Abstract
Background
Prediabetes is a condition preceding the development of diabetes and is associated with an increased risk of a number of complications. The primary mode of management is thought to be lifestyle modification. Pharmacological therapy, such as glucagon-like peptide-1 receptor agonists (GLP-1RAs), were not well addressed in the literature and were only evaluated in trials as secondary and exploratory outcomes with a limited sample size. Here, GLP-1RAs are evaluated as a comprehensive therapy approach for patients with prediabetes.
Methods
A comprehensive search of Web of Science, SCOPUS, PubMed, and Cochrane was performed on May 5, 2023, to retrieve randomized controlled trials (RCTs) comparing the effect of GLP-1RAs to placebo and/or lifestyle modification on prediabetes reversion to normoglycemia, prevention of overt diabetes, glycemic control, anthropometric parameters, and lipid profiles. Review Manager (RevMan) version 5.4 was used. The quality of RCTs was assessed using the revised version of the Cochrane Risk of Bias Tool. GRADE was performed to evaluate the certainty of evidence.
Results
Twelve trials involving 2903 patients in the GLP-1RAs group and 1413 in the control group were included in the meta-analysis. Low quality of evidence revealed that GLP-1RAs significantly increased the incidence of prediabetes reversion to the normoglycemic state [RR = 1.76, 95% CI (1.45, 2.13), P < 0.00001] and moderate quality of evidence showed that GLP-1RAs significantly prevented new-onset diabetes [RR = 0.28, 95% CI (0.19, 0.43), P < 0.00001]. Significant reductions in HbA1c, fasting plasma glucose, body weight, waist circumference, triglycerides, and LDL were observed in the GLP-1RAs arm (P < 0.05). However, higher incidences of gastrointestinal disorders were reported in the GLP-1RAs group (P < 0.05).
Conclusions
GLP-1RAs combined with lifestyle modification proved to be a more effective therapy for managing prediabetic patients than lifestyle modification alone, with a tolerable safety profile. Future guidelines should consider GLP-1RAs as an adjunct to lifestyle modification in the management of prediabetic patients to provide better management and improve treatment adherence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-024-01371-3.
Keywords: Diabetes, Liraglutide, Prediabetes, Reversion, Regression, Normoglycemia, Impaired, Tolerance, Lifestyle
Background
Prediabetes is a condition characterized by impaired glucose tolerance and/or fasting glucose levels that are higher than normal but not high enough to be diagnosed as diabetes [1]. Prediabetes is defined by the World Health Organization (WHO) as impaired glucose tolerance (IGT), impaired fasting glucose (IFG), or a combination of the 2 [2]. IGT is plasma glucose of 7.8–11.0 mmol/L (140–200 mg/dL) two hours after ingestion of 75 g of glucose. IFG is defined as fasting plasma glucose (FPG) levels ranging from 6.1–6.9 mmol/L (110–125 mg/dL) [2]. The American Diabetes Association (ADA) uses the same criteria but with a lower cut-off value for IFG (100–125 mg/dL) and adds HbA1c level of 5.7% to 6.4% to define prediabetes [3].
According to the impaired fasting glucose criteria, around 10.6% of adults globally (541 million individuals) are estimated to have prediabetes in 2021, which will increase to 11.4% (730 million people) by 2045 [4]. Prediabetes progresses to diabetes in 25% of cases within 3–5 years, and 70% of prediabetics develop overt diabetes in their lifetime [1]. In addition to an increased risk of diabetes progression and diabetes-related complications, prediabetes is associated with an increased risk of cardiovascular disease, chronic kidney disease, retinopathy, and other complications [1, 5–8].
Lifestyle interventions, such as diet and exercise, play a pivotal role in preventing or delaying the progression of prediabetes to diabetes [9, 10]. Their implementation and long-term adherence, however, pose significant challenges. Diabetes prevention program revealed that adherence to lifestyle interventions was lower than adherence to metformin. While only 38% of patients in the lifestyle group retained their weight loss, medication adherence over 4 years was around 70% [11]. This highlights the importance of investigating alternative strategies for managing prediabetes, such as pharmacological interventions.
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a class of drugs that mimic the effects of GLP-1, a hormone that stimulates insulin secretion, inhibits glucagon secretion, delays gastric emptying, and reduces appetite [12]. GLP-1RAs stimulate GLP-1 receptors in the pancreas, increasing insulin release and alleviating hyperglycemia. Stimulation of GLP-1 receptors in the hypothalamus reduces appetite and increases satiety, which aids in weight loss [13]. A 5% to 7% weight loss can significantly reduce the risk of type 2 diabetes [14, 15]. GLP-1 receptor agonists have been shown to improve glycemic control, decrease body weight, and cardiometabolic parameters [16–18], improve atherosclerotic risk [19], lower cardiovascular risk [20], improve endothelial dysfunction [21], and improve dyslipidemia [22]. Furthermore, GLP-1 receptor agonists showed positive effects on factors that trigger prediabetes, such as oxidative stress [23, 24] and inflammation [24, 25]. Therefore, GLP-1RAs may be a viable intervention for the management of prediabetes.
The effect of GLP-1RAs on patients with impaired glucose tolerance has not been properly addressed in the literature. Several trials explored the effect of GLP-1RAs inhibitors on preventing the development of type 2 diabetes in prediabetic subjects as secondary or exploratory analyses [26–29]; however, some of the results were not statistically significant, which may be due to small sample sizes.
In this systematic review and meta-analysis, we aim to address this gap in knowledge by evaluating the efficacy and safety of GLP-1 receptor agonists in patients with prediabetes. We will also examine their effects on other diabetes-related parameters, such as HbA1c, fasting plasma glucose (FPG), hypoglycemia, body weight, and lipids. We will conduct subgroup analyses to examine the effect of different GLP-1RA treatment durations, types, and doses. The findings of this review have the potential to inform clinical practice, guidelines, and future research directions in the management of prediabetes.
Methods
The authors followed the PRISMA guidelines for reporting systematic reviews and meta-analyses of randomized controlled trials (RCTs) [30]. This systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration ID CRD42023456814.
Eligibility criteria
Studies were included if they met the PICOS criteria: patients, intervention, control, outcomes, and study design. The patients of interest were prediabetic or had impaired glucose tolerance or impaired fasting plasma glucose with or without obesity or overweight. The intervention was GLP-1RAs or dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonists such as tirzepatide, alone or combined with lifestyle modification. The control group included prediabetic patients who received a placebo and/or lifestyle modification. The studies must measure and report the results of the outcomes of interest separately for the prediabetic patients to be included. The authors included only randomized controlled trials comparing. If multiple publications exist for a single eligible study, they will be included if they provide new data, such as results for more treatment durations; otherwise, only the publication with the most data about prediabetic patients and their baseline data will be included.
Non-randomized trials, animal studies, conference abstracts, non-English papers, and single-arm studies were all excluded. Studies in which both diabetic and prediabetic patients were included, but the results of the prediabetic patients were not reported separately were excluded. Studies in which patients had polycystic ovary syndrome or received GLP-1RAs in combination with other drugs that raise blood glucose levels were excluded.
Information sources
PubMed, Web of Science, Scopus, and Cochrane were searched for the relevant articles on February 20, 2023, and we updated the search again on May 5, 2023. The references of the eligible papers were also searched for other relevant studies.
Search strategy
PubMed, Web of Science, Scopus, and Cochrane were searched using a combination of the following terms: “Glucagon Like Peptide”, “Prediabetic”, “State” “Trial”, “Exenatide”, “Semaglutide”, “Efpeglenatide”, “lixisenatide”, “Liraglutide”, and “Tirzepatide”. No filters were applied. The full search strategy is shown in Table S1.
Selection process
Endnote was used to gather articles from various databases. Afterward, the articles were exported to an Excel spreadsheet and screened in 2 stages for studies that met our inclusion criteria. The first stage entailed screening the title and abstract of the retrieved records, with those who passed progressing to the second stage, which entailed screening the full text. Two independent reviewers carried out the screening, and disagreements were settled through discussion or by a third author.
Data collection process
The lead author prepared formatted Excel sheets for extracting patients’ baseline data, study characteristics, risk of bias (ROB) assessment, and outcomes of interest. The two authors extracted data from each study independently and then discussed it. A third senior author settled any disagreements. Methods recommended in the Cochrane Handbook [31] were used to deal with any incomplete or incompatible data.
Data items (outcomes)
The primary outcomes were the incidence of prediabetes reversion to normoglycemia and the incidence of developing overt diabetes. The secondary outcomes were fasting plasma glucose (FPG), HbA1c, weight loss, waist circumference, lipid profile outcomes, which are triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), and safety outcomes, which are any adverse event (AEs), any serious AEs, any gastrointestinal disorders, nausea, vomiting, diarrhea, hypoglycemia, and headache.
Data items (other variables)
Two authors independently extracted study characteristics and baseline data. The characteristics of the studies included study ID, country and center, criteria of prediabetes diagnosis, inclusion and exclusion criteria, name and dose of the intervention and control, ample size, and follow-up duration. Patients baseline data included age, gender, weight, BMI, fasting plasma glucose (FPG), HbA1c, TG, and LDL.
Study risk of bias assessment
The Cochrane risk-of-bias tool version 2 [32] was used to assess the quality of the included studies in the following domains: (A) bias arising from the randomization process, B) bias resulting from deviations from intended interventions, (C) bias resulting from missing outcome data, (D) bias in outcome measurement, and (E) bias in the selection of the reported results. The domains were classified as low, moderate, or high risk. Two authors conducted the evaluation independently, with discussions with a third author in the event of disagreements. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria were used to evaluate the quality of evidence.
Effect measures and synthesis methods
Review Manager (RevMan) version 5.4 [33] was used to conduct all the analyses. Continuous data were extracted as means and standard deviation (SD) or mean difference against placebo and standard error, while dichotomous data were extracted as event and total. The dichotomous outcomes, including the incidence of prediabetes reversion to normoglycemia, the incidence of new-onset diabetes, and safety outcomes, were pooled using the Mantel-Haensze equation and reported as risk ratio (RR) and 95% confidence interval (CI). The continuous outcomes, including FPG, HbA1c, body weight reduction, waist circumference, TG, HDL, and LDL, were pooled using the generic inverse variance statistic method and reported as mean differences with a 95% CI.
