Abstract
Amphotropic murine leukemia virus (A-MuLV) utilizes the Pit-2 sodium-dependent phosphate transporter as a cell surface receptor to infect mammalian cells. Previous studies established that infection of cells with A-MuLV resulted in the specific down-modulation of phosphate uptake mediated by Pit-2 and in resistance to superinfection with A-MuLV. To study the mechanisms underlying these phenomena, we constructed plasmids capable of efficiently expressing ɛ epitope- and green fluorescent protein (GFP)-tagged human Pit-2 proteins in mammalian cells. Overexpression of ɛ-epitope-tagged Pit-2 transporters in NIH 3T3 cells resulted in a marked increase in sodium-dependent Pi uptake. This increase in Pi uptake was specifically blocked by A-MuLV infection but not by infection with ecotropic MuLV (E-MuLV) (which utilizes a cationic amino acid transporter, not Pit-2, as a cell surface receptor). These data, together with the finding that the tagged Pit-2 transporters retained their A-MuLV receptor function, indicate that the insertion of epitope tags does not affect either retrovirus receptor or Pi transporter function. The overexpressed epitope-tagged transporters were detected in cell lysates, by Western blot analysis using both ɛ-epitope- and GFP-specific antibodies as well as with Pit-2 antiserum. Both the epitope- and GFP-tagged transporters showed almost exclusive plasma membrane localization when expressed in NIH 3T3 cells, as determined by laser scanning confocal microscopy. Importantly, when NIH 3T3 cells expressing these proteins were productively infected with A-MuLV, the tagged transporters and receptors were no longer detected in the plasma membrane but rather were localized to a punctate structure within the cytosolic compartment distinct from Golgi, endoplasmic reticulum, endosomes, lysosomes, and mitochondria. The intracellular Pit-2 pool colocalized with the virus in A-MuLV-infected cells. A similar redistribution of the tagged Pit-2 proteins was not observed following infection with E-MuLV, indicating that the redistribution of Pit-2 is not directly attributable to general effects associated with retroviral infection but rather is a specific consequence of A-MuLV–Pit-2 interactions.
The amphotropic murine leukemia virus (A-MuLV) has the ability to infect a variety of mammalian cell lines. This broad tropism together with its relatively simple organization has made this retrovirus a particularly promising vector for gene therapy. Although A-MuLV-derived vectors are now broadly used for gene therapy purposes (1, 4, 30), very little is known about the biology of their receptor. Cell surface receptors for A-MuLV have been cloned (17, 24, 29) and demonstrated to serve as sodium-dependent phosphate (Na+/Pi) transporters in the normal physiology of diverse cell types (12, 28). Based on their structural and functional characteristics, these molecules, together with the gibbon ape leukemia virus (GALV) receptor, were classified as type III Na+/Pi transporters (11) and were designated Pit-2 and Pit-1, respectively (9, 12).
The activity and protein levels of the Pit-2 phosphate transporter/viral receptor are highly regulated in cells. Pit-2-mediated Na+/Pi uptake can be specifically blocked by infection of cells with A-MuLV (28), and expression of amphotropic envelope protein (Env) in murine cells also has been shown to inhibit phosphate transport (12). A similar loss of the Pit-1 transporter functions has been described for GALV-infected cells (19). Phosphate concentration changes also have been shown to regulate Pi uptake activity. Depletion of extracellular phosphate was found to increase Pit-2 and Pit-1 expression three- to fivefold in fibroblasts (12). Moreover, removal of phosphate from the culture media was shown to both increase the amount of Pit-2 mRNA and the quantity of a 71-kDa protein specifically recognized by antibodies against Pit-2. This increase in Pit-2 mRNA levels observed in response to Pi depletion appeared to be regulated not at a transcriptional but rather at a posttranscriptional level due to enhanced mRNA stability (6). In a more recent study carried out with CHO cells, the levels of Pit-2 at the cell surface remained unchanged following variations of the phosphate supply, but the efficiency of phosphate uptake and retrovirus entry was found to be inversely related to the extracellular phosphate concentration (22). These results suggested that Pit-2 activities may be modulated by posttranslational modifications of the cell surface Pit-2 proteins in response to changes in phosphate concentration and that such modifications are required to activate phosphate transporter and retrovirus receptor functions. In addition, our earlier studies established that activation of protein kinase C (PKC) by treatment of cells with phorbol 12-myristate 13-acetate (PMA) enhanced Na+/Pi uptake (18). More recent studies have established that PMA treatment of cells enhances Na+/Pi uptake via stimulation of Pit-2 and that this effect is specifically mediated through PMA activation of the PKCɛ isoform (10).
Cells infected by retroviruses display a strong resistance to superinfection by viruses that utilize the same receptor as the preinfecting virus but retain susceptibility to viruses that use a different receptor. This phenomenon, termed superinfection interference, is thought to arise from interaction of the viral envelope protein with the receptor (7). However, the level and site of this interaction remain obscure. While superinfection and receptor down-regulation phenomena are widely recognized, and methods based on viral superinfection interference are commonly used in murine retrovirus research (8, 16), the mechanisms underlying the loss of transporter and receptor functions are largely unknown (7). To determine the fate of Pit-2 in A-MuLV-producing infected and control uninfected cells, we constructed plasmids capable of efficiently expressing ɛ-epitope- and green fluorescent protein (GFP)-tagged human Pit-2 proteins in mammalian cells. The results presented in this report demonstrate that the tagged Pit-2 receptors are localized to the plasma membrane in uninfected NIH 3T3 cells. However, when NIH 3T3 cells expressing these tagged proteins are infected with A-MuLV, the tagged receptors are no longer detectable in the plasma membrane but are found redistributed to punctate structures within the cytosolic compartment. This loss of Pit-2 viral receptors from the cell membrane apparently is responsible for superinfection interference induced with A-MuLV infection.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium (DMEM) and fetal calf serum were purchased from Biofluids Inc. (Rockville, Md.). PMA was purchased from Calbiochem (San Diego, Calif.). 32P-labeled monopotassium phosphate was obtained from ICN (Costa Mesa, Calif.). The pEGFP-N1 enhanced GFP (EGFP) tagging vector is available from Clontech (Palo Alto, Calif.), and the Xpress protein expression system (pTrcHis) and plasmids coding for endoplasmic reticulum (ER)- and mitochondrion-targeted GFP were obtained from Invitrogen (San Diego, Calif.). The early endosomal protein (EEA1) fragment (amino acids 1252 to 1411)-EGFP chimera was a gift from Tamás Balla (National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md.). The pɛMTH expression vector was developed in our laboratory as described previously (20). LysoTracker DND-99, Texas red-conjugated transferrin, Alexa 488 phalloidin, and dextran 10 were purchased from Molecular Probes, Inc. (Eugene, Oreg.).
