Abstract
Background
Lung cancer is the second most diagnosed cancer and the leading cause of cancer death in 2020, representing approximately one in 10 (11.4 %) cancers diagnosed and one in 5 (18.0 %) deaths. There are currently very few studies evaluating the prevalence and related factors of lung cancer detected using low-dose CT scans.
Objective
Evaluate the prevalence and related factors of lung cancer using low-dose CT scans in high-risk populations in Vietnam.
Materials and methods
A cross-sectional analysis study of 169 high-risk patients was conducted to assess the lung cancer prevalence and related factors. Enrolled patients received a physical examination, low-dose computerized tomography scan, and biopsy if abnormalities were found through the CT scan. Univariable and Multivariable analysis through Odd Ratio (OR) to assess the related risk of lung cancer.
Results
A total of 169 high-risk patients with a mean age of 62.93 ± 9.31 (years), and the majority were male (91.7 %). Of which 4 cases (2.37 %) were recorded with lung cancer, 3 cases of adenocarcinoma, and 1 case of squamous cell carcinoma. A history of smoking and Chronic Obstructive Pulmonary Disease (COPD) were associated with an increased risk of abnormalities on lung CT scans. Multivariate regression analysis revealed that smoking over 30 pack-years and COPD significantly increased the risk of abnormalities on lung CT scans, p < 0.05.100 % of lung cancer-detected cases were male and smoking ≥30 pack – years.
Conclusion
The prevalence of lung cancer in the Vietnamese high-risk population was relatively high. Relative factors such as smoking ≥30 pack - years, and COPD had increased risk of CTscan abnormalities.
Keywords: Lung cancer, Adenocarcinoma, Squamous cell carcinoma, Related factors, Prevalence
Highlights
-
•
Lung cancer prevalence was high in Vietnamese high-risk groups.
-
•
Age, smoking, T2DM, history of pulmonary tuberculosis, and COPD increase lung cancer risk.
-
•
More research on lung cancer genetics is needed for better population risk assessment.
1. Introduction
Lung cancer nowadays is one of the five types of cancer with the highest prevalence and mortality rate in the world and is also the second-highest-diagnosed disease in the US and Europe [1]. According to the GLOBOCAN report with an estimated 2.2 million new cancer cases and 1.8 million deaths, lung cancer is the second most commonly diagnosed cancer and the leading cause of cancer death in 2020, representing approximately one in 10 (11.4 %) cancers diagnosed and one in 5 (18.0 %) deaths. Among men, lung cancer is the most commonly diagnosed cancer in 36 countries, while it is the leading cause of cancer death in 93 countries [2]. Particularly in Vietnam, lung cancer has a prevalence rate of 14.4 % and a mortality rate of 18 %, ranking second after liver cancer (15.48 %, 19.2 %), and in women of 9.4 % after breast cancer and colon cancer [3]. However, only about 15 % of lung cancer cases are diagnosed in the early stages, without metastasis [4].
Studies have shown that early diagnosis of lung cancer patients can reduce the mortality rate, significantly improve the treatment effectiveness, and the 10-year survival proportion in stage I lung cancer patients with 88 % [4]. Chronic Obstructive Pulmonary Disease (COPD) and smoking have long been proven to be huge risk factors for lung cancer [5,6]. Many studies have shown that computerized tomography (CT) scan has a high value in early diagnosis of lung cancer [1,4,[7], [8], [9]]. However, in high-risk populations of Vietnam, there are currently very few studies evaluating the prevalence and related factors of lung cancer detected using low-dose CT scans. Therefore, our study aimed to evaluate the prevalence and related factors of lung cancer using low-dose CT scans in high-risk populations in Vietnam.
