Abstract
BACKGROUND:
The extent of cardiac damage and its association with clinical outcomes in patients undergoing transcatheter edge-to-edge repair (TEER) for degenerative mitral regurgitation remains unclear. This study was aimed to investigate cardiac damage in patients with degenerative mitral regurgitation treated with TEER and its association with outcomes.
METHODS:
We analyzed patients with degenerative mitral regurgitation treated with TEER in the Optimized Catheter Valvular Intervention-Mitral registry, which is a prospective, multicenter observational data collection in Japan. The study subjects were classified according to the extent of cardiac damage at baseline: no extravalvular cardiac damage (stage 0), mild left ventricular or left atrial damage (stage 1), moderate left ventricular or left atrial damage (stage 2), or right heart damage (stage 3). Two-year mortality after TEER was compared using Kaplan-Meier analysis.
RESULTS:
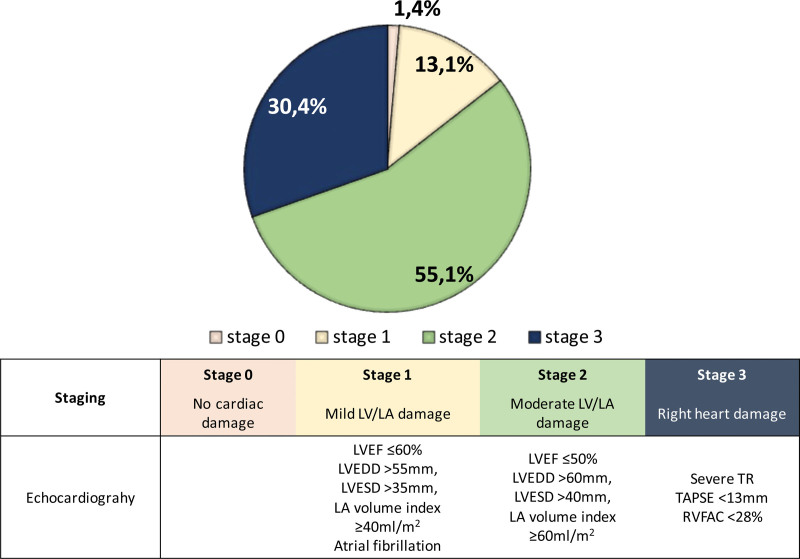
Out of 579 study participants, 8 (1.4%) were classified as stage 0, 76 (13.1%) as stage 1, 319 (55.1%) as stage 2, and 176 (30.4%) as stage 3. Two-year survival was 100% in stage 0, 89.5% in stage 1, 78.9% in stage 2, and 75.3% in stage 3 (P=0.013). Compared with stage 0 to 1, stage 2 (hazard ratio, 3.34 [95% CI, 1.03–10.81]; P=0.044) and stage 3 (hazard ratio, 4.51 [95% CI, 1.37–14.85]; P=0.013) were associated with increased risk of 2-year mortality after TEER. Significant reductions in heart failure rehospitalization rate and New York Heart Association functional scale were observed following TEER (both, P<0.001), irrespective of the stage of cardiac damage.
CONCLUSIONS:
Advanced cardiac damage is associated with an increased risk of mortality in patients undergoing TEER for degenerative mitral regurgitation.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: UMIN000023653.
Keywords: cardiac damage, degenerative mitral regurgitation, heart failure, risk stratification, transcatheter edge-to-edge repair
WHAT IS KNOWN
Degenerative mitral regurgitation is a disease of the mitral valve apparatus, developing heart failure symptoms and excess mortality risk. Although the introduction of transcatheter edge-to-edge repair has changed the landscape of the clinical practice of degenerative mitral regurgitation, data on risk prediction in this new treatment entity remain limited.
WHAT THE STUDY ADDS
The staging classification of cardiac damage in patients with degenerative mitral regurgitation offers prognostic implications for clinical outcomes.
Advanced cardiac damage is associated with an increased risk of mortality after transcatheter edge-to-edge repair.
Transcatheter edge-to-edge repair may mitigate the risk of mortality rehospitalization and improve their symptomatic status, irrespective of the extent of cardiac damage.
Degenerative mitral regurgitation (DMR) is a disease of the mitral valve apparatus, and the pathologies of the mitral valve components (ie, leaflets, chordae, papillary muscles) cause incompetence of valves, with regurgitant blood flow from the left ventricle to the left atrium. DMR is a serious condition, developing heart failure symptoms and excess mortality risk. The incidence of DMR is high in elderly patients and increases in the global community owing to its aging society.1,2 Although surgical mitral valve correction is an established standard therapy for young, low-surgical risk patients,3,4 a large amount of the elderly population is often deemed at high-surgical risk because of advanced age and multiple comorbidities.2 The introduction of transcatheter edge-to-edge repair (TEER) has changed the landscape of the clinical practice of DMR,5 which is a safe alternative to surgery if high perioperative risks are expected.3,4 However, data on risk prediction in this new treatment entity remain to be investigated.