Primary antibodies.
PKCɛ isotype-specific polyclonal antibodies (Gibco BRL, Gaithersburg, Md.) were used to detect ɛ-epitope-tagged Pit-2 proteins. Anti-GFP polyclonal antibody was purchased from Chemicon International Inc. (Temecula, Calif.). The Pit-2-specific rabbit antiserum was produced in our laboratory using a recombinant HaPit-2 (the hamster homolog of Pit-2) cytoplasmic loop fragment (amino acids 272 to 462) as antigen (29). The bacterial recombinant vector, pTrcHisA-EARcA21, expressing this fragment was overexpressed in Escherichia coli and then affinity purified using a His-Ni2+ column (Novagen, Madison, Wis.). One and one-half milligrams of the approximately 95% pure cytosolic loop fragment protein was used as antigen for immunization of two rabbits. A-MuLV pig antiserum (lot no. 77S000445) and purified goat anti-Rausch leukemia virus gp69/71 antibodies (lot no. 80S000018) were obtained from the NCI/BCB Reagents Repository (Camden, N.J.).
Secondary antibodies.
Cy3-conjugated anti-rabbit and anti-mouse immunoglobulin (IgG), fluorescein-conjugated anti-pig IgG, and Texas red-conjugated anti-goat IgG secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, Pa.). Peroxidase-labeled goat anti-rabbit and anti-mouse IgGs were from Kirkegaard & Perry Laboratories, Inc. (Gaithersburg, Md.).
Cell culture.
Retrovirus-infected and vector-transduced NIH 3T3 cells were cultured in DMEM supplemented with 10% fetal calf serum. After the cells reached confluency, the medium was changed to serum-free DMEM for 24 h. To induce overexpression of ɛ-epitope-tagged ectopic gene products, transfected cells were incubated in the presence or absence of 20 μM zinc acetate, as indicated, to induce the up-regulation of the metallothionein promoter of pɛMTH (20).
DNA constructs. (i) Primers.
The PCR primers used were as follows: 1, GAGGTCGACATGGCCATGGATGAGTATTTGTGG; 2, TCCTACTTTGGTGAAGACCTGATGCCCACAGGCAAATTACAAAAAGAAGGTGC; 3, GTCTTCACCAAAGTAGGAGAAGCCTTTTATTTTCCTCCGCATCCACGG; 4, GTATACGCGTCACATATGGAAGGATCCCATACATG; 5, GAAGCCCCGGGCCACATATGGAAGGATCCCATACATG. Recognition sites for restriction endonucleases used for cloning are underlined; overlapping sequences are italicized.
(ii) pTrcHisA-EARcA21.
The SacI-BamHI restriction fragment of hamster Pit-2 (EAR, HaPit-2) was ligated into the pTrcHisA bacterial expression vector cut with SacI-BglII.
(iii) pPit2ɛ-ɛMTH.
Insertion of the ɛ-epitope tag into the cytoplasmic loop of Pit-2 was accomplished in two steps by overlapping PCR using primer pairs 1-2 and 3-4 in the first rounds of PCRs. The products of these reactions then were purified, annealed, and used as template for a final PCR using primers 1 and 4. The purified product was cut with SalI-MluI and ligated into the pɛMTH vector cut with XhoI-MluI. A second ɛ-epitope tag was added at the C-terminal end of this cytosolic loop-tagged Pit-2ɛ, with insertion and expression of this construct in the pɛMTH vector.
(iv) pPit2-EGFPN1.
Construction of human Pit-2 C-terminally tagged with GFP was accomplished using primers 1 and 5 to amplify human Pit-2 cDNA. The PCR-amplified product was cut with restriction endonucleases SalI-XmaI and then ligated into the pEGFP-N1 vector.
Generation of overexpressor cell lines.
NIH 3T3 cells were transfected with either the control expression vector pɛMTH or pPit2ɛ-ɛMTH, expressing the ɛ-epitope-tagged Pit-2, using electroporation as previously described (10). Stably transfected cell lines were selected with G418 (0.8 mg/ml). Individually picked colonies (10 from each transfection) were selected and pooled for further studies to minimize potential artifacts attributable to anomalies associated with special clones. The mixed populations of stable Pit-2ɛɛ overexpressor cells were used only through 12 to 14 passages in culture to negate possible outgrowth of one particular population of cells. pɛMTH contains a Zn2+-inducible promoter. Thus, pɛMTH v-transfected cells were incubated in the presence and absence of 20 μM zinc acetate, as noted, to induce synthesis of the indicated recombinant proteins. NIH 3T3 and CHO-K1 cells also were transiently transfected by electroporation with either the control vector pEGFP-N1 or the pPit2-EGFPN1 expression vector. Cells transiently expressing Pit-2–GFP were used for further studies 24 h after transfection.
Phosphate uptake measurement.
Na+/Pi uptake was determined as described previously (18).
Retrovirus infections.
NIH 3T3 fibroblasts were maintained in DMEM supplemented with 10% (vol/vol) fetal bovine serum and were infected with wild-type A-MuLV strain 4070A or with ecotropic Moloney MuLV as previously described (10, 19). Productive infection was monitored by measuring the reverse transcriptase activity found in media of the infected cells (27) and/or by superinfection interference studies. Cells productively infected with A-MuLV were demonstrated to be resistant to challenge infection with A-MuLV envelope vectors but not with E-MuLV envelope vectors. Cells productively infected with E-MuLV were resistant to E-MuLV envelope vectors but retained susceptibility to A-MuLV infectivity.
Western blot analysis.