2. Materials and Methods
2.1. Study design and population
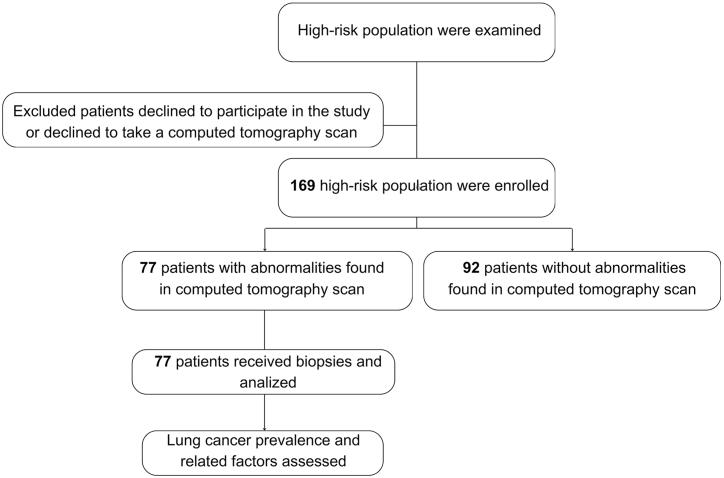
A cross-sectional analysis study in 169 high-risk outpatients for screening in Ho Chi Minh City from November 2019 to April 2022 at Gia Dinh Hospital of the People (Fig. 1).
Fig. 1.
Study population flowchart.
Including criteria: Patients age ≥50 and: (1) smoking ≥30 pack-years or (2) smoking ≥20 pack-years and more than 1 passive smoking risk factor (Living with a smoking person or smoking area) or (3) history of COPD according to GOLD 1–3 [10].
Excluding criteria: Pregnant and lactating women; Inability to answer questions such as not being able to speak, not being able to hear, having difficulty communicating, mental illness; Patients with new pulmonary tuberculosis and currently undergoing tuberculosis treatment; CT scan contrast drug allergy; cirrhosis, severe heart failure; chronic kidney disease (CKD); atrial fibrillation, uncontrolled heart rhythm disturbances; Refuse to participate in the study.
2.2. Sample size
Based on the study by Olivier Leleu et al. (2020) on the high-risk population group with smoking ≥30 pack-years [11] recorded a cancer prevalence of 2.7 % (p = 0.027). We use the one proportion formula estimate sample, a calculated sample size of 41 patients with d = 0.06, Z = 1.96 (with α = 0.05). Our study collected much more than estimated with 169 patients to rule out the possibility of sample loss.
2.3. Study variables and data collection methods
All outpatients who meet the sampling criteria will be enrolled in the study using the non-probability sampling method. Patients enrolled had a clinical examination by respiratory specialists and conducted a CT scan to screen for lung cancer. Patients with abnormalities on the CT-scan film will continue to conduct a biopsy of the abnormal tissue to confirm the diagnosis of lung cancer. Clinical examination recorded: Age; Sex; symptoms (Including chest pain, dyspnea, non-productive coughs or hemoptysis; fever; loss of appetite; weight loss; hoarseness); signs (Including edema, peripheral lymphadenopathy, pleural effusion sign, consolidation syndrome, pulmonary rales, chest deformity); Smoke; Hypertension ((based on ESC/ESH 2018 [12]); Type 2 Diabetes Mellitus (T2DM) (according to American Diabetes Association (ADA) recommendations 2020 [13]); Old pulmonary tuberculosis; Interstitial lung disease; Asthma; Chronic Obstructive Pulmonary Disease (COPD) [10]; Occupational lung disease.
Multi-slice CT scanner (MSCT) on a volume with low tube voltage (80 or 100 kVp), 30 mA, 15 mAs, Pitch = 1.07, collimation 0.625, thickness 3–5 mm, produces 1 mm window images. The survey field is from the apex to the base of the lung. Effective dose <1.5 mSv is pre-set according to Lung Low Dose software to reduce routine dose for patients. The CT scan results were abnormal when there were signs such as localized or free pneumothorax or pleural effusion; Pneumothorax; Infiltration; Cavernous tuberculosis; Consolidation nodule or tumor; Clear edges nodule or tumor; Multi-arc-shaped nodule or tumor; Dendritic shape nodule or tumor; Necrotic nodule or tumor; Calcified nodule or tumor. CT scan results were read and classified by radiologists and respiratory specialists and conflict was resolved by a consultation record with 3 or more specialists in the field. In fact, throughout our study period, there was no conflict in the diagnosis of the CT scan. Biopsy is performed when abnormalities were founded on CT scan, including abnormalities on biopsy: Squamous cell carinoma; Bronchopulmonary gland carcinoma, Carcinoma bronchioloalveolar, Small-cell carcinoma, Large cell carcinoma; Mucoepidermoid carcinomas; Neuroendocrine cancer; Metastasis; Pneumonitis; Endobronchial fibrous histiocytoma; bronchial mucous gland adenoma; Pleural mucinous fibrous tumor; Chronic inflammatory granuloma; Hamartoma; Carcinoid. Spirometry was conducted on COPD patients to measure Forced expiratory volume in the first second (FEV1) (%), Forced Vital Capacity (FVC) (%), and Tiffeneau-Pinelli index (FEV1/FVC).