A staging classification of cardiac damage has been proposed and applied in patients with chronic heart failure or aortic stenosis, showing a good ability for risk prediction of mortality.6,7 Namely, the involvements of extravalvular damages are found to be associated with outcomes. A similar association was recently demonstrated in patients with DMR treated with surgery.8 However, no study has investigated a staging classification of cardiac damage in patients undergoing TEER for DMR and its association with outcomes. If significant volume overload of DMR persists, it may provoke myocardial damage,9 resulting in heart failure and death. Vice versa, TEER may regress cardiac damage by reducing mitral regurgitation (MR), thereby improving clinical outcomes.
The purpose of this study is to assess the extent of cardiac damage in patients undergoing TEER for DMR and to investigate its association with clinical outcomes.
METHODS
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was designed as a retrospective analysis of data from the Optimized Catheter Valvular Intervention-Mitral registry, which is a multicenter, consecutive, prospective data collection of patients undergoing transcatheter mitral valve interventions in Japan.10 MitraClip (Abbott Vascular Inc, Santa Clara, CA) has been the only commercially available transcatheter system for treating MR since April 2018, starting with the G2 system, whereas the MitraClip G4 system has also been launched since September 2020. In the present study, pooled data of patients with DMR treated with the MitraClip system from April 2018 to June 2021 were reviewed for analysis. The study registry was approved by the institutional review committee of Keio University and recorded with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000023653). All subjects received informed consent. The data collection and analysis were conducted in accordance with the provisions of the Declaration of Helsinki and the guidelines for epidemiological studies issued by the Ministry of Health, labor, and Welfare of Japan.
Definition of Staging Classification of Cardiac Damage
Parameters in the staging classification were selected based on the previous literature and current guidelines for DMR.3,4,6,9 We also considered the simplicity of acquisition. All patients were categorized into 4 stages according to the presence or absence of extravalvular cardiac remodeling or dysfunction as assessed by transthoracic echocardiography at baseline—stage 0: no cardiac damage detected; stage 1: mild left-entricular (LV) or atrial (LA) damage as defined by the presence of LV ejection fraction ≤60%, LV end-diastolic diameter >55 mm, LV end-systolic diameter >35 mm, LA volume index ≥40 mL/m2, or atrial fibrillation; stage 2: moderate LV or LA damage as defined by the presence of LV ejection fraction ≤50%, LV end-diastolic diameter >60 mm, LV end-systolic diameter >40 mm, or LA volume index ≥60 mL/m2; stage 3: right heart damage as defined by the presence of tricuspid regurgitation (TR) severe or severe right ventricle dysfunction (tricuspid annular plane systolic excursion <13 mm or right ventricle fractional area change <28%).3,4,6,11,12
Follow-Up
After the procedure, clinical and echocardiographic data were prospectively collected during scheduled outpatient clinic visits and registered on the internet-based system. The database was checked by self-audit on each site. Data committee members regulated the completeness and consistency of the database and regularly sent queries to each center. Telephone interviews directed to patients or their families were alternatively performed, if necessary.
Study End Point
The primary end point of the present study was all-cause mortality within 2 years after TEER. The secondary end points included 2-year cardiac death and noncardiac death. Also, we assessed changes in the rate of heart failure rehospitalization and the New York Heart Association (NYHA) functional class within 1 year before after TEER.
Statistical Analysis
All statistical analyses were performed using EZR version 1.37 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), R version 4.3.0 (R Foundation for Statistical Computing), or IBM SPSS Statistics 26 (IBM Corporation, New York).
We compared baseline demographics and clinical outcomes among the cardiac damage staging. The Cochran-Armitage test was applied to test whether there was a linear trend in categorical variables according to the stages, whereas the Jonckheere-Terpstra test was used to assess a trend in continuous variables. Continuous variables are presented as mean and SD or median and interquartile range. Categorical variables are expressed as numbers and percentages.
Time-to-event curves are depicted using the Kaplan-Meier method and compared between the cardiac stages using the log-rank test to assess the clinical outcomes according to the cardiac damage staging. The risk of 2-year mortality according to cardiac staging classification was assessed using Cox proportional hazard model. Age, sex, coronary artery disease, estimated glomerular filtration ratio, NYHA functional class, and residual MR ≥2+ were included in the multivariable models to adjust the association.13–15 Proportional hazards assumptions were tested for all models, and no violations were found. As a sensitivity analysis, a Cox proportional hazard model stratified by age, sex, NYHA functional class, and history of heart failure was conducted. Interaction P values are provided. Furthermore, we assessed the associations of each variable in the staging classification with outcomes. Finally, we applied logistic regression analysis to examine the association of each factor in the staging classification with the risk of residual MR after TEER.