For protein extraction, cells were washed with ice-cold phosphate-buffered saline (PBS), harvested by scraping into lysis buffer (20 mM Tris-HCl [pH 7.4], 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 20 mM leupeptin), and disrupted in a Dounce homogenizer. Cell homogenates were fractionated by differential centrifugation into nuclear (pellet of 800 × g centrifugation for 10 min), particulate (plasma membrane-enriched; 16,000 × g, 1-h pellet of the nucleus-free 800 × g supernatant), and cytosolic (16,000 × g supernatant) fractions. Proteins of each fraction were separated by precast sodium dodecyl sulfate–4 to 20% polyacrylamide gel (Owl Separation Systems, Portsmouth, N.H.) electrophoresis and electrophoretically transferred from the gel onto Protran membranes (Schleicher & Schuell, Keene, N.H.); immunoreactive proteins were detected as described elsewhere (20).
Laser scanning confocal microscopy.
NIH 3T3 cells were grown on glass multichamber slides. Vector control cells and cells overexpressing Pit-2ɛɛ (human Pit-2 protein double tagged with the ɛ epitope) were fixed in 4% buffered formaldehyde solution (Sigma) for 30 min at 4°C and then blocked with PBS containing 1% bovine serum albumin (BSA) and 0.6% Triton X-100 for 1 h at room temperature. Immunostaining was carried out with the indicated primary antibodies in the same PBS solution for 2 to 3 h at room temperature. After three 5-min washes in PBS, the appropriate secondary antibodies were applied for 1 h at room temperature and washed with PBS. The slides then were mounted with Vectashield antifade reagent (Vector Laboratories, Burlingame, Calif.) and covered with glass coverslips. Cells transiently overexpressing Pit-2–EGFP were labeled with cell organelle markers and mounted without fixation. For the LysoTracker, dextran 10, and transferrin receptor localization experiments, NIH 3T3 fibroblasts transiently transfected with pPit2-EGFPN1 by electroporation were plated on glass chamber slides and labeled with the markers 24 h posttransfection. Lysosomes were labeled with LysoTracker Red DND-99 according to the manufacturer's suggestions. To visualize the recycling endosome pool, cells were washed three times with serum-free DMEM and then incubated in the same medium containing 20 μg of Texas red-conjugated transferrin per ml for 30 min. The cells then were washed, chased with unlabeled transferrin (20 μg/ml) for 30 min, and mounted for fluorescence microscopy as described above. For labeling the late endosomal compartment, cells were incubated with 1 mg of Texas red-labeled dextran 10 per ml for 45 min, rinsed with medium, and incubated in fresh medium for 60 min to wash out the dextran prior to imaging. Confocal fluorescent images were collected with a Bio-Rad MRC 1024 confocal scan head mounted on a Nikon Optiphot microscope with a 40× or 60× planapochromat lens. A krypton-argon gas laser provided excitation at 488 and 568 nm. Emission filters of 598/40 and 522/32 were used for collecting red and green fluorescence, respectively, in channels 1 and 2, while phase-contrast images of the same cell were collected in the third channel using a transmitted light detector. After sequential excitation, red and green fluorescent images of the same cell were merged for colocalization of GFP with cellular organelle markers.
RESULTS AND DISCUSSION
ɛ-Epitope-tagged human Pit-2 overexpressed in NIH 3T3 fibroblasts and GFP-tagged human Pit-2 overexpressed in CHO-K1 cells retain their functional and regulatory properties.
PCR products of Pit-2 were produced and then cloned into either the pɛMTH or pEGFP-N1 vector as described in Materials and Methods. The resulting Pit2ɛ-ɛMTH construct (Fig. 1A) was used to transfect NIH 3T3 cells, and cell lines stably overexpressing Pit-2ɛɛ were selected. Overexpression of Pit-2ɛɛ in NIH 3T3 fibroblasts resulted in only a slight increase in Pi uptake. However, following Zn2+ induction of the metallothionein promoter to enhance expression of the Pit-2ɛɛ protein, Pi uptake was significantly increased (160%) compared to vector control. Moreover, ɛ-epitope-tagged Pit-2 transporters retained the ability to be up-regulated by PMA, as demonstrated by a proportional increase in Pi uptake detected with the tagged Pit-2 transporters following PMA treatment of the overexpressor cells (Fig. 1C). A-MuLV infection of the Pit-2ɛɛ overexpressor cells resulted in a significant (60%) decrease in Pi uptake activity, indicating that the Pi transporter activity of the tagged Pit-2 molecules was blocked by A-MuLV infection in a manner similar to that observed with the native or wild-type Pit-2 transporters (Fig. 1D). CHO-K1 cells normally are resistant to A-MuLV infection due to the presence of inhibitors secreted by CHO-K1 cells that can inhibit endogenous HaPit-2 receptor function (5). This resistance can be overcome by expressing the human form of the Pit-2 transporters/receptors in CHO-K1 cells. To test the Pit-2–EGFP chimera (Fig. 1B) for A-MuLV receptor function, we transiently transfected plasmid pPit2-EGFP into CHO-K1 cells and then infected the transiently transfected cells with A-MuLV vectors carrying a β-galactosidase reporter gene as described elsewhere (5). CHO-K1 cells transfected with pPit2-EGFP were found to be sensitive to A-MuLV, as indicated by the formation of blue foci in response to expression of the β-galactosidase reporter gene. No blue foci were detected in CHO-K1 cells transfected with the control pEGFP-N1 and A-MuLV vectors (data not shown). As expected, since CHO-K1 cells are resistant to A-MuLV infection, the virus titer obtained for A-MuLV vectors on CHO-K1 control was 0 infectious unit/ml. The titer obtained with CHO-K1 cells expressing Pit-2 was 2,000 infectious units/ml, which compared to a value of 1,800 infectious units/ml with CHO-K1 cells expressing epitope-tagged Pit-2. Further, expression of the Pit-2–EGFP chimera significantly increased Pi uptake compared to vector control, and PMA treatment enhanced Pit-2–EGFP-mediated Pi transport (data not shown). These results indicate that the ɛ-epitope-tagged Pit-2 and the Pit-2–EGFP chimera both retain A-MuLV receptor function and that both Pit-2ɛɛ and Pit-2–EGFP retain all of the functional and regulatory characteristics of wild-type Pit-2 phosphate transporter activity.
FIG. 1.