Evaluate the proportion of CT abnormalities in the group of high-risk patients and the prevalence of cancer recorded through biopsy in the group of high-risk patients and the group with CT abnormalities. Relative analyzes were conducted to clearly show risk factors for lung cancer such as: Age >60; Male; Smoke; Hypertension; T2DM; Old pulmonary tuberculosis; Asthma; COPD.
2.4. 2.4Data bias control methods and data analysis
Controlling bias in 2 areas includes (1) Bias due to data collection: standardized measuring equipment, training of researcher skills with a detailed explanation of each problem and content. The study was conducted as a pilot study on 20 subjects to gain experience before being included in the official study. Research information was re-checked after the end of each case study so that it could be supplemented promptly; (2) Recall error: using questions with content about a short time ago along with comparison questions to limit the error.
Data was collected and processed by Statistical Package for the Social Sciences (SPSS) 20.0 software. Quantitative variables with normal distribution were described by mean ± standard deviation (SD), and non-normal distribution variables were characterized by the median, and interquartile range (IQR). Qualitative variables are represented by rate and percentage. The difference between two qualitative variables described by the Chi-squared test, normal distribution quantitative variables by simple t-test (if 2 groups analyzed), and quantitative variables with non-normal distribution by Mann-Whitney test, p < 0.05 considered to be statistically significant. Univariable and Multivariable analysis through Odd Ratio (OR) to assess the related risk of abnormalities of CT scan images and lung cancer dectected. OR1 for compared between normal and abnormal CTscaner results, OR2 for compared between normal CTscaner results and cancer detected through biopsy, and OR3 for compared between abnormal CTscaner results and cancer detected through biopsy.
Ethical approval
Study participants were voluntary and received clear explanations before enrolling in the study. The collected data is kept completely confidential and used only for scientific research purposes. This study had been approved by the protocol review board and Ethics Committee in Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City.
3. Results
3.1. 3.1Population characteristics of the study
A total of 169 high-risk patients were included in the analysis, of which 92 patients had no abnormalities on the CT scan (54.44 %). The study population had a high mean age of 62.93 ± 9.31 (years), the majority were male (91.7 %), and there was no difference in age and sex between groups (Table 1). However, our study found a difference in the smoking condition and COPD prevalence with higher packet-year or prevalence in the abnormalities of CT scan images. Most patients have underlying diseases, of which hypertension is the largest with 37.9 %, followed by COPD with 30.2 % (Table 1).
Table 1.
Clinical characteristics of the study populations.
