Highlights
-
•
EE-SWAS is associated with developmental regression and neurocognitive deterioration.
-
•
EE-SWAS is often drug-resistant.
-
•
Anakinra may be beneficial in cases of EE-SWAS of unknown etiology.
Keywords: Epileptic encephalopathy with spike wave activation in sleep, Anakinra, CSWS, ESES, Epilepsy, Drug-resistant epilepsy, EE-SWAS, DEE-SWAS
Abstract
Patients with epileptic encephalopathy with spike wave activation in sleep (EE-SWAS) often display drug-resistant epilepsy. The activation of epileptic activity during sleep is associated temporally with neurocognitive impairment and causes a spectrum of disorders within the epilepsy-aphasia syndrome. The prognosis is dependent on promptness of treatment and etiology. However, there is no clear consensus with regards to the optimal management for patients with EE-SWAS. We queried our Pediatric Epilepsy Outcome-Informatics Project (PEOIP) database for all patients treated with anakinra in our centre. We herein report a case of a female with EE-SWAS, who demonstrated remarkable neurocognitive improvement with anakinra. We suggest that a trial of anakinra may be an option for patients with EE-SWAS due to non-structural and possibly inflammatory etiology.
1. Introduction
Developmental and epileptic encephalopathy with spike wave activation in sleep (DEE-SWAS) and epileptic encephalopathy with spike wave activation in sleep (EE-SWAS) encompass a spectrum of epileptic disorders with variable cognitive, language, behavioral, and motor regression in childhood. The developmental regression may involve any of the cognitive domains and is temporally associated with a marked increase of the burden of epileptiform abnormality present during sleep compared to the awake state. The presence of a previous developmental abnormality prior to the onset of SWAS distinguishes DEE-SWAS from EE-SWAS. Epilepsy-aphasia spectrum of disorders range from atypical self-limited epilepsy with centrotemporal spikes (mild cases), epileptic encephalopathy with continuous spike-and-wave in sleep (CSWS), and the severe Landau-Kleffner syndrome. DEE-SWAS and EE-SWAS account for 0.5–0.6 % of all epilepsy cases at tertiary centers [1]. Onset of seizure ranges from 2 to 12 years of age (peak of 4–5 years). Seizures are focal in onset and infrequent. Electroencephalogram is characterized by focal or generalized slow (1.5 to 2 Hz) spike-and-wave discharges in NREM sleep, previously termed electrical status epilepticus in sleep (ESES), and this pattern often arise within 1–2 years of seizure onset.
The etiology is unknown in most cases of DEE-SWAS and EE-SWAS, but risk factors for DEE-SWAS include early thalamic injury [2] and malformations such as bilateral perisylvian polymicrogyria [3]. The most common identified monogenic cause is due to heterozygous pathogenic variants in GRIN2A, encoding for the 2A subunit of the NMDA-type glutaminergic receptor [4].
The diagnosis and management for this heterogeneous group of disorder is unclear. For example, there had been considerable debate as to what is the burden of the spike-wave epileptiform abnormalities required to cause cognitive disturbance. A spike-wave index (SWI) threshold of 85 % had been used extensively to diagnose SWAS, although it was noted that a SWI of 50 % may be enough to cause cognitive impairments. In addition, optimal quantification of SWI is a subject of research. Various antiseizure medications (ASMs) have been tried with the aim of abolishing seizures and the SWAS EEG pattern and improvement of cognitive and developmental regression, although the condition is often drug-resistant. Although the SWAS pattern remits by adolescence [5], significant neurocognitive impairment persists [6], and the prognosis depends heavily on the duration and etiology of the DEE-SWAS and EE-SWAS [1]. Thus, early diagnosis and treatment are important to improve outcome. Here, we present a case of idiopathic EE-SWAS whose neurocognitive impairments demonstrated marked and sustained improvement on anakinra.
2. Methods
The Pediatric Epilepsy Outcome-Informatics Project (PEOIP) was created on January 1, 2016 with a vision to provide comprehensive clinical care and research that leads to improved outcome and education for all stakeholders in a manner that optimizes societal resources [7]. The project entails a standardized, point of care data entry, near-time data analysis and availability of outcome dashboards. Common clinical data elements were entered by physicians and nurses into a note available in our electronic health records. Some of the clinical data elements included seizure description (duration, frequency, semiology, ILAE diagnoses), treatment (ASMs, side effects, non-medication therapies), and psychological comorbidities. The patient data is then aggregated, analyzed, and presented as population and patient-specific dashboards available to clinicians at the point of care.
