Abstract
Disease-specific mechanisms underlying emotion recognition difficulties in behavioural-variant frontotemporal dementia (bvFTD), Alzheimer’s disease (AD), and Parkinson’s disease (PD) are unknown. Interoceptive accuracy, accurately detecting internal cues (e.g., one’s heart beating), and cognitive abilities are candidate mechanisms underlying emotion recognition.
One hundred and sixty-eight participants (52 bvFTD; 41 AD; 24 PD; 51 controls) were recruited. Emotion recognition was measured via the Facial Affect Selection Task or the Mini-Social and Emotional Assessment Emotion Recognition Task. Interoception was assessed with a heartbeat detection task. Participants pressed a button each time they: 1) felt their heartbeat (Interoception); or 2) heard a recorded heartbeat (Exteroception-control). Cognition was measured via the Addenbrooke’s Cognitive Examination-III or the Montreal Cognitive Assessment. Voxel-based morphometry analyses identified neural correlates associated with emotion recognition and interoceptive accuracy.
All patient groups showed worse emotion recognition and cognition than controls (all P’s ≤ .008). Only the bvFTD showed worse interoceptive accuracy than controls (P < .001). Regression analyses revealed that in bvFTD worse interoceptive accuracy predicted worse emotion recognition (P = .008). Whereas worse cognition predicted worse emotion recognition overall (P < .001). Neuroimaging analyses revealed that the insula, orbitofrontal cortex, and amygdala were involved in emotion recognition and interoceptive accuracy in bvFTD.
Here, we provide evidence for disease-specific mechanisms for emotion recognition difficulties. In bvFTD, emotion recognition impairment is driven by inaccurate perception of the internal milieu. Whereas, in AD and PD, cognitive impairment likely underlies emotion recognition deficits. The current study furthers our theoretical understanding of emotion and highlights the need for targeted interventions.
Keywords: Behavioural-variant frontotemporal dementia, Interoception, Emotion, Social cognition, Insula
Navigating the social world is a complex undertaking and is proposed to involve the continual integration of information from the world around us with cues from within our own bodies (Barrett, 2017; Barrett & Simmons, 2015; Damasio, 1996, 2006; James, 1884, 1894; Schachter & Singer, 1962). Interoception is the ongoing processing of one’s internal body environment and encompasses a range of cues from autonomic, hormonal, visceral and immunological sources (Cameron, 2001; Craig, 2002, 2003, 2009). While these processes occur without full awareness, interoceptive accuracy requires, at least in part, conscious perception and accurate monitoring of this internal milieu, such as changes in heart rate, feelings of fullness and fluctuations in breathing (Khalsa et al., 2018). Despite increasing recognition of the role of interoception in emotion processing (Adolfi et al., 2017; Couto et al., 2015; Critchley & Garfinkel, 2017; Critchley & Harrison, 2013; Fittipaldi et al., 2020; Salamone et al., 2021; Yoris et al., 2020), the extent to which deficits in interoceptive accuracy contribute to the well-recognised emotion recognition impairments in some dementia has been surprisingly under-explored. Moreover, whether other potential mechanisms, such as cognitive impairment may better explain emotion recognition difficulties in some dementia syndromes warrants investigation. Understanding syndrome-specific mechanisms contributing to emotion recognition deficits, and in turn the social and behavioural symptoms observed in dementia, is essential to inform differential diagnosis, alleviate carer distress, and develop disease-specific therapeutic interventions.
It is increasingly recognised that people with behavioural-variant frontotemporal dementia (bvFTD) are severely impaired on tasks of facial emotion recognition, particularly for negative emotions (see for meta analysis Bora, Velakoulis, & Walterfang, 2016; Boeve, Boxer, Kumfor, Pijnenburg, & Rohrer, 2022; Chiu et al., 2018; Goodkind et al., 2015; Kumfor et al., 2011; Piguet & Kumfor, 2020; Salamone et al., 2021). These emotional deficits contribute substantially to carer burden and distress (Miller et al., 2013). Clinically, bvFTD is characterised by profound changes to behaviour and personality, with early brain atrophy observed in the ventromedial prefrontal cortex and insula (Rascovsky et al., 2011), extending to the temporal lobes and subcortical regions with disease progression (Landin-Romero et al., 2017). The insula is a key brain structure involved in interoception (Craig, 2002, 2003, 2008) and is also one of the earliest sites of atrophy in bvFTD (Seeley et al., 2008). Neurobiological models of social interaction, such as the Social Context Network Model propose that the insula modulates social contextual understanding by integrating information arising from the body with the external environment (Baez, García, & Ibañaez, 2016; Ibáñez, 2018, 2019; Ibañaez & Manes, 2012; Ibanez & Schulte, 2020; Legaz et al., 2021), and is involved in assigning value to internal and external information as part of the salience network (Menon & Uddin, 2010; Salamone et al., 2021; Seeley et al., 2007). Recent updates of this model suggest that allostatic interoceptive overload, or the imbalanced weighting of interoceptive cues with external demands, underlie the neural and behavioural deficits in bvFTD (Birba et al., 2022). Moreover, given the role of interoception in emotion (see for review Critchley & Garfinkel, 2017), it is plausible that deficits in interoceptive processing also contribute to emotion recognition impairments routinely observed in bvFTD. Emerging studies have shown that bvFTD patients have impaired interoceptive accuracy (Abrevaya et al., 2020; García-Cordero et al., 2016; Salamone et al., 2021; but see Marshall et al., 2017), although results are mixed, and limited by small sample sizes. Of relevance here, no study has investigated whether impaired interoceptive accuracy underlies the profound emotion recognition deficits in bvFTD, or if these abilities have a shared neurobiological basis.
In contrast, while patients with Alzheimer’s disease (AD) and Parkinson’s disease (PD) show impairment on emotion recognition tasks, these impairments may be secondary to more generalised cognitive impairment (see for meta-analysis Bora et al., 2016; Baggio et al., 2012; Gray & Tickle-Degnen, 2010; Kumfor, Sapey-Triomphe, et al., 2014; Torres Mendonça De Melo Fadel, Santos De Carvalho, Belfort Almeida Dos Santos, & Dourado, 2019). AD patients present with predominant episodic memory loss and impaired executive functioning, with atrophy observed in the medial temporal lobe, most notably in the hippocampus and posterior cingulate (McKhann et al., 2011). PD patients present with tremors, bradykinesia and rigidity, with atrophy initially observed in the substantial nigra and brainstem, progressing to the basal ganglia and related subcortical regions over the course of the disease (Postuma et al., 2015). Importantly, in both AD and PD, the insula is relatively preserved, and emotion recognition deficits tend to be milder compared with those observed in bvFTD (see for meta analysis Bora et al., 2016; Goodkind et al., 2015). In addition, there is some emerging evidence of impaired interoceptive accuracy in both AD and PD (Abrevaya et al., 2020; García-Cordero et al., 2016; Ricciardi et al., 2016; Santangelo et al., 2018), however this is not consistently observed (Salamone et al., 2021) Altered interoceptive accuracy in these patient groups, however, may be due to the cognitive demands of the tasks used, which required sustained attention and working memory (Marshall et al., 2017; Ricciardi et al., 2016; Santangelo et al., 2018).
The current study aimed to examine disease-specific mechanisms underlying facial emotion recognition in bvFTD, AD and PD. Based on the neuroanatomical profile, we hypothesised that bvFTD patients would be impaired on a continuous monitoring interoceptive accuracy task. We further hypothesised that interoceptive accuracy would predict emotion recognition performance in bvFTD. Secondly, we hypothesised that AD and PD patients would not show interoceptive accuracy deficits using a continuous monitoring interoception task, where cognitive demands of the interoception tasks used were minimised and multiple biases of previous interoceptive accuracy methods were avoided (de la Fuente et al., 2019; Fittipaldi et al., 2020). Moreover, we hypothesised that cognitive impairment would predict emotion recognition performance in AD and PD. Finally, we aimed to investigate the neural correlates of interoceptive accuracy and emotion recognition. We hypothesised that interoceptive accuracy and emotion recognition tasks would be associated with reduced integrity of shared brain structures underpinning both processes, specifically the insula as a key interoceptive brain region involved in integrating internal and external social cues.
1. Methods
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
1.1. Participants
One hundred and sixty-eight participants (52 behavioural-variant frontotemporal dementia; 41 Alzheimer’s disease; 24 Parkinson’s disease; 51 controls) were recruited from three international dementia centres: FRONTIER, the younger onset dementia research clinic in Sydney, Australia; the Institute of Cognitive Neurology (INECO) in Buenos Aires, Argentina; and the Latin American Brain Health Institute (BrainLat) of Universidad Adolfo Ibáñez in Santiago, Chile. All participants underwent a comprehensive examination that included a neuropsychological assessment, an anatomical brain MRI acquisition, and examination by an experienced behavioural neurologist. All assessments were conducted in either English (Australia) or Spanish (Argentina, Chile). Diagnosis was established by the multidisciplinary teams at each research institute based on current consensus diagnostic criteria (McKhann et al., 2011; Postuma et al., 2015; Rascovsky et al., 2011). All healthy controls scored >88/100 on the Addenbrooke’s Cognitive Examination-III (ACE-III) (Hsieh, Schubert, Hoon, Mioshi, & Hodges, 2013) or >26/30 on the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005). Patients and healthy controls were excluded for the following: lifelong and untreated history of psychiatric disorder; significant head injury; alcohol or substance abuse; presence of other neurological disorder; or limited language proficiency for that country. All participants provided informed consent in accordance with the Declaration of Helsinki. The study was approved by the Ethical Research Committee of INECO (Argentina), the South Eastern Sydney Local Health District ethics committee, the University of New South Wales, and the University of Sydney ethics committees (Australia), and Universidad Adolfo Ibáñez (Chile).