| Patient characteristic | Total (n = 169) |
CT scan abnormality (n = 77) |
None CT scan abnormality (n = 92) |
pa | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| General characteristics | |||||||
| Aged (years), Mean ± SD | 63.03 ± 9.24 | 63.26 ± 9.59 | 62.84 ± 8.98 | 0.767b | |||
| Male | 155 | 91.7 | 74 | 96.1 | 81 | 88.0 | 0.058 |
| Smoking (Pack – years), Median (IQR) | 30.00 (20.00–40.00) | 30.00 (20.00–44.00) | 22.50 (15.00–40.00) | 0.014c | |||
| Hypertension | 64 | 37.9 | 27 | 35.1 | 37 | 40.2 | 0.559 |
| T2DM | 31 | 18.3 | 15 | 19.5 | 16 | 17.4 | 0.673 |
| Old pulmonary tuberculosis | 12 | 7.1 | 6 | 7.8 | 6 | 6.5 | 0.717 |
| Interstitial lung disease | 1 | 0.6 | 0 | 0.0 | 1 | 1.1 | 0.364 |
| Asthma | 40 | 23.7 | 14 | 18.2 | 26 | 28.3 | 0.142 |
| COPD | 51 | 30.2 | 32 | 41.6 | 19 | 20.7 | 0.002 |
T2DM: type 2 diabetes mellitus; CT: computerized tomography; COPD: Chronic obstructive pulmonary disease; a: Chi-squared test; b: Independence Sample T-test; c: Mann-Whitney U test; SD: standard deviation; IQR: interquartile range.
3.2. Lung functional test and lung cancer prevalence detected through biopsy
Table 2 shows that the average forced expiratory volume (FEV1) was 76.8 ± 18.6 (%), and the mean of Tiffeneau was 0.8 ± 0.1. CT scans recorded the most abnormality as emphysema (19.5 %), followed by tumors (18.3 %) (Table 2). 4 cases (2.4 %) were recorded with lung cancer, of which 4 cases were cancer with 3 cases of adenocarcinoma, and 1 case of squamous cell carcinoma (Table 2).
Table 2.
Computerized tomography scan, biopsy, and spirometry results.
| Paraclinical results | Total Mean ± SD | CT scan abnormality Mean ± SD |
None CT scan abnormality Mean ± SD |
pa | |||
|---|---|---|---|---|---|---|---|
| Spirometry | FEV1 (%) | 76.8 ± 18.6 | 76.0 ± 18.3 | 79.8 ± 21.6 | 0.696 | ||
| FVC (%) | 91.1 ± 13.8 | 91.9 ± 14.5 | 88.2 ± 11.3 | 0.603 | |||
| Tiffeneau | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.438 | |||
| CT scan | Tumor | 31 | 18.3 | 31 | 40.2 | – | – |
| Nodule | 4 | 2.3 | 4 | 5.2 | – | – | |
| Tuberculosis | 3 | 2.7 | 3 | 3.9 | – | – | |
| Emphysema | 33 | 19.5 | 33 | 42.8 | – | – | |
| Interstitial lung disease | 2 | 1.2 | 2 | 2.6 | – | – | |
| Others | 7 | 4.1 | 7 | 9.1 | – | – | |
| Biopsy | Adenocarcinoma (%) | 3 | 1.7 | 3 | 3.9 | – | – |
| Squamous cell carcinoma (%) | 1 | 0.6 | 1 | 1.3 | – | – | |
| Total (%) | 4 | 2.4 | 4 | 5.2 | – | – | |
CT: computerized tomography; SD: standard deviation; FEV1: Forced expiratory volume; FVC: Forced vital capacity; a: Independence Sample T-test.
3.3. Related factors assessment of lung cancer and abnormalities in computerized tomography scan results
Table 3 showed that related factors with computed tomography abnormalities recorded that smoking ≥30 pack-years and COPD significantly increased the risk with an OR of 2.35 (95 % CI: 1.11–4.96, p = 0.025); 2.33 (95 % CI: 1.01–5.34, p = 0.046), respectively. All lung cancer cases were male and had smoked ≥30 pack-years. Evaluation of related factors to the risk of lung cancer showed that age ≥60, T2DM, old pulmonary tuberculosis, and COPD had odd >1 but were not statistically significant (Table 3).
Table 3.
