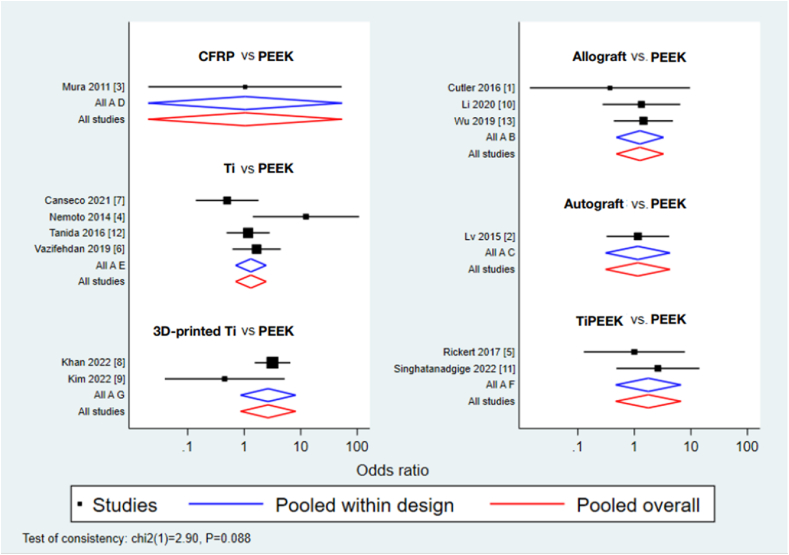
Altogether were six types of head-to-head trials, which included PEEK vs. allograft (n = 106 patients), PEEK vs. autograft (n = 180), PEEK vs. CFRP (n = 100), PEEK vs. Ti (n = 721), PEEK vs. TiPEEK (n = 122) and PEEK vs. 3D-printed Ti (n = 311) (Fig. 2). A summary of the characteristics of each surgical approach including types of bone graft used, operated lumbar levels, lumbar fusion rates and functional outcomes, is presented in Table 2. The pooled results with PEEK material as the reference comprise the following: allograft (RR 1.26, 95 % CI 0.48 to 3.27), autograft (RR 1.15, 95 % CI 0.31 to 4.27), CFRP (RR 1.04, 95 % CI 0.02 to 53.69), Ti (RR 1.31, 95 % CI 0.71 to 2.45), TiPEEK (RR 1.77, 95 % CI 0.47 to 6.66) and 3D-printed Ti (RR 2.65, 95 % CI 0.86 to 8.13). Moreover, no statistically significant difference was found among the allograft, autograft, CFRP, Ti, TiPEEK and 3D-printed Ti (Fig. 3).
Fig. 2.
Network geometry of the consistency model of the different interbody materials. The size of each node is proportional to the total number of randomly assigned participants, and the width of each line is proportional to the number of studies comparing each pair of treatment strategies.
Fig. 3.
Interval plots of the different interbody materials.
We performed the NMA with multiple treatment comparisons simultaneously in a single analysis including corresponding OR and 95 % CIs. The fusion assessment at the postoperative follow-up in the trials ranged between 12 and 50 months. We described the bias assessment using the Newcastle–Ottawa quality assessment form for nonrandomized study.13 Two of 13 studies were a prospective randomized controlled trial (RCT). Another 11 studies were assessed in the selection domain of which three studies demonstrated four stars, while eight studies demonstrated three stars. All 11 nonRCT studies demonstrated one star in the comparability domain and three stars in the outcome/exposure domain. Thus, eight studies received a total of seven stars and three studies received a total of eight stars. In other words, all studies revealed a low risk of bias (Table 3).
Table 3.
The NewCastle-Ottawa scale (NOS) of the include studies.