The changes in categorical variables from baseline to follow-up were tested using Wilcoxon’s signed rank test. In addition to heart failure hospitalization and NYHA functional scale, we explored the echocardiographic follow-up within 1 year, including the severity of MR and repeat mitral valve treatment, according to the extent of cardiac damage. The log-rank test was applied to compare 2-year mortality between patients with MR ≥3+ or repeat mitral valve treatment during the follow-up.
RESULTS
Study Population and Baseline Characteristics
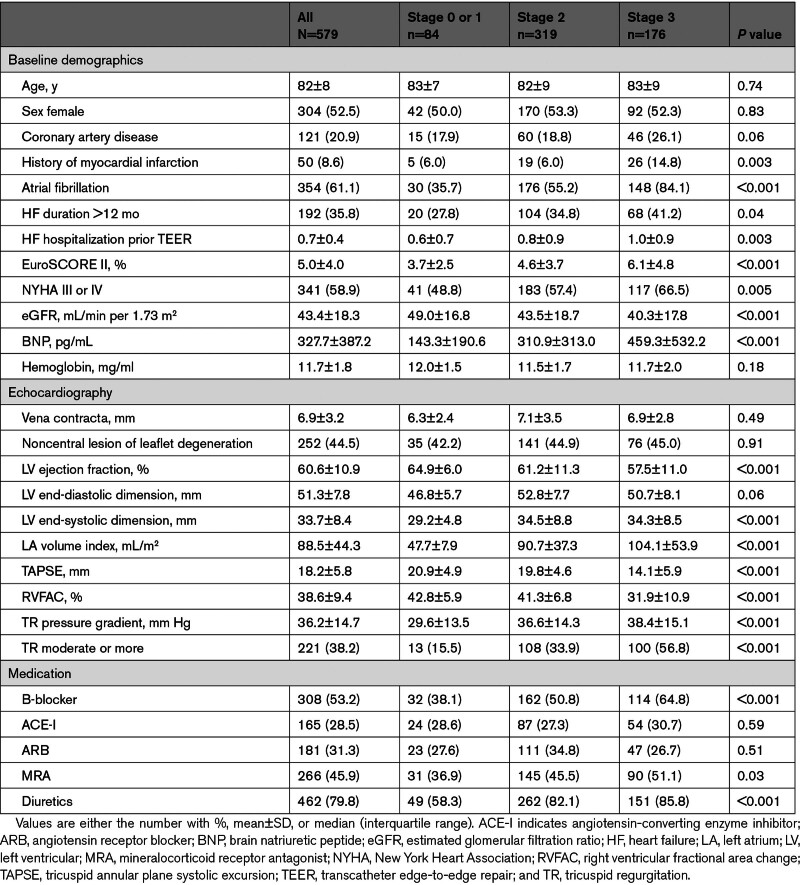
From the Optimized Catheter Valvular Intervention-Mitral registries, 639 patients with DMR were treated with TEER. After excluding patients due to the incomplete data collection regarding cardiac damage assessment, 579 patients were included in the present analysis. The study participants were old (mean age: 82.5±8.4) and predominantly female (52.5%). At the time of TEER, 8 (1.4%) were in stage 0 (no cardiac damage), 76 (13.1%) were in stage 1 (mild LV or LA damage), 319 (55.1%) were in stage 2 (moderate LV or LA damage), and 176 (30.4%) were in stage 3 (right heart damage). The prevalence of the cardiac stages is shown in Figure 1. There were significant trends in baseline characteristics according to the cardiac stage classifications (Table 1). Compared with patients in stages 0 to 1, patients in stages 2 and 3 more often had a history of myocardial infarction, longer heart failure duration, higher NYHA functional scale, lower estimated glomerular filtration ratio, and elevated brain natriuretic peptide.
Figure 1.
Staging stratification of degenerative mitral regurgitation based on the extent of cardiac damage. Cardiac stratification of degenerative mitral regurgitation based on the extent of cardiac damage. LA indicates left atrial; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion; and TR, tricuspid regurgitation.
Table 1.