Functional and regulatory properties of recombinant human Pit-2 proteins Pit-2ɛɛ (A) and Pit-2–GFP (B). Short-term (2-min) Pi transport was determined in control pɛMTH and pPit2ɛ-ɛMTH-transfected NIH 3T3 fibroblasts as indicated in Materials and Methods. Cells were grown in serum-free medium with 20 μM zinc acetate to up-regulate the metallothionein promoter and then treated with 1 μM PMA for 10 min as indicated (C). (D) Pi uptake of pɛMTH vector- and pPit2ɛ-ɛMTH vector-transfected fibroblasts, as well as in these transfected cells productively infected with A-MuLV. Each column represents the mean ± standard error of the mean of three independent experiments performed in duplicate.
Western blot analysis of overexpressed ɛ-epitope- and GFP-tagged human Pit-2 phosphate transporter/retrovirus receptor proteins in NIH 3T3 cell lysates.
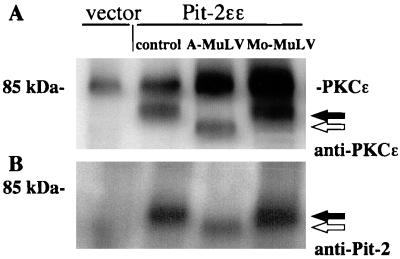
Immunostaining of protein blots of a plasma membrane enriched particulate fraction prepared from Pit-2ɛɛ overexpressor cells with anti-ɛ antibody revealed an immunoreactive 70-kDa band (Fig. 2A, lane 2). Interestingly, the ɛ-epitope-tagged Pit-2 protein detected in the 16,000 × g particulate fraction prepared from A-MuLV-infected cells was found to migrate as a lower-molecular-mass protein of about 60 kDa (Fig. 2A, lane 3). The change in Pit-2 receptor migration rate was specific for A-MuLV-infected cells. Pit-2 detected in the particulate fraction prepared from cells infected with ecotropic Moloney MuLV (which binds to the cationic amino acid transporter, M-CAT1), migrated at a molecular weight similar to that observed with the uninfected control cells (Fig. 2A, lane 4). No immunoreactive Pit-2ɛɛ band was detected in the protein sample prepared from the vector control cell line (Fig. 2A, lane 1). Endogenous PKCɛ was detected with the anti-PKCɛ antibody as a 90-kDa band and served as an internal control of the amount of protein loaded per lane. Western blot analysis using anti-HaPit-2 rabbit antiserum in place of the ɛ-tag antibody resulted in a similar pattern (Fig. 2B). Again, Pit-2 was found to migrate as a lower-molecular-weight band in A-MuLV-infected NIH 3T3 cells compared to uninfected and ecotropic Moloney MuLV-infected cells. These results indicate that infection of NIH 3T3 cells expressing epitope-tagged Pit-2 with A-MuLV (which utilizes Pit-2 as its cell surface receptor) correlates with the presence of a more rapidly migrating form of Pit-2 and results in the loss of Pi transporter function. One explanation for this observation is that the Pit-2 present in A-MuLV-infected cells is more susceptible to a peptidase, which may catalyze the proteolytic processing of Pit-2. A second possibility is that the band shift noted with Pit-2 concomitant with A-MuLV superinfection may be due to the blockage of required posttranslational modifications such as phosphorylation and/or glycosylation which may take place before or at the time of Pit-2 trafficking to the plasma membrane. In a related study using Moloney MuLV-infected NIH 3T3 cells, the MCAT-1 ecotropic virus receptor was observed to also migrate as a lower-molecular-weight protein (13). This increase in electrophoretic mobility noted with MCAT-1 from infected cells was found to be due to a blockage of normal N-linked glycosylation. It was proposed that binding of newly synthesized ecotropic virus envelope surface protein, gp70, with the MCAT-1 receptor in the ER acted to prevent normal glycosylation of MCAT-1 (13).
FIG. 2.
Western blot analysis of cell lysates prepared from ɛ-epitope-tagged Pit-2 overexpressor fibroblasts productively infected with A-MuLV and E-MuLV. A 16,000 × g plasma membrane-enriched particulate fraction was prepared as described in Materials and Methods from control vector-transfected and Pit-2ɛɛ overexpressor fibroblasts, either uninfected or infected with A-MuLV or E-MuLV, as indicated. ɛ-Tag antibodies (1:2,000) (A) and rabbit Pit-2 antiserum (1:500) (B) were used to detect Pit-2 in the particulate fraction of stable overexpressor cell lines. Closed arrows indicate Pit-2-specific bands in uninfected and E-MuLV-infected cells; open arrows indicate Pit-2 specific bands in A-MuLV-infected cells.
Subcellular redistribution of Pit-2ɛɛ in NIH 3T3 cells productively infected with A-MuLV.