Risk assessment via univariable and multivariabe analysis.
| Factors | OR1 (95 % CI) | OR2 (95 % CI) | OR3 (95 % CI) |
|---|---|---|---|
| Risk assessment via univariable analysis | |||
| Aged ≥60 | 1.04 (0.56–1.94) | 1.92 (0.19–18.86) | 1.909 (0.19–19.27) |
| Male | 3.35 (0.89–12.47) | – | – |
| Smoked ≥30 pack - years | 2.33 (1.17–4.60)a | – | – |
| Hypertension | 0.83 (0.44–1.56) | 0.49 (0.05–4.84) | 0.54 (0.05–5.45) |
| T2DM | 1.28 (0.59–2.79) | 1.34 (0.13–13.67) | 1.18 (0.11–12.16) |
| Old pulmonary tuberculosis | 1.24 (0.38–4.03) | 4.39 (0.42–45.83) | 4.20 (0.37–48.16) |
| Asthma | 0.57 (0.27–1.20) | 1.00 (0.10–9.89) | 1.41 (0.14–14.67) |
| COPD | 2.90 (1.46–5.78)a | 2.18 (0.29–15.96) | 1.27 (0.17–9.52) |
| Risk assessment via multivariable analysis | |||
| Aged ≥60 | 0.69 (0.31–1.54) | 2.02 (0.17–23.26) | 2.55 (0.19–34.55) |
| Male | 2.28 (0.19–27.14) | – | – |
| Smoked ≥30 pack - years | 2.35 (1.11–4.96)a | – | – |
| Hypertension | 0.73 (0.32–1.65) | 0.23 (0.01–3.54) | 0.33 (0.02–5.25) |
| T2DM | 1.78 (0.67–4.69) | 2.02 (0.14–30.04) | 1.99 (0.10–37.69) |
| Old pulmonary tuberculosis | 1.42 (0.29–6.87) | 6.12 (0.42–90.21) | 7.94 (0.36–173.58) |
| Asthma | 0.75 (0.30–1.85) | 0.84 (0.05–13.51) | 1.53 (0.06–35.94) |
| COPD | 2.33 (1.01–5.34)a | 1.30 (0.10–16.53) | 1.09 (0.06–17.93) |
COPD: Chronic obstructive pulmonary disease; T2DM: Type 2 diabetes mellitus; OR: odd ratio; OR1: Compared between normal and abnormal CTscaner results, OR2: Compared between normal CTscaner results and cancer detected through biopsy; OR3: Compared between abnormal CTscaner results and cancer detected through biopsy.
p < 0.05.
4. Discussion
4.1. Principal findings
Our study assessed the prevalence of lung cancer and related factors affecting the prevalence hazard the Vietnamese population. In the high-risk population of smoking and older people, there were 45.56 % abnormal CTscan results, and the prevalence of lung cancer was detected at 2.4 % (4/169) (Table 1, Table 2). There were 100 % of lung cancer-detected cases were male and smoking ≥30 pack - years. Risk assessment via univariable and multivariable analysis has shown smoking ≥30 pack - years, and COPD had increased odds of abnormal CTscan detected. Therefore our study showed the lung cancer prevalence and related factors that must be intervened to enhance in the Vietnamese high-risk population.
4.2. Possible explanations and comparison with other studies
Our study population with the majority of males, and the mean age of 63.03 ± 9.24. The results consistent with the lung cancer population studies such as S Sone et al. (2001) [14]; Thierry Blanchon et al. (2007) [15]; Chih-Yu Chen et al. (2016) [16]; Maurizio Infante et al. (2015) [17]; Olivier Leleu et al. (2020) [11]. The lung cancer prevalence was 2.36 %, which consistent with the study of Thierry Blanchon et al. (2007) [15] and Olivier Leleu et al. (2020) [11]. Our prevalence was higher than S Sone et al. (2001) [14] and Chih-Yu Chen et al. (2016) [16], the difference was due to the high-risk population of criteria for enrollment (Table 4).
Table 4.