Patient Characteristics
Procedural Outcomes
Procedural characteristics are summarized in Table S1. Compared with patients with cardiac damage in stage 0 to 1, patients with advanced cardiac damage (stages 2 or 3) were less likely to achieve a reduction in MR to ≤1+(81.5% in stage 0–1; 68.7% in stage 2; 66.7% in stage 3) and had a longer duration of hospital stay (9 days [interquartile range, 7–15 days] in stages 0–1; 11 days [interquartile range, 7–20 days] in stage 2; 16 days [interquartile range, 9–30 days] in stage 3). The associations of each cardiac damage factor with residual MR are listed in Table S2. There were no significant differences in the type of implanted devices or postprocedural transmitral pressure gradient.
Extent of Cardiac Damage and Its Association With Clinical Outcome
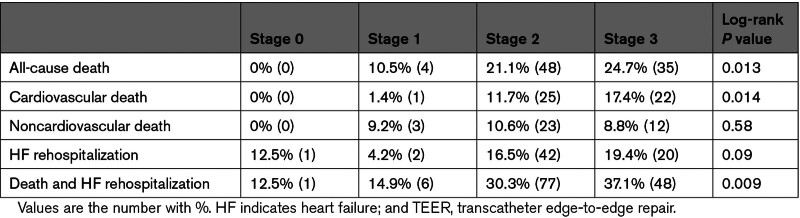
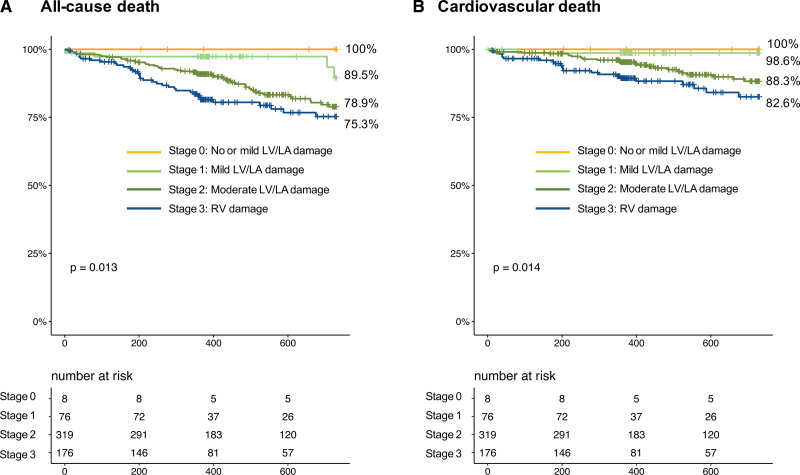
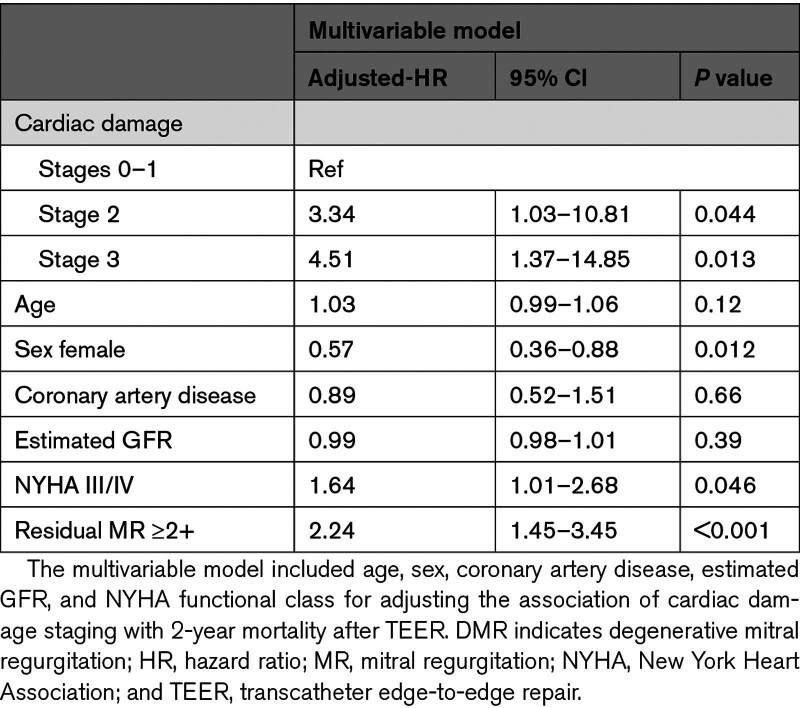
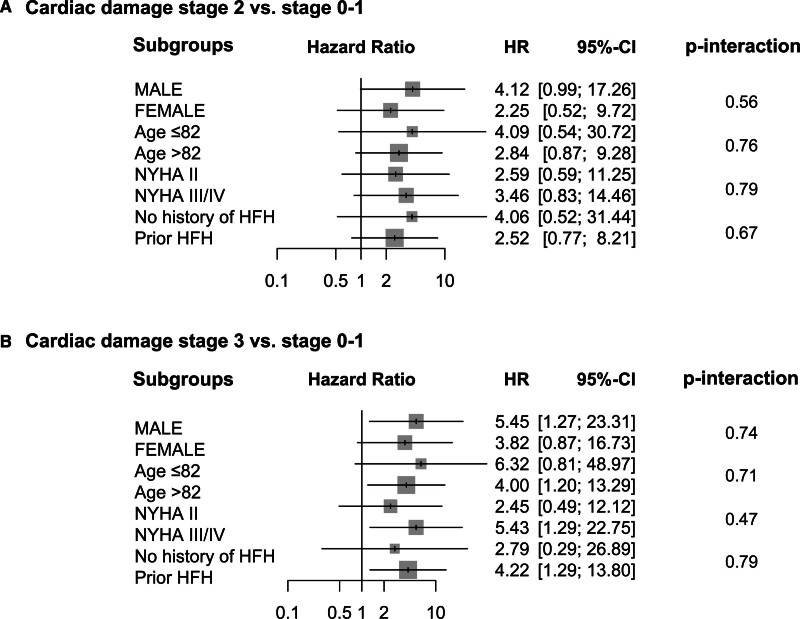
With the median follow-up duration of 527 days (interquartile range, 365–739 days), 87 patients died, with cardiovascular causes identified in 48 patients and noncardiovascular death in 39 patients. At 2 years, all-cause mortality increased with the advancement of cardiac damage (Table 2; Figure 2): 1-year survival was 100% in stage 0, 89.5% in stage 1, 78.9% in stage 2, and 75.3% in stage 3 (log-rank P=0.013). The difference was mainly derived from cardiovascular mortality (log-rank P=0.014). In contrast, noncardiovascular death was comparable among the staging groups (log-rank P=0.58). The associations of each cardiac damage factor with 2-year mortality are listed in Table S3. After the multivariable adjustment, the advanced stages of cardiac damage were independently associated with excess mortality compared with the lower degree of cardiac damage (stage 2 versus stages 0–1: adjusted hazard ratio, 3.34 [95% CI, 1.03–10.81]; P=0.044; stage 