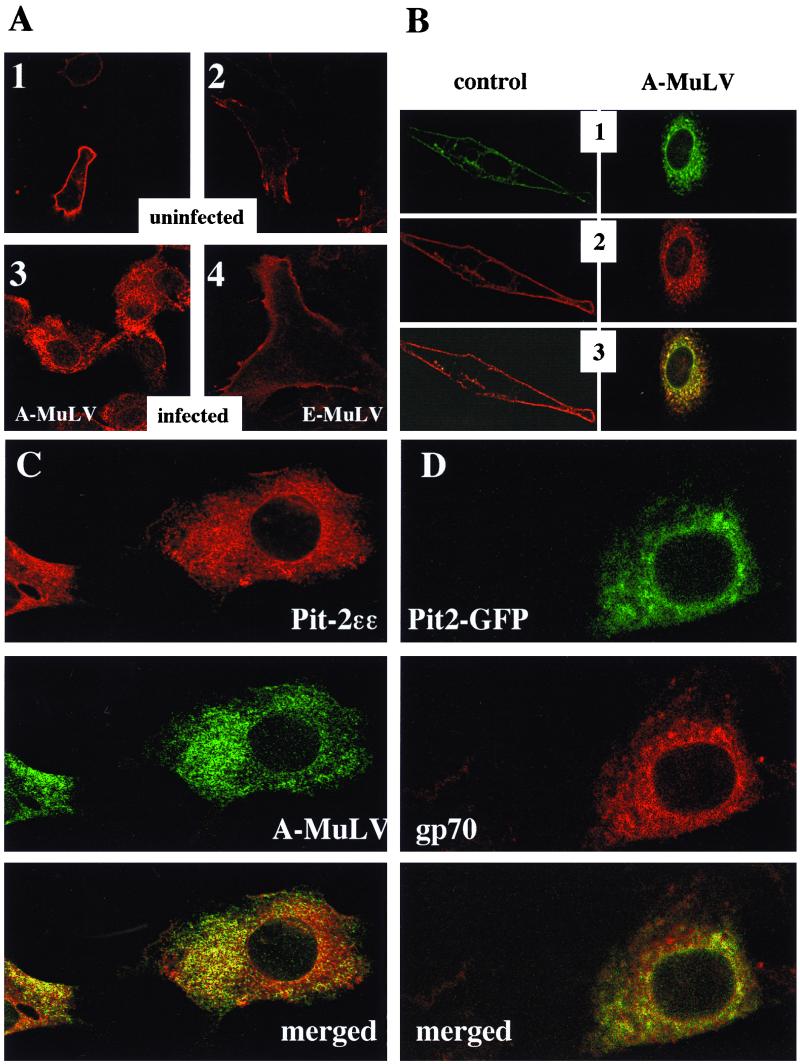
Fluorescence laser confocal microscopy was used to provide optical sectioning of cells. Basically, the image obtained with confocal laser microscopy is equivalent to a thin section across the entire length or width of the cell and does not represent the cell as a whole. The results obtained revealed that Pit-2ɛɛ localized mainly to the plasma membrane when overexpressed in control NIH 3T3 cells (Fig. 3A, 1 and 2; Fig. 3B, 2 control). In addition, faint staining also was detected at a perinuclear location in some of those uninfected cells. This may represent a Golgi-localized pool of newly synthesized Pit-2ɛɛ. Likewise, Pit-2–GFP transiently coexpressed in the Pit-2ɛɛ overexpressor cells also exhibited plasma membrane localization (Fig. 3B, 1 control). Importantly, the epitope-tagged Pit-2 was not detected at the plasma membrane following productive infection of the overexpressor cells with A-MuLV; rather, it was found predominantly redistributed to an unidentified intracellular location (Fig. 3A, 3; Fig. 3B, 2 A-MuLV). Some staining was detected at an apparent nuclear membrane location in A-MuLV-infected cells. A similar pattern of Pit-2–GFP redistribution was observed when A-MuLV producer Pit-2ɛɛ overexpressor cells were transiently transfected with the pPit2-EGFPN1 construct (Fig. 3B, 1 A-MuLV). Merging of Fig. 3B pictures 1 and 2 resulted in areas of yellow signal indicating colocalization of Pit-2ɛɛ and Pit-2–GFP (Fig. 3B, 3). In both infected and uninfected NIH 3T3 fibroblasts, the ɛ-epitope-tagged Pit-2 transporters colocalized with the Pit-2–GFP chimeras, indicating that the presence of the bulky GFP protein at the C terminus did not affect the localization of Pit-2–GFP (Fig. 3B, 3). The epitope-tagged Pit-2 transporters remained localized to the plasma membrane in ecotropic Moloney MuLV (Fig. 3A, 4)- and 57A Friend MuLV (not shown)-producing cells, indicating that the observed redistribution of Pit-2 was A-MuLV specific and not attributable to general effects associated with retroviral infection.
FIG. 3.
Immunocytochemical localization of ɛ-epitope-tagged Pit-2 transporter in uninfected (A, 1 and 2), and A-MuLV-infected (A, 3), and E-MuLV-infected (A, 4) overexpressor NIH 3T3 cells. ɛ-Tag antibodies (1:1,000 dilution) and Cy3-labeled anti-rabbit IgG (1:1,000 dilution) were used as primary and secondary antibodies, respectively, to stain for overexpressed Pit-2ɛɛ transporters. Views 1 and 2 in panel B depict dual imaging of control and A-MuLV-infected cells cooverexpressing both Pit-2–GFP chimeras and Pit-2ɛɛ, respectively. The coexpressed ɛ-epitope-tagged Pit-2 and the GFP-tagged Pit-2 were detected using fluorescent labeling and confocal laser microscopy as described in Materials and Methods. Panel C represents dual immunostaining of A-MuLV producer Pit-2ɛɛ-overexpressing NIH 3T3 fibroblasts with ɛ-tag antibodies and with A-MuLV pig antiserum. Cells were prepared and fixed on glass microscope slides as described in Materials and Methods. Pit-2ɛɛ staining with ɛ-tag antibodies was carried out as described in the legend to Fig. 2. The same cells were also stained for A-MuLV, using 1:500-diluted pig antiserum and 1:1,000-diluted fluorescein-conjugated anti-pig IgG secondary antibodies. Similarly stained uninfected and untransfected control NIH 3T3 cells were prepared to establish background levels for both the red and the green signals. Panel D shows A-MuLV-infected NIH 3T3 cells transiently transfected for 24 h with the pPit2-EGFPN1 construct and then stained for A-MuLV gp70 envelope protein with 1:500-diluted goat anti-Rausch murine leukemia virus gp69/71 antibody and 1:500-diluted Texas red-conjugated anti-goat IgG secondary antibodies. The distribution of Pit-2–GFP and A-MuLV gp70 protein was visualized under laser scanning confocal microscopy as described in Materials and Methods. The merged pictures of panels C and D resulted from merging the red and green signals.
