Abstract
This article examines technical use of Fitbit during an intervention for pulmonary hypertension (PAH)‐patients. Technical issues with the device led to data being unavailable(37.5%). During intervention objective daily physical activity (DPA) decreased and subjective DPA increased. This emphasizes that an assessment of DPA in PAH requires incorporating both objective and subjective measurements.
Keywords: daily physical activity, pulmonary hypertension, smartwatch, technical use
INTRODUCTION
Patients with pulmonary arterial hypertension (PAH) often have a decreased daily physical activity (DPA), 1 , 2 , 3 resulting in a lower physical quality of life (QoL) and overall well‐being. 4 Several studies have shown smartwatches as useful tool to assess DPA. 2 , 5 , 6 , 7 However, the evaluation on the prolonged technical use of these devices and patients' subjective perception of DPA remains largely unexplored.
The UPHILL study (A nutrition and lifestyle intervention to improve quality of life for patients with pulmonary arterial hypertension) focused on nutritional adaptations and its effect on QoL 8 during a 1‐year period. The most effective part of this study was the e‐learning component, which expressed nutritional adaptations and an improvement in QoL, even though QoL was already high at baseline. In this study patients from the intervention group were asked to voluntary wear a Fitbit Charge3 to assess objective DPA with daily step counts. Additionally, they were asked to complete questionnaires to assess subjective DPA (Short Form Healthy Survey 36 (SF‐36) and Short QUestionnaire to ASsess Health‐enhancing physical activity (SQUASH)).
In this article, we delineate the extended use of a Fitbit within e‐learning intervention, alongside an evaluation of the congruence between objective and subjective assessments of DPA.
METHODS
Data was used from the UPHILL study, from baseline upon e‐learning intervention. In this study, patients were included with the following inclusion criteria: idiopathic, hereditary or drug‐related PAH, age <80 and >18 years, NYHA II or III, and stable for at least 3 months, determined by a stable 6‐min walk test (6MWT) with a difference of <10%, an estimated glomerular filtration rate (eGFR) of >60 mL/min and willing and able to sign the informed consent form. All participants provided written informed consent before any study‐related procedures. The UPHILL study was approved by the medical ethics committee with approval number 2018.538 and complies with the Declaration of Helsinki. From the 17 patients in the intervention group that started and completed the study, 16 signed the informed consent to wear the Fitbit Charge3.
To assess objective DPA, the daily step count collected with Fitbit Charge3 was utilized. Patients were instructed to wear the device as much as possible, only removing it for battery recharge or during showering.
DPA data was collected from Fitabase, the management platform from Fitbit, encompassing collected data. Before analysis, data was subjected to a strict selection process. To calculate the mean steps per day, three consecutive weeks were chosen. Each week had to include a minimum of four full days of data. 9 , 10 , 11 A day was considered full when the device was worn for more than 20 h a day, as indicated by the heart rate display recorded every 15 min. The mean steps per hour were then calculated and averaged to obtain the mean steps per day for each individual. Finally, the overall mean daily steps were computed from all the selected data points.
To assess subjective DPA, two questionnaires were applied. The SQUASH questionnaire was used to evaluate DPA, following the Dutch physical activity guidelines. This questionnaire requires patients to self‐report their daily activities in minutes, including activities such as walking, cycling, exercise, gardening, and household chores. 12 Before the analysis, data was processed to calculate the mean self‐reported active minutes per day. To evaluate the mean physical QoL, the SF‐36 questionnaire was utilized. The SF‐36 is a set of generic, coherent, and easily administered QoL measures. 13 Both questionnaires are available in Supporting Information S1 and S1: [Link], [Link].
Data are presented as mean ± (standard deviation or SD) for normally distributed data or as median (interquartile range or IQR) for non‐normally distributed data. For non‐normally distributed data, logarithmic transformation was performed before the analysis. Relationships between two continuous variables were assessed with a T‐test. p‐interaction is determined using two‐way analysis of variance with post hoc comparison. A p‐value of <0.05 was considered statistically significant. Statistical analyses and graphical illustrations were generated in R studio (version 3.5.2).