A priori power analyses were conducted using G*Power 3.1 and indicated that a total sample of 48 participants would be appropriate for a repeated measures (within-between) subjects design with 2 repeated measures and 4 groups (effect size f = 0.25, power = 0.80, α = 0.05). Additionally, a sample size of 131 participants would be appropriate for a multiple linear regression with 13 predictors (effect size f = 0.25, power = 0.80, α = 0.05). Our sample size exceeded this estimation and therefore our study was sufficiently powered. No part of the study procedures or analyses were pre-registered prior to the research being conducted.
1.2. Measures
1.2.1. Disease severity and duration
The Frontotemporal dementia Rating Scale (FRS) was used to provide an index of disease severity and an associated Rasch score, with higher Rasch scores indicating less severe disease stage (Mioshi, Hsieh, Savage, Hornberger, & Hodges, 2010). Disease duration was determined by the number of years since symptoms were noticed by the carer/informant. The FRS is available freely online here: https://www.sydney.edu.au/brain-mind/resources-for-clinicians/dementia-test.html.
1.2.2. Global cognitive ability
Participants completed either the ACE-III (Hsieh et al., 2013) or the MoCA (Nasreddine et al., 2005). The ACE-III was completed by Australian participants in English, and the MoCA was completed by Argentinean and Chilean participants in Spanish. Each measure assesses attention, memory, language, and visuospatial abilities. To account for potential differences in the tests used, z-scores were calculated relative to controls scores on the ACE-III or MoCA.
The ACE-III is available freely online here: https://www.sydney.edu.au/brain-mind/resources-for-clinicians/dementia-test.html. The MoCA is available freely online here: https://mocacognition.com/paper/.
1.2.3. Emotion assessment
Participants completed either the Facial Affect Selection Task (FAST) (Kumfor, Sapey-Triomphe, et al., 2014; Miller et al., 2012) or Emotion Recognition subtest of the Mini-Social and Emotional Assessment (SEA) (Bertoux et al., 2012). The FAST was completed by Australian participants in English and the Mini-SEA was completed by Argentinean and Chilean participants in Spanish. For the FAST, participants viewed an array of seven faces of the same individual posing several different emotional expressions (fear, sadness, disgust, surprise, anger, happiness and neutral). On the Mini-SEA Emotion Perception task, participants selected an emotional label to match a presented emotion showing the same six basic emotion categories presented in the FAST (i.e., fear, sadness, disgust, surprise, anger and happiness) as well as a neutral expression. To account for potential differences in the tests used, z-scores were calculated relative to controls scores on the FAST or the mini-SEA. Legal copyright restrictions prevent public archiving of FAST and mini-SEA which can be obtained from the copyright holders in the cited references.
1.2.4. Interoception task
Interoception was assessed using a validated motor-tracking heartbeat detection task with two, 2-min experimental conditions, where attention was directed to either external or internal cues (García-Cordero et al., 2017; Gonzalez Campo et al., 2020; Richter et al., 2021; Salamone et al., 2021) available online here: http://bit.ly/2EpfGrq. In the exteroception condition, participants responded by tapping a button or key press each time they heard a heartbeat played by an external audio recording. The recorded heartbeat was presented at a varied rate equivalent to 60 beats per minute. Participants were instructed to respond immediately after hearing the sound, without anticipating the next beat. This condition was used as a control condition, to ensure all participants were able to follow instructions and respond appropriately. In the interoception condition, participants responded via button or key press each time they felt their heart beating. They were instructed not to rely on external cues (e.g., not checking their pulse).
1.3. Physiological recording
Electrocardiographic (ECG) signals were recorded using an 8/35 Powerlab Data Acquisition System (ADinstruments, Castle Hill, Australia) with LabChart Pro for Windows (v. 7.3.7) (Australia) or an AD620 amplifier (Analog Devices, Massa-chusetts, U.S.A) with PsychToolbox (Brainard & Vision, 1997), script via MATLAB (MathWorks) (Argentina and Chile). ECG was measured via a 3-lead placement using 3 Ag/AgeCl electrodes and ECG signals were digitised at a sampling rate of 1000 Hz/sec. R-wave values from the ECG signal were identified using peak detection functions in LabChart Pro or in MATLAB and were verified manually.
1.4. Data reduction and analyses
1.4.1. Interoceptive accuracy
Performance accuracy for both conditions was calculated using the mean distance index (See supplementary material for more details; Abrevaya et al., 2020; de la Fuente et al., 2019; Fittipaldi et al., 2020; Salamone et al., 2020). In brief, alignment was tested by comparing the frequencies of the event in the corresponding condition (i.e., audio cue in exteroception; actual heartbeat in interoception) and the frequencies of the participant’s response in that condition (i.e., button/key press) in 10-s overlapping windows across the task. Accuracy within each window was then averaged over the duration of the task to give a mean distance index for each condition. The lowest possible score for the mean distance index is 0 and reflects an exact match between frequencies of the condition’s cue and the participant’s response. Higher scores indicate larger distances between frequencies, and therefore, worse performance. This method is free of the bias present in other approaches (regarding average estimation, number of tracked responses, time-locking assessment, and heart rate fluctuations), and yields more robust responses (de la Fuente et al., 2019; Fittipaldi et al., 2020). To investigate the regularity of participant’s responses and to account for any potential lapses in attention, a coefficient of variation was also calculated as a ratio of the standard deviation and the mean response frequency over the duration of the condition (segmented into 10-s individual overlapping windows). Response windows that did not meet the specified threshold of regularity (coefficient of variation >0.5) were not included in the final mean distance index calculation (see supplementary materials; de la Fuente et al., 2019; Fittipaldi et al., 2020). Five participants (2 AD, 2 PD, and 1 control) were identified as significant outliers on the exteroception condition (>3 box lengths above 75th percentile) and were removed from subsequent analyses. Twenty-four participants did not complete the exteroception condition (bvFTD = 11; AD = 12; PD = 1), due to an update in the protocol at the Argentinian centre, but these participants were retained in the interoception analyses to maximise power.
1.5. Neuroimaging
1.5.1. Data acquisition
Whole-brain structural MRI data were obtained using the standard imaging protocols of each research centre (see Supplementary Table 1). MRI images were collected from four MRI scanners (1: Phillips Ingenia 3.0 T, 224 × 224, 160 slices, echo time/repetition time = 3.8/8.3 ms; 2: Phillips Intera 1.5 T, 256 × 256, 196 slices, echo time/repetition time = 3.4/7.49 ms; 3: GE 3 T 256 × 256, 200 slices, echo time/repetition time = 2.5/6.7 ms; Scanner 4: Siemens Skyra 3.0 T, 256 × 256, 192 slices, echo time/repetition time = 2/2.4 ms) with 1 mm3 isotropic resolution and a flip angle α = 8°. To account for potential differences in acquisition, MRI scanner was included as a covariate in the imaging analyses. MRI scans were available for 138 participants (40 healthy controls, 34 AD patients, 46 bvFTD patients and 18 PD patients). Participants who had an MRI did not differ significantly to those who did not have an MRI in relevant demographics or cognitive ability: age (t (159) = 1.00, P = .32), education (t (158) = 1.51, P = .13), sex (χ2 = 0.27, P = .60) or overall cognition (t (153) = −1.00, P = .32)).
1.5.2. Pre-processing
MRI data were analysed using the FSL suite (http://fsl.fmrib.ox.ac.uk) (Ashburner & Friston, 2000; Mechelli, Price, Friston, & Ashburner, 2005; Smith et al., 2004; Woolrich et al., 2009). First, structural images were brain-extracted using BET, then tissue segmentation was conducted via automatic segmentation (Zhang, Brady, & Smith, 2001). Next, grey matter partial volume maps were aligned to Montreal Neurological Institute (MNI) standard space (MNI152) using non-linear registration (FNIRT) which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). A study specific template was created using participants representative of each research centre and diagnostic group, and the native grey matter images were non-linearly re-registered. Modulation of the registered partial volume maps were carried out by dividing them by the Jacobian of the warp field. Finally, the modulated, segmented images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
1.6. Statistical analyses
1.6.1. Behavioural analyses
Clinical and behavioural data were analysed using IBM SPSS (v. 28). One-way ANOVAs were conducted for continuous variables (e.g., age, education, heart rate) and chi-square analyses for categorical variables (e.g., sex). Analyses were also conducted between centres for each diagnostic group on demographic and cognitive variables (see Supplementary Table 2).
Two separate one-way ANCOVA’s were used to determine group differences in emotion recognition performance (z-score) or cognitive ability (z-score) controlling for relevant demographics that differed significantly between samples (e.g., age, education) and test used.
Next, a linear mixed effects model (LME) was used to analyse performance on the interoception task. The mean distance index for the exteroception and interoception condition was the outcome variable with the following fixed effects: (i) diagnostic group (controls, AD, bvFTD, PD), (ii) condition (exteroception vs. interoception), (iii) the interaction between diagnostic group and condition. Covariates included in the model were (iv) age, (v) education (years), (vi) centre (Argentina, Australia, Chile); together with (vii) the intercept, and (viii) individual participant variability as a random effects. Condition was specified as a repeated effect. Significant continuous main effects (e.g., age) were investigated using Pearson’s correlation (two-tailed, Bonferroni corrected for multiple comparison).