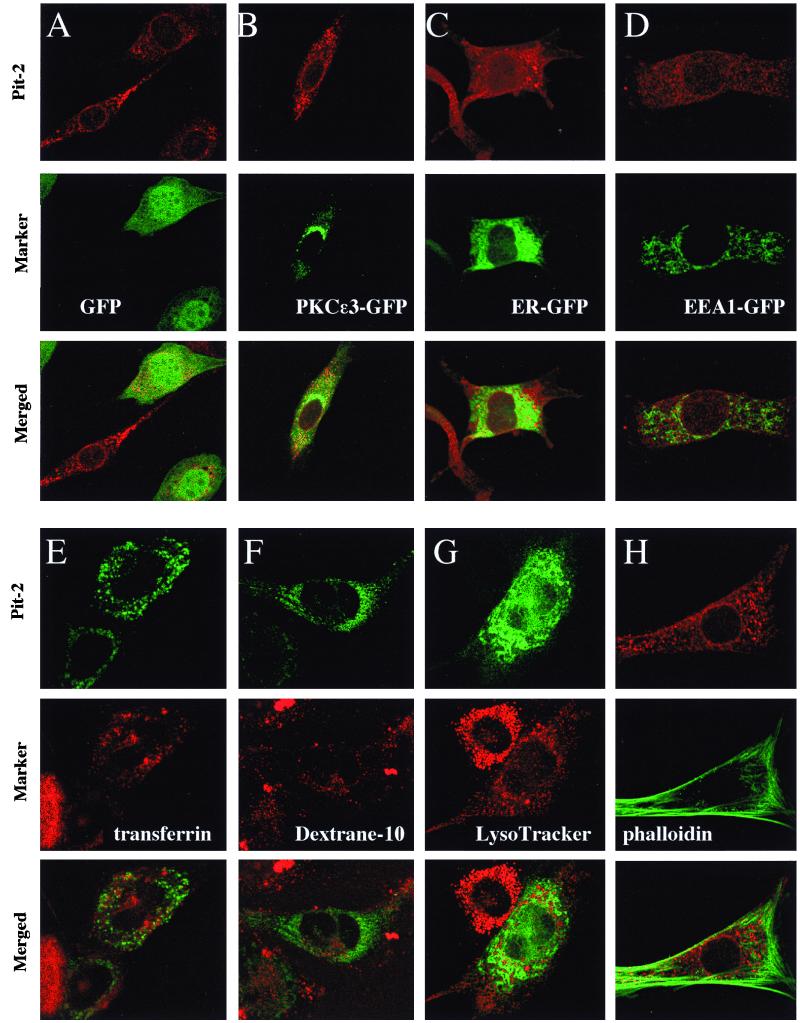
FIG. 4.
Dual immunostaining of overexpressed Pit-2 transporters with intracellular compartment markers in A-MuLV producer NIH 3T3 fibroblasts. A-MuLV producer, ɛ-epitope-tagged Pit-2 overexpressor cells were transiently transfected with pEGFP-N1 (A), pPKCɛ3-EGFPC3 (Golgi-localizing chimera) (B), pER-GFP (coding for ER-targeted GFP) (C), and pEEA1-GFP (early endosome marker) (D), fixed, and stained with anti ɛ-tag antibody as described in Materials and Methods. Alternatively A-MuLV-infected fibroblasts were transiently transfected with the pPit2-EGFP construct by electroporation and then labeled with transferrin (for recycling; endosomes) (E), dextran 10 (for late endosomes) (F), and LysoTracker (for lysosomes) (G) as detailed in Materials and Methods. A-MuLV producer, ɛ-epitope-tagged Pit-2ɛɛ overexpressor cells were fixed and double stained with ɛ-epitope antibody and fluorescein-conjugated phalloidin (for actin staining) (H). Control untransfected NIH 3T3 cells were prepared in the same manner to establish background levels for the ɛ-tag antibody signal. Top panels within each experimental set represent Pit-2; middle panels represent different intracellular compartment markers within the same cells; lower panels show the results obtained with merging of the red and green signals.
Colocalization of A-MuLV envelope protein with the overexpressed epitope-tagged Pit2 transporter/receptor in productively infected Pit-2 overexpressor NIH 3T3 cells.
To define the relationship between infection of cells with A-MuLV and the redistribution of Pit-2 receptor within the virus producer fibroblasts, double immunostaining experiments were performed with fluorescent labeling and confocal microscopy using A-MuLV-specific pig antiserum and antibodies against the ɛ-epitope tag of Pit-2ɛɛ as described in Materials and Methods. Anti-ɛ-epitope staining for Pit-2ɛɛ in the A-MuLV-infected cells using Cy3-conjugated secondary antibodies resulted in the characteristic punctate intracellular (internalized) pattern noted previously for Pit-2ɛɛ in productively infected cells (Fig. 3C, Pit-2ɛɛ). The distribution of A-MuLV within the infected NIH 3T3 cells was visualized by fluorescein-conjugated goat anti-pig IgG secondary antibodies. Uninfected cells were stained in the same way to set the background signal to zero. A-MuLV particles also appeared as disperse punctate structures throughout the cytoplasm, with denser distribution occurring in areas similar to the patterns represented by Pit-2ɛɛ (Fig. 3C, A-MuLV). Merging of the pictures resulted in areas of yellow signal indicating colocalization of Pit-2 and A-MuLV in punctate structures throughout the cytosol (Fig. 3C, merged). Alternatively, A-MuLV-infected NIH 3T3 cells were transiently transfected with the pPit2-EGFPN1 construct and stained for A-MuLV gp70 envelope protein with goat anti-Rausch murine leukemia virus gp69/71 antibody using Texas red-conjugated anti-goat IgG secondary antibodies. This goat anti-Rausch murine leukemia virus gp69/71 antibody has been shown to recognize the proline-rich region of MuLV protein, including the A-MuLV gp70 envelope protein (26). The distribution of Pit-2–GFP and A-MuLV gp70 protein was visualized under laser scanning confocal microscopy as described in Materials and Methods. Again, Pit-2–GFP showed subcellular localization similar to A-MuLV gp70, and merging the pictures resulted in yellow areas indicating colocalization of Pit-2–GFP and A-MuLV gp70 envelope protein (Fig. 3D).
Pit-2ɛɛ overexpressed in A-MuLV producer fibroblasts did not colocalize with commonly used markers for intracellular organelles.