3 versus stage 0–1: adjusted hazard ratio, 4.51 [95% CI, 1.37–14.85]; P=0.013; Table 3). Sex female, NYHA functional class III/IV, and residual MR ≥2+ were the other factors associated with 2-year mortality after TEER. Also, no interaction was detected between age, sex, NYHA functional class, or prior history of heart failure with the stage of cardiac damage in regard to the 2-year mortality (Figure 3).
Table 2.
Two-Year Outcomes After TEER
Figure 2.
Two-year outcomes after transcatheter edge-to-edge repair according to the extent of cardiac damage. A, Survival curves according to cardiac damage. B, Event-free survival from cardiovascular mortality. LA indicates left atrial; LV, left ventricular; and RV, right ventricular.
Table 3.
Association of Cardiac Damage Staging With 2-Year Mortality After TEER for DMR
Figure 3.
Association of cardiac stage with mortality in selected subgroups. Forrest plots show the association of cardiac damage with all-cause mortality in each subgroup. HFH indicates heart failure hospitalization; HR, hazard ratio; and NYHA, New York Heart Association.
Additionally, we incorporated residual MR into the analysis. Overall, postprocedural MR severity was assessed in 571 patients, whereas residual MR ≥2+ was observed in 171 patients (29.9%). At 2 years, survival rates were 85.1% (95% CI, 80.0%–89.0%) in patients with residual MR ≤1+ and 65.7% (95% CI, 55.2%–74.2%) in those with residual MR ≥2+ (P<0.001). The superior outcomes of residual MR ≥2+ over residual MR ≤1+ were consistent among the staging of cardiac damage, while, with the limited sample size, no significant difference was seen in stage 0 to 1 (92.3% [95% CI, 72.6%–98.0%] versus 92.9% [95% CI, 59.1%–99.0%]; P=0.29) but in stage 2 (84.3% [95% CI, 77.3%–89.3%] versus 65.4% [95% CI, 50.8%–76.6%]; P=0.011) and stage 3 (82.5% [95% CI, 71.7%–89.5%] versus 61.5% [95% CI, 45.2%–74.2%]; P<0.001; Figure S1).