We conducted subgroup analysis based on the treatment duration and type and dose of the GLP-1RAs whenever the number of studies included in the analysis was sufficient.
Cochrane's Q test and the I2 statistic were used to assess heterogeneity. If the P value was less than 0.1, the heterogeneity was considered significant, and a random-effects model was used; otherwise, a fixed-effects model was used. A sensitivity analysis using the leave-one-out method was used to identify the source of heterogeneity and investigate the robustness of our results.
TSA was performed with a 5% risk of type I error and a 10% risk of type II error (90% power). The TSA was conducted in chronological order by year of publication. TSA was performed using the TSA Viewer, version 0.9 beta (Copenhagen Trial Unit, Copenhagen, Denmark).
A funnel plot, as well as Egger's and Begg's tests, were used to investigate publication bias, and P < 0.05 was judged significant [34, 35]. Trim-and-fill statistical analysis was used to account for potential publication bias [36].
Results
Literature search results
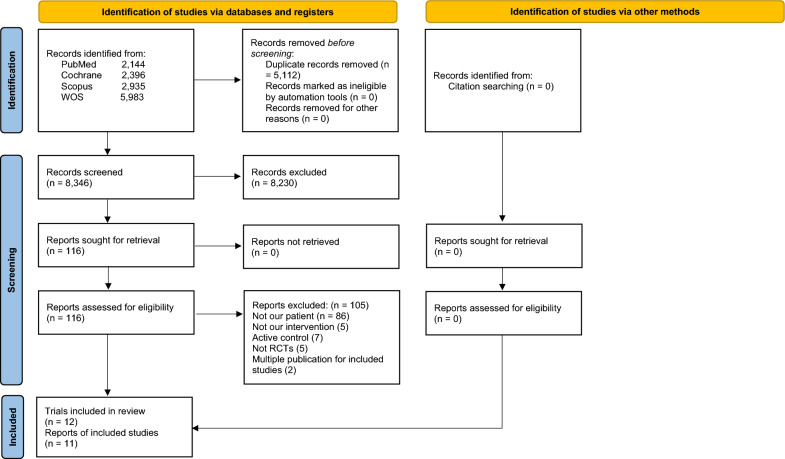
The search produced 13,458 results. After duplicates were removed, the total number was 8,346. Only 116 papers were eligible for full-text screening after title and abstract screening. Finally, 12 trials with a total of 11 publications [26–29, 37–43] were determined to be eligible for the final analysis. The paper of Perreault et al. 2022 [28] reported the results of the outcomes of interest for prediabetic patients from three trials. Astrup et al. [37] and [42] were two publications for one trial. Figure 1 demonstrates a PRISMA flow diagram.
Fig. 1.
PRISMA flow diagram shows the detailed process of search strategy results and study selection
Study characteristics
The meta-analysis included a total of 2903 prediabetic patients in the GLP-1RAs group and 1,413 in the control group. Most of the included studies were international multicenter studies [26, 28, 37, 39, 42], while six studies were single-center: three were conducted in the United States [40, 41, 43], one in China [38], and one in Sweden [27]. Of the GLP-1RAs used, Liraglutide was administered in six trials: Astrup et al. [37, 42] examined four doses of 1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg once/day, while three trials investigated dose 1.8 mg/day [40, 41, 43], one trial investigated dose 1.2 mg/day [38], and the remaining trial gave 3 mg/day [26]. Semaglutide at a dose of 2.4mg QW was given in the three trials published in the paper of Perreault et al. 2022 [28]. Efpeglenatide at doses of 4 mg QW, 6 mg QW, 6 mg Q2W, or 8 mg Q2W was administered in Pratley et al. 2021 [39]. Exenatide at a dose of 10 µg twice a day was administered in one study [29] and at a dose of 2 mg QW combined with Dapagliflozin in another study [27]. All studies gave GLP-1RAs in combination with lifestyle modification, except Mashayekhi et al. 2022 [40], where they compared the GLP-1RAs alone to the hypocaloric diet. The detailed characteristics of the included studies and the follow-up durations are shown in Table 1. All studies included obese patients ≥ 18 years old, except Zhou et al. 2017, which included obese prediabetic children. The inclusion and exclusion criteria applied by each study are shown in Table S2. The mean BMI of the included patients in all studies was > 30 kg/m2. Patients’ baseline characteristics are shown in Table 2.
Table 1.
Study characteristics
| Study ID | Country/Center | Criteria for prediabetes diagnosis | Definition of new-onset diabetes | Prediabetic sample size/Total sample size | Follow-up duration | Intervention group | Control group | Comedications status |
|---|---|---|---|---|---|---|---|---|
| Ariel et al. 2014 | USA (Single-center) | FPG ≥ 100 and < 126 mg/dL and/or a plasma glucose concentration ≥ 140 and < 190 mg/dL 2-h after a 75-g oral glucose challenge | NA | 68/68 | 14 weeks | Liraglutide, 1.8 mg/day, SC, starting at 0.6 mg daily, increased by 0.6 mg weekly to 1.8 mg/day + calorie-restricted diet | Placebo + calorie-restricted diet | Patients were excluded if they were taking any medications that affect lipoprotein or carbohydrate metabolism or promote weight loss |
| Rosenstock et al. 2010 | USA | IGT (fasting glucose < 7 mmol/L and 2-h postprandial glucose ≥ 7.8 and < 11.1 mmol/L), IFG (fasting glucose 6.1– 6.9 mmol/l and 2-h postprandial glucose < 7.8 mmol/l) | NA | 33/152 | 24 weeks | Exenatide, 10 µg, twice/day + a structured program of diet and physical activity | Placebo + a structured program of diet and physical activity | Patients with previous use of glucose-lowering medications for more than 3 months were excluded |
| Mashayekhi et al. 2022 | USA (Single-center) | FPG between 100–125 mg/dL or IGT after a 75-g glucose challenge of 140–199 mg/dL or HbA1c = 5.7 to 6.4% | NA | 88/88 | 14 weeks | Liraglutide, 1.8 mg/day, SC, starting at 0.6 mg daily, increased by 0.6 mg weekly to 1.8 mg/day | Sitagliptin 100 mg/day or a hypocaloric diet | NA |
| Pratley et al. 2021 | Five countries (Multicenter) | HbA1c = 5.7% to 6.4% or FPG = 100 to 125 mg/dL | NA | 140/140 | 20 weeks | Efpeglenatide, 4 mg QW, 6 mg QW, 6 mg Q2W, or 8 mg Q2W, SC + reducing calorie intake by approximately 500 kcal each day and increasing physical activity | Placebo + reducing calorie intake by approximately 500 kcal each day and increasing physical activity | NA |
| Kim et al. 2013 | USA (Single-center) | FPG = 5.6 to 6.9 mmol/L or elevated 2-h glucose (7.8–11.0 mmol/L) concentration after a 75 g OGTT | NA | 68/68 | 14 weeks | Liraglutide, 1.8 mg/day, SC, starting at 0.6 mg daily, increased by 0.6 mg weekly to 1.8 mg/day + decreasing total caloric intake by 500 kcal/day, and keeping baseline physical activity | Placebo + decreasing total caloric intake by 500 kcal/day and keeping baseline physical activity | Patients using medications that affect carbohydrate metabolism or promote weight loss were excluded |
| Lundkvist et al. 2017 | Sweden (Single-center) |
Prediabetes was defined as any IFG or IGT. IFG (FPG ≥ 5.6 mmol/L measured just before the OGTT), IGT ≥ 7.8 mmol/L measured 120 min after the start of the OGTT) |