The pattern of subcellular redistribution of Pit-2ɛɛ in A-MuLV-infected cells was similar in some respect to that reported for the human immunodeficiency virus type 1-induced redistribution of CD4 molecules to the ER (2). Therefore, we used ER-targeted GFP to determine a possible colocalization with Pit-2ɛɛ in A-MuLV-infected fibroblasts. Double-channel fluorescent laser confocal imaging of Pit-2ɛɛ-overexpressing A-MuLV producer cells showed no colocalization of Pit-2ɛɛ with the ER marker GFP (Fig. 4C). Furthermore, no colocalization of Pit-2ɛɛ was observed either with GFP alone (which exhibits both cytoplasmic and nuclear staining) (Fig. 4A) or with a PKCɛ zinc finger fragment 3-GFP chimera (which localizes predominantly to the Golgi complex [14]) (Fig. 4B). To test the possibility that a modified Pit-2 may be redistributed to the lysosomes rather than transported to the plasma membrane, we assessed the subcellular localization of Pit-2–GFP chimeras in live cells stained with the lysosome marker LysoTracker as described in Materials and Methods. Again, no colocalization of Pit-2–GFP with the lysosome marker was observed in the A-MuLV-infected cells (Fig. 4G). Experiments using Texas red-conjugated transferrin (a marker for recycling endosomes) or dextran 10 (a marker for late endosomes) together with Pit-2–GFP showed no colocalization with Pit-2–GFP in A-MuLV-infected live fibroblasts (Fig. 4E and F). Moreover, the subcellular localization of the epitope-tagged Pit-2ɛɛ pool was found to be different from that for mitochondria (with mitochondrion-targeted GFP as a marker) (data not shown) and from that for early endosomes with EEA1-GFP chimeras as a marker (Fig. 4D).
Recently, it was reported that Pit-2 formed a physical association with actin (22). Further, the formation of actin stress fibers was implicated in determining the cell surface distribution of Pit-2, the internalization of the receptor in response to virus binding, and the capacity to process retrovirus entry. To determine whether the reported association between Pit-2 and actin was conserved after A-MuLV infection, A-MuLV producer Pit-2ɛɛ-overexpressing cells were double stained with ɛ-tag antibody to detect Pit-2ɛɛ- and fluorescein-conjugated phalloidin to detect actin stress fibers. We were unable to detect colocalization of Pit-2ɛɛ and the actin network within A-MuLV producer fibroblasts (Fig. 4H). In addition, no apparent loss of actin cytoskeletal integrity was detected under those conditions, which might result from the loss of actin stress fibers or aggregation of actin near the cell surface as described for other oncoretroviral transformation (3).
Cells infected with one retrovirus are typically resistant to superinfection with the same virus or other viruses that utilize the same receptor. This phenomenon of superinfection interference often involves the down-modulation of a specific cell surface viral receptor. For several retroviruses, interference involves depletion of receptors from the surface of infected cells. However, other mechanisms for superinfection interference have been proposed. For example, E-MuLV has been reported to bind to a conformationally mobile site on the mouse MCAT-1 receptor (25). Binding of the envelope glycoprotein gp70 to the MCAT-1 viral receptor/basic amino acid transporter appears to slow down a conformational transition of the empty transporter required to move the viral binding site from the inside back to the outside of the cell. This results in a significant decrease in further viral infection mediated through this receptor. Yet the infected cells retain basic amino acid transport activity, which is required for cell viability. It has been suggested that interference in response to human immunodeficiency virus type 1 infection is more complex than with simpler retroviruses such as A-MuLV. In addition to env, at least two other genes also have been reported to contribute to this process. The product of vpu interacts with newly synthesized CD4 and causes its degradation if it is associated with the envelope protein, while nef expression causes loss of the CD4 receptor from the cell surface (2). Infection by cytopathic retroviruses such as certain strains of feline leukemia virus is not associated with superinfection interference. It has been suggested that this delay or failure to establish superinfection interference may be responsible for the cell killing noted with infection by such cytopathic viruses (23). However, infection with other subgroups of FeLV does lead to superinfection interference. This FeLV subgroup-specific superinfection interference does not appear to be due to a blockade or down-regulation of other cell components required for virus entry (21).
Infection of cells with A-MuLV does induce resistance to superinfection, and it has been proposed that this interference may in part involve down-modulation of the Pit-2 receptor (11). Previously, it was established that Pit-2-mediated Na+/Pi uptake can be specifically blocked by infection of cells with A-MuLV (28), and expression of the amphotropic envelope protein in murine cells also was shown to inhibit phosphate transport mediated by Pit-2 (12). However, the mechanism responsible for the loss of Pit-2 transporter and viral receptor functions with A-MuLV infection has not yet been elucidated. Here we have presented experimental findings obtained with an epitope-tagged form of human Pit-2 designed to determine the fate of Pit-2 in A-MuLV-infected cells. Results are presented to show that the tagged Pit-2 receptors are localized to the plasma membrane in uninfected NIH 3T3 cells. However, when cells expressing the tagged Pit-2 were productively infected with A-MuLV, the tagged protein was no longer present at the plasma membrane. Rather, tagged Pit-2 now was found distributed to punctate structures within the cytosolic compartment, where it was found to colocalize with the A-MuLV gp70 envelope protein. The intracellular pool of epitope-tagged Pit-2 phosphate transporter/viral receptor present in A-MuLV-infected cells was shown to be a more rapidly migrating, apparently lower-molecular-weight form of Pit-2. A-MuLV, but not E-MuLV, appears to infect cells by releasing nucleocapsid into the cytoplasm following direct fusion at the plasma membrane and not through an endocytic pathway as a complex with Pit-2 receptor (15). Thus, the loss of Pit-2 from the plasma membrane in A-MuLV-infected cells does not appear to be due to a virus-induced endocytic process. Rather, it is conceivable that in A-MuLV-infected cells, complexes of newly synthesized Pit-2 receptor and the A-MuLV envelope glycoprotein may form shortly after protein synthesis. The newly synthesized Pit-2 found in these complexes may not be available for required covalent modifications or may be more susceptible to proteolytic modification. In turn, the posttranslational modifications blocked with the formation of intracellular Pit-2-A-MuLV envelope protein complexes may be required for normal Pit-2 processing and trafficking to the plasma membrane. The absence of Pit-2 receptors from the plasma membrane appears to be responsible for the superinfection interference observed in cells productively infected with A-MuLV.