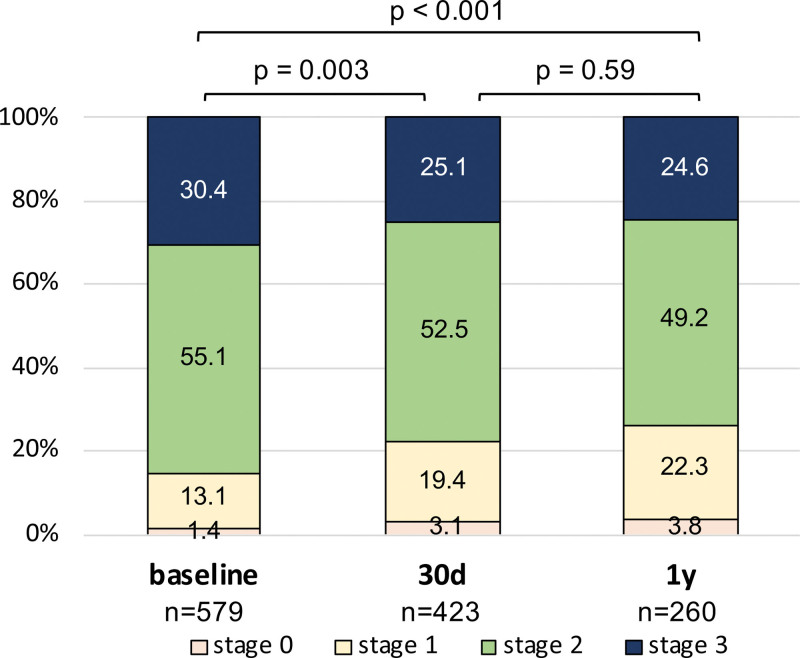
Furthermore, we assessed the development of cardiac damage over time. A downgrade of the staging classification was modestly observed following TEER (Figure 4). At baseline, 85.5% of patients were deemed as having stages 2 or 3, which declined to 77.6% at 30 days (P=0.003) and 73.8% at 1 year (P<0.001) after TEER.
Figure 4.
Regress in staging classification after transcatheter edge-to-edge repair. Distribution of the cardiac damage stages at baseline, 30 d, and 1 y.
Heart Failure Hospitalization Prior and After TEER
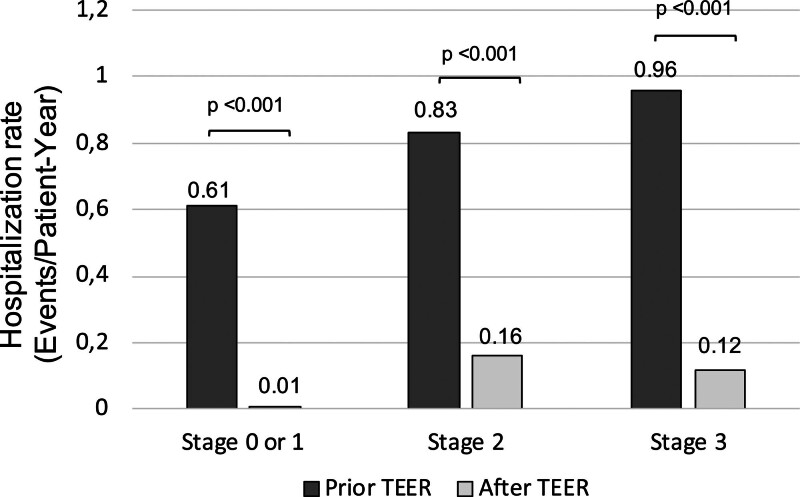
The heart failure hospitalization rates before and after TEER were assessed among patients with 1-year follow-up (n=539). The hospitalization rate decreased from 0.83±0.86 to 0.13±0.47 events/patient-year from before and after TEER, with a reduction rate of 84%. The heart failure hospitalization rates consistently reduced in each stage of cardiac damage (Figure 5). Patients with advanced cardiac damage (ie, stages 2 or 3) remained to have a higher incidence of hospitalization after TEER compared with stages 0 or 1.
Figure 5.
Reduction in hospitalization after transcatheter edge-to-edge repair (TEER). Hospitalization rates (events per patient-year) before and after TEER.