Finally, a backwards stepwise multiple linear regression analysis was conducted to identify significant predictors of emotion recognition performance using the emotion recognition z-score. Thirteen predictors were included in the regression model: age, education (years), diagnosis (dummy coded with reference to controls), interoception accuracy, cognitive z-score, the interaction terms between interoception and each diagnostic group (dummy coded), and the interaction terms between cognition and each diagnostic group (dummy coded). The backwards stepwise multiple linear regression procedure was employed. Statistical significance was set to P < .05 for behavioural analyses, unless otherwise stated. Analysis codes is available here: https://osf.io/x28ky/.
1.6.2. Neuroimaging analyses
Voxel-wise general linear models (GLM) were created to investigate grey matter intensity differences between groups with permutation-based, non-parametric testing, correcting for multiple comparisons across space, with 5000 permutations per contrast (Nichols & Holmes, 2002). Then, to examine the neural correlates of interoception and emotion recognition, interoception accuracy and emotion recognition scores were entered into separate GLMs to determine the regions associated with each task in each patient group combined with controls. Combining patients with controls has been shown to achieve greater variance in scores, increasing the statistical power to detect behavioural correlations in neuroimaging (Sollberger et al., 2009). Scatterplots between the peak voxel and the relevant score (interoceptive accuracy; emotion recognition z-score) were checked for linearity. Inclusive masking analysis was conducted to identify common brain regions associated with interoceptive accuracy and emotion. Each GLM was Family-Wise Error corrected for multiple comparisons using threshold free cluster enhancement (TFCE), and a conservative cluster threshold of 100 voxels reported. In each GLM, demographic information (age, years of education), and MRI scanner (dummy coded) were included as covariates. Relevant t scores for each GLM are listed beneath each table and figure. The statistical threshold for neuroimaging analyses was set at P < .05 unless otherwise specified. Anatomical locations of significant results were overlaid on the MNI standard brain. Anatomical labels were determined with reference to the HarvardeOxford probabilistic cortical and subcortical atlases.
1.7. Data availability
The conditions of our ethics approval do not permit public archiving of the data supporting this study. Readers seeking access to this data should contact the senior author, Fiona Kumfor to discuss a formal data sharing agreement.
2. Results
2.1. Demographics
Significant group differences were observed for age and education (both P ≤ .001; Table 1). Control participants and bvFTD patients were significantly younger than the AD and PD groups (all P values < .005) consistent with typically observed demographics. Control participants had more years of education than AD (P < .001), bvFTD (P = .023) and PD patients (P = .003). No significant differences were observed for sex (P = .16). Cognitive ability differed between groups (P < .001), with all patient groups performing worse than controls (all P values < .001), and AD worse than bvFTD and PD patients (both P’s < .001). No significant differences in heart rate (bpm) were observed between groups during the interoception condition (P = .14).
Table 1.
Comparison of demographic, overall cognition, and emotion across diagnostic groups.
| Controls | bvFTD | AD | PD | Statistic | p | Post-hoc | |
|---|---|---|---|---|---|---|---|
| n = 50 | n = 52 | n = 39 | n = 22 | ||||
| Age (years) | 65.00 ± 5.44 | 66.53 ± 9.50 | 72.89 ± 8.23 | 72.23 ± 6.78 | 11.33 | <.001 | Controls = bvFTD < AD = PD |
| Education (years) | 15.72 ± 2.17 | 13.52 ± 4.11 | 12.42 ± 4.29 | 11.95 ± 4.52 | 8.04 | <.001 | Patients < Controls |
| Sex (M:F) | 24:26 | 34:18 | 16:23 | 12:10 | 5.14 | .16 | |
| Disease duration(years) | - | 5.74 ± 4.73 | 3.57 ± 1.91 | - | 2.71 | .11 | |
| FRS (Rasch score) | - | −0.26 ± 1.36 | −0.51 ± 1.69 | 1.43 ±1.28 | 9.32 | <.001 | AD = bvFTD < PD |
| Cognition (z-score) | −0.00 ± 1.00 | −6.74 ± 3.74 | −9.55 ± 4.45 | −5.45 ± 3.59 | 51.65a | <.001 | Patients < Controls; AD < bvFTD &PD |
| Heart rate (bpm)b | 66.00 ± 7.38 | 72.87 ± 22.25 | 66.45 ± 12.88 | 71.50 ± 17.73 | 1.87 | .14 |
Note. Values are mean ± standard deviation.
ANCOVA F Statistic, covariates included: Age (years), Education (years), Cognition z-score (ACE-III or MOCA);
Interoception condition;
bvFTD = behavioural-variant frontotemporal dementia; AD = Alzheimer’s disease; PD = Parkinson’s disease. Disease duration = years since symptom onset; FRS = Frontotemporal dementia Rating Scale; Global cognition % (ACE-III: MoCA = 77:78); Available data: Age (years): 50/50 Controls, 51/52 bvFTD, 38/39 AD, 22/22 PD; Education (years): 50/50 Controls, 50/52 bvFTD, 38/39 AD; Disease Duration (years): 31/52 bvFTD, 14/39 AD, 22/22 PD; FRS: 38/52 bvFTD, 24/39 AD, 15/22 PD; Global cognition (%): 46/50 Controls, 50/52 bvFTD, 38/39 AD, 21/22 PD; Heart rate (bpm): 50/50 Controls; 46/52 bvFTD; 38/39 AD; 22/22 PD.
2.2. Behavioural results
Emotion recognition differed significantly across diagnostic groups, F (3, 128) = 13.32, P < .001, partial η2 = .239 (Fig. 1A), after controlling for demographics and emotion test used. Post-hoc comparisons revealed that all patient groups performed worse than controls (bvFTD vs controls: P < .001; AD vs controls: P < .001; PD vs controls: P = .008), with no differences observed between patient groups (all P values > .35).
Fig. 1 –
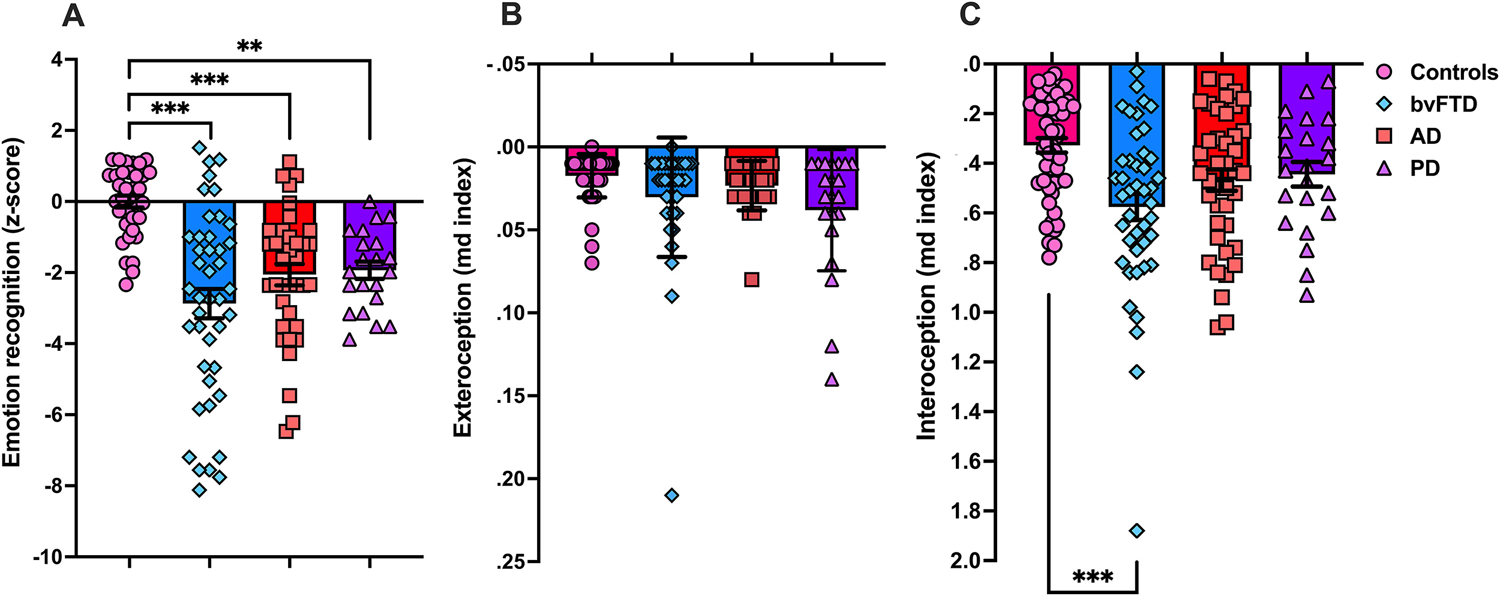
Behavioural performance in each diagnostic group. (A) Emotion recognition performance using z-scores relative to controls. Available data for emotion recognition: 45/52 bvFTD, 37/39 AD, 21/22 PD, 35/50 Controls. (B) Exteroception accuracy. Available data for exteroception task: 38/52 bvFTD; 27/39 AD; 21/22 PD; Controls: 50/50. (C) Interoceptive accuracy. Available data for interoception task: 46/52 bvFTD; 38/39 AD, 22/22 PD, 50/50 Controls. In (B) and (C), lower mean distance shows better performance. **P < .01 ***P < .001. bvFTD = behavioural-variant frontotemporal dementia; AD = Alzheimer’s disease; PD = Parkinson’s disease; md index = mean distance index.