REFERENCES
- 1.Batra R K, Olsen J C, Pickles R J, Hoganson D K, Boucher R C. Transduction of non-small cell lung cancer cells by adenoviral and retroviral vectors. Am J Respir Cell Mol Biol. 1998;18:402–410. doi: 10.1165/ajrcmb.18.3.2784. [DOI] [PubMed] [Google Scholar]
- 2.Bour S, Geleziunas R, Wainberg M A. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Microbiol Rev. 1995;59:63–93. doi: 10.1128/mr.59.1.63-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burridge K. Substrate adhesions in normal and transformed fibroblasts: organization and regulation of cytoskeletal and extracellular matrix components at focal contact points. Cancer Rev. 1986;4:18–78. [Google Scholar]
- 4.Cardinali M, Jakus J, Shah S, Ensley J F, Robbins K C, Yeudall W A. p21(WAF1/Cip1) retards the growth of human squamous cell carcinomas in vivo. Oral Oncol. 1998;34:211–218. doi: 10.1016/s1368-8375(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 5.Chaudry G J, Farrell K B, Ting Y-T, Schmitz C, Lie Y S, Petropoulos C J, Eiden M V. Gibbon ape leukemia virus receptor functions of type III phosphate transporters from CHOK1 cells are disrupted by two distinct mechanisms. J Virol. 1999;73:2916–2920. doi: 10.1128/jvi.73.4.2916-2920.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien M L, O'Neill E, Garcia J V. Phosphate depletion enhances the stability of the amphotropic murine leukemia virus receptor mRNA. Virology. 1998;240:109–117. doi: 10.1006/viro.1997.8933. [DOI] [PubMed] [Google Scholar]
- 7.Coffin J M. Retroviridae. In: Knipe D M, Howley P M, Fields B N, editors. Field's virology. Vol. 2. Philadelphia, Pa: Lippincott; 1996. pp. 1767–1848. [Google Scholar]
- 8.Eglitis M A, Eiden M V, Wilson C A. Gibbon ape leukemia virus and the amphotropic murine leukemia virus 4070A exhibit an unusual interference pattern on E36 Chinese hamster cells. J Virol. 1993;67:5472–5477. doi: 10.1128/jvi.67.9.5472-5477.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiden M V, Farrell K B, Wilson C A. Substitution of a single amino acid residue is sufficient to allow the human amphotropic murine leukemia virus receptor to also function as a gibbon ape leukemia virus receptor. J Virol. 1996;70:1080–1085. doi: 10.1128/jvi.70.2.1080-1085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jobbagy Z, Olah Z, Petrovics G, Eiden M V, Leverett B D, Dean N M, Anderson W B. Up-regulation of the Pit-2 phosphate transporter/retrovirus receptor by protein kinase C epsilon. J Biol Chem. 1999;274:7067–7071. doi: 10.1074/jbc.274.11.7067. [DOI] [PubMed] [Google Scholar]
- 11.Kavanaugh M P, Kabat D. Identification and characterization of a widely expressed phosphate transporter/retrovirus receptor family. Kidney Int. 1996;49:959–963. doi: 10.1038/ki.1996.135. [DOI] [PubMed] [Google Scholar]
- 12.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J W, Cunningham J M. N-linked glycosylation of the receptor for murine ecotropic retroviruses is altered in virus-infected cells. J Biol Chem. 1993;268:16316–16320. [PubMed] [Google Scholar]
- 14.Lehel C, Olah Z, Jakab G, Anderson W B. Protein kinase C epsilon is localized to the Golgi via its zinc-finger domain and modulates Golgi function. Proc Natl Acad Sci USA. 1995;92:1406–1410. doi: 10.1073/pnas.92.5.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller A D, Wolgamot G. Murine retroviruses use at least six different receptors for entry into Mus dunni cells. J Virol. 1997;71:4531–4535. doi: 10.1128/jvi.71.6.4531-4535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olah Z, Lehel C, Anderson W B. Differential effects of activation of protein kinase C and cyclic-AMP-dependent protein kinase on sodium-dependent phosphate uptake in NIH 3T3 cells. Biochim Biophys Acta. 1993;1176:333–338. doi: 10.1016/0167-4889(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 19.Olah Z, Lehel C, Anderson W B, Eiden M V, Wilson C A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 20.Olah Z, Lehel C, Jakab G, Anderson W B. A cloning and epsilon-epitope-tagging insert for the expression of polymerase chain reaction-generated cDNA fragments in Escherichia coli and mammalian cells. Anal Biochem. 1994;221:94–102. doi: 10.1006/abio.1994.1384. [DOI] [PubMed] [Google Scholar]
- 21.Reinhart T A, Ghosh A K, Hoover E A, Mullins J I. Distinct superinfection interference properties yet similar receptor utilization by cytopathic and noncytopathic feline leukemia viruses. J Virol. 1993;67:5153–5162. doi: 10.1128/jvi.67.9.5153-5162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues P, Heard J M. Modulation of phosphate uptake and amphotropic murine leukemia virus entry by posttranslational modifications of PIT-2. J Virol. 1999;73:3789–3799. doi: 10.1128/jvi.73.5.3789-3799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Temin H M. Mechanism of cell killing/cytopathic effects of nonhuman retroviruses. Rev Infect Dis. 1986;10:399–405. doi: 10.1093/clinids/10.2.399. [DOI] [PubMed] [Google Scholar]
- 24.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O'Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Dechant E, Kavanaugh M, North R A, Kabat D. Effects of ecotropic murine retroviruses on the dual-function cell surface receptor/basic amino acid transporter. J Biol Chem. 1992;267:23617–23624. [PubMed] [Google Scholar]
- 26.Weimin Wu B, Cannon P M, Gordon E M, Hall F L, Anderson W F. Characterization of the proline-rich region of murine leukemia virus envelope protein. J Virol. 1998;72:5383–5391. doi: 10.1128/jvi.72.7.5383-5391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson C A, Eiden M V. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J Virol. 1991;65:5975–5982. doi: 10.1128/jvi.65.11.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson C A, Eiden M V, Anderson W B, Lehel C, Olah Z. The dual-function hamster receptor for amphotropic murine leukemia virus (MuLV), 10A1 MuLV, and gibbon ape leukemia virus is a phosphate symporter. J Virol. 1995;69:534–537. doi: 10.1128/jvi.69.1.534-537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson C A, Farrell K B, Eiden M V. Properties of a unique form of the murine amphotropic leukemia virus receptor expressed on hamster cells. J Virol. 1994;68:7697–7703. doi: 10.1128/jvi.68.12.7697-7703.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S, Delgado R, King S R, Woffendin C, Barker C S, Yang Z Y, Xu L, Nolan G P, Nabel G J. Generation of retroviral vector for clinical studies using transient transfection. Hum Gene Ther. 1999;10:123–132. doi: 10.1089/10430349950019255. [DOI] [PubMed] [Google Scholar]