There were 3979 patients in our registry between Jan 1, 2016 and May 28, 2022. Using the outcome dashboard, we identified five patients treated with anakinra. Two patients were diagnosed with febrile infection-related epilepsy syndrome. One patient had subcortical band heterotopia, and another patient had non-lesional multifocal epilepsy with possible inflammatory contribution to their epilepsies. The last patient had EE-SWAS and neurocognitive deterioration, whose case we will present below. Off-label drug usage in Canada does not require institutional review board (IRB) approval. The case was identified from the database associated with PEOIP as part of developing and implementing best practices for epilepsy treatment at the Alberta Children’s Hospital (ACH). This falls under the umbrella of quality assessment (QA) and quality improvement (QI). The PEOIP program and associated QA/QI initiatives were reviewed by the Conjoint Health Research Ethics Board (CHREB) and considered to fall outside the REB mandate. The PEOIP program was recommended for a second opinion review by A Project Ethics Community Consensus Initiative (ARECCI), which was followed and the study received a favorable review.
3. Case report
An 8-year-old right hand dominant female was born at term to a 30-year-old mother (G2), following an unremarkable delivery and perinatal course. There was maternal aspirin 81 mg daily usage due to a history of preeclampsia in her first pregnancy. Both parents are of German and English descent and are non-consanguineous. Up until the time of her first seizure, she had met her developmental milestones on time.
She first presented with seizures at 2 year 9 months of age. She had independent left and right focal motor seizures with impaired awareness. Her first seizure type is characterized by left facial droop, left arm weakness, slurred speech, left eyelid clonus during wakefulness or sleep. Her second seizure type is characterized by right facial twitching during sleep. In addition, she also had bilateral tonic-clonic seizures with unknown onset, starting at 3 years 9 months of age. Her seizures were short in duration (less than 5 min) and occurred with a frequency of one to three seizures per month. Her head circumference, weight, and length were 67 percentile, 96 percentile, and 58 percentile, respectively. She had a normal neurological and general physical examination. Brain MRI, performed at the age of 3, did not reveal any intracranial abnormality. Electroencephalography (EEG) performed a few days after her first seizure demonstrated frequent epileptiform discharges over the right centrotemporal, left central, and left occipital head region, with activation of the centrotemporal epileptiform discharges during sleep. This occupied 50–80 % of the sleep record (SWI 50–80 %). She was initially started on Clobazam nightly. A repeat EEG performed two months later showed bilateral independent centrotemporal epileptiform discharges with spike wave activation in sleep (SWI >90 %) with normal awake background (Fig. 1). She continued to have 1–3 seizures per month even after Clobazam was maximized and Levetiracetam was added. Despite the addition of divalproex and ethosuximide, EEG showed almost continuous left frontocentral epileptiform discharges during wakefulness and sleep.
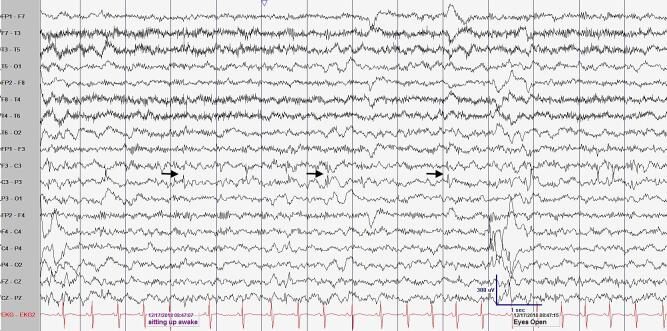
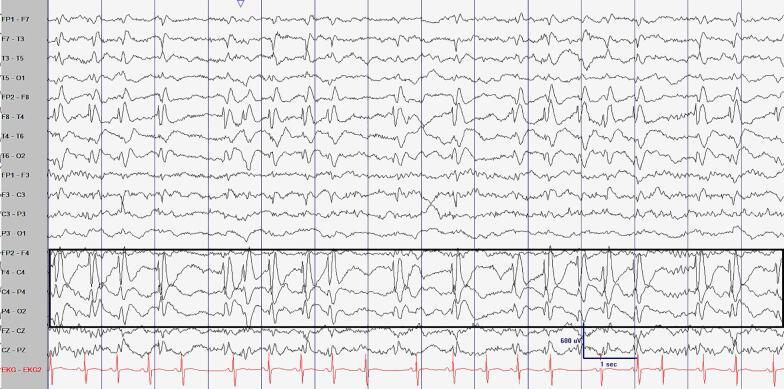
Fig. 1.