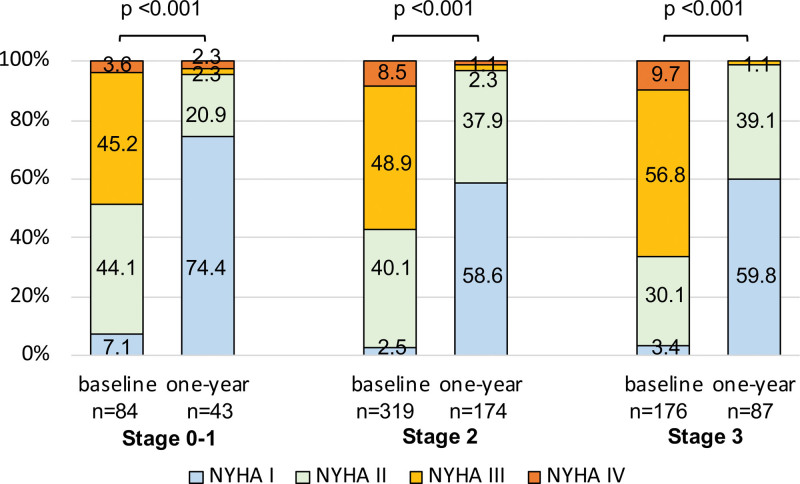
Improvement in NYHA Functional Scale According to Cardiac Damage
After TEER, a significant improvement in heart failure symptoms was consistently observed regardless of cardiac damage (Figure 6). Although patients with advanced cardiac damage were more likely severely symptomatic at baseline (NYHA III or IV: 48.8% in stage 0–1; 57.4% in stage 2; 66.5% in stage 3; P=0.018), there were no significant differences in NYHA functional scale at follow-up (NYHA III or IV: 4.6% in stages 0–1; 3.4% in stage 2; 1.1% in stage 3).
Figure 6.
Change in New York Heart Association (NYHA) functional scale according to cardiac damage. NYHA functional scale at baseline and 1 y according to each stage of cardiac damage.
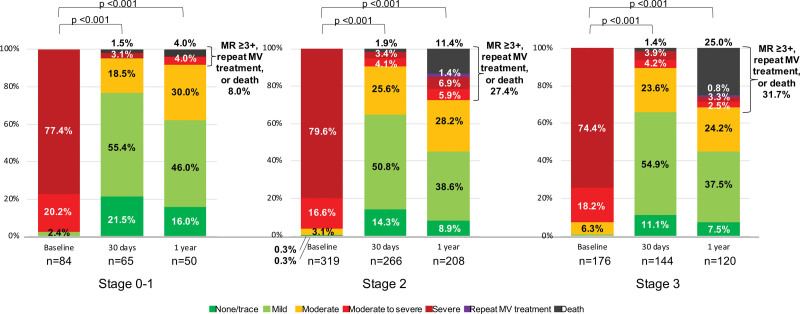
Severity of MR During Follow-Up
Echocardiographic follow-up was performed in 511 patients within 1 year after TEER. At 1 year, a composite of MR ≥3+, repeat mitral valve treatment, and all-cause mortality was observed in 8.0% of patients in stage 0 to 1, 27.4% in stage 2, and 31.7% in stage 3 (Figure 7). Detailed percentages are labeled on each graph. Furthermore, patients who experienced MR ≥3+ or repeat mitral valve treatment during the follow-up showed a lower 2-year survival compared with those without (65.8% [95% CI, 50.7%–77.3%] versus 86.1% [95% CI, 81.3%–89.7%], log-rank P<0.001).
Figure 7.
Echocardiographic follow-up after transcatheter edge-to-edge repair (TEER). Severity of mitral regurgitation (MR), repeat mitral valve (MV) treatment, or mortality at 1-mo and 1-y follow-up after TEER.
DISCUSSION
The main findings of the present study can be summarized as follows: (1) The extent of cardiac damage was associated with 2-year mortality. (2) Significant reductions in hospitalization rate and NYHA functional scale were consistently observed, irrespective of the extent of cardiac damage. (3) A downgrade of the staging classification was modestly observed following TEER, with a sustained improvement over time.
The term degenerative corresponds to the heterogenous process of valve alternations, and DMR is a mechanical problem of the leaflet coaptation, which can be treated either with surgery or transcatheter approaches. The ideal timing for mitral valve interventions may be when patients have heart failure symptoms. However, symptoms do not always coincide with the advancement of the disease, especially in elderly patients. The idea of the staging classification of cardiac damage is developed to aim to assess the extent of extravalvular involvements and refine the risk stratification of patients with aortic stenosis, while its utility for patients with DMR treated with TEER remained to be studied.
In the present study, the mean age was 82 years old, and the mean EuroSCORE II was 5.0%, implying that this less invasive technology has been applied not only to the elderly population but also to the outside of high-surgical risk patients. We found that 85.5% of patients were deemed as having advanced cardiac damage (stages 2 or 3) at baseline, showing signs of moderate LV or LA damage or right heart involvement. Patients with advanced cardiac damage displayed a longer duration of heart failure and multiple hospitalizations before TEER.
Regarding procedural results, device-related complications were infrequent among each group, whereas patients with an advanced stage of cardiac damage were less likely to achieve a reduction in MR to ≤1+. These findings suggest that TEER is safe, regardless of the extent of cardiac damage, whereas functional outcomes of TEER may be altered by the disease progression. For instance, a larger LA volume can pose a challenging morphology for TEER (eg, displacement of the posterior mitral annulus, the restricted motion of the posterior leaflet).16 We demonstrated that LA volume was positively associated with the risk of residual MR after TEER.