2.3. Interoceptive accuracy
On the heartbeat detection task, a main effect of diagnosis was observed, F (3, 88.87) = 6.62, P < .001, with bvFTD patients showing worse accuracy than controls (P < .001) (Fig. 1C). No other post hoc comparisons were significant (all P values > .15). A main effect of condition was also present, F (1, 144.55) = 314.97, P < .001, with greater accuracy in the exteroception than in the interoception condition. Importantly, a significant interaction between condition and diagnosis was observed, F (3,144.40) = 6.33, P < .001. On the interoceptive condition, bvFTD showed worse performance than controls (P < .001), whereas no difference was seen between AD, PD, and controls (AD vs controls: P = .09; PD vs controls: P = .73) or between patient groups (bvFTD vs AD: P = .43; bvFTD vs PD: P = .16; AD vs PD: P = .98). On the exteroception condition, no significant difference between controls or patients (controls vs AD: P = .96; controls vs bvFTD: P = .34; controls vs PD: P = 1.0) or between patient groups (bvFTD vs AD: P = .10; bvFTD vs PD: P = .87; AD vs PD: P = .87) were present (Fig. 1B). Within group analyses showed that all groups performed worse on the interoception than exteroception condition (all P values < .001). A main effect of age was present, F (1, 103.78) = 5.17, P = .025. Correlation analyses showed that older age was associated with worse exteroception, r (136) = .233, P < .006, with no significant correlation observed between age and interoception, r (151) = −.143, P = .08. No other covariates included in the model were significant: education, F (1, 99.63) = 2.94, P = .09, centre, F (2, 98.33) = 0.58, P = .56.
2.4. Predictors of emotion recognition performance
Backwards step-wise multiple linear regression analyses revealed a significant model F (5, 121) = 16.00, P < .001, which explained 39.8% of the variance in emotion recognition performance (Table 2; Fig. 2). Overall cognitive performance was a significant predictor of emotion recognition performance in all participants. Additionally, interoception in bvFTD (relative to controls) was a significant predictor of emotion recognition performance. In the final model, the interaction between bvFTD and cognition, AD and cognition and PD and cognition contributed to the overall regression equation but were not a significant predictors of emotion recognition. Additionally, age, education, interoception, AD x interoception, PD x interoception were not significant predictors and were removed from the model at earlier stages of the backwards regression procedure.
Table 2.
Unstandardised (B) and standardised regression (β) coefficients for predictors of emotion recognition performance.
| Variables | B(SE) | β | t | p |
|---|---|---|---|---|
| (Constant) | −0.04(0.26) | - | −0.14 | .893 |
| Overall cognition (%) | 0.29(0.07) | 0.65 | 4.51 | <.001 |
| bvFTD × Interoception | −2.11(0.78) | −0.27 | −2.71 | .008 |
| AD × Cognition | −0.09(0.07) | −0.20 | −1.27 | .207 |
| PD × Cognition | −0.15(0.14) | −0.16 | −1.10 | .274 |
| PD diagnosis | −1.30(0.78) | −0.22 | −1.67 | .100 |
Note. SE = Standard Error of B; bvFTD = behavioural variant frontotemporal dementia; AD = Alzheimer’s disease; PD = Parkinson’s disease. Model Summary: Model 1: R2 = .404, p < .001; Model 2: ΔR2 =.000, p = .953; Model 3: ΔR2 =.000, p = .919; Model 4: ΔR2 = .000, p = .907, Model 5: ΔR2 = .000, p = .823; Model 6: ΔR2 = − .002, p = .519, Model 7: ΔR2 = .000, p = .771, Model 8: ΔR2 = −.002, p = .521, Model 9 (Final): ΔR2 = −.003, p = .449.
Fig. 2 –
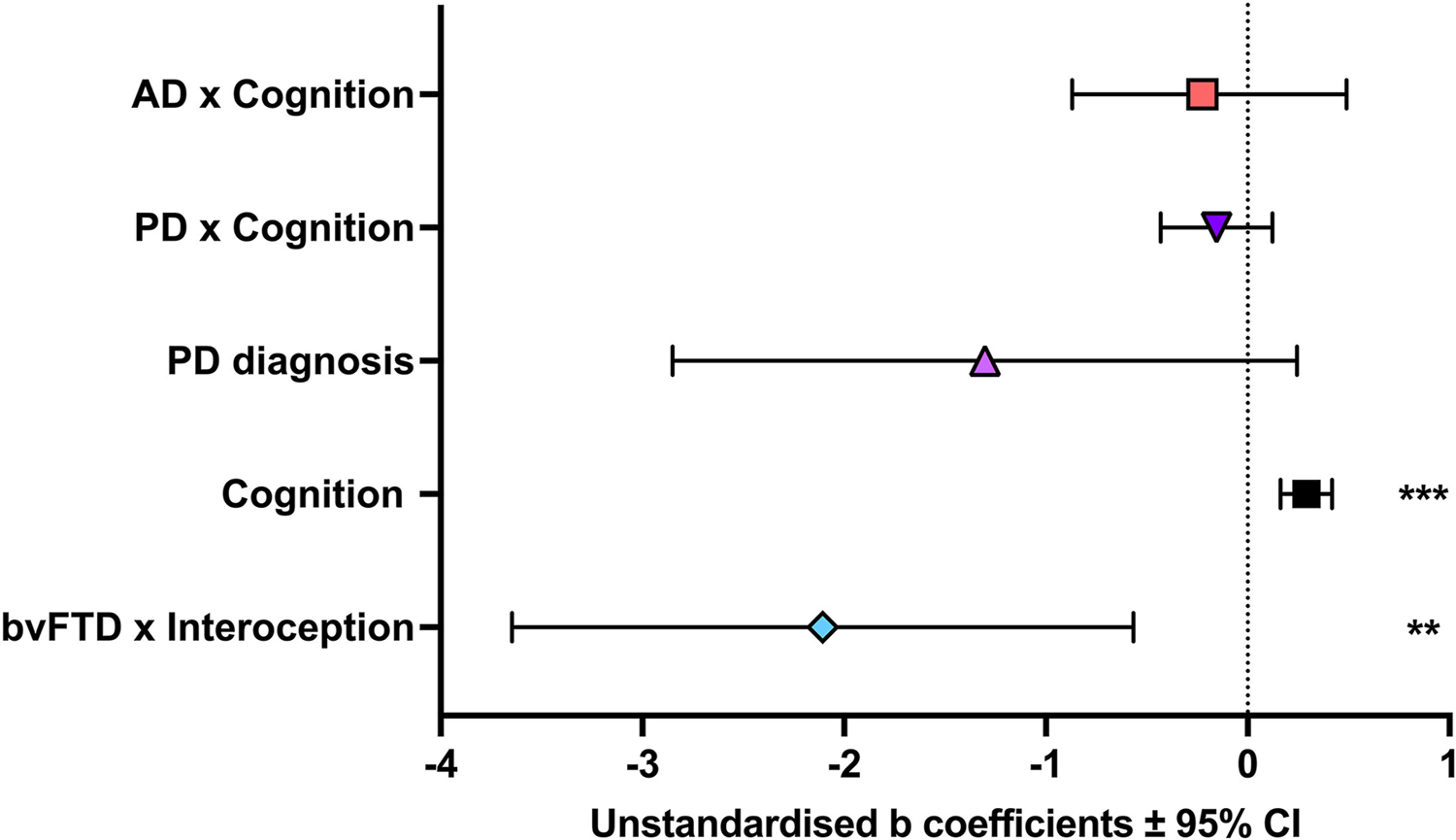
Predictors of emotion recognition. Note. **P < .01; ***P < .001.
2.5. Neuroimaging results
Patterns of differences in grey matter integrity in bvFTD and AD compared to controls were consistent with expectations and reported in Supplementary Figure 1 and Supplementary Table 3. No significant clusters were observed in PD.
2.5.1. Neural correlates of emotion recognition
In bvFTD, emotion recognition performance was associated with integrity in frontal (frontal pole, orbitofrontal cortex, medial prefrontal cortex) and temporal (middle temporal and superior temporal gyrus) regions, as well as the insula, striatum (putamen and caudate), precentral gyrus and central opercular gyrus, bilaterally (Fig. 3A and Supplementary Table 4). In AD, emotion recognition was associated with integrity of parietal (bilateral angular gyrus and supramarginal gyrus) and temporal (middle temporal, inferior temporal) brain regions bilaterally (Supplementary Table 5). No significant clusters were identified in PD.