Example of spike wave activation in sleep seen in routine electroencephalography at 3 years of age. (A) An awake epoch of EEG showing frequent left central epileptiform discharges (black arrow showing phase reversal at C3). LFF 1 Hz, HFF 70 Hz, Sensitivity 20 uV/mm, Timebase 30 mm/sec. (B) A sleep epoch of EEG showing near continuous, high amplitude, right centrotemporal epileptiform discharges. Boxed area highlighting the focal epileptiform discharges. Bipolar longitudinal montage, LFF 1 Hz, HFF 70 Hz, Sensitivity 30 uV/mm, Timebase 30 mm/sec.
Approximately one year after seizure onset, her parents started noticing developmental regression. Specifically, her speech was more difficult for parents to understand, and she started to gesture instead of speaking. She also had worsening of attention. Despite being four years old, she was not toilet trained. A neuropsychological evaluation revealed severe attention problems, widespread cognitive challenges, and she was diagnosed with “Major Neurocognitive Disorder”, requiring substantial supports. In terms of her language assessment, although she had relative strengths in basic naming abilities, her receptive and expressive language skills were far below expectations.
A 8-week tapering course of prednisolone (initial: 2 mg/kg) was started. She had transient, but substantial, improvement in her attention, speech, and displayed less aggression. Her EEG also showed reduced burden of epileptiform discharges and resolved spike wave activation in sleep. However, as the steroids wore off, she once again showed regression in her cognition, speech, and attention. Intravenous immunoglobulins 2 mg/kg monthly did not improve her cognition. She did not tolerate a trial of ketogenic diet.
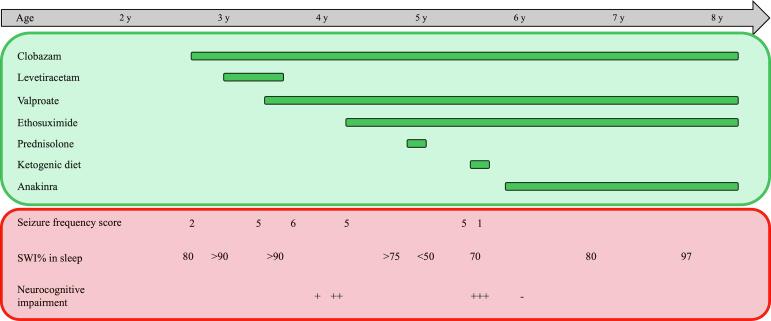
Serum cytokine and chemokine profile showed elevation of eotaxin (70 pg/mL, ref 4.0–58.9), IL-7 (10.8 pg/mL, ref 0–10.5), MCP-1 (467 pg/mL, ref 36–379 pg/mL), indicating activation in several proinflammatory pathways. Serum IL-1 was not detected. These findings, along with a previous, definite, but transient, response to oral corticosteroids, prompted a trial of anakinra (an IL-1 receptor antagonist). At 4 months follow up after anakinra was started, she made excellent developmental progress and was performing well in school. Interestingly, subsequent EEGs continue to show SWAS with SWI between 60–97 % despite a resolution of her developmental regression/stagnation. A comprehensive epilepsy panel showed two variants of uncertain significance in HCN2 (c.1739_1741del, p.Lys580del, in addition to c.2162C > T, p.Pro721Leu) and a pathogenic variant in VARS (p.Trp726*). Both genes may cause autosomal recessive forms of epilepsy that did not match the patient’s phenotype. A depiction of patient treatment course is shown in Fig. 2.
Fig. 2.
Schematic of patient treatment course. The horizontal axis depicting the age of patient can be seen at the top. Below the age axis in green, various antiseizure treatments and duration are depicted. Patient treatment responses are in red. Seizure frequency scores: 1 = seizure free, requires ASM to remain. 2 = 1–3 seizures per year, 3 = 4–11 seizures per year, 4 = 1–3 seizures per month, 5 = 1–6 seizures per week, 6 = 1–3 seizures per day. Spike wave index (SWI) of routine EEGs during sleep is shown. Subjective neurocognitive impairment as reported by the family is shown. + = mild. ++ = moderate, +++ = severe.