RESULTS
Sixteen patients were initially enrolled in the Fitbit and DPA component of the UPHILL study. However, for two patients data transfer to Fitabase was insufficient due to issues with their mobile phones. One patient provided unreliable data by use of a mobility scooter. Furthermore, three patients experienced incomplete data due to technical anomalies in the Fitbit device itself. Consequently, a complete set of data was available from 10 patients. The general characteristic of these patients were: mean age 40 (±9) years, the NYHA class was II, with an average mean arterial pressure of 49 (±9) and a median NTproBNP of 147 [84; 419]. The study group consisted mostly of females (eight females and two males).
As depicted in Figure 1, during the intervention period, 38% of the Fitbits experienced technical issues. Additionally, there was an inverse relationship observed between objective daily physical activity (DPA) measured in steps and subjective DPA reported as active minutes per day. The overall mean daily step count suggests a trend indicating a decrease in objective DPA. Conversely, the mean data from the SF36 and SQUASH questionnaires indicate a trend suggesting an increase in subjective DPA, with respective increments of 11% and 9%.
Figure 1.
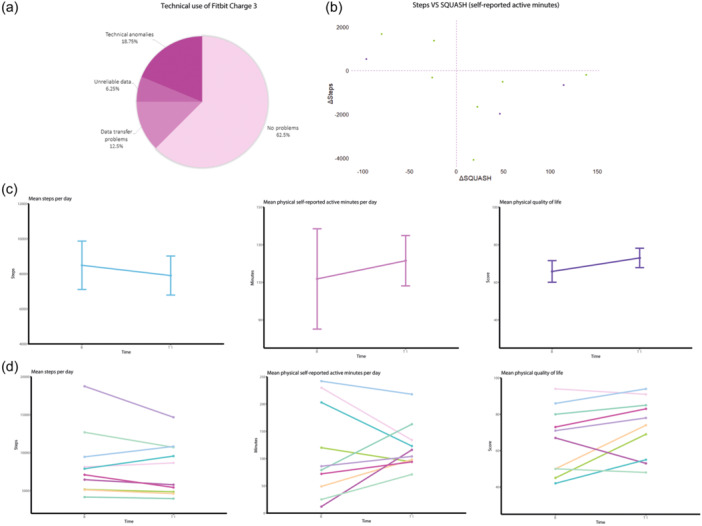
(a) presents the technical use of the Fitbit Charge3 during a period of 3 months, with a 37.5% of technical issues. In 12.5% data transfer to Fitabase was insufficient due to issues with mobile phones. There was unreliable data due to an artificially inflated step count in 6.25%. Furthermore, 18.75% had incomplete data due to technical anomalies in the Fitbit device itself. (b) Shows a negative relation between objective DPA in daily steps and subjective DPA, in self‐reported activity in minutes per day. When objective DPA increased, self‐reported DPA decreased and conversely. The purple dots represent a decrease in physical QoL. In (c), the total mean daily step count is displayed, indicating a signal of decrease of 7% (587 daily steps) in the objective DPA during intervention. The mean data of the SQUASH is revealing a signal of increase with 9% (11 min of self‐reported activity per day) of subjective DPA during intervention. The SF‐36 shows the mean of this data, indicating a signal of increase with 11% (9 points in physical QoL score) of subjective DPA during intervention. (d) Shows the individual mean daily step count, representing the objective DPA. The individual subjective DPA is presented measured with the SQUASH questionnaire in self‐reported active minutes per day and the individual subjective DPA is measured as physical QoL with the SF‐36 questionnaire. DPA, daily physical activity; QoL, quality of life; SF‐36, Short Form Healthy Survey 36; SQUASH, Short QUestionnaire to ASsess Health‐enhancing physical activity.
DISCUSSION
The technical challenges observed in the Fitbit Charge3 during intervention raised concerns about reliability, as evidenced by inadequate or missing data in 37.5% of the participants.
The adherence towards wearing the Fitbit device was very high. Even the patients providing incomplete data worn the Fitbit for the entire 1‐year period, as stated in the signed informed consent. However, it is essential to acknowledge certain limitations associated with the use of a Fitbit Charge3 for measuring objective DPA.
The study encountered challenges with some Fitbit Charge3 devices for data collection. Anomalies in the devices and connectivity issues with mobile phones led to missing or incomplete data in some cases. Additionally, eight devices required wristband replacements due to damage during the intervention period. Moreover, some patients occasionally forgot to wear the device after activities such as showering or recharging battery, leading to data gaps. Furthermore, a notable bias as observed in the movement registration for daily steps, particularly affecting the participant which used a mobility scooter. This bias resulted in an artificially inflated daily step count, with a mean objective DPA of >10.000 steps per day, in this specific patient and was therefore excluded from this study.