FPG > 7.0 mmol/L, 2-h OGTT > 11.1 mmol/L, and/or HbA1c > 48 mmol/mol | 33/49 | 24 weeks | Dapagliflozin 10 mg, once/day, oral + Exenatide 2 mg QW, SC + balanced diet and moderate exercise. However, diet and exercise modifications were not mandated or documented | Placebo + balanced diet and moderate exercise. However, diet and exercise modification were not mandated or documented | Remained on the same medication without alternations |
| Zhou et al. 2017 | China (Single-center) |
IFG: FPG (5.6–6.9 mmol/L), while 2 h OGTT < 7.8 mmol/L, IGT: OGTT 2 h blood glucose (7.8–11.0) while FPG < 5.6 |
FPG ≥ 7.0 or OGTT 2 h blood glucose > 11.1 | 42/42 | 12 weeks | Liraglutide, 1.2 mg/day, SC, starting at 0.6 mg daily, increased to 1.2 mg/day after one week + a unified diet and exercise prescription with regular telephone conversations and outpatient follow-up | A unified diet and exercise prescription with regular telephone conversations and outpatient follow-up | NA |
| Astrup et al. 2009 | 19 clinical research sites in eight European countries | Either IFG (5·6–6·9 mmol/L) or IGT (7·8–11·0 mmol/L) during OGTT | Fasting plasma glucose ≥ 7·0 mmol/L or ≥ 11·1 mmol/L during OGTT | 187/564 | 20 weeks | Liraglutide, 1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg, once/day, SC + decreasing total caloric intake by 500 kcal/day, and increasing physical activity | Placebo or orlistat + decreasing total caloric intake by 500 kcal/day, and increasing physical activity | Patients were excluded weight-lowering pharmacotherapy or participation in a clinical weight control study |
| Astrup et al. 2012 |
19 clinical research sites in eight European countries |
Either IFG (5·6–6·9 mmol/L) or IGT (7·8–11·0 mmol/L) during OGTT | Fasting plasma glucose ≥ 7·0 mmol/L or ≥ 11·1 mmol/L during OGTT | 187/564 | 52 weeks | Liraglutide, 1.2 mg, 1.8 mg, 2.4 mg, or 3.0 mg, once/day, SC + decreasing total caloric intake by 500 kcal/day, and increasing physical activity | Placebo or orlistat + decreasing total caloric intake by 500 kcal/day, and increasing physical activity | Patients were excluded if they used weight-lowering pharmacotherapy or participation in a weight-control stud |
| Le Roux et al. 2017 | 191 clinical research sites in 27 countries | One of the three American Diabetes Association (ADA) 2010 criteria: HbA1c = 5·7 − 6·4%, or FPG = 5·6 mmol/L to 6·9 mmol/L, or 2-h OGTT = 7·8 mmol/L to 11·0 mmol/L | Two consecutive measurements of HbA1c ≥ 6.5%, or FPG ≥ 126 mg/dl (7.0 mmol/liter), or 2-h OGTT ≥ 200 mg/dl (11.1 mmol/liter) | 2254/2254 | 160 weeks | Liraglutide, 3.0 mg, once/day, SC, starting at 0.6 mg with weekly 0.6-mg increments to 3.0 mg + reduced-calorie diet, and increased physical activity | Placebo + reduced-calorie diet, and increased physical activity | Patients were excluded medications causing significant weight gain or loss |
| Perreault et al. 2022a | 16 countries (multicenter) | American Diabetes Association criteria. Prediabetes was defined as FPG 5.6–6.9 mmol/L or HbA1c 5.7–6.4% (39–47 mmol/mol) | HbA1c = 48 mmol/L (6.5%) or greater | 856/1961 | 68 weeks | Semaglutide, 2.4 mg, QW, SC, starting with 0.25 mg weekly for the first 4 weeks, increased every 4 weeks to reach 2.4 mg weekly + decreasing total caloric intake by 500 kcal/day, and increasing physical activity by 150 min/week of physical activity. Both diet and activity were recorded daily | Placebo + decreasing total caloric intake by 500 kcal/day, and increasing physical activity. Both diet and activity were recorded daily | The individual has not had any prior surgical obesity treatment or use of antiobesity medication within 90 days prior to enrollment |
| Perreault et al. 2022b | USA (multicenter) | American Diabetes Association criteria. Prediabetes was defined as FPG 5.6–6.9 mmol/L or HbA1c 5.7–6.4% (39–47 mmol/mol) | HbA1c = 48 mmol/L (6.5%) or greater | 304/611 | 68 weeks | Semaglutide, 2.4 mg, QW, SC, starting with 0.25 mg weekly for the first 4 weeks, increased every 4 weeks to reach 2.4 mg weekly + intensive behavioral intervention (low-calorie diet (1000–1200 kcal/d) in the initial 8 weeks + hypocaloric diet (1200–1800 kcal/d) for the rest of the 68 weeks) + 100 min of physical activity per week, which increased by 25 min every 4 weeks to reach 200 min/week | Placebo + intensive behavioral intervention (low-calorie diet (1000–1200 kcal/d) in the initial 8 weeks + hypocaloric diet (1200–1800 kcal/d) for the rest of 68 weeks) + 100 min of physical activity per week, which increased by 25 min every 4 weeks, to reach 200 min/week | NA |
| Perreault et al. 2022c | 10 countries (multicenter) | American Diabetes Association criteria. Prediabetes was defined as FPG 5.6–6.9 mmol/L or HbA1c 5.7–6.4% (39–47 mmol/mol) | HbA1c = 48 mmol/L (6.5%) or greater | 376/803 | 48 weeks | Semaglutide, 2.4 mg, QW, SC, starting with 0.25 mg weekly for the first 4 weeks, increased every 4 weeks to reach 2.4 mg weekly + decreasing total caloric intake by 500 kcal/day, and increasing physical activity by 150 min/week of physical activity. Both diet and activity were recorded daily | Placebo + decreasing total caloric intake by 500 kcal/day, and increasing physical activity. Both diet and activity were recorded daily | NA |
IGT: impaired glucose tolerance; IFG: impaired fasting plasma glucose; FPG: fasting plasma glucose; OGTT: oral glucose tolerance test; SC: subcutaneous; QW: once weekly; Q2W: once every 2 weeks
Table 2.
Patients baseline characteristics
| Study ID | Groups | Sample size | Age (years) | Female | Weight (kg) | BMI (kg/m2) | HbA1c (%) | FPG (mg/dl) | TG (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | N (%) | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Ariel et al. 2014 | Liraglutide 1.8 mg daily | 23 | 58 ± 7 | 15 (67) | 88.9 ± 11.2 | 31.9 ± 2.8 | NA | 105 ± 8 | 144.4 ± 73.7 | 113.3 ± 25.9 |
| Placebo | 27 | 58 ± 8 | 17 (63) | 88.7 ± 11.2 | 31.9 ± 3.5 | NA | 107 ± 8 | 129.8 ± 62.6 | 117.4 ± 31.5 | |
| Rosenstock et al. 2010 # | Exenatide 10ug BID | 73 | 46 ± 12 | 124 (82) | 109.5 ± 2.7 | 39.6 ± 7.0 | NA | NA | NA | NA |
| Placebo | 79 | 107.6 ± 2.6 | NA | NA | NA | NA | ||||
| Mashayekhi et al. 2022 | Liraglutide 1.8 mg daily | 44 | 49.8 ± 10.1 | 31 (70.5) | 108.8 ± 20.9 | 38.8 ± 6.1 | 5.7 ± 0.3 | 96.2 ± 10.1 | 122.2 ± 51.4 | 119.1 ± 31.9 |
| Hypocaloric diet | 22 | 49.2 ± 12.5 | 14 (63.6) | 111.3 ± 21.5 | 38.4 ± 5.9 | 5.7 ± 0.3 | 96.7 ± 11.8 | 115.0 ± 67.1 | 111.5 ± 35.7 | |
| Pratley et al. 2021 | Efpeglenatide 4 mg QW | 28 | 45.6 ± 11.0 | 18 (64.3) | 104.9 ± 22.0 | 35.9 ± 4.7 | 5.7 ± 0.3 | 100.3 ± 9.9 | NA | NA |