4. Discussion
The Pediatrics Epilepsy Outcome – Informatics Project registry included 3979 patients in southern Alberta, southeastern British Columbia, and southwestern Saskatchewan, Canada. 40 patients were diagnosed with DEE- or EE-SWAS, accounting for 1 % of patients in our registry. This is slightly higher than the reported 0.6 % of epilepsy presentations at other pediatric tertiary epilepsy centers [1]. One possible explanation for the higher rates of SWAS diagnoses is that we have high rates (estimated to be ∼90 %) of capturing at least stage N2 sleep on routine EEGs in our center due to some routine EEGs being extended to capture sleep. We also cannot rule out the possibility of any underlying differences in patient population captured in our registry.
We reported a patient with infrequent, but drug-resistant, seizures, neurocognitive impairment, and epileptiform discharges with SWAS on EEG. Despite trials of several ASMs, SWAS persisted. Neurocognitive impairment became apparent one year into her epilepsy. Given that her cytokine profile showed proinflammatory changes, corticosteroids was administered with remarkable, but transient, neurocognitive improvement. Anakinra was used as a steroid-sparing immunomodulatory agent and led to similar, but sustained, neurocognitive improvement, despite persistence of the SWAS pattern. These data suggest that anakinra led to an uncoupling of the SWAS and neurocognitive impairment. Given that the patient was discharged from our tertiary centre, no follow up neuropsychological testing was performed. Thus, only substantial subjective neurocognitive improvement was documented. As such, this is a main limitation of our study.
There are considerable practice variations and uncertainty in the management of DEE-SWAS and EE-SWAS. First, it is not straightforward when determining whether cognitive difficulties can be directly attributed to the epileptic activity during sleep. This may be due to lack of documented formal neuropsychological testing before the onset and during SWAS. Parental reporting is often reliable, but the domains and the degree of cognitive impairment is not as objective as formal testing. In addition, in patients with previous neurocognitive impairment (developmental encephalopathy) and SWAS, it is difficult to determine whether SWAS is incidental or whether SWAS contributed to further neurocognitive impairment.
Some studies suggest that a SWI of 50 % is sufficient to cause cognitive impairment, although a threshold of 85 % is the most widely used to diagnosis SWAS [8]. Furthermore, marked activation of epileptiform abnormalities (SWI > 85 %) can also be seen in patients with self-limited focal epilepsies of childhood without obvious neurocognitive impairments [1]. Therefore, the SWI threshold to initiate treatment of SWAS is unclear.
The most common treatment for DEE-SWAS and EE-SWAS is high dose benzodiazepines such as diazepam and clobazam. Other ASMs commonly used for DEE-SWAS and EE-SWAS are valproic acid, ethosuximide, and levetiracetam [9]. Corticosteroids, IVIg, and ketogenic diet have been tried with various degrees of success. Epilepsy surgery have been performed in patients with resectable structural brain lesions. In a systematic review of 575 DEE-SWAS and EE-SWAS cases in the literature, 81 %, 68 %, and 49 % of patients treated with steroids, benzodiazepines, and ASMs, respectively, showed either cognitive or EEG improvement [8].
The efficacy of corticosteroids and IVIg in some patients with DEE-SWAS, EE-SWAS, raised the possibility that immune activation may play a role in its pathogenesis [10], [11]. Indeed, patients with DEE-SWAS and EE-SWAS showed higher levels of monocyte interleukin (IL)-1α, IL-6, IL-10, CCL2, and IL8 compared to controls [12]. A recent case report of a 6 year old with EE-SWAS demonstrate improvement of behavioral symptoms, sleep patterns with anakinra, with persistence of SWAS pattern. IVIg and sirolimus were subsequently added resulting in resolution of SWAS. Anakinra is a recombinant interleukin 1 receptor antagonist and is FDA approved for the treatment of moderate to severe active rheumatoid arthritis in patients who failed at least one disease-modifying antirheumatic drug [13]. Anakinra has also been used in other autoinflammatory diseases such as systemic juvenile idiopathic arthritis, cryopyrin-associated periodic syndromes and Still’s disease [14]. For the treatment of epilepsy, anakinra has been mainly used for the acute and chronic treatment of febrile infection-related epilepsy syndrome (FIRES), a subcategory of new-onset refractory status epilepticus (NORSE) in which a febrile illness within 2 weeks to 24 h precedes a super-refractory status epilepticus [15]. Although safe and well tolerated, reported adverse effects of anakinra include injection site reactions and susceptibility to infection [16]. In our registry, 5 of 3979 epilepsy patients were treated with anakinra, representing ∼0.1 % of all epilepsy patients. Out of the 40 patients with DEE and EE-SWAS, only the present patient was treated with anakinra.