Lung cancer prevalence and types compared with other studies.
| Study | Nation | n | Tobaco criteria (pack – years) | Total cancer |
Squamous cell carcinoma |
Adenocarcinoma |
Others |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| S Sone et al. (2001) [14] | Japan | 13786 | None | 60 | 0.43 | 6 | 0.04 | 51 | 0.37 | 3 | 0.02 |
| Thierry Blanchon et al. (2007) [15] | France | 385 | #20 | 8 | 2.08 | 2 | 0.52 | 5 | 1.29 | 1 | 0.26 |
| Chih-Yu Chen et al. (2016) [16] | Taiwan | 3339 | None | 34 | 1.02 | 0 | 0.0 | 30 | 88.2 | 4 | 11.8 |
| Maurizio Infante et al. (2015) [17] | Italy | 1264 | ≥20 | 104 | 8.23 | 25 | 1.98 | 44 | 3.48 | 14 | 1.11 |
| Olivier Leleu et al. (2020) [11] | French | 1307 | ≥30 | 26 | 2.7 | 22 | 1.68 | 3 | 0.23 | 6 | 0.46 |
| Our study | Vietnam | 169 | ≥30 or ≥ 20 with aged ≥50 | 4 | 2.3 | 1 | 0.6 | 3 | 1.7 | 0 | 0.0 |
Our results showed that 100 % of lung cancer-detected cases were male and smoking ≥30 pack - years. Risk assessment via univariable and multivariable analysis has shown smoking ≥30 pack - years, and COPD had increased odds of abnormal CTscan detected. The results are consistent with medical literature [18]. In addition, the study by Roushney Fatima Mukti et al. (2014) [19] in Bangladesh showed consistent outcomes with smoking (OR = 9.707) and previous lung disease (OR = 7.095) increased lung cancer prevalence. Other studies also showed smoker population with >20 pack-years increased the prevalence of lung cancer consistent with our outcomes. Charles Faselis et al. (2022) [20] a cohort study with 432 current smokers ≥20 pack-years indicated a multivariable-adjusted hazard ratio at 30.37 (95 % CI: 15.42–59.84). Lap Ah Tse et al. (2022) [21] study showed smokers with pack-years from 20 to 40 had OR = 2.40 (95 % CI: 1.36–4.22), and the OR increased to 3.09 (95 % CI: 1.80–5.26) with >40 pack-years populations. Therefore, prevention or enhanced living routine interventions are required in high-risk populations such as males smoking and COPD. Clinicians must thoroughly examine and review this at-risk population, increase early disease screening proportion, and improve regional health quality.
4.3. Strengths and weaknesses of the study
Our study had a clear design of the sample collection process, including, and excluding criteria. Our data clearly and significantly showed the prevalence and related factors of CTscan abnormalities in high-risk populations. Therefore, our study outcomes could be used as medical evidence for further studies.
However, our study was evaluated only in 1 hospital, which might lead to bias in baseline characteristics, and the sample size in our study is still minimal based on the prevalence of the disease as a minor than 10 percent. Therefore, a multicenter study with the larger sample size is required to represent the study population better. Since our data collection didn't account for excluded patients, selection bias can't be completely excluded. Our study only assessed clinical-related factors of lung cancer, genes have been shown to affect the prevalence hazard [18,22]. In addition, the logistic regression models are not robust because there was no exploration of the assumptions of logistic regression.
5. Conclusions
The prevalence of lung cancer in the Vietnamese high-risk population was relatively high. Relative factors such as smoking ≥30 pack - years, and COPD had increased odds of CTscan abnormalities. Further studies assessing gene issues related to lung cancer are required for a better view of the population risk.
Ethics statement
Our study was approved by the Institutional review board of Ho Chi Minh University of Medicine and Pharmacy (616/HDDD-ĐHYD, Date: 29/10/2019). All participants provided written informed consent for the use and publication of their data and images for scientific research purposes.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Duy Hoang Tran, upon reasonable request.
Funding
None.
CRediT authorship contribution statement
Duy Hoang Tran: Writing – review & editing, Writing – original draft, Data curation, Conceptualization. Tho Van Nguyen: Conceptualization. Linh Thi My Luong: Supervision, Software, Formal analysis. Hoang Minh Phan: Writing – original draft, Methodology. To To To: Writing – original draft. Thuy Thi Cam Bui: Formal analysis, Data curation. Ngoc Thi Minh Nguyen: Writing – original draft, Software, Resources. Phuong Minh Nguyen: Writing – original draft, Resources. Lan Thi Tuyet Le: Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the Rectorate Board of Can Tho University of Medicine and Pharmacy and Ho Chi Minh University of Medicine and Pharmacy for creating favorable conditions for this study to be carried out. Specially, we sincerely thank Toan Hoang Ngo (MD, PhD student, Can Tho University of Medicine and Pharmacy) and Bao Lam Thai Tran (MD, Can Tho University of Medicine and Pharmacy) for their support in helping us complete the initial manuscript for publication of this research project.