To address these limitations and improve data collection future research need to consider adopting new and more sensitive devices. Specifically, devices such as the Oura‐ring or the AxivityAX3. Both accelerometers are deemed user friendly and provide promising data for research purposes. 14 , 15 , 16 Implementing such advanced technologies may mitigate the issues encountered in this study and provide more accurate and comprehensive data for analysis.
During intervention period objective DPA showed a signal of 7% decrease in daily steps. In contrast, both questionnaires on subjective DPA and QoL showed a signal of increase with 9% and 11%, respectively. When daily steps increased, self‐reported DPA decreased and conversely. Despite the insufficient statistical power in this study, these findings proffer intriguing facets warranting contemplation in future and more expansive studies.
The signal of decrease of daily steps during intervention, suggest a lower DPA. However, it is important to note that the subjective assessment of DPA, as reflected in self‐reported active minutes per day and physical QoL, showed a signal of increase. These results suggest that the objective DPA, by itself, did not appear as a major component in patients' QoL and overall well‐being.
Instead, it appears that certain daily activities, such as gardening, house chores, and regular exercise, as reported in the SQUASH‐questionnaire, are more related to QoL outcomes. This implies that the patients' perception and self‐reporting of their DPA and engagement in various activities may have a stronger influence on their QoL compared to the objective DPA alone. Additionally, prolonged utilization of a smartwatch may be subject to the influence of weather conditions on the resulting outcomes of objective DPA. 17 Combining objective and subjective assessments can provide a more comprehensive and nuanced understanding of the factors influencing patients' overall well‐being and QoL.
This group of patients had already a high physical QoL at baseline, with a mean physical QoL score of 66, compared to a mean score of 37 in the overall population. 18 Additionally, this group demonstrated a high level of objective DPA, demonstrated by its daily step count. Despite a 7% reduction in the objective DPA after intervention, the mean daily step count in this cohort remained above mean of the average population. 19 , 20
In conclusion, in the context of prolonged monitoring, the Fitbit Charge3 exhibits shortcomings. Therefore, in future interventions other devices should be considered. It is imperative to emphasize that a comprehensive assessment of DPA in PAH requires the consideration of both objective and subjective measurements. Integrating these two types of measurements provides a holistic understanding of the impact of DPA on patients' well‐being. The simultaneous presentation of objective and subjective DPA data is crucial to gain a comprehensive insight into this matter.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Documented informed consent for publication has been obtained from each patient.
Supporting information
Supporting information.
Supporting information.
Kwant CT, Man FS, Bogaard HJ, Vonk Noordegraaf A. Evaluating the technical use of a Fitbit during an intervention for patients with pulmonary arterial hypertension with quality of life as primary endpoint: lessons learned from the UPHILL study. Pulm Circ. 2024;14:e12381. 10.1002/pul2.12381
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Matura LA, Shou H, Fritz JS, Smith KA, Vaidya A, Pinder D, Archer‐Chicko C, Dubow D, Palevsky HI, Sommers MS, Kawut SM. Physical activity and symptoms in pulmonary arterial hypertension. Chest. 2016;150:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minhas J, Shou H, Hershman S, Zamanian R, Ventetuolo CE, Bull TM, Hemnes A, Chakinala MM, Mathai S, Al‐Naamani N, Ellenberg S, Matura LA, Kawut SM, Shcherbina A. Physical activity and its association with traditional outcome measures in pulmonary arterial hypertension. Ann Am Thorac Soc. 2022;19:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cascino TM, McLaughlin VV, Richardson CR, Behbahani‐Nejad N, Moles VM, Visovatti SH, Jackson EA. Barriers to physical activity in patients with pulmonary hypertension. Pulm Circ. 2019;9:1–8. 10.1177/2045894019847895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakazato L, Mendes F, Paschoal IA, Oliveira DC, Moreira MM, Pereira MC. Association of daily physical activity with psychosocial aspects and functional capacity in patients with pulmonary arterial hypertension: a cross‐sectional study. Pulm Circ. 2021;11:1–9. 10.1177/2045894021999955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sehgal S, Chowdhury A, Rabih F, Gadre A, Park MM, Li M, Wang X, Highland KB. Counting steps: a new way to monitor patients with pulmonary arterial hypertension. Lung. 2019;197:501–508. [DOI] [PubMed] [Google Scholar]