| Study | Selection | Comparability | Outcome/Exposure |
|---|---|---|---|
| Cutler 2016 | ★★★ | ★ | ★★★ |
| Lv 2015 | ★★★ | ★ | ★★★ |
| Mura 2011 | ★★★ | ★ | ★★★ |
| Nemoto 2014 | ★★★ | ★ | ★★★ |
| Vazifehdan 2019 | ★★★ | ★ | ★★★ |
| Canseco 2021 | ★★★ | ★ | ★★★ |
| Khan 2022 | ★★★★ | ★ | ★★★ |
| Kim 2022 | ★★★ | ★ | ★★★ |
| Li 2020 | ★★★★ | ★ | ★★★ |
| Tanida 2016 | ★★★ | ★ | ★★★ |
| Wu 2019 | ★★★★ | ★ | ★★★ |
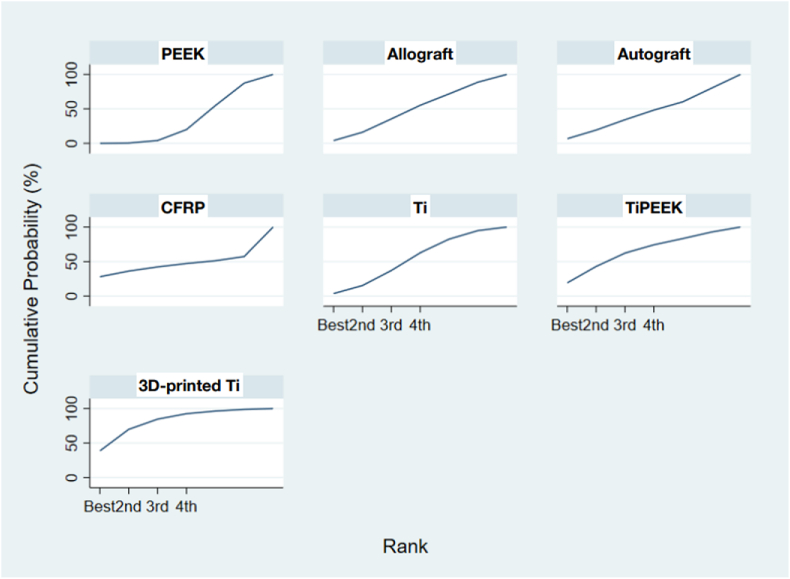
The design-by-treatment interaction model did not show significant inconsistency in the NMA of the fusion rate (Chi-square = 2.90; p = 0.088; Fig. 4). These results were ranked based on the cumulative probability plots and the SUCRA values were determined (Fig. 5). Comparing PEEK with the other six materials, = no significant different rate of lumbar fusion was noted. The SUCRA scores of the seven materials revealed that 3D-printed Ti (SUCRA = 0.8) had the highest probabilities of being the best interbody material for fusion rates. The second and third rank belonged to TiPEEK (SUCRA = 0.6), Ti (SUCRA = 0.5) and allograft (SUCRA = 0.5), respectively. The fifth rank belonged to autograft (SUCRA = 0.4) and CFRP (SUCRA = 0.4). However, the opposite to rank cumulative probability, the SUCRA suggested that PEEK (SUCRA = 0.3) to be the material with the least success rate of fusion in TLIF.
Fig. 4.
Network forest plot of the different interbody materials.
Fig. 5.
Results of the network rank test of the different interbody materials by the surface under the cumulative ranking curve (SUCRA) values.
4. Discussion
Lumbar interbody fusion plus posterior instrumentation has proved to provide superior fusion rates than posterolateral fusion alone.16,17 According to the TLIF approach, the unilateral working zone to perform discectomy and endplate preparation is narrow. As a result, TLIF accommodates a smaller interbody footprint compared with others.18 This would make a successful interbody fusion crucial because of the smaller interbody surface area. In addition to biocompatible and load carrying capacity, the ideal material interbody spacers should have 1) elastic modulus similar to cortical bone (18 GPa)19; 2) surface modification to improve bone on-growth and ingrowth20, 21, 22, 23; 3) radiolucency for easily assess radiographic fusion19 and 4) high level of support for osteogenic tissues.24
When compared Titanium (Ti) and PEEK, the commonly used interbody materials in clinical practice. Titanium has more favorable fusion rates than PEEK24,25 because of its high strength and elastic modulus which could potentially cause a stress shielding effect on the peri-implant bone and periprosthetic loosening.26 In vitro study demonstrated more human osteoblast differentiation and potential for improving cell adhesion on bone-titanium interface.27 PEEK is radiolucency allowing visualization of bone healing by normal radiographs and indicated less elastic modulus than cortical bone.28 Also, PEEK is hydrophobic in nature making it difficult to bond to bone. This lack of implant osteo-integration has been demonstrated in animal models.25
Hybrid technologies and surface coating have been made to combine the advantages of Ti and PEEK. TiPEEK composite with titanium endplate and a PEEK central portion demonstrated the osseo-integrative potential of titanium and radiolucency as well as favorable elastic modulus of PEEK.29 Walsh et al.30 demonstrated the histology of bone-implant interface of adult sheep comparing plasma-sprayed TiPEEK and PEEK implants at 4 and 12 weeks. The TiPEEK implants had direct bone ongrowth, whereas PEEK presented a fibrous union. Mechanical testing also confirmed at 4 and 12 weeks that hybrid implants had shear stress 8.0 and 18.1 MPa, while PEEK had 1.7 and 1.8 MPa, respectively. In term of subsidence rates, the titanium-coated PEEK also proved similar outcomes compared with the pre-existing modest subsidence of PEEK.31
CFRP PEEK had been developed and introduced during 1990(28). This material has the mechanical support, biocompatible and radiolucent properties as well as elastic modulus approximate to cortical bone.32,33 In addition, the material is compatible with CT and magnetic resonance imaging because of its nonmetal characteristics.34 Clinical studies have reported the interbody fusion success rates ranged from 70 to 100 %.35, 36, 37, 38 Although the fusion rates of CFRP are comparable with PEEK and Ti, the brittleness of carbon fiber constitutes a disadvantage.33 Tullberg reported the carbon cage could break if nonunion occurred.39 This could be the reason for the lower popularity of this material over time.