The serum cytokine profile performed in our patient showed evidence of a systemic proinflammatory state, including an elevation of monocyte chemoattractant protein-1 (MCP1). However, the relevance of a systemic proinflammatory profile to SWAS pattern and neurocognitive impairment is unclear. Indeed, several reports demonstrated elevated CSF, but not serum, cytokines in patients with FIRES, indicating that CSF cytokines may be a more relevant measure of neuroinflammation in the context of FIRES [17]. Whether patients with DEE-SWAS and EE-SWAS of presumed inflammatory etiology have intrathecal production of cytokines is unknown. Elevation of serum MCP1 occurs during inflammation and is able to disrupt the integrity of blood–brain-barrier [18]. There is accumulating evidence indicating blood–brain-barrier alterations, albumin extravasation, and entrance of peripheral leukocyte into central nervous system can contribute to epileptogenesis [19], [20]. Innate immunity including the prototypical inflammatory cytokines are implicated in the maintenance of a chronic neuroinflammatory state [21]. In our patient, it is possible that Anakinra can mediate its anti-inflammatory action in the brain in the context of MCP1-mediated blood–brain-barrier dysfunction. One limitation of this study is that no cytokine profile in the CSF in our patient was obtained due to the invasiveness of this test.
The reason for a remarkable improvement in cognition after anakinra without a corresponding reduction in SWI is unknown. Anakinra may have the ability to cross the blood–brain-barrier and inhibit all effects of IL-1 downstream signaling in the CNS compartment. In rodents, aberrant levels of brain IL-1 is associated with cognitive dysfunction [22], [23]. The improvement seen with anakinra on neurocognition but not SWAS challenges the correlation between spike-wave activation and impact on cognition in some patients with DEE-SWAS. This finding supports that Interleukin 1 receptor antagonists (IL-1ra) has neurotropic effects independent of its action on seizures/spikes, which has similarly been reported in a preclinical study of the ketogenic diet in rodent seizure models [24].
5. Conclusion
We reported anakinra treatment led to a remarkable improvement in neurocognition in a patient with EE-SWAS of unknown etiology. Given that anakinra is a safe and tolerable drug, this may represent a novel therapy for patient with drug-resistant EE-SWAS.
Ethical statement
Informed consent was obtained from the patient.
CRediT authorship contribution statement
Andy Cheuk-Him Ng: Writing – review & editing, Writing – original draft, Conceptualization. Morris Scantlebury: Writing – review & editing, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Canadian Institutes of Health Research, Grant/Award Number: PJT 461929. Stollery Clinical Research Fellowship. Alberta Children’s Hospital Research Institute Clinical Research Fellowship.