The vital question would be the timing of TEER for patients with DMR. We found that the staging classification of cardiac damage stratified the risk of mortality after TEER. Two-year mortality was 0% in patients without any sign of cardiac damage (stage 0), while the mortality increased to 10.5% in patients with mild LV or LA damage (stage 1), 21.1% in patients with moderate LV or LA damage (stage 2), 24.7% in patients with right heart damage (stage 3). The association between advanced stage cardiac damage and an increased risk of 2-year mortality was independent of baseline demographics, inferring that the extent of the extravalvular damage of DMR patients plays an important role in the prognosis. Based on the findings, it may be conceivable to conclude that the timing of MR treatment is critical in patients with DMR, so those patients may need Heart team evaluation perhaps earlier in the disease process.
Another question is whether an MR treatment is particularly effective or futile. The association between the extent of cardiac damage and survivals persisted after accounting for residual MR. Moreover, the superior outcomes of MR reduction to ≤1+ over residual MR ≥2+ were consistently observed among each stage of cardiac damage. Although the present study was neither a propensity-score matching analysis nor a randomized control trial, our findings indicate that TEER for the correction of MR may merit survival in patients with DMR, even if they suffer from advanced cardiac damage. Indeed, significant reductions in hospitalization rate and NYHA functional scale following TEER were also consistently observed among each stage of cardiac damage. Benfari et al17 reported the potential survival benefit of TEER over medical therapy alone was consistent among subgroups for atrial fibrillation, LV ejection fraction, or LV end-diastolic diameter. Treating MR by TEER may be beneficial to mitigate the risk of hospitalization and improve their symptoms even in cases with advanced stages of cardiac damage.
We found that the stage of cardiac damage was modestly downgraded following TEER, with a sustained improvement over time. Correction of MR and thereby unloading the left chambers might lead to restoring LV or LA function.18–20 Ideally, MR is to be treated before the onset of cardiac damage before irreversible changes occur.13 Moreover, right heart response to TEER is not always favorable. Patients with right heart damage with TR may benefit from concomitant tricuspid surgery.21 In contrast, whether a concomitant transcatheter approach for MR and TR has an impact on right heart damage and its prognostic benefit remains to be seen.
Given the decent MR reduction in the present study (residual MR ≤1+: 68.9%) and in the EVEREST II trial ([Endovascular Valve Edge-to-Edge Repair Study]; residual MR ≤1+: 52.6%),5 one might argue that the correction of MR using TEER for DMR is indispensable but still not perfect. Three-fourths of the devices in the present study were the second generation of the MitraClip system. In contrast, the recent literature investigating the procedural outcomes of the MitraClip G3 system reported higher MR reduction rates in patients with DMR (residual MR ≤1+: 82.4%).22 Furthermore, the fourth generation of the MitraClip platform demonstrated further enhancements in TEER results,23 which are likely attributable to the improved capabilities of independent leaflet grasping and the availability of wider clip sizes.
Furthermore, the initial achievement of MR reduction to ≤1+ may translate to durable results of TEER over time.24 We found a higher incidence of the recurrence of MR (ie, MR ≥3+) and repeat mitral valve treatment in patients with advanced cardiac damage, which may be associated with long-term mortality. Besides the extent of cardiac damage as shown in the present analysis, optimal device selection for patients with DMR is to be investigated.25,26 The PASCAL system (Edwards Lifesciences, Irvine, CA) is a novel, tool of TEER, which uses a central spacer and a single horizontal row of leaflet retention features designed to reduce leaflet stress and optimize leaflet clasping while minimizing the risk of leaflet damage. According to the recent clinical evidence from the CLASP IID trial (Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical), each platform (ie, PASCAL and MitraClip systems) provides a safe and effective procedural outcome in patients with DMR, whereas they indicated that the initially seemingly comparable MR reduction might provide different sustainability during follow-up.27
The procedural result of TEER improves along with the device iterations, physician’ experience, patient selection, and knowledge of imaging modalities. Moreover, providing the less invasive option has improved the prognosis of patients with DMR treated with surgery.28 Further refinement of the risk evaluation and patient selection is a pivotal milestone before expanding the transcatheter technologies to lower-risk and younger-age categories.
Limitations
The present study is subject to inherent limitations due to its observational, nonrandomized design. However, this is one of the largest multicenter cohort studies of patients with DMR undergoing TEER. All measurements were conducted prospectively by independent sonographers and cardiologists within the routine practice for DMR evaluation and quantitation using transthoracic echocardiography, which should increase the generalizability of the findings. Second, the described staging