Fig. 3 –
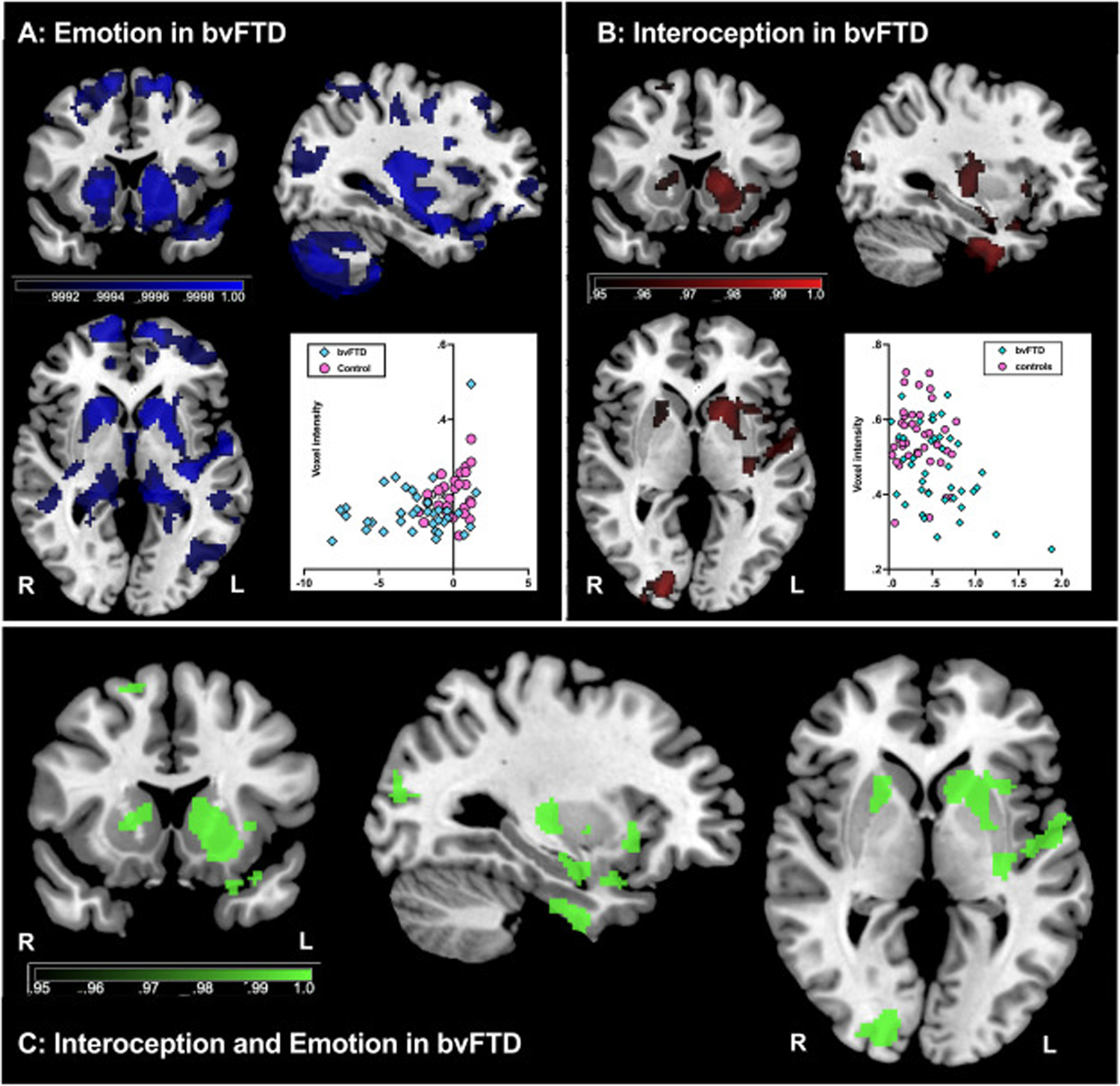
Voxel-based morphometry analyses of brain regions associated with emotion and interoception. (A) Emotion recognition performance in bvFTD (blue) (B) Interoceptive accuracy in bvFTD (red); and (C) overlap analyses for emotion and interoceptive accuracy in bvFTD (green). Coloured voxels show regions identified via in a threshold free cluster enhancement (TFCE) contrasts that reached statistical significance at either P < .05 or P < .001 as displayed on figure. Scatterplots are included showing the linear relationship between the peak voxel and (A) emotion or (B) interoception. All analyses were Family-Wise Error corrected for multiple comparisons with a cluster extent threshold of 100 voxels, t (85) > 1.66. Covariates included in each GLM included: age (years), education (years), and Scanner used. Note, images are in radiological orientation (i.e., right hemisphere appears on left side of page). MNI coordinates: X = −33, Y = 15; Z = 0. R = right, L = left. bvFTD = behavioural variant frontotemporal dementia; AD = Alzheimer’s disease.
2.5.2. Neural correlates of interoceptive accuracy
In bvFTD, interoceptive accuracy was associated with reduced integrity of the insula, superior frontal gyrus, inferior and superior temporal gyrus, temporal fusiform cortex, bilateral caudate, putamen, amygdala, hippocampus, and parahippocampal gyrus (Fig. 3B, Supplementary Table 4). No significant clusters were identified in AD or PD.
2.5.3. Shared neural correlates of emotion recognition and interoceptive accuracy
The overlap analysis revealed shared regions associated with emotion recognition and interoceptive accuracy in bvFTD including the left insula, left temporal pole, left orbitofrontal cortex, left frontal opercular cortex, bilateral striatum, left inferior temporal gyrus, left temporal fusiform gyrus, left parahippocampal gyrus, left amygdala, left hippocampus, as well as right supplementary motor cortices, right superior frontal gyrus, and the right occipital lobe region (Fig. 3C, Supplementary Table 4).
3. Discussion
This study is the first to investigate the contribution of interoceptive accuracy and cognitive ability as potential mechanisms underlying emotion recognition impairments in three unique dementia syndromes. We found that bvFTD patients showed impaired interoceptive accuracy, and that this impairment uniquely contributed to their emotion recognition ability. In contrast, individuals diagnosed with AD and PD performed similarly to healthy controls with respect to interoceptive accuracy. In line with previous reports, emotion recognition in these syndromes is likely secondary to cognitive impairment. Neuroimaging analyses in bvFTD revealed that both interoceptive accuracy and emotion recognition shared common neural correlates in the insula as well as being associated with a broader network of frontal and temporal brain regions. Taken together, these findings provide further evidence that the social cognitive profile seen in bvFTD is underpinned by a compromised ability to sense internal body signals which impacts on their capacity to interpret external social cues. In contrast, in AD and PD, emotion recognition deficits reflect broader cognitive decline. In the following sections, we discuss how these findings advance our current knowledge of these dementia syndromes as well as informing the theoretical constructs of interoception, cognition, and emotion.
We found that bvFTD patients showed profound impairment in the ability to recognise emotions and that this impairment was associated with widespread atrophy to frontal, temporal, and insular cortices in line with previous reports (see for meta-analysis Bora et al., 2016; Chiu et al., 2018; Goodkind et al., 2015; Kumfor et al., 2011). Moreover, we found that bvFTD had a unique impairment in interoceptive accuracy. These results are consistent with emerging reports of reduced interoceptive accuracy in bvFTD (Abrevaya et al., 2020; Birba et al., 2022; García-Cordero et al., 2016; Salamone et al., 2021) and fit with previous evidence of altered body schemas in bvFTD, particularly in patients with the C9orf72 gene expansion (Downey et al., 2014). Taken together, our findings suggest that in everyday life, bvFTD patients are not able to interpret relevant interoceptive cues and integrate these cues with external environmental demands, in line with recent accounts of allostatic interoceptive overload (Birba et al., 2022). It is possible that these patients become overly reliant on external contextual cues to compensate for insufficient internal cues (Kumfor et al., 2018). Another possible explanation for the emotional disturbances in bvFTD is that the strength of the interoceptive signal is dampened. Indeed, bvFTD patients have previously shown blunted physiological responses to social situations (Sturm, Ascher, Miller, & Levenson, 2008), as well as when viewing emotionally evocative stimuli (Kumfor, Hazelton, Rushby, Hodges, & Piguet, 2019) suggesting that interoceptive signals from the body may be compromised as well. While we found no differences in heart rate in our study, future research is necessary to disentangle whether emotional disturbances in bvFTD are due to a lack of interoceptive accuracy (top-down processing) and/or dampened interoceptive signals (bottom-up processing). Future studies should consider comparing interoceptive accuracy at rest and when manipulating interoceptive cues, such as via vagus nerve stimulation (Richter et al., 2021; Villani, Tsakiris, & Azevedo, 2019; Weng et al., 2021).
For the first time, we have shown that impaired interoceptive accuracy uniquely predicts impaired emotion recognition in bvFTD patients only. Previous research in healthy adults found that the heart evoked potential, a neural marker of interoception, is amplified when viewing emotional content (Couto et al., 2015), as well as following brief periods of interoception (Ernst, Northoff, Böker, Seifritz, & Grimm, 2013). Our study complements a recent EEG study in bvFTD (Salamone et al., 2021), which found that reduced emotion recognition co-occurs with reduced heart-evoked potentials following interoceptive attention. Importantly, bvFTD patients experience a myriad of social cognitive deficits beyond the emotion recognition difficulties observed here, including reduced empathy (Carr & Mendez, 2018), inappropriate social behaviours (Piguet, Kumfor, & Hodges, 2017), reduced perspective taking (Henry, Phillips, & Von Hippel, 2014; Strikwerda-Brown, Ahmed, Piguet, & Irish, 2022), and difficulties identifying their own emotional state (Mendez, 2021). In healthy people, these fundamental social cognitive abilities have been linked to interoception (Fukushima, Terasawa, & Umeda, 2011; Terasawa, Moriguchi, Tochizawa, & Umeda, 2014). Therefore, the impact of compromised interoception in bvFTD likely goes beyond impaired emotion recognition and also may account for the broader social functioning deficits characteristic of this syndrome.