The structural allograft interbody provides an osteoconductive scaffold, which serves an essential biologic role to promote bone growth. Fatima et al.12 reported the structural allograft had 2.59-fold higher probability of fusion compared with PEEK. However, the subsidence rate of PEEK was lower than that of structural allografts. In term of cost effectiveness, related literature reported PEEK cages were more costly than structural allografts. The estimated cost for PEEK spacers were $4930 to $5246, whereas the structural allograft spacers were $1220 to $3640.40
The lamina, articular and spinous processes that were obtained during posterior decompression could be used as the morselized impacted bone grafts in the interbody space.41 Although this technique is less expensive, the increment in the disc height was significantly worse than using the cage (p < 0.012).42 It was explained in that the interbody cage is able to support the compression better than the morselized bone grafts.42 Autogenous iliac bone graft might be better in term of restore disc height; however, the method is not commonly used because of donor site pain and requiring more surgical intervention.41
3D-printed Ti is a recent development technology to improve osteo-integration of the cage by titrating the porosity, strut widths and orientation of surface modifications.43,44 McGilvray et al.43 demonstrated the histologic sections of bony ingrowth in ovine lumbar fusion model comparing PEEK, plasma sprayed porous titanium-coated PEEK (PSP) and 3D-printed Ti. PEEK implants were surrounded with poorly fibrous connective tissue, whereas the PSP implants had better neovascularization and slightly decreased fibrous union. The best osteo-integration was found in 3D-printed Ti, revealing increasing osteoblastic and osteoclastic remodeling as well as complete bony filling without fibrous union at the implant pores. Kim et al.45 reported no difference in terms of fusion rate between PEEK and 3D-printed Ti (95.0 % and 93.0 %) at 12 months. However, they found the quality or fusion grade I was better in the 3D-printed Ti than PEEK cage (37.5 vs. 16.3 % at 6 months, 77.5%vs. 51.2 % at 1 year). Furthermore, the subsidence rate of 3D-printed Ti indicates lower incidence than PEEK (23.5 vs. 40.2 %).46 The 3D-printed technology has a unique porous structure which could adjust the size of the porosity leading to maintaining the elastic modulus similar to the physiologic level.
The limitation in our review is that it only investigated studies using TLIF as the surgical approach without BMP application. Only 13 head-to-head studies with 1919 cases were included in our NMA. This small sample size may lead to underpowered pooled estimates and wide confidence intervals.
5. Conclusion
The NMA within the confines of our clinical study showed no significant difference in lumbar interbody fusion rate between materials of the TLIF interbody cage. However, 3D-printed titanium interbody cage statistically appeared to have the highest probability of being the best material for promoting fusion in TLIF. Future prospective comparative studies are required to confirm these conclusions and may help guide selecting the appropriate interbody material.