| Efpeglenatide 6 mg QW | 26 | 46.2 ± 12.2 | 20 (76.9) | 104.5 ± 23.3 | 36.8 ± 5.0 | 5.7 ± 0.3 | 101.7 ± 9.6 | NA | NA | |
| Efpeglenatide 6 mg Q2W | 32 | 48.5 ± 10.9 | 23 (71.9) | 100.9 ± 19.5 | 35.9 ± 5.6 | 5.7 ± 0.3 | 103.4 ± 10.0 | NA | NA | |
| Efpeglenatide 8 mg Q2W | 24 | 47.5 ± 9.3 | 22 (91.7) | 94.5 ± 11.3 | 34.8 ± 3.3 | 5.7 ± 0.3 | 103.3 ± 8.5 | NA | NA | |
| Placebo | 30 | 45.0 ± 11.3 | 23 (76.7) | 96.9 ± 10.9 | 35.1 ± 3.1 | 5.7 ± 0.4 | 102.3 ± 12.0 | NA | NA | |
| Kim et al. 2013 | Liraglutide 1.8 mg daily | 24 | 58 ± 7 | 16 (67) | 88.4 ± 11.2 | 31.9 ± 2.7 | NA | 106.2 ± 7.2 | 141.7 ± 70.9 | 112.14 ± 27.07 |
| Placebo | 27 | 58 ± 8 | 17 (63) | 88.7 ± 11.2 | 31.9 ± 3.5 | NA | 109.8 ± 7.2 | 132.9 ± 62 | 116 ± 30.94 | |
| Lundkvist et al. 2017 # | Dapagliflozin 10 mg daily + Exenatide 2 mg QW | 25 | 53.3 ± 13.5 | 15 (60) | 106.43 ± 15.55 | 35.8 ± 2.9 | 5.6 ± 0.35 | 106.2 ± 11.34 | 124 ± 50.49 | 136.12 ± 35.19 |
| Placebo | 24 | 50 ± 11.8 | 15 (62.5) | 102.72 ± 17.26 | 35 ± 3.7 | 5.6 ± 0.3 | 104.94 ± 7.74 | 140.83 ± 52.3 | 133.02 ± 34.42 | |
| Perreault et al. 2022a | Semaglutide 2.4 mg QW | 593 | 48.5 ± 12.5 | 407 (68.6) | 106.9 ± 22.4 | 38.4 ± 6.6 | 5.9 ± 0.2 | 98.7 ± 11.0 | NA | NA |
| Placebo | 263 | 49.4 ± 12.1 | 196 (74.5) | 106.9 ± 21.1 | 38.9 ± 6.5 | 5.9 ± 0.2 | 97.6 ± 11.8 | NA | NA | |
| Perreault et al. 2022b | Semaglutide 2.4 mg QW | 196 | 48.8 ± 11.9 | 153 (78.1) | 108.7 ± 22.9 | 39.0 ± 6.8 | 6.0 ± 0.2 | 96.8 ± 9.4 | NA | NA |
| Placebo | 108 | 49.7 ± 12.3 | 88 (81.5) | 106.9 ± 23.3 | 38.5 ± 6.9 | 6.0 ± 0.2 | 96.7 ± 9.2 | NA | NA | |
| Perreault et al. 2022c | Semaglutide 2.4 mg QW | 262 | 49.9 ± 11.6 | 208 (79.4) | 109.7 ± 25.1 | 39.3 ± 7.8 | 5.9 ± 0.2 | 100.9 ± 11.5 | NA | NA |
| Placebo | 114 | 50.0 ± 10.6 | 81 (71.1) | 109.6 ± 24.6 | 39.1 ± 7.6 | 5.9 ± 0.2 | 98.5 ± 9.3 | NA | NA | |
| Zhou et al. 2017 | Liraglutide 1.2 mg daily | 21 | 11.19 ± 2.27 | 7 (33) | NA | 30.98 ± 4.97 | 5.87 ± 1.35 | NA | 100.09 ± 63.77 | 94.74 ± 38.67 |
| Lifestyle intervention | 21 | 11.12 ± 2.10 | 6 (28.57) | NA | 30.89 ± 5.02 | 5.85 ± 1.33 | NA | 100.97 ± 54.92 | 95.51 ± 37.12 | |
| Astrup et al. 2009 # | Liraglutide 1.2 mg daily | 95 | 47·2 ± 9·7 | 73 (77) | 96·2 ± 13·5 | 34·8 ± 2·6 | 5.6 ± 0.3 | 95.4 ± 10.8 | 127.55 ± 74.4 | 130.7 ± 29.776 |
| Liraglutide 1.8 mg daily | 90 | 45·5 ± 10·9 | 68 (76) | 98·0 ± 12·5 | 35·0 ± 2·6 | 5.6 ± 0.4 | 95.4 ± 10.8 | 126.66 ± 87.69 | 136.5 ± 34.03 | |
| Liraglutide 2.4 mg daily | 93 | 45·0 ± 11·1 | 70 (76) | 98·4 ± 13·0 | 35·0 ± 2·8 | 5.5 ± 0.3 | 95.4 ± 10.8 | 121.35 ± 56.69 | 135.73 ± 33.26 | |
| Liraglutide 3.0 mg daily | 93 | 45·9 ± 10·7 | 70 (75) | 97·6 ± 13·7 | 34·8 ± 2·8 | 5.6 ± 0.4 | 97.2 ± 10.8 | 125.78 ± 69.09 | 131.48 ± 30.16 | |
| Placebo | 98 | 45·9 ± 10·3 | 73 (75) | 97·3 ± 12·3 | 34·9 ± 2·8 | 5.6 ± 0.4 | 97.2 ± 14.4 | 138.18 ± 69.09 | 136.5 ± 34.42 | |
| Le Roux et al. 2017 | Liraglutide 3.0 mg daily | 1505 | 47·5 ± 11·7 | 1141 (76) | 107.5 ± 21.6 | 38.8 ± 6.4 | 5.8 ± 0.3 | 99 ± 10.8 | 132.86 ± 54.1 | 112.14 ± 27.9 |
| Placebo | 747 | 47·3 ± 11·8 | 573 (77) | 107.9 ± 21.8 | 39 ± 6.3 | 5.7 ± 0.3 | 99 ± 9 | 132.86 ± 66.6 | 116 ± 28 |
QW once per week; Q2W once every 2 weeks; BID twice per day
#data are for patients with and without prediabetes, as no data were reported for prediabetes separately at baseline
Quality assessment
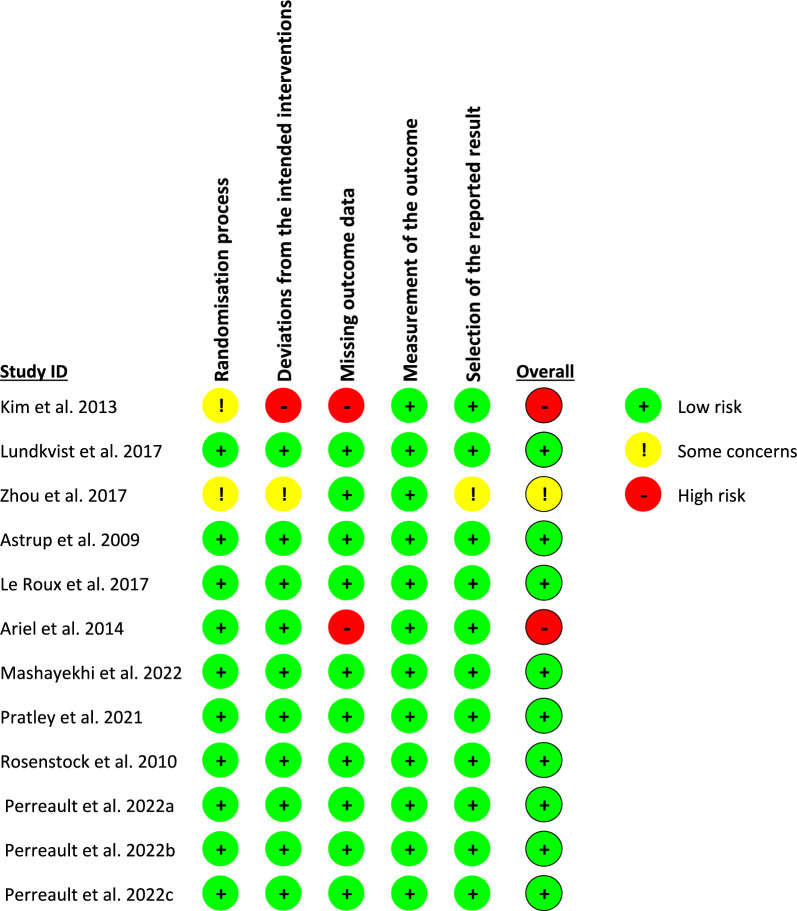
The quality of the included studies was evaluated using the revised Cochrane risk of bias tool, as shown in Fig. 2. Nine trials had a low risk of bias in all domains. Two trials showed a high risk of bias resulting from missing outcome data, and one of them showed a high risk of bias resulting from deviation from the intended intervention as well. Zhou et al. 2017 [38] showed some concern regarding the randomization process, deviation from the intended intervention, and selection of the reported results due to a lack of information. The quality of evidence was determined via GRADE instructions (Table S3).
Fig. 2.
Summery of the risk of bias of the included studies
Primary outcomes
The incidence of prediabetes reversion to normoglycemia
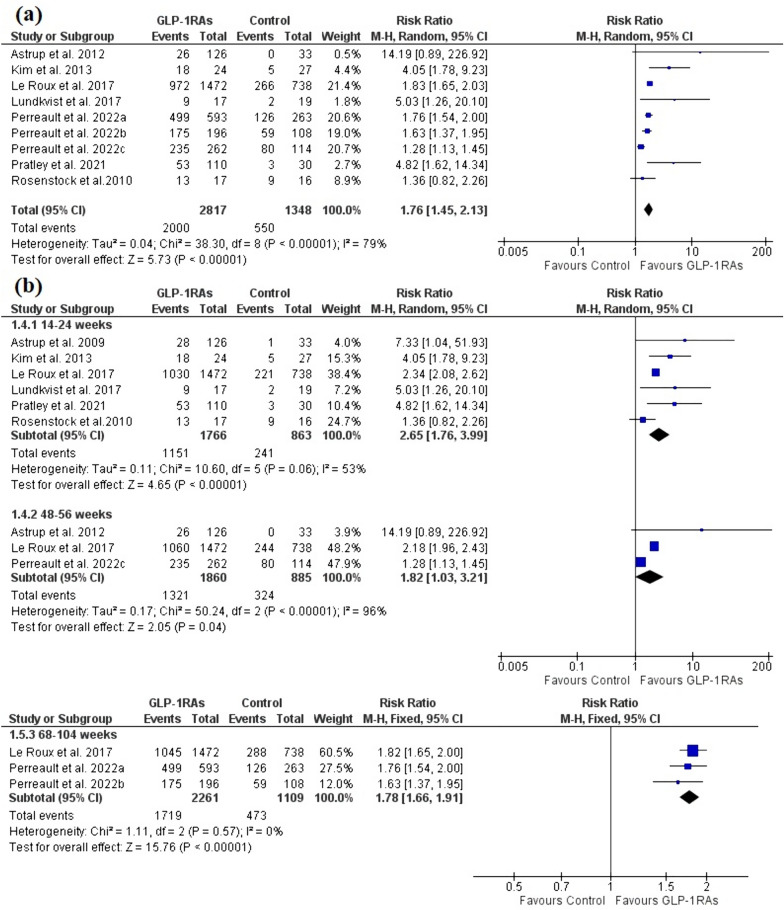
The analysis included nine trials with seven publications [26–29, 39, 42, 43] with a total of 2817 patients in the GLP-1RAs arm and 1348 patients in the control arm. Evidence with a low degree of certainty demonstrated that GLP-1RAs significantly revert prediabetic patients to a normoglycemia state compared to the control group [RR = 1.76, 95% CI (1.45, 2.13), P < 0.00001] (Fig. 3a). High heterogeneity was observed [I2 = 79%, P < 0.00001], which was partially resolved after the exclusion of Perreault et al. 2022c [I2 = 51%, P = 0.05] without significant change in the pooled analysis [RR = 1.83, 95% CI (1.57, 2.12), P < 0.00001].
Fig. 3.
A forest plot shows the effect of GLP-1RAs on the incidence of prediabetes reversion to normoglycemic state. a Overall effect. b subgroup analysis based on the treatment duration
Subgroup analysis revealed that the effect of GLP-1RAs on prediabetes reversion was accomplished as early as 14–24 weeks [RR = 2.65, 95% CI (1.76, 3.99), P < 0.00001] and lasted up to 48–56 weeks [RR = 1.82, 95% CI (1.03, 3.21), P = 0.04] and 68–104 weeks [RR = 1.78, 95% CI (1.66, 1.91), P < 0.00001] (Fig. 3b). The pooled analysis was homogenous for 68–104 weeks [I2 = 0%, P = 0.57], but heterogeneous for 14–24 weeks [I2 = 53%, P = 0.06] and 48–56 weeks [I2 = 96%, P < 0.00001], which was resolved after the exclusion of Rosenstock et al.2010 [I2 = 31%, P = 0.22], and Perreault et al. 2022c [I2 = 44%, P = 0.18], respectively, without significant change in the pooled analysis [RR = 2.44, 95% CI (2.18, 2.74), P < 0.00001], [RR = 2.21, 95% CI (1.98, 2.46), P < 0.00001].
Subgroup analysis revealed that semaglutide 2.4mg once weekly (QW) significantly outperformed the control group [RR = 1.54, 95% CI (1.24, 1.91), P < 0.00001]. The pooled analysis showed heterogeneity [I2 = 85%, P = 0.001]. Heterogeneity was eliminated by excluding Perreault et al. 2022c without significant change in the pooled analysis [RR = 1.71, 95% CI (1.54, 1.90), P < 0.00001]. Furthermore, Liraglutide 1.8mg once daily (QD) and 3mg were significantly effective [RR = 5.68, 95% CI (2.52, 12.82), P < 0.00001], [RR = 1.85, 95% CI (1.67, 2.05), P < 0.00001], and the pooled analyses were homogenous [I2 = 33%, P = 0.22], [I2 = 52%, P = 0.15] (Figure S1).