Our neuroimaging results support a shared neurobiological mechanism underpinning both interoceptive accuracy and emotion recognition in bvFTD, with the left insula emerging as a key region in both processes. The insula is responsible for receiving and interpreting ascending signals from within the body (Craig, 2002, 2003) and plays a key role in the salience network (Seeley et al., 2007). Previously, the insula has also been implicated in emotion recognition, particularly for negative emotions (Adolphs, 2002; Bora et al., 2016; Kumfor, Irish, Hodges, & Piguet, 2013) and is thought to integrate internal and external contextual information (Baez et al., 2016; Birba et al., 2022; Ibañez & Manes, 2012). Both interoceptive accuracy and emotion were also associated with reduced structural integrity of salience network regions, such as the left orbitofrontal cortex, left amygdala and bilateral striatum (Seeley et al., 2007) as well as the parahippocampal gyrus which is thought to be crucial for deciphering context (Baumann & Mattingley, 2016) and the hippocampus which plays a role in emotional memory (Kumfor, Irish, Hodges, & Piguet, 2014; Phelps, 2004). It is plausible that degeneration of these key areas means that bvFTD patients are no longer able to assign appropriate value to internal interoceptive cues or external social stimuli or use this information to update their behaviours over time (Birba et al., 2022). Of note, we observed relatively left-lateralised involvement of several key interoceptive regions. Although interoception has been proposed to involve right-lateralised structures, such as the right anterior insula and anterior cingulate cortex, left hemisphere involvement has also been observed in experimental studies of healthy people (Dobrushina et al., 2021; Fittipaldi et al., 2020; Wiebking et al., 2014) and is also observed in meta-analyses on interoception, together with right hemisphere structures (see for meta-analyses Adolfi et al., 2017; Schulz, 2016). Further research is needed in lesion models characterised by left- or right-predominant atrophy to further investigate lateralisation of interoception and emotion. Importantly, the regions identified in our neuroimaging analyses are highly connected to the insula via white matter tracts (Ghaziri et al., 2018). Therefore, degeneration of these underlying white matter pathways in each hemisphere may also relate to the interoceptive accuracy and emotion recognition deficits and warrants future research.
In both AD and PD patients, emotion recognition ability was impaired, however the performance was likely due to a disruption in cognitive ability rather than interoceptive accuracy. Notably, in contrast with previous reports interoceptive accuracy remained largely intact (García-Cordero et al., 2016; Ricciardi et al., 2016; Salamone et al., 2021; Santangelo et al., 2018). This discrepancy is likely due to differences in task design. The task employed here, allowed participants to adjust their responding over time and only considered time periods where patients were actively attending to the task (de la Fuente et al., 2019; Fittipaldi et al., 2020). This adaptation reduced attentional and memory-related task demands. We found instead that cognitive impairment was a significant predictor of emotion recognition performance, in line with previous reports (see for meta-analysis Bora et al., 2016; Baggio et al., 2012; Gray & Tickle-Degnen, 2010; Kumfor, Sapey-Triomphe, et al., 2014; Torres Mendonça De Melo Fádel et al., 2019). Therefore, in AD and PD patients, difficulties in understanding others’ emotions do not appear to be necessarily dependent on the failure to integrate internal interoceptive cues, but rather arise from impairments in cognitive processes, such as memory and attention, which impact on task performance.
Our neuroimaging results support our findings of different underlying mechanisms driving reduced emotion recognition in AD to those observed in bvFTD. In AD, impaired emotion recognition was associated with reduced structural integrity of bilateral temporal and parietal structures, and not interoception-related brain structures (e.g., insula). Indeed, the structures identified as important for emotion recognition in AD are commonly implicated in memory processing, with the middle temporal gyrus and angular gyrus being involved in autobiographical memory, semantic memory, and future thinking (Davey et al., 2016; Ramanan, Piguet, & Irish, 2018; Seghier, 2013). Here, it is likely that the emotion recognition impairments observed in AD reflect memory-related task demands rather than a primary emotion recognition deficit.
Our neuroimaging results in PD patients are less clear, with no brain regions associated with interoceptive accuracy or emotion recognition performance. These findings echo previous neuroimaging research in PD. For instance, previous research using whole brain VBM analyses in PD found no associations between grey matter integrity and emotion (Baggio et al., 2012), emotional learning (Legaz et al., 2021), or cognition (Dalaker et al., 2010). Of note, the PD patients in the current study were at a relatively mild disease stage. Future research using advanced imaging tools that can accurately measure brain regions involved in mild PD, such as the brain stem nuclei and basal ganglia may be necessary to understand the neural bases of impaired cognition and emotion recognition in these patients, particularly early in the disease course.
Although our study is the first to identify syndrome-specific mechanisms underlying emotion recognition impairments in separate dementia syndromes, some caveats should be noted. The multi-centre approach taken by this study meant that different tests were used as part of routine clinical assessment (i.e., different tests of cognition and emotion across research centres). We mitigated this limitation by using z-scores relative to controls to account for any potential differences. Future prospective studies will benefit from the establishment of gold-standard tests of cognition and emotion, validated cross-culturally. In addition, our study focused on cardiac interoceptive accuracy. Other interoceptive cues, however, such as changes in breathing rate or sensations of hunger, may also be affected in bvFTD, as they rely on the same interoceptive neural network (Craig, 2002, 2008). For instance, changes to eating behaviours, including binge eating, are commonly observed in bvFTD (see for review Ahmed et al., 2018), and are associated with integrity of the insula, anterior cingulate, thalamus, hypothalamus, orbitofrontal cortex and striatum (Ahmed et al., 2016; Piguet, 2011; Woolley et al., 2007). These changes in eating may reflect impaired recognition of fullness via gastric interoceptive accuracy, which warrants future investigation. Finally, the patients included in the current study had an average disease duration of more than three years. It is possible that interoceptive impairments precede observable clinical symptoms in bvFTD, due to the early degeneration of the insula (Seeley et al., 2008). Indeed, atrophy to the insula has also been observed in pre-symptomatic gene carriers of bvFTD (Cash et al., 2018). Therefore, future research is needed to determine whether interoception can be used as a potential pre-clinical biomarker of disease onset.
4. Conclusion
Our study is the first to identify disease-specific mechanisms contributing to emotion recognition in three of the most common dementia syndromes. Over the past decade, impairment in emotion recognition has been established as a characteristic diagnostic feature (Boeve et al., 2022; Ducharme et al., 2020). However, the mechanisms driving these impairments remain poorly understood. While the potential role of interoceptive accuracy has been previously hypothesised (Van den Stock & Kumfor, 2017), this study is the first to our knowledge to empirically demonstrate this link. Clinically, attempts to rehabilitate emotion perception in bvFTD have focused on directing attention to emotionally salient features, with limited success (Kumfor et al., 2011). Indeed, bvFTD patients do not show abnormalities in directing attention to these emotional cues (Hutchings et al., 2018). Our results suggest that a more effective intervention will likely involve the modulation of internal cues to enable improved detection and decoding of the internal body state. This represents an unexplored opportunity for interventions which target the underlying mechanism. From a theoretical perspective, our results support theories of emotion which emphasise the critical role of physiological state in guiding our interactions with the social world.
Supplementary Material
Acknowledgements
We would like to acknowledge the patients and their families for participating in this research. We would also like to acknowledge the technical assistance provided by the Sydney Informatics Hub, a Core Research Facility of the University of Sydney.
Funding
This work was supported in part by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council (NHMRC) (GNT1037746). JH is supported by a National Health and Medical Research Council (NHMRC) Postgraduate scholarship (GNT1168597). SF is supported by CONICET, Universidad de San Andres, and BrainLat Postdoctoral Fellowship. MS is supported by the Australian Research Council grant (DP170101815). AI is supported by grants of Takeda CW2680521; CONICET; FONCYT-PICT (2017-1818, 2017-1820); ANID/FONDECYT Regular (1,210,195, 1,210,176, 1,220,995); ANID/FONDAP (15,150,012); ANID/PIA/ANILLOS ACT210096; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), funded by the National Institutes of Aging of the National Institutes of Health under award number R01AG057234, an Alzheimer’s Association grant (SG-20-725707-ReDLat), the Rainwater Foundation, and the Global Brain Health Institute. OP is supported by a NHMRC Senior Research Fellowship (GNT1103258). FK is supported by a NHMRC Career Development Fellowship (GNT1158762). The content is solely the responsibility of the authors and does not represent the official views of these institutions.
Abbreviations:
- ACE-III
Addenbrooke’s Cognitive Examination – III
- AD
Alzheimer’s disease
- bvFTD
behavioural-variant frontotemporal dementia
- FAST
Facial Affect Selection Task
- FRS
Frontotemporal dementia Rating Scale
- mini-SEA
mini-Social and Emotional Assessment
- MoCA
Montreal Cognitive Examination
- PD
Parkinson’s disease
- VBM
Voxel Based Morphometry
Footnotes
Open Practices
The study in this article earned Open Material badges for transparent practices. The materials for this study are available at: https://osf.io/x28ky/
CRediT author statement
Jessica L. Hazelton: Conceptualisation, Methodology, Investigation, Formal analysis, Writinge–Original draft, Visualization; Sol Fittipaldi: Software, Data curation, Investigation, Writinge–Reviewing & editing; Matias Fraile-Vazquez: Software, Writinge–Reviewing & editing; Marion Sourty: Software, Formal analysis, Writinge–Reviewing & editing; Agustina Legaz: Investigation, Writinge–Reviewing & editing; Anna L. Hudson: Writinge–Reviewing & editing; Indira Garcia Cordero: Investigation, Writinge–Reviewing & editing; Paula C. Salamone: Investigation, Writinge–Reviewing & editing; Adrian Yoris: Investigatio, Writinge–Reviewing & editing; Agustín Ibañez: Conceptualisation, Methodology, Resources, Supervision, Funding Acquisition, Writinge–Reviewing & editing; Olivier Piguet: Conceptualisation, Methodology, Resources, Supervision, Funding Acquisition, Writinge–Reviewing & editing; Fiona Kumfor: Conceptualisation, Methodology, Resources, Supervision, Funding Acquisition, Writinge–Reviewing & editing.