- 6. Lachant D, Light A, Hannon K, Abbas F, Lachant M, White RJ. Comparison of chest‐and wrist‐based actigraphy in pulmonary arterial hypertension. Eur Heart J ‐ Digital Health. 2022;3:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard LS, Rosenkranz S, Frantz RP, Hemnes AR, Pfister T, Hsu Schmitz SF, Skåra H, Humbert M, Preston IR. Assessing daily life physical activity by actigraphy in pulmonary arterial hypertension. Chest. 2023;163:407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwant CT, de Man F, van der Horst FAL, Bogaard HJ, Vonk Noordegraaf A. The UPHILL study: a nutrition and lifestyle intervention to improve quality of life for patients with pulmonary arterial hypertension. Pulm Circ. 2023;13:e12243. 10.1002/pul2.12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van der Meij E, Van der Ploeg HP, Van Den Heuvel B, Dwars BJ, Meijerink WJHJ, Bonjer HJ, Huirne JAF, Anema JR. Assessing pre‐ and postoperative activity levels with an accelerometer: a proof of concept study. BMC Surg. 2017;17:56. 10.1186/s12893-017-0223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Middelweerd A, Van Der Ploeg HP, Van Halteren A, Twisk JWR, Brug J, Te Velde SJ. A validation study of the fitbit one in daily life using different time intervals. Med Sci Sports Exerc. 2017;49:1270–1279. [DOI] [PubMed] [Google Scholar]
- 11. Stevens ML, Lin CWC, van der Ploeg HP, De Sousa M, Castle J, Nicholas MK, Maher CG. Feasibility, validity, and responsiveness of self‐report and objective measures of physical activity in patients with chronic pain. PM&R. 2019;11:858–867. [DOI] [PubMed] [Google Scholar]
- 12. Wendel‐Vos W, Schuit J SQUASH Short QUestionnaire to ASses Health enhancing physical activity . 2004. [DOI] [PubMed]
- 13. Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF‐36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinnunen H, Rantanen A, Kenttä T, Koskimäki H. Feasible assessment of recovery and cardiovascular health: accuracy of nocturnal HR and HRV assessed via ring PPG in comparison to medical grade ECG. Physiol Meas. 2020;41:04NT01. 10.1088/1361-6579/ab840a [DOI] [PubMed] [Google Scholar]
- 15. Nawab KA, Storey BC, Staplin N, Walmsley R, Haynes R, Sutherland S, Crosbie S, Pugh CW, Harper CHS, Landray MJ, Doherty A, Herrington WG. Accelerometer‐measured physical activity and functional behaviours among people on dialysis. Clin Kidney J. 2021;14:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Niela‐Vilen H, Azimi I, Suorsa K, Sarhaddi F, Stenholm S, Liljeberg P, Rahmani AM, Axelin A. Comparison of oura smart ring against ActiGraph accelerometer for measurement of physical activity and sedentary time in a free‐living context. CIN: Comput Inform Nursing. 2022;40:856–862. [DOI] [PubMed] [Google Scholar]
- 17. Ferguson T, Curtis R, Fraysse F, Olds T, Dumuid D, Brown W, Esterman A, Maher C. Weather associations with physical activity, sedentary behaviour and sleep patterns of Australian adults: a longitudinal study with implications for climate change. Int J Behav Nutr Phys Act. 2023;20:30. 10.1186/s12966-023-01414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarzyńska K, Świątoniowska‐Lonc N, Dudek K, Jonas K, Kopeć G, Gajek J, Jankowska‐Polańska B. Quality of life of patients with pulmonary arterial hypertension: a meta‐analysis. Eur Rev Med Pharmacol Sci. 2021;25(15):4983–4998. [DOI] [PubMed] [Google Scholar]
- 19. Marvin‐Peek J, Hemnes A, Huang S, Silverman‐Loyd L, MacKinnon G, Annis J, Martin SS, Blaha MJ, Brittain EL. Daily step counts are associated with hospitalization risk in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204:1338–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stens NA, Bakker EA, Mañas A, Buffart LM, Ortega FB, Lee D, Thompson PD, Thijssen DHJ, Eijsvogels TMH. Relationship of daily step counts to all‐cause mortality and cardiovascular events. J Am Coll Cardiol. 2023;82:1483–1494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