References
- 1.Pineiro B., et al. Smoking cessation interventions within the context of Low-Dose Computed Tomography lung cancer screening: a systematic review. Lung Cancer. 2016;98:91–98. doi: 10.1016/j.lungcan.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Dajac J., et al. To screen or not to screen: low dose computed tomography in comparison to chest radiography or usual care in reducing morbidity and mortality from lung cancer. Cureus. 2016;8(4):e589. doi: 10.7759/cureus.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., et al. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:3767–3776. doi: 10.2147/COPD.S168048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber M.F., et al. Cancer incidence and cancer death in relation to tobacco smoking in a population-based Australian cohort study. Int. J. Cancer. 2021;149(5):1076–1088. doi: 10.1002/ijc.33685. [DOI] [PubMed] [Google Scholar]
- 7.Li J., et al. New recommendation and coverage of low-dose computed tomography for lung cancer screening: uptake has increased but is still low. BMC Health Serv. Res. 2018;18(1):525. doi: 10.1186/s12913-018-3338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberle D.R., Abtin F., Brown K. Computed tomography screening for lung cancer: has it finally arrived? Implications of the national lung screening trial. J. Clin. Oncol. 2013;31(8):1002–1008. doi: 10.1200/JCO.2012.43.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayo J.R., Aldrich J., Muller N.L. Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology. 2003;228(1):15–21. doi: 10.1148/radiol.2281020874. [DOI] [PubMed] [Google Scholar]
- 10.GOLD . 2015. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease; pp. 1–44. [Google Scholar]
- 11.Leleu O., et al. Lung cancer screening by low-dose CT scan: baseline results of a French prospective study. Clin. Lung Cancer. 2020;21(2):145–152. doi: 10.1016/j.cllc.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Thompson B., et al. Durability of tobacco control efforts in the 22 Community Intervention Trial for Smoking Cessation (COMMIT) communities 2 years after the end of intervention. Health Educ. Res. 2000;15(3):353–366. doi: 10.1093/her/15.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Association A.D. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–s31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 14.Sone S., et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br. J. Cancer. 2001;84(1):25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchon T., et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR) Lung Cancer. 2007;58(1):50–58. doi: 10.1016/j.lungcan.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Chen C.Y., et al. Lung cancer screening with low-dose computed tomography: experiences from a tertiary hospital in Taiwan. J. Formos. Med. Assoc. 2016;115(3):163–170. doi: 10.1016/j.jfma.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Infante M., et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am. J. Respir. Crit. Care Med. 2015;191(10):1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 18.Kanwal M., Ding X.J., Cao Y. Familial risk for lung cancer. Oncol. Lett. 2017;13(2):535–542. doi: 10.3892/ol.2016.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukti R.F., et al. Score based risk assessment of lung cancer and its evaluation for Bangladeshi people. Asian Pac. J. Cancer Prev. APJCP. 2014;15(17):7021–7027. doi: 10.7314/apjcp.2014.15.17.7021. [DOI] [PubMed] [Google Scholar]
- 20.Faselis C., et al. Assessment of lung cancer risk among smokers for whom annual screening is not recommended. JAMA Oncol. 2022;8(10):1428–1437. doi: 10.1001/jamaoncol.2022.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tse L.A., et al. Risk assessment and prediction for lung cancer among Hong Kong Chinese men. BMC Cancer. 2022;22(1):585. doi: 10.1186/s12885-022-09678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pham K.H., et al. Single nucleotide Polymorphisms of FAM13A Gene in chronic obstructive pulmonary disease. A Case Control Study in Vietnam. 2023;91(3):268–277. doi: 10.3390/arm91030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Duy Hoang Tran, upon reasonable request.