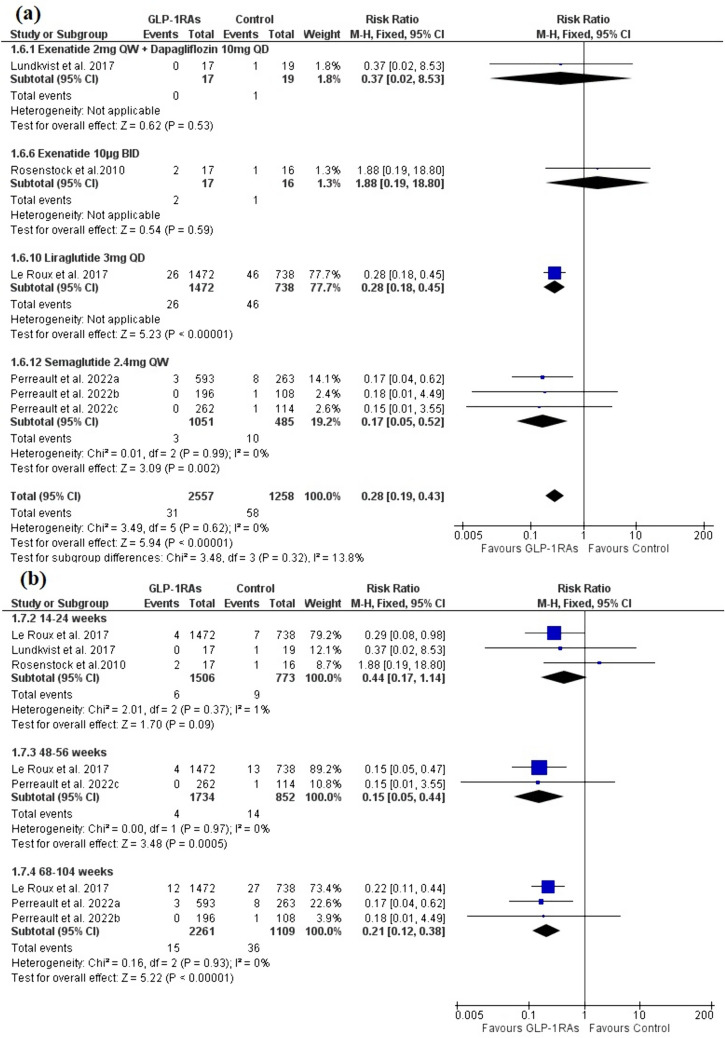
The incidence of new-onset diabetes
The analysis included six trials with four publications [26–29] with a total of 2557 patients in the GLP-1RAs arm and 1258 patients in the control arm. Evidence with a moderate degree of certainty demonstrated that prediabetic patients who progressed to a diabetes state were significantly fewer in the GLP-1RAs group compared to the control [RR = 0.28, 95% CI (0.19, 0.43), P < 0.00001]. Semaglutide 2.4mg QW significantly outperformed the control group in the subgroup analysis [RR = 0.17, 95% CI (0.05, 0.52), P = 0.002]. Both pooled analyses were homogenous [I2 = 0%, P = 0.62], and [I2 = 0%, P = 0.99] (Fig. 4a).
Fig. 4.
A forest plot shows the effect of GLP-1RAs on the incidence of new-onset diabetes. a Overall effect and subgroup analysis based on the type and dose of GLP-1RAs. b subgroup analysis based on the treatment duration
The protective effect of GLP-1RAs was noticed after 48–56 weeks and 68–104 weeks of the treatment [RR = 0.15, 95% CI (0.05, 0.44), P = 0.0005], [RR = 0.21, 95% CI (0.12, 0.38), P < 0.00001], but not as early as 14–24 weeks [RR = 0.44, 95% CI (0.17, 1.14), P = 0.09]. All the results were homogenous (P > 0.1) (Fig. 4b).
Secondary outcomes
FPG (mg/dl)
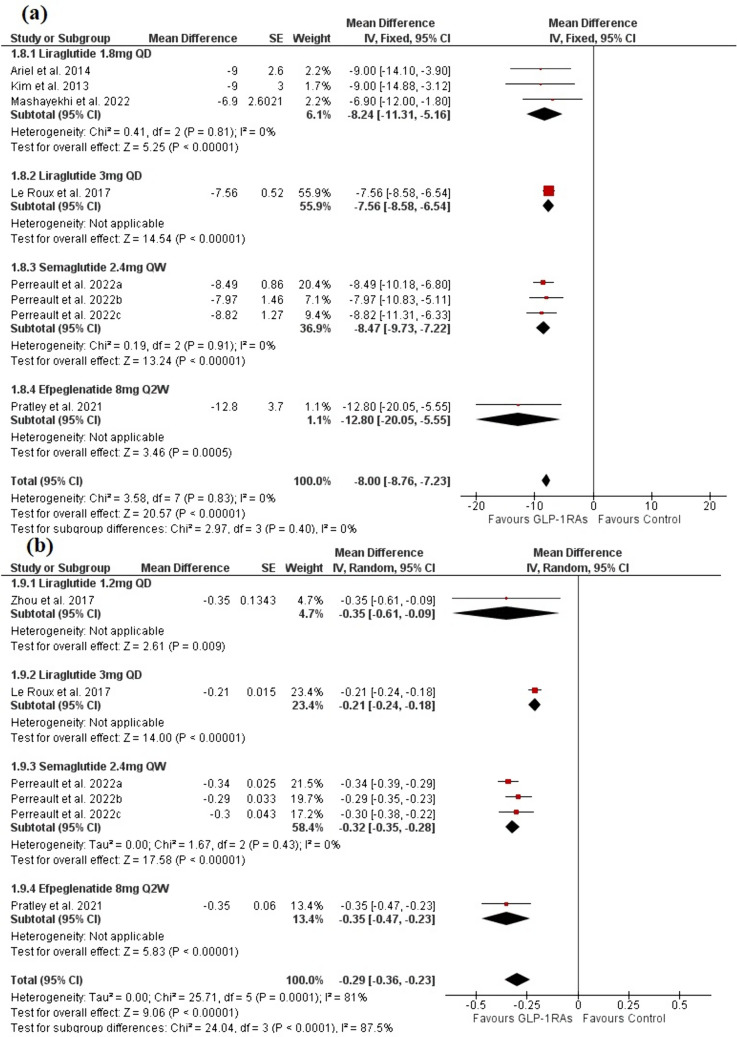
The analysis included eight trials with 6 publications [26, 28, 39–41, 43] with a total of 2636 patients in the GLP-1RAs arm and 1297 patients in the control arm. Evidence with a high degree of certainty demonstrated that the GLP-1 group significantly decreased FPG compared to the control group [MD = -8.00, 95% CI (-8.76, -7.23), P < 0.00001]. The pooled analysis was homogenous (P > 0.1). The liraglutide 1.8mg and semaglutide 2.4mg QW significantly outperformed the control group in the subgroup analysis [MD = − 8.24, 95% CI (− 11.31, − 5.16), P < 0.00001], and [MD = − 8.47, 95% CI (− 9.73, − 7.22), P < 0.00001], respectively, and the pooled analysis was homogenous for both (P > 0.1) (Fig. 5a).
Fig. 5.
A forest plot shows the overall effect and subgroup analysis based on the type and dose of GLP-1RAs on a FPG and b HbA1c
HbA1c (%)
The analysis included six trials with four publications [26, 28, 38, 39] with a total of 2568 patients in the GLP-1RAs arm and 1274 patients in the control arm. Evidence with a low degree of certainty demonstrated that the GLP-1 group significantly reduced HbA1c levels when compared to the control group [MD = − 0.29, 95% CI (− 0.36, − 0.23), P < 0.00001] (Fig. 5b). High heterogeneity was observed [I2 = 81%, P = 0.0001]. The heterogeneity was eliminated by excluding Le Roux et al. 2017 [26] [I2 = 0%, P = 0.74] without significant change in the pooled analysis [MD = − 0.32, 95% CI (− 0.35, − 0.29), P < 0.00001]. Semaglutide 2.4mg QW significantly outperformed the control group in the subgroup analysis [MD = − 0.32, 95% CI (− 0.35, − 0.28), P < 0.00001], and the pooled analysis was homogenous (P > 0.1) (Fig. 5b).
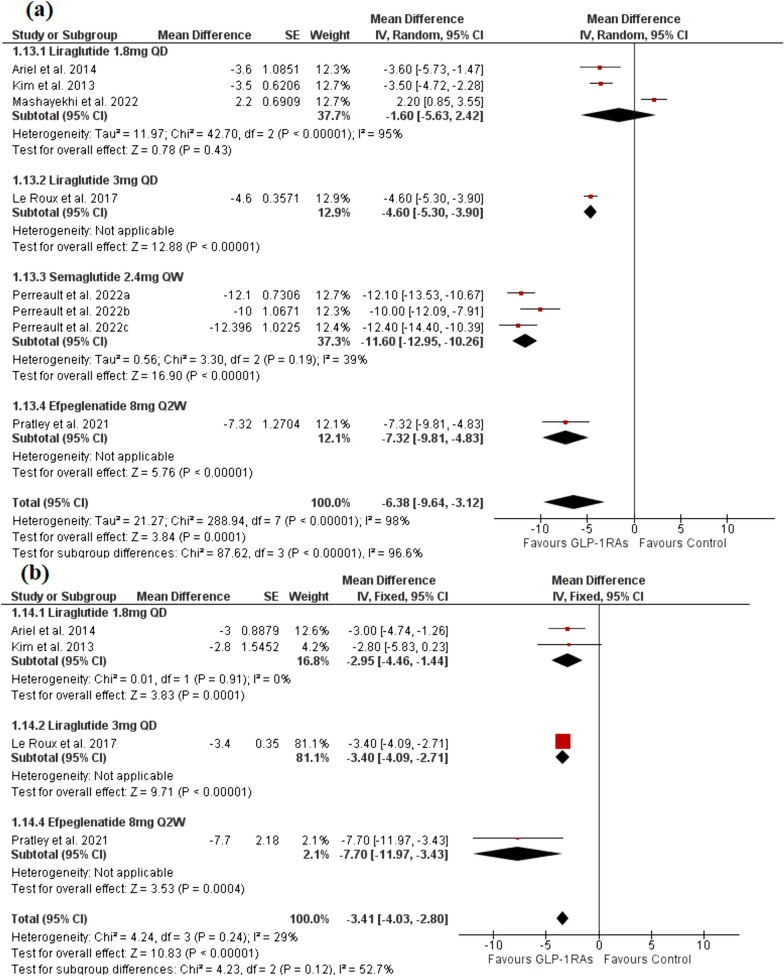
Weight loss (kg)
The analysis included eight trials with six publications [26, 28, 39–41, 43] with a total of 2636 patients in the GLP-1RAs arm and 1297 patients in the control arm. Evidence with a very low degree of certainty demonstrated that the GLP-1 group significantly reduced body weight when compared to the control group [MD = − 6.38, 95% CI (− 9.64, − 3.12), P = 0.0001] (Fig. 6a). There was significant heterogeneity [I2 = 98%, P < 0.00001], which could not be resolved with the statistical analysis. Semaglutide 2.4mg QW significantly outperformed the control group in the subgroup analysis [MD = − 11.60, 95% CI (− 12.95, − 10.26), P < 0.00001] with the pooled analysis being homogenous (P > 0.1) while, liraglutide 1.8mg showed no significant difference compared to the control group in the subgroup analysis [MD = -1.60, 95% CI (− 5.63, 2.42), P = 0.43] (Fig. 6a). The pooled analysis was heterogeneous (P < 0.00001), which was resolved by the exclusion of Mashayekhi et al. 2022 (P > 0.01), after which the pooled analysis significantly favored the liraglutide 1.8mg group [MD = − 3.52, 95% CI (− 4.58, − 2.47), P = 0.43].