Declaration of competing interest
The authors report no competing interests.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2023.02.009.
REFERENCES
- Abrevaya S, Fittipaldi S, García AM, Dottori M, Santamaria-Garcia H, Birba A, et al. (2020). At the heart of neurological dimensionality: Cross-nosological and multimodal cardiac interoceptive deficits. Psychosomatic medicine, 82(9), 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, et al. (2017). Convergence of interoception, emotion, and social cognition: A twofold fMRI meta-analysis and lesion approach. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior, 88, 124–142. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2002). Neural systems for recognizing emotion. Current opinion in neurobiology, 12(2), 169–177. [DOI] [PubMed] [Google Scholar]
- Ahmed RM, Irish M, Henning E, Dermody N, Bartley L, Kiernan MC, et al. (2016). Assessment of eating behavior disturbance and associated neural networks in frontotemporal dementia. JAMA Neurology, 73(3), 282–290. 10.1001/jamaneurol.2015.4478 [DOI] [PubMed] [Google Scholar]
- Ahmed RM, Ke YD, Vucic S, Ittner LM, Seeley W, Hodges JR, et al. (2018). Physiological changes in neurodegeneration—mechanistic insights and clinical utility. Nature Reviews Neurology, 14(5), 259–271. [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2000). Voxel-based morphometrydthe methods. Neuroimage, 11(6), 805–821. [DOI] [PubMed] [Google Scholar]
- Baez S, García AM, & Ibañez A (2016). The social context network model in psychiatric and neurological diseases. Social Behavior from Rodents to Humans, 379–396. [DOI] [PubMed] [Google Scholar]
- Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti M, Compta Y, et al. (2012). Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia, 50(8), 2121–2128. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017). The theory of constructed emotion: An active inference account of interoception and categorization. [Social Cognitive and Affective Neuroscience Electronic Resource], 12(1), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature reviews neuroscience, 16(7), 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, & Mattingley JB (2016). Functional organization of the parahippocampal cortex: Dissociable roles for context representations and the perception of visual scenes. Journal of Neuroscience, 36(8), 2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoux M, Delavest M, de Souza, Funkiewiez A, Lépine JP,… Sarazin M (2012). Social cognition and emotional assessment differentiates frontotemporal dementia from depression. Journal of Neurology, Neurosurgery & Psychiatry, 83(4), 411–416. [DOI] [PubMed] [Google Scholar]
- Birba A, Santamaría-García H, Prado P, Cruzat J, Ballesteros AS, Legaz A, et al. (2022). Allostatic interoceptive overload in frontotemporal dementia. Biological Psychiatry, 92(1), 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Boxer AL, Kumfor F, Pijnenburg Y, & Rohrer JD (2022). Advances and controversies in frontotemporal dementia: Diagnosis, biomarkers, and therapeutic considerations. Lancet Neurology, 21(3), 258–272. [DOI] [PubMed] [Google Scholar]
- Bora E, Velakoulis D, & Walterfang M (2016). Meta-analysis of facial emotion recognition in behavioral variant frontotemporal dementia: Comparison with alzheimer disease and healthy controls. Journal of Geriatric Psychiatry and Neurology, 29(4), 205–211. [DOI] [PubMed] [Google Scholar]
- Brainard DH, & Vision S (1997). The psychophysics toolbox. Spatial vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Cameron OG (2001). Visceral sensory neuroscience: Interoception. Oxford University Press. [Google Scholar]
- Carr AR, & Mendez MF (2018). Affective empathy in behavioral variant frontotemporal dementia: A meta-analysis. The Florida Nurse, 9, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash DM, Bocchetta M, Thomas DL, Dick KM, van Swieten JC, Borroni B, et al. (2018). Patterns of gray matter atrophy in genetic frontotemporal dementia: Results from the GENFI study. Neurobiology of Aging, 62, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I, Piguet O, Diehl-Schmid J, Riedl L, Beck J, Leyhe T, et al. (2018). Facial emotion recognition performance differentiates between behavioral variant frontotemporal dementia and major depressive disorder. The Journal of clinical psychiatry, 79(1). [DOI] [PubMed] [Google Scholar]
- Couto B, Adolfi F, Velasquez M, Mesow M, Feinstein J, Canales-Johnson A, et al. (2015). Heart evoked potential triggers brain responses to natural affective scenes: A preliminary study. Autonomic Neuroscience, 193, 132–137. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature reviews neuroscience, 3(8), 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003). Interoception: The sense of the physiological condition of the body. Current opinion in neurobiology, 13(4), 500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD (2008). Interoception and emotion: A neuroanatomical perspective. Handbook of emotions, 3(602), 272–288. [Google Scholar]
- Craig AD (2009). How do you feel–now? The anterior insula and human awareness. Nature reviews neuroscience, 10(1). [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Garfinkel SN (2017). Interoception and emotion. Current opinion in psychology, 17, 7–14. [DOI] [PubMed] [Google Scholar]
- Critchley HD, & Harrison NA (2013). Visceral influences on brain and behavior. Neuron, 77(4), 624–638. [DOI] [PubMed] [Google Scholar]
- Dalaker TO, Zivadinov R, Larsen JP, Beyer MK, Cox JL, Alves G, et al. (2010). Gray matter correlations of cognition in incident Parkinson’s disease. Movement disorders, 25(5), 629–633. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 351(1346), 1413–1420. [DOI] [PubMed] [Google Scholar]
- Damasio AR (2006). Descartes’ error. Random House. [Google Scholar]
- Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, et al. (2016). Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage, 137, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrushina OR, Arina GA, Dobrynina LA, Novikova ES, Gubanova MV, Belopasova AV, et al. (2021). Sensory integration in interoception: Interplay between top-down and bottom-up processing. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior, 144, 185–197. [DOI] [PubMed] [Google Scholar]
- Downey LE, Fletcher PD, Golden HL, Mahoney CJ,Agustus JL, Schott JM, et al. (2014). Altered body schema processing in frontotemporal dementia with C9ORF72 mutations. Journal of Neurology, Neurosurgery, and Psychiatry, 85(9), 1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Dols A, Laforce R, Devenney E, Kumfor F, Van Den Stock J, et al. (2020). Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain: a Journal of Neurology, 143(6), 1632–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente A, Sedeño L, Vignaga SS, Ellmann C, Sonzogni S, Belluscio L, et al. (2019). Multimodal neurocognitive markers of interoceptive tuning in smoked cocaine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 44(8), 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Northoff G, Böker H, Seifritz E, & Grimm S (2013). Interoceptive awareness enhances neural activity during empathy. Human brain mapping, 34(7), 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi S, Abrevaya S, de la Fuente A, Pascariello GO, Hesse E, Birba A, et al. (2020). A multidimensional and multi-feature framework for cardiac interoception. Neuroimage, 212, Article 116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima H, Terasawa Y, & Umeda S (2011). Association between interoception and empathy: Evidence from heartbeat-evoked brain potential. International Journal of Psychophysiology, 79(2), 259–265. [DOI] [PubMed] [Google Scholar]
- García-Cordero I, Esteves S, Mikulan EP, Hesse E, Baglivo FH, Silva W, et al. (2017). Attention, in and out: Scalp-level and intracranial EEG correlates of interoception and exteroception. The Florida Nurse, 11, 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cordero I, Sedeño L, De La Fuente L, Slachevsky A, Forno G, Klein F, et al. (2016). Feeling, learning from and being aware of inner states: Interoceptive dimensions in neurodegeneration and stroke. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), Article 20160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Boucher O, Houde J-C, Descoteaux M, … Nguyen DK (2018). Subcortical structural connectivity of insular subregions. Scientific reports, 8(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Campo C, Salamone PC, Rodríguez-Arriagada N, Richter F, Herrera E, Bruno D, et al. (2020). Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities. Multiple Sclerosis: Clinical and Laboratory Research, 26(14), 1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sturm VE, Ascher EA, Shdo SM, Miller BL, Rankin KP, et al. (2015). Emotion recognition in frontotemporal dementia and Alzheimer’s disease: A new film-based assessment. Emotion, 15(4), 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray HM, & Tickle-Degnen L (2010). A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychology, 24(2), 176. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, & Von Hippel C (2014). A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia, 56, 53–62. [DOI] [PubMed] [Google Scholar]
- Hsieh S, Schubert S, Hoon C, Mioshi E, & Hodges JR (2013). Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 36(3–4), 242–250. [DOI] [PubMed] [Google Scholar]
- Hutchings R, Palermo R, Bruggemann J, Hodges JR, Piguet O, & Kumfor F (2018). Looking but not seeing: Increased eye fixations in behavioural-variant frontotemporal dementia. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior, 103, 71–81. [DOI] [PubMed] [Google Scholar]
- Ibañez A (2018). Brain oscillations, inhibition and social inappropriateness in frontotemporal degeneration. Brain: a Journal of Neurology, 141(10), e73. e73. [DOI] [PubMed] [Google Scholar]
- Ibañez A (2019). Insular networks and intercognition in the wild. Cortex; a journal devoted to the study of the nervous system and behavior, 115, 341–344. [DOI] [PubMed] [Google Scholar]
- Ibañez A, & Manes F (2012). Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology, 78(17), 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, & Schulte M (2020). Situated minds: Conceptual and emotional blending in neurodegeneration and beyond. Brain: a Journal of Neurology, 143(12), 3523–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W (1884). What is emotion?.