CRediT authorship contribution statement
Sutipat Pairojboriboon: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Supranee Niruthisard: Writing – review & editing, Conceptualization. Chandhanarat Chandhanayingyong: Conceptualization. Chalinee Monsereenusorn: Conceptualization. Siwaporn Poopan: Methodology, Formal analysis, Data curation, Conceptualization. Sheng-Fu Larry Lo: Conceptualization, Methodology, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- TLIF
Transforaminal lumbar interbody fusion
- PEEK
polyetheretherketone
- CFRP
carbon fiber reinforcement to PEEK
- Ti
titanium
- NMA
network meta-analysis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SP1
Sutipat Pairojboriboon
- SP2
Siwaporn Poopan
- SN
Supranee Niruthisard
- BMP
bone morphogenic protein
- CT
computed tomography
- CI
confidence interval
- OR
odds ratio
- SUCRA
surface under the cumulative ranking
- NOS
NewCastle-Ottawa Scale
- RCT
randomized controlled trial
- PSP
plasma sprayed porous titanium-coated PEEK
- SD
standard deviation
- GPa
Gigapascal
- MRI
magnetic resonance imaging
- DBM
demineralized bone matrix
- F/U
follow up
- mo
month
- VAS
Visual Analogue Scale
- ODI
Oswestry Disability Index
- Pre-op
preoperative
- Post-op
postoperative
- 3D
three dimensional
- n
number
- HA
hydroxyapatite
- B-TCP
beta-tricalcium phosphate
- n-HA/PA66
nanohydroxyapatite/polyamide-66
- N/A
not available
Contributor Information
Sutipat Pairojboriboon, Email: sutipat.pb@pmk.ac.th, sutipat.pairoj@gmail.com.
Supranee Niruthisard, Email: drsupranee@hotmail.com.
Chandhanarat Chandhanayingyong, Email: Chandhanarat@gmail.com.
Chalinee Monsereenusorn, Email: chalinee_monsereenusorn@pedpmk.org.
Siwaporn Poopan, Email: siwaporn.poo@mahidol.ac.th.
Sheng-Fu Larry Lo, Email: larrylo@northwell.edu.
References
- 1.Harms J., Rolinger H. [A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl)] Z Orthop Ihre Grenzgeb. 1982;120(3):343–347. doi: 10.1055/s-2008-1051624. [DOI] [PubMed] [Google Scholar]
- 2.Tsahtsarlis A., Wood M. Minimally invasive transforaminal lumber interbody fusion and degenerative lumbar spine disease. Eur Spine J. 2012;21(11):2300–2305. doi: 10.1007/s00586-012-2376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kunder S.L., van Kuijk S.M.J., Rijkers K., et al. Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J. 2017;17(11):1712–1721. doi: 10.1016/j.spinee.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S.D., Chen J.W., Jiang L.S. Which procedure is better for lumbar interbody fusion: anterior lumbar interbody fusion or transforaminal lumbar interbody fusion? Arch Orthop Trauma Surg. 2012;132(9):1259–1266. doi: 10.1007/s00402-012-1546-z. [DOI] [PubMed] [Google Scholar]
- 5.Parajón A., Alimi M., Navarro-Ramirez R., et al. Minimally invasive transforaminal lumbar interbody fusion: meta-analysis of the fusion rates. What is the optimal graft material? Neurosurgery. 2017;81(6):958–971. doi: 10.1093/neuros/nyx141. [DOI] [PubMed] [Google Scholar]
- 6.Derman P.B., Singh K. Surgical strategies for the treatment of lumbar pseudarthrosis in degenerative spine surgery: a literature review and case study. HSS J. 2020;16(2):183–187. doi: 10.1007/s11420-019-09732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert T.J., Pinto M., Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion: a functional and radiographic outcome study. Spine. 2000 Jan 1;25(1):123. doi: 10.1097/00007632-200001010-00021. [DOI] [PubMed] [Google Scholar]
- 8.Rao P.J., Pelletier M.H., Walsh W.R., Mobbs R.J. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seaman S., Kerezoudis P., Bydon M., Torner J.C., Hitchon P.W. Titanium vs. polyetheretherketone (PEEK) interbody fusion: meta-analysis and review of the literature. J Clin Neurosci. 2017;44:23–29. doi: 10.1016/j.jocn.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Wang X., Lu X., et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J. 2013;22(7):1539–1546. doi: 10.1007/s00586-013-2772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabraja M., Oezdemir S., Koeppen D., Kroppenstedt S. Anterior cervical discectomy and fusion: comparison of titanium and polyetheretherketone cages. BMC Musculoskelet Disord. 2012;13:172. doi: 10.1186/1471-2474-13-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatima N., Massaad E., Shankar G.M., Shin J.H. Structural allograft versus polyetheretherketone implants in patients undergoing spinal fusion surgery: a systematic review and meta-analysis. World Neurosurg. 2020;136:101–109. doi: 10.1016/j.wneu.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Peterson J., Welch V., Losos M., Tugwell P.J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute. 2011;2(1):1–2. [Google Scholar]