Fig. 6.
A forest plot shows the overall effect and subgroup analysis based on the type and dose of GLP-1RAs on a body weight and b waist circumference
Waist circumference (cm)
The analysis included four trials [26, 39, 41, 43] with a total of 1543 patients in the GLP-1RAs arm and 822 patients in the control arm. Evidence with a moderate degree of certainty demonstrated that t00he GLP-1 group significantly reduced waist circumference compared to the control group [MD = − 3.41, 95% CI (− 4.03, − 2.80), P < 0.00001]. The pooled analysis was homogenous (P > 0.1). The liraglutide 1.8mg group significantly outperformed the control group in the subgroup analysis [MD = − 2.95, 95% CI (− 4.46, − 1.44), P = 0.0001]. The pooled analysis was homogenous (P > 0.1) (Fig. 6b).
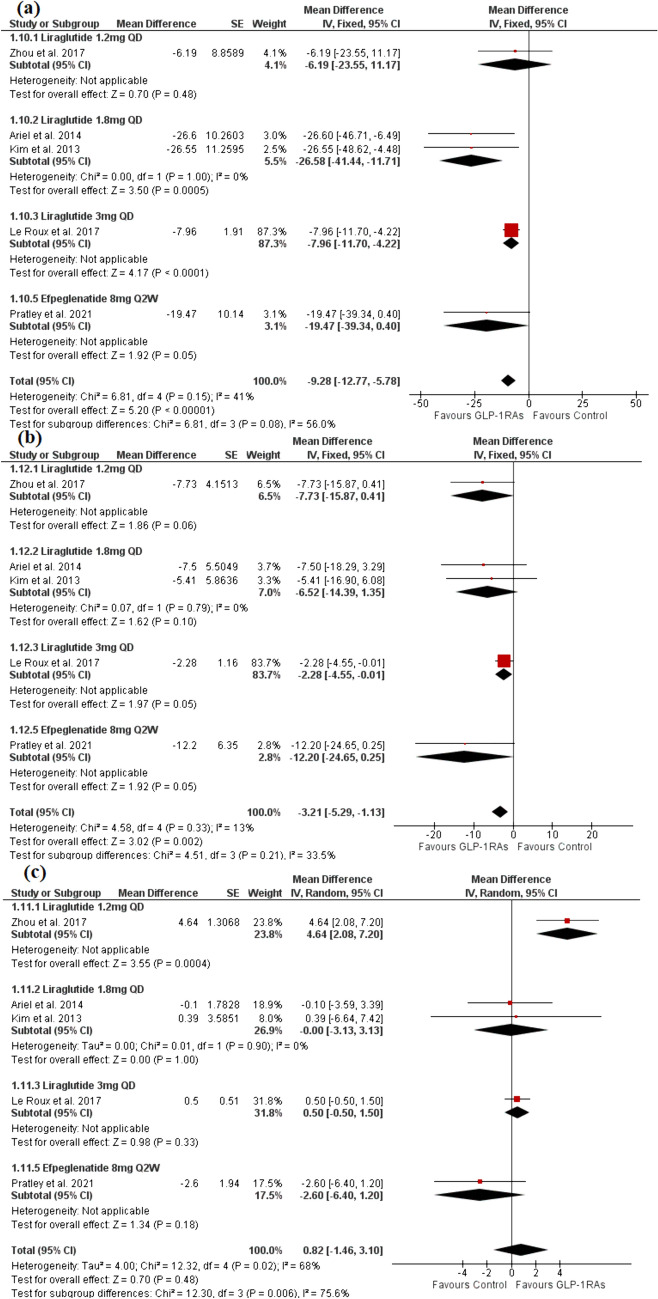
Lipid profile (mg/dl)
The analysis included five trials [26, 38, 39, 41, 43] with a total of 1564 patients in the GLP-1RAs arm and 843 patients in the control arm. The GLP-1 group significantly reduced TG and LDL compared to the control group [MD = − 9.28, 95% CI (− 12.77, − 5.78), P < 0.00001], [MD = − 3.21, 95% CI (− 5.29, − 1.13), P = 0.02] (Fig. 7a and b). The pooled analysis was homogeneous for both outcomes (P > 0.1). However, no significant difference was observed between the control group and the GLP-1 group regarding HDL [MD = 0.82, 95% CI (− 1.46, 3.10), P = 0.48] (Fig. 7c). The pooled analysis was heterogeneous (P = 0.02), which was resolved after the exclusion of Zhou et al. [38] (P > 0.1) without significant effect on the pooled analysis [MD = 0.27, 95% CI (− 0.65, 1.20), P = 0.56]. The evidence was of moderate, low, and very low degrees of certainty for TG, LDL, and HDL, respectively.
Fig. 7.
A forest plot shows the overall effect and subgroup analysis based on the type and dose of GLP-1RAs on a TG, b LDL, and c HDL
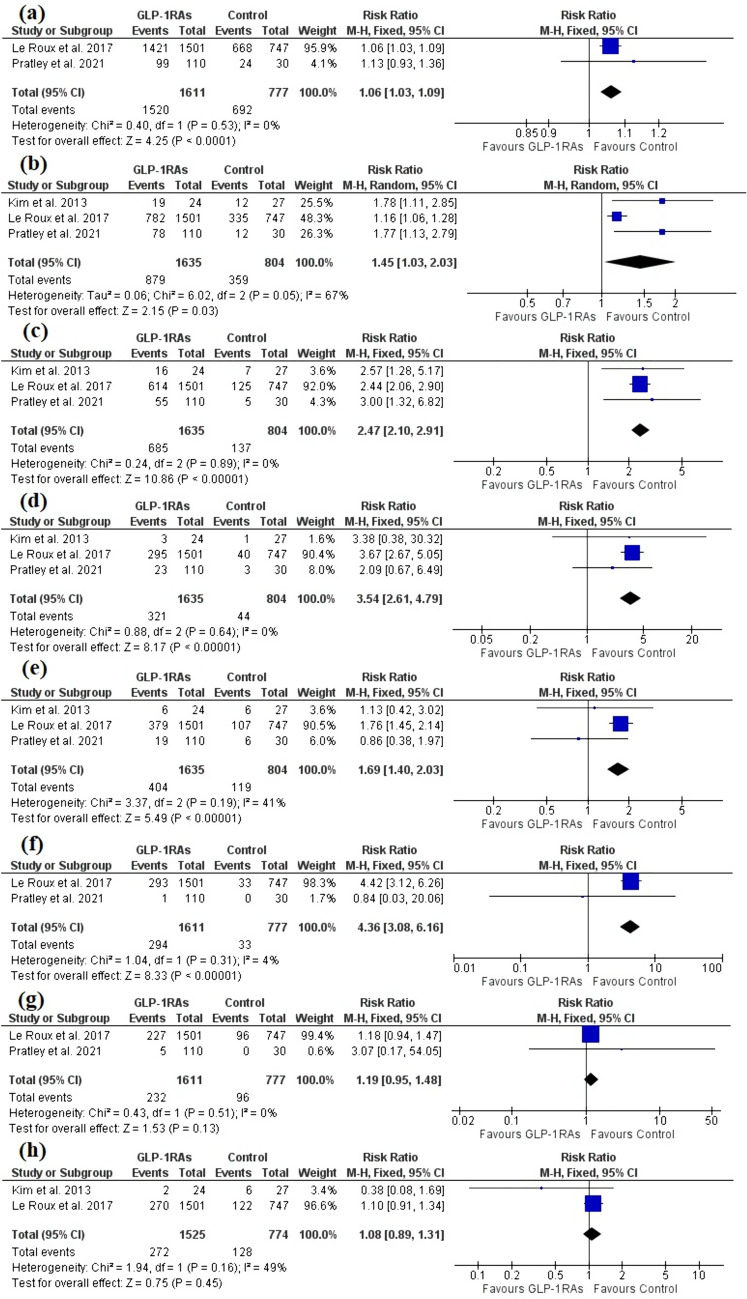
Safety outcomes
Any gastrointestinal disorders, nausea, vomiting, and diarrhea adverse events were reported for prediabetic patients by three trials [26, 39, 43] with a total of 1635 patients in the GLP-1RAs arm and 804 patients in the control arm, and GLP-1RAs revealed a higher incidence of the aforementioned adverse events compared to the control [RR = 1.45, 95% CI (1.03, 2.03), P < 0.00001], [RR = 2.47, 95% CI (2.10, 2.91), P < 0.00001], [RR = 3.54, 95% CI (2.61, 4.79), P < 0.00001], [RR = 1.69, 95% CI (1.40, 2.03), P < 0.00001], respectively (Fig. 8b–e). All the pooled analyses were homogenous (P > 0.1) except for any gastrointestinal disorders, where significant heterogeneity was present [I2 = 67%, P = 0.05]. The heterogeneity was resolved by excluding Le Roux et al. 2017 [I2 = 0%, P = 0.99] without significant change in the pooled analysis [RR = 1.78, 95% CI (1.27, 2.48), P = 0.0008].
Fig. 8.