- James W (1894). The physical basis of emotion.
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. (2018). Interoception and mental health: A roadmap. Biological psychiatry: cognitive neuroscience and neuroimaging, 3(6), 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Hazelton JL, Rushby JA, Hodges JR, & Piguet O (2019). Facial expressiveness and physiological arousal in frontotemporal dementia: Phenotypic clinical profiles and neural correlates. Cognitive, Affective & Behavioral Neuroscience, 19(1), 197–210. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Ibañez A, Hutchings R, Hazelton JL, Hodges JR, & Piguet O (2018). Beyond the face: How context modulates emotion processing in frontotemporal dementia subtypes. Brain: a Journal of Neurology, 141(4), 1172–1185. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, & Piguet O (2013). Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. Plos One, 8(6), Article e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, & Piguet O (2014a). Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Frontiers in behavioral neuroscience, 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Miller L, Lah S, Hsieh S, Savage S, Hodges JR, et al. (2011). Are you really angry? The effect of intensity on facial emotion recognition in frontotemporal dementia. Social Neuroscience, 6(5–6), 502–514. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Sapey-Triomphe L-A, Leyton CE, Burrell JR, Hodges JR, & Piguet O (2014b). Degradation of emotion processing ability in corticobasal syndrome and Alzheimer’s disease. Brain: a Journal of Neurology, 137(11), 3061–3072. 10.1093/brain/awu246 [DOI] [PubMed] [Google Scholar]
- Landin-Romero R, Kumfor F, Leyton CE, Irish M, Hodges JR, & Piguet O (2017). Disease-specific patterns of cortical and subcortical degeneration in a longitudinal study of Alzheimer’s disease and behavioural-variant frontotemporal dementia. Neuroimage, 151, 72–80. [DOI] [PubMed] [Google Scholar]
- Legaz A, Abrevaya S, Dottori M, Campo CG, Birba A, Caro MM, et al. (2021). Multimodal mechanisms of human socially reinforced learning across neurodegenerative diseases. Brain: a Journal of Neurology, 145(3), 1052–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Hardy CJ, Russell LL, Clark CN, Dick KM, Brotherhood EV, et al. (2017). Impaired interoceptive accuracy in semantic variant primary progressive aphasia. The Florida Nurse, 8, 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. (2011). The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimer’s & dementia, 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, & Ashburner J (2005). Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging, 1(2), 105–113. [Google Scholar]
- Mendez MF (2021). Frontotemporal dementia: A window to alexithymia. The Journal of Neuropsychiatry and Clinical Neurosciences, 33(2), 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Hsieh S, Lah S, Savage S, Hodges JR, & Piguet O (2012). One size does not fit all: Face emotion processing impairments in semantic dementia, behavioural-variant frontotemporal dementia and alzheimer’s disease are mediated by distinct cognitive deficits. Behavioural neurology, 25(1), 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Mioshi E, Savage S, Lah S, Hodges JR, & Piguet O (2013). Identifying cognitive and demographic variables that contribute to carer burden in dementia. Dementia and Geriatric Cognitive Disorders, 36(1–2), 43–49. [DOI] [PubMed] [Google Scholar]
- Mioshi E, Hsieh S, Savage S, Hornberger M, & Hodges JR (2010). Clinical staging and disease progression in frontotemporal dementia. Neurology, 74(20), 1591–1597. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. [DOI] [PubMed] [Google Scholar]
- Nichols TE, & Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: A primer with examples. Human brain mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA (2004). Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current opinion in neurobiology, 14(2), 198–202. [DOI] [PubMed] [Google Scholar]
- Piguet O (2011). Eating disturbance in behavioural-variant frontotemporal dementia. Journal of Molecular Neuroscience: MN, 45(3), 589–593. [DOI] [PubMed] [Google Scholar]
- Piguet O, & Kumfor F (2020). Frontotemporal dementias: Main syndromes and underlying brain changes. Current Opinion in Neurology, 33(2), 215–221. 10.1097/wco.0000000000000792 [DOI] [PubMed] [Google Scholar]
- Piguet O, Kumfor F, & Hodges J (2017). Diagnosing, monitoring and managing behavioural variant frontotemporal dementia. Medical Journal of Australia, 207(7), 303–308. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Movement disorders, 30(12), 1591–1601. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Piguet O, & Irish M (2018). Rethinking the role of the angular gyrus in remembering the past and imagining the future: The contextual integration model. The Neuroscientist: a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 24(4), 342–352. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain: a Journal of Neurology, 134(9), 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi L, Ferrazzano G, Demartini B, Morgante F, Erro R, Ganos C, et al. (2016). Know thyself: Exploring interoceptive sensitivity in Parkinson’s disease. Journal of the neurological sciences, 364, 110–115. [DOI] [PubMed] [Google Scholar]
- Richter F, García AM, Rodriguez Arriagada N, Yoris A, Birba A, Huepe D, et al. (2021). Behavioral and neurophysiological signatures of interoceptive enhancements following vagus nerve stimulation. Human brain mapping, 42(5), 1227–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, & Hawkes DJ (1999). Nonrigid registration using free-form deformations: Application to breast MR images. IEEE transactions on medical imaging, 18(8), 712–721. [DOI] [PubMed] [Google Scholar]
- Salamone PC, Legaz A, Sedeño L, Moguilner S, Fraile-Vazquez M, Campo CG, et al. (2021). Interoception primes emotional processing: Multimodal evidence from neurodegeneration. Journal of Neuroscience, 41(19), 4276–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone PC, Sedeño L, Legaz A, Bekinschtein T, Martorell M, Adolfi F, et al. (2020). Dynamic neurocognitive changes in interoception after heart transplant. Brain communications, 2(2). fcaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo G, Vitale C, Baiano C, D’Iorio A, Longo K, Barone P, et al. (2018). Interoceptive processing deficit: A behavioral marker for subtyping Parkinson’s disease. Parkinsonism & Related Disorders, 53, 64–69. [DOI] [PubMed] [Google Scholar]
- Schachter S, & Singer J (1962). Cognitive, social, and physiological determinants of emotional state. Psychological review, 69(5), 379. [DOI] [PubMed] [Google Scholar]
- Schulz SM (2016). Neural correlates of heart-focused interoception: A functional magnetic resonance imaging meta-analysis. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), Article 20160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, et al. (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Archives of neurology, 65(2), 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML (2013). The angular gyrus: Multiple functions and multiple subdivisions. The Neuroscientist: a Review Journal Bringing Neurobiology, Neurology and Psychiatry, 19(1), 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208eS219. [DOI] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, et al. (2009). Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia, 47(13), 2812–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikwerda-Brown C, Ahmed RM, Piguet O, & Irish M (2022). Try to see it my way–Examining the relationship between visual perspective taking and theory of mind in frontotemporal dementia. Brain and Cognition, 157, Article 105835. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, & Levenson RW (2008). Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion, 8(6), 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa Y, Moriguchi Y, Tochizawa S, & Umeda S (2014). Interoceptive sensitivity predicts sensitivity to the emotions of others. Cognition & Emotion, 28(8), 1435–1448. [DOI] [PubMed] [Google Scholar]
- Torres Mendonça De Melo Fádel B, Santos De Carvalho RL, Belfort Almeida Dos Santos TT, & Dourado MCN (2019). Facial expression recognition in Alzheimer’s disease: A systematic review. Journal of clinical and experimental neuropsychology, 41(2), 192–203. [DOI] [PubMed] [Google Scholar]
- Van den Stock J, & Kumfor F (2017). Behavioural variant frontotemporal dementia: At the interface of interoception, emotion and social cognition. Cortex; a Journal Devoted To the Study of the Nervous System and Behavior, 115, 335–340. [DOI] [PubMed] [Google Scholar]
- Villani V, Tsakiris M, & Azevedo R (2019). Transcutaneous vagus nerve stimulation improves interoceptive accuracy. Neuropsychologia, 134, Article 107201. [DOI] [PubMed] [Google Scholar]
- Weng HY, Feldman JL, Leggio L, Napadow V, Park J, & Price CJ (2021). Interventions and manipulations of interoception. Trends in neurosciences, 44(1), 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjaǹska M, Doyon J, et al. (2014). GABA in the insula—a predictor of the neural response to interoceptive awareness. Neuroimage, 86, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Gorno-Tempini M-L, Seeley WW, Rankin K, Lee SS, Matthews BR, et al. (2007). Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology, 69(14), 1424–1433. 10.1212/01.wnl.0000277461.06713.23 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. (2009). Bayesian analysis of neuroimaging data in FSL. Neuroimage, 45(1), S173eS186. [DOI] [PubMed] [Google Scholar]
- Yoris A, Legaz A, Abrevaya S, Alarco S, Peláez JL, Sánchez R, et al. (2020). Multicentric evidence of emotional impairments in hypertensive heart disease. Scientific reports, 10(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE T Med Imaging, 20, 45–57 (In: English). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The conditions of our ethics approval do not permit public archiving of the data supporting this study. Readers seeking access to this data should contact the senior author, Fiona Kumfor to discuss a formal data sharing agreement.