- 14.Bridwell K.H., Lenke L.G., McEnery K.W., Baldus C., Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20(12):1410–1418. [PubMed] [Google Scholar]
- 15.Bridwell K.H., O'Brien M.F., Lenke L.G., Baldus C., Blanke K. Posterior spinal fusion supplemented with only allograft bone in paralytic scoliosis. Does it work? Spine. 1994;19(23):2658–2666. [PubMed] [Google Scholar]
- 16.Luo J., Cao K., Yu T., et al. Comparison of posterior lumbar interbody fusion versus posterolateral fusion for the treatment of isthmic spondylolisthesis. Clin Spine Surg. 2017;30(7):E915. doi: 10.1097/BSD.0000000000000297. e22. [DOI] [PubMed] [Google Scholar]
- 17.Levin J.M., Tanenbaum J.E., Steinmetz M.P., Mroz T.E., Overley S.C. Posterolateral fusion (PLF) versus transforaminal lumbar interbody fusion (TLIF) for spondylolisthesis: a systematic review and meta-analysis. Spine J. 2018;18(6):1088–1098. doi: 10.1016/j.spinee.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Spiker W.R., Goz V., Brodke D.S. Lumbar interbody fusions for degenerative spondylolisthesis: review of techniques, indications, and outcomes. Global Spine J. 2019;9(1):77–84. doi: 10.1177/2192568217712494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtz S.M., Devine J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirazi-Adl A., Dammak M., Paiement G. Experimental determination of friction characteristics at the trabecular bone/porous-coated metal interface in cementless implants. J Biomed Mater Res. 1993;27(2):167–175. doi: 10.1002/jbm.820270205. [DOI] [PubMed] [Google Scholar]
- 21.Dos Santos M.V., Elias C.N., Cavalcanti Lima J.H. The effects of superficial roughness and design on the primary stability of dental implants. Clin Implant Dent Relat Res. 2011;13(3):215–223. doi: 10.1111/j.1708-8208.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 22.Vandrovcová M., Bačáková L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol Res. 2011;60(3):403–417. doi: 10.33549/physiolres.932045. [DOI] [PubMed] [Google Scholar]
- 23.Rosa A.L., Beloti M.M. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz Dent J. 2003;14(1):16–21. doi: 10.1590/s0103-64402003000100003. [DOI] [PubMed] [Google Scholar]
- 24.Olivares-Navarrete R., Gittens R.A., Schneider J.M., et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12(3):265–272. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelletier M.H., Cordaro N., Punjabi V.M., Waites M., Lau A., Walsh W.R. PEEK versus Ti interbody fusion devices: resultant fusion, bone apposition, initial and 26-week biomechanics. Clin Spine Surg. 2016;29(4):E208–E214. doi: 10.1097/BSD.0b013e31826851a4. [DOI] [PubMed] [Google Scholar]
- 26.Ma R., Tang T. Current strategies to improve the bioactivity of PEEK. Int J Mol Sci. 2014;15(4):5426–5445. doi: 10.3390/ijms15045426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sagomonyants K.B., Jarman-Smith M.L., Devine J.N., Aronow M.S., Gronowicz G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials. 2008;29(11):1563–1572. doi: 10.1016/j.biomaterials.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Rao P.J., Pelletier M.H., Walsh W.R., Mobbs R.J. Spine interbody implants: material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop Surg. 2014;6(2):81–89. doi: 10.1111/os.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enders J.J., Coughlin D., Mroz T.E., Vira S. Surface technologies in spinal fusion. Neurosurg Clin N Am. 2020;31(1):57–64. doi: 10.1016/j.nec.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Walsh W.R., Bertollo N., Christou C., Schaffner D., Mobbs R.J. Plasma-sprayed titanium coating to polyetheretherketone improves the bone-implant interface. Spine J. 2015;15(5):1041–1049. doi: 10.1016/j.spinee.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Assem Y., Mobbs R.J., Pelletier M.H., Phan K., Walsh W.R. Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: a systematic review and preclinical evaluation. Eur Spine J. 2017;26(3):593–605. doi: 10.1007/s00586-015-4353-8. [DOI] [PubMed] [Google Scholar]