References
- 1.Specchio N., Wirrell E.C., Scheffer I.E., Nabbout R., Riney K., Samia P., et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1398–1442. doi: 10.1111/epi.17241. [DOI] [PubMed] [Google Scholar]
- 2.Kersbergen K.J., de Vries L.S., Leijten F.S., Braun K.P., Nievelstein R.A., Groenendaal F., et al. Neonatal thalamic hemorrhage is strongly associated with electrical status epilepticus in slow wave sleep. Epilepsia. 2013;54:733–740. doi: 10.1111/epi.12131. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsuka Y., Tanaka A., Kobayashi K., Ohta H., Abiru K., Nakano K., et al. Childhood-onset epilepsy associated with polymicrogyria. Brain Dev. 2002;24:758–765. doi: 10.1016/s0387-7604(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 4.Carvill G.L., Regan B.M., Yendle S.C., O'Roak B.J., Lozovaya N., Bruneau N., et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45:1073–1076. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraballo R., Pavlidis E., Nikanorova M., Loddenkemper T. Encephalopathy with continuous spike-waves during slow-wave sleep: evolution and prognosis. Epileptic Disord. 2019;21:15–21. doi: 10.1684/epd.2019.1052. [DOI] [PubMed] [Google Scholar]
- 6.Roulet Perez E., Davidoff V., Despland P.A., Deonna T. Mental and behavioural deterioration of children with epilepsy and CSWS: acquired epileptic frontal syndrome. Dev Med Child Neurol. 1993;35:661–674. doi: 10.1111/j.1469-8749.1993.tb11711.x. [DOI] [PubMed] [Google Scholar]
- 7.Buchhalter J.R., Scantlebury M.H., D'Alfonso S., Pablo Appendino J., Bello Espinosa L., Brooks B.L., et al. Creation and implementation of an electronic health record note for quality improvement in pediatric epilepsy: Practical considerations and lessons learned. Epilepsia Open. 2021;6:345–358. doi: 10.1002/epi4.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Munckhof B., van Dee V., Sagi L., Caraballo R.H., Veggiotti P., Liukkonen E., et al. Treatment of electrical status epilepticus in sleep: A pooled analysis of 575 cases. Epilepsia. 2015;56:1738–1746. doi: 10.1111/epi.13128. [DOI] [PubMed] [Google Scholar]
- 9.Baumer F.M., McNamara N.A., Fine A.L., Pestana-Knight E., Shellhaas R.A., He Z., et al. Treatment practices and outcomes in continuous spike and wave during slow wave sleep: A Multicenter Collaboration. J Pediatr. 2021;232:220–228 e3. doi: 10.1016/j.jpeds.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nevsimalova S., Tauberova A., Doutlik S., Kucera V., Dlouha O. A role of autoimmunity in the etiopathogenesis of Landau-Kleffner syndrome? Brain Dev. 1992;14:342–345. doi: 10.1016/s0387-7604(12)80157-4. [DOI] [PubMed] [Google Scholar]
- 11.Fayad M.N., Choueiri R., Mikati M. Landau-Kleffner syndrome: consistent response to repeated intravenous gamma-globulin doses: a case report. Epilepsia. 1997;38:489–494. doi: 10.1111/j.1528-1157.1997.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 12.van den Munckhof B., de Vries E.E., Braun K.P., Boss H.M., Willemsen M.A., van Royen-Kerkhof A., et al. Serum inflammatory mediators correlate with disease activity in electrical status epilepticus in sleep (ESES) syndrome. Epilepsia. 2016;57:e45–e50. doi: 10.1111/epi.13274. [DOI] [PubMed] [Google Scholar]
- 13.Mertens M., Singh J.A. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36:1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli G., Dinarello C.A. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol. 2018;9:1157. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh Z., Hirsch L.J. A practical approach to in-hospital management of new-onset refractory status epilepticus/febrile infection related epilepsy syndrome. Front Neurol. 2023;14:1150496. doi: 10.3389/fneur.2023.1150496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedaiwi M.K., Almaghlouth I., Omair M.A. Effectiveness and adverse effects of anakinra in treatment of rheumatoid arthritis: a systematic review. Eur Rev Med Pharmacol Sci. 2021;25:7833–7839. doi: 10.26355/eurrev_202112_27630. [DOI] [PubMed] [Google Scholar]
- 17.Kenney-Jung D.L., Vezzani A., Kahoud R.J., LaFrance-Corey R.G., Ho M.L., Muskardin T.W., et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80:939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao Y., Tsirka S.E. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–697. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loscher W., Friedman A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: A cause, consequence, or both? Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchi N., Banjara M., Janigro D. Blood-brain barrier, bulk flow, and interstitial clearance in epilepsy. J Neurosci Methods. 2016;260:118–124. doi: 10.1016/j.jneumeth.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vezzani A. Brain inflammation and seizures: evolving concepts and new findings in the last 2 decades. Epilepsy Curr. 2020;20:40S–43S. doi: 10.1177/1535759720948900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skelly D.T., Griffin E.W., Murray C.L., Harney S., O'Boyle C., Hennessy E., et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. 2019;24:1533–1548. doi: 10.1038/s41380-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitazawa M., Cheng D., Tsukamoto M.R., Koike M.A., Wes P.D., Vasilevko V., et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer's disease model. J Immunol. 2011;187:6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scantlebury M.H., Chun K.C., Ma S.C., Rho J.M., Kim D.Y. Adrenocorticotropic hormone protects learning and memory function in epileptic Kcna1-null mice. Neurosci Lett. 2017;645:14–18. doi: 10.1016/j.neulet.2017.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]