A forest plot shows the overall effect of GLP-1RAs on a Any adverse events, b Any gastrointestinal disorders, c nausea, d vomiting, e diarrhea, f hypoglycemia, g serious adverse event, h headache
Any adverse events, hypoglycemia, and any serious adverse events were reported by two trials [26, 39] with a total of 1611 patients in the GLP-1RAs arm and 777 patients in the control arm, and GLP-1RAs revealed a higher incidence of Any adverse events and hypoglycemia [RR = 1.06, 95% CI (1.03, 1.09), P < 0.00001], [RR = 4.36, 95% CI (3.08, 6.16), P < 0.00001], while no significant difference was found regarding any serious adverse events [RR = 1.19, 95% CI (0.95, 1.48), P = 0.13] (Fig. 8a, f, and g). The pooled analyses were homogenous (P > 0.1).
GLP-1RAs did not show significant difference in terms of headache compared to the control [RR = 1.08, 95% CI (0.89, 1.31), P = 0.45], as reported by 2 studies [26, 43]. The pooled analysis was homogenous (P > 0.1) (Fig. 8h).
Sensitivity analysis
Due to the differences in the included studies and patient characteristics, we performed a sensitivity analysis to see the robustness of our analysis. Lundkvist et al. [27] administered GLP-1RA combined with a sodium-glucose co-transporter 2 (SGLT2) inhibitor. Mashayekhi et al. [40] compared GLP-1RAs without lifestyle modification to the hypocaloric diet. Zhou et al. [38] included only patients aged 6 to 18 years old. As a result, these studies were excluded from the analysis, which revealed no significant change on the results.
Trial sequential analysis
The TSA demonstrates that the cumulative z-curve had crossed the trial sequential monitoring boundary for the beneficial effect of GLP-1RAs therapy on the incidence of prediabetes reversion to normoglycemia (Figure S2) and the incidence of new-onset diabetes (Figure S3), and the actual cumulative sample size exceeded the required information size. This showed that the sample size was sufficient to draw firm conclusions regarding the beneficial effect of GLP-1RAs therapy on prediabetes reversion to normoglycemia and reducing the incidence of new-onset diabetes.
Publication bias
The funnel plots are shown in figures S4 to S12. No publication bias was observed in any of the efficacy outcomes as indicated by Egger’s (P > 0.05) and Begg’s (P > 0.05) tests, except for LDL, where there was significant publication bias as indicated by Egger’s test (P = 0.023). After applying the trim-and-fill method, it was revealed that after trimming three studies, no significant effect was noticed on the pooled estimate [MD = − 2.39, 95% CI (− 4.35, − 0.44)] (Figure S9).
Discussion
The present systematic review and meta-analysis aim to evaluate the efficacy and safety of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in the management of prediabetic patients. Our analysis included 11 trials comprising a total of 4,316 prediabetic patients, with follow-up durations ranging from 14 to 104 weeks. The findings of this study provide valuable insights into the potential benefits and limitations of GLP-1RAs in the context of prediabetes management. Our meta-analysis consistently demonstrates the positive effects of GLP-1RAs on prediabetes reversion to normoglycemia, prevention of overt diabetes, glycemic control, anthropometric parameters, and lipid profiles. These results collectively suggest that GLP-1RAs hold promise as a comprehensive therapeutic approach for prediabetic patients.
Our meta-analysis revealed that GLP-1RAs significantly increased the incidence of prediabetes reversion to normoglycemia in prediabetic patients compared to the control group. Prediabetes itself is associated with an increased risk of a spectrum of complications. Prediabetes increases the risk of cardiovascular events such as stroke, myocardial infarction, and cardiovascular cause-specific mortality [44]. Schlesingerc et al. [45] discovered that prediabetes was associated with an increased risk of all-cause mortality and incident cardiovascular events, coronary heart disease, stroke, heart failure, atrial fibrillation, chronic kidney disease, total cancer, total liver cancer, hepatocellular carcinoma, breast cancer, and all-cause dementia, with a moderate certainty of evidence. Furthermore, retinopathy, nephropathy, and peripheral neuropathy were all observed among individuals with prediabetes [46–48]. The mechanism underlying a higher likelihood of the aforementioned complications is proposed to be hypoglycemia-induced modifications in polyol, hexosamine, and protein kinase C (PKC) [49]. Our findings suggest that GLP-1RAs can effectively reverse prediabetes to normoglycemia as early as 14–24 weeks and last up to 68–104 weeks, potentially protecting against prediabetic complications.
Our analysis demonstrated a significant reduction in the incidence of new-onset diabetes in the GLP-1RAs group compared to the control group. Prediabetes has a yearly progression rate to diabetes of 10% [11]. The progression of prediabetic patients to diabetes adds an additional risk of complications, increasing the incidence of cardiovascular diseases such as hypertension, cardiac insufficiency, and coronary heart disease, as well as neurological diseases, renal failure, recurrent infections, retinopathy, and digestive disorders compared to non-diabetic patients [50]. Diabetes was responsible for 6.7 million deaths worldwide in 2021 [4]. Diabetes-related worldwide health spending was estimated to be 966 billion USD [51]. Therefore, pharmacological interventions such as GLP-1RAs can help reduce global economic burden, morbidity, and mortality by preventing the progression of prediabetes to diabetes. The protective effect of GLP-1RAs was observed after 48–56 weeks and lasted for 68–104 weeks; however, no significant effect was observed at 14–24 weeks, indicating that the preventive benefits of GLP-1RAs may take some time to manifest fully. The delayed onset of effect might be attributed to the gradual physiological impact of GLP-1RAs on insulin sensitivity, pancreatic function, and overall glycemic control [52, 53]. These findings are consistent with the natural progression of prediabetes to diabetes and highlight the importance of sustained intervention to achieve significant preventive effects.
GLP-1RAs demonstrated favorable effects on glycemic control, as evidenced by significant reductions in FPG and HbA1c levels compared to the control group. This was consistent across all the included studies. Lowering FPG and HbA1c levels contributes to reduced cardiovascular risk and improved overall health outcomes in diabetic and prediabetic patients [54, 55]. Furthermore, waist circumference has a stronger association with the risk of prediabetes [56]. Moreover, obese men and women have a sevenfold and 12-fold increase in the risk of developing diabetes, respectively [57]. Obesity increases adipose tissue fatty acid release, and visceral fat is related to increased circulating inflammatory cytokines and adhesion molecules, which have been linked to the development of insulin resistance and beta-cell dysfunction [58]. Our findings showed that GLP-1RAs had significant benefits for weight management and waist circumference reduction. This emphasizes their potential as comprehensive interventions for addressing prediabetes and its associated comorbidities and risk factors.
Although GLP-1RAs had favorable effects on body weight and waist circumference across all of the studies included, only Mashayekhi et al. [40] reported the opposite finding, which can be attributed to the fact that it was the only study that compared GLP-1RAs without lifestyle modification to lifestyle modification. This is consistent with earlier research in which lifestyle changes outperformed metformin in terms of bodyweight loss [11]. However, higher adherence to medication may offer comparable effect on the long term.
Our analysis also explored the impact of GLP-1RAs on lipid profiles, revealing notable effects on TG and LDL levels. For TG, this finding was consistent across all five studies that studied lipid profiles except for Zhou et al. [38], who studied liraglutide 1.2mg QD. For LDL, consistent lower values were observed across all the included studies except Rosenstock et al. [29], which could be attributed to the type and dose of GLP-1RAs or the small sample size. These findings suggest that beyond their glycemic benefits, GLP-1RAs may contribute to favorable changes in lipid metabolism, potentially lowering cardiovascular risk in prediabetic patients. The homogeneity observed in the pooled analyses for these outcomes indicates consistent effects across the studies. However, it's worth noting that there was no significant difference in high-density lipoprotein cholesterol (HDL-C) levels between the GLP-1RA group and the control group. These findings warrant further investigation into the underlying mechanisms and the possible dose-dependent or GLP-1RAs type-specific effect on the LDL. While these lipid-lowering effects are encouraging, the clinical implications need to be considered in the context of the broader cardiovascular risk profile of prediabetic patients. The observed reductions in TG and LDL-C levels, when coupled with improvements in glycemic control and other anthropometric measures, could collectively contribute to a more favorable cardiovascular risk profile in this population.
The safety analysis revealed that GLP-1RAs were associated with a higher incidence of gastrointestinal adverse events, such as nausea, vomiting, and diarrhea, compared to the control group. These side effects are consistent with the known gastrointestinal effects of GLP-1RAs, which are generally considered tolerable and transient in nature. These events often occur due to the effects of GLP-1RAs on gastric emptying and satiety. Although they may lead to treatment discontinuation in some cases, appropriate patient education and management strategies can mitigate their impact and improve treatment adherence. It is essential for clinicians to be aware of these potential adverse events and to counsel patients accordingly to ensure adherence to treatment. Importantly, our analysis did not find a significant increase in serious adverse events associated with GLP-1RA use in prediabetic patients. This finding is reassuring and suggests that GLP-1RAs can be tolerable for use in this population with an overall favorable benefit-risk profile.
According to the American Diabetes Association, the current recommended treatment for prediabetes is metformin along with lifestyle modification [59]. We revealed that GLP-1RAs combined with lifestyle modification resulted in better management than lifestyle modification alone. Our findings guide future guidelines to include GLP-1RAs as an addition to lifestyle modification instead of metformin in the management of prediabetic individuals who are intolerant to metformin. Further comparative studies are needed to identify the most effective medication along with lifestyle modification.
Strengths and limitations
A comprehensive, large-scale search was conducted in the present study to retrieve all potentially relevant studies. Furthermore, the majority of the studies included were of high quality, and no publication bias was found. The majority of the pooled effects of the outcomes were homogenous, adding to the robustness of the findings. We performed a sensitivity analysis by omitting studies with different characteristics to evaluate how robust our conclusions are, and the results showed no significant changes. However, there are some limits. Some of the included studies did not mention if patients did not take any therapies that could directly or indirectly influence circulating glucose values. However, the majority reported that they excluded patients receiving weight loss medications or medications affecting carbohydrate metabolism. Our primary outcome had a high degree of heterogeneity that could only be partially resolved. However, subgroup analysis revealed that the duration of the treatment could explain this. Furthermore, due to limited data, we were unable to draw firm conclusions about the effects of different doses and types of GLP-1RAs.