- 32.Brantigan J.W., Steffee A.D. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18(14):2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 33.Brantigan J.W., McAfee P.C., Cunningham B.W., Wang H., Orbegoso C.M. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone. An investigational study in the Spanish goat. Spine. 1994;19(13):1436–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Koh Y.G., Park K.M., Lee J.A., Nam J.H., Lee H.Y., Kang K.T. Total knee arthroplasty application of polyetheretherketone and carbon-fiber-reinforced polyetheretherketone: a review. Mater Sci Eng C Mater Biol Appl. 2019;100:70–81. doi: 10.1016/j.msec.2019.02.082. [DOI] [PubMed] [Google Scholar]
- 35.Brantigan J.W., Neidre A., Toohey J.S. The Lumbar I/F Cage for posterior lumbar interbody fusion with the variable screw placement system: 10-year results of a Food and Drug Administration clinical trial. Spine J : official journal of the North American Spine Society. 2004;4(6):681–688. doi: 10.1016/j.spinee.2004.05.253. [DOI] [PubMed] [Google Scholar]
- 36.Mofidi A., Sedhom M., O'Shea K., Fogarty E.E., Dowling F. Is high level of disability an indication for spinal fusion? Analysis of long-term outcome after posterior lumbar interbody fusion using carbon fiber cages. J Spinal Disord Tech. 2005;18(6):479–484. doi: 10.1097/01.bsd.0000145481.92783.e7. [DOI] [PubMed] [Google Scholar]
- 37.Kroeze R.J., Verberne S.J., Graat H., Slot K., Pluymakers W.J., Temmerman O. Mid-term and long-term clinical and radiological outcomes of a carbon I/F stand-alone cage in anterior lumbar interbody fusion. Int J Spine Surg. 2020;14(5):665–670. doi: 10.14444/7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaura H., Ohnishi A., Yamagishi A., Ohwada T. Early fusion status after posterior lumbar interbody fusion with cortical bone trajectory screw fixation: a comparison of titanium-coated polyetheretherketone cages and carbon polyetheretherketone cages. Asian Spine J. 2019;13(2):248–253. doi: 10.31616/asj.2018.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tullberg T. Failure of a carbon fiber implant. A case report. Spine. 1998;23(16):1804–1806. doi: 10.1097/00007632-199808150-00016. [DOI] [PubMed] [Google Scholar]
- 40.Epstein N.E. Iliac crest autograft versus alternative constructs for anterior cervical spine surgery: pros, cons, and costs. Surg Neurol Int. 2012;3(Suppl 3):S143–S156. doi: 10.4103/2152-7806.98575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lv C., Li X., Zhang H., Lv J., Zhang H. Comparative effectiveness of two different interbody fusion methods for transforaminal lumbar interbody fusion: cage versus morselized impacted bone grafts. BMC Musculoskelet Disord. 2015;16:207. doi: 10.1186/s12891-015-0675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdul Q.R., Qayum M.S., Saradhi M.V., Panigrahi M.K., Sreedhar V. Clinico-radiological profile of indirect neural decompression using cage or auto graft as interbody construct in posterior lumbar interbody fusion in spondylolisthesis: which is better? J Craniovertebr Junction Spine. 2011;2(1):12–16. doi: 10.4103/0974-8237.85308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGilvray K.C., Easley J., Seim H.B., et al. Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018;18(7):1250–1260. doi: 10.1016/j.spinee.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma R., Virk S., Qureshi S. Interbody fusions in the lumbar spine: a review. HSS J. 2020;16(2):162–167. doi: 10.1007/s11420-019-09737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D.Y., Kwon O.H., Park J.Y. Comparison between 3-DimensionalPrinted titanium and polyetheretherketone cages: 1-year outcome after minimally invasive transforaminal interbody fusion. Neurospine. 2022;19(3):524–532. doi: 10.14245/ns.2244140.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan I., Parker S.L., Bow H., et al. Clinical and cost-effectiveness of lumbar interbody fusion using tritanium posterolateral cage (vs. Propensity-matched cohort of PEEK cage) Spine Surg Relat Res. 2022;6(6):671–680. doi: 10.22603/ssrr.2021-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]