Conclusions
GLP-1RAs added to lifestyle modification achieved better effects on prediabetes reversion to normoglycemia, prevention of overt diabetes, glycemic control, anthropometric parameters, and lipid profiles, with a tolerable safety profile without significant serious adverse events. Future guidelines may propose the use of GLP-1RAs along with lifestyle modification to improve prediabetic patient management and therapy adherence. In addition, for people with metformin intolerance, GLP-1RAs may be a feasible alternative therapy. Future studies are needed to establish the most effective medication that can be used in combination with lifestyle modification.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ADA
American Diabetes Association
- AEs
Adverse events
- BMI
Body mass index
- CI
Confidence intervals
- FPG
Fasting plasma glucose
- GLP-1RAs
Glucagon-like peptide 1 receptor agonists
- HbA1c
Glycated hemoglobin
- HDL
High-density lipoprotein
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- LDL
Low-density lipoprotein
- MD
Mean difference
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCTs
Randomized controlled trials
- ROB
Risk of bias
- RR
Risk ratio
- SD
Standard deviation
- T2DM
Type 2 diabetes mellitus
- TG
Triglyceride
Author contributions
H.M.S. validated the idea, conducted the search, performed the analysis and wrote the results and methods sections, revised the whole manuscript, participated in the two phases of screening, formulated the extraction sheets, and drafted the tables and figures. A.M. wrote the introduction, discussion, and conclusions sections. M.A. took part in the data extraction and the two phases of screening. K.A.A. took part in the data extraction and the two phases of screening. S.E. took part in the data extraction. W.A.F.E. took part in the quality assessment. K.A.A. took part in the quality assessment. D.M. took part in the data extraction. J.D. took part in the quality assessment. R.B. took part in the quality assessment. A.T. revised and performed language editing. L.I. participated in the two phases of screening. Z.Z. revised the manuscript and performed language editing. A.S. revised the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data analyzed during this study are included in this published article or listed in references.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH, Federation ID. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia : report of a WHO/IDF consultation. Geneva: World Health Organization.
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 4.Magliano DJ, Boyko EJ, committee IDA 10th edition scientific. IDF DIABETES ATLAS. International Diabetes Federation; 2021; 54–5.
- 5.Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: the PROMISE cohort. Diabetes Care. 2015;38:793–800. doi: 10.2337/dc14-2585. [DOI] [PubMed] [Google Scholar]
- 6.Kirthi V, Nderitu P, Alam U, Evans JR, Nevitt S, Malik RA, et al. The prevalence of retinopathy in prediabetes: a systematic review. Surv Ophthalmol. 2022;67:1332–1345. doi: 10.1016/j.survophthal.2022.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Echouffo-Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta-analysis. Diabet Med. 2016;33:1615–1624. doi: 10.1111/dme.13113. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016 doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42:S29–33. [DOI] [PubMed]
- 10.Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res Clin Pract. 2017;132:169–170. doi: 10.1016/j.diabres.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 13.Pedrosa MR, Franco DR, Gieremek HW, Vidal CM, Bronzeri F, de Cassia RA, et al. GLP-1 agonist to treat obesity and prevent cardiovascular disease: what have we achieved so far? Curr Atheroscler Rep. 2022;24:867–884. doi: 10.1007/s11883-022-01062-2. [DOI] [PubMed] [Google Scholar]
- 14.Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346: 393–403. [DOI] [PMC free article] [PubMed]
- 15.Prediabetes—your chance to prevent type 2 diabetes|CDC. https://www.cdc.gov/diabetes/basics/prediabetes.html. Accessed 12 Jul 2023.
- 16.Rizzo M, Rizvi AA, Patti AM, Nikolic D, Giglio RV, Castellino G, et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol. 2016;15:162. doi: 10.1186/s12933-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalra S, Baruah M, Sahay R, Unnikrishnan A, Uppal S, Adetunji O. Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes: past, present, and future. Indian J Endocrinol Metab. 2016;20:254. doi: 10.4103/2230-8210.176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolic D, Patti AM, Giglio RV, Chianetta R, Castellino G, Magán-Fernández A, et al. Liraglutide improved cardiometabolic parameters more in obese than in non-obese patients with type 2 diabetes: a real-world 18-month prospective study. Diabetes Ther. 2022;13:453–464. doi: 10.1007/s13300-022-01217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giglio RV, Pantea Stoian A, Al-Rasadi K, Banach M, Patti AM, Ciaccio M, et al. Novel therapeutical approaches to managing atherosclerotic risk. Int J Mol Sci. 2021;22:4633. doi: 10.3390/ijms22094633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patti AM, Rizvi AA, Giglio RV, Stoian AP, Ligi D, Mannello F. Impact of glucose-lowering medications on cardiovascular and metabolic risk in type 2 diabetes. J Clin Med. 2020;9:912. doi: 10.3390/jcm9040912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patti AM, Nikolic D, Magan-Fernandez A, Giglio RV, Castellino G, Chianetta R, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: An 8-month prospective study. Diabetes Res Clin Pract. 2019;149:163–169. doi: 10.1016/j.diabres.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Patti AM, Giglio RV, Papanas N, Rizzo M, Rizvi AA. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expert Rev Clin Pharmacol. 2019;12:129–143. doi: 10.1080/17512433.2019.1567328. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo M, Abate N, Chandalia M, Rizvi AA, Giglio RV, Nikolic D, et al. Liraglutide reduces oxidative stress and restores heme oxygenase-1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J Clin Endocrinol Metab. 2015;100:603–606. doi: 10.1210/jc.2014-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giglio RV, Nikolic D, Volti GL, Stoian AP, Banerjee Y, Magan-Fernandez A, et al. Liraglutide increases serum levels of microRNA-27b, -130a and -210 in patients with type 2 diabetes mellitus: a novel epigenetic effect. Metabolites. 2020;10:391. doi: 10.3390/metabo10100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo M, Nikolic D, Banach M, Giglio RV, Patti AM, Di BV, et al. The effects of liraglutide on glucose, inflammatorymarkersandlipoprotein metabolism: current knowledge and future perspective. Clin Lipidol. 2013;8:173–181. doi: 10.2217/clp.13.8. [DOI] [Google Scholar]
- 26.le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 27.Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once-daily and exenatide once-weekly dual therapy: a 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19:49–60. doi: 10.1111/dom.12779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perreault L, Davies M, Frias JP, Laursen PN, Lingvay I, Machineni S, et al. Changes in glucose metabolism and glycemic status with once-weekly subcutaneous semaglutide 2.4 mg among participants with prediabetes in the STEP program. Diabetes Care. 2022;45:2396–405. doi: 10.2337/dc21-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstock J, Klaff LJ, Schwartz S, Northrup J, Holcombe JH, Wilhelm K, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33:1173–1175. doi: 10.2337/dc09-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook.
- 32.Higgins JP, Savović J, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) Full Guidance Document. Br Med J. 2019;1–72.
- 33.Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020.
- 34.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 36.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 37.Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q-X, Wang Z-Y, Zhao H-F, Wang S. The effects of GLP-1 analogues on pre-diabetes of the children. Exp Ther Med. 2017;13:1426–1430. doi: 10.3892/etm.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratley RE, Jacob S, Baek S, Trautmann ME, Hompesch M, Han O, et al. Efficacy and safety of efpeglenatide in key patient subgroups from the BALANCE randomized trial, stratified by pre-diabetes status, BMI, and age at baseline. BMJ Open Diabetes Res Care. 2022;10:e002207. doi: 10.1136/bmjdrc-2021-002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mashayekhi M, Beckman JA, Nian H, Garner EM, Mayfield D, Devin JK, et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: a randomized controlled trial. Diabetes, Obes Metab. 2023;25:570–580. doi: 10.1111/dom.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ariel D, Kim SH, Abbasi F, Lamendola CA, Liu A, Reaven GM. Effect of liraglutide administration and a calorie-restricted diet on lipoprotein profile in overweight/obese persons with prediabetes. Nutr Metab Cardiovasc Dis. 2014;24:1317–1322. doi: 10.1016/j.numecd.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean MEJ, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36:3276–3282. doi: 10.2337/dc13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation. 2014;130:1374–1382. doi: 10.1161/CIRCULATIONAHA.114.010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlesinger S, Neuenschwander M, Barbaresko J, Lang A, Maalmi H, Rathmann W, et al. Prediabetes and risk of mortality, diabetes-related complications and comorbidities: umbrella review of meta-analyses of prospective studies. Diabetologia. 2022;65:275–285. doi: 10.1007/s00125-021-05592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahar A, Makhlough A, Yousefi A, Kashi Z, Abediankenari S. Correlation between prediabetes conditions and microalbuminuria. Nephrourol Mon. 2013;5:741–744. doi: 10.5812/numonthly.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol. 2011;7:682–690. doi: 10.1038/nrendo.2011.113. [DOI] [PubMed] [Google Scholar]
- 48.Lamparter J, Raum P, Pfeiffer N, Peto T, Höhn R, Elflein H, et al. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: the Gutenberg health study. J Diabetes Complications. 2014;28:482–487. doi: 10.1016/j.jdiacomp.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Brannick B, Wynn A, Dagogo-Jack S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp Biol Med. 2016;241:1323–1331. doi: 10.1177/1535370216654227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 51.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brunton SA, Wysham CH. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. 2020; 132:3–14. [DOI] [PubMed]
- 53.Kaneto H, Kimura T, Shimoda M, Obata A, Sanada J, Fushimi Y, et al. Favorable effects of GLP-1 receptor agonist against pancreatic β-cell glucose toxicity and the development of arteriosclerosis: “the earlier, the better” in therapy with incretin-based medicine. Int J Mol Sci. 2021;22:7917. doi: 10.3390/ijms22157917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7:e015949. doi: 10.1136/bmjopen-2017-015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu PC, Bosnyak Z, Ceriello A. The importance of glycated haemoglobin (HbA(1c)) and postprandial glucose (PPG) control on cardiovascular outcomes in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:1–9. doi: 10.1016/j.diabres.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Li S, Xiao J, Ji L, Weng J, Jia W, Lu J, et al. BMI and waist circumference are associated with impaired glucose metabolism and type 2 diabetes in normal weight Chinese adults. J Diabetes Complications. 2014;28:470–476. doi: 10.1016/j.jdiacomp.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Algoblan A, Alalfi M, Khan M. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes Targets Ther. 2014;4:587. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American Diabetes Association 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S34–9. doi: 10.2337/dc21-S003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this published article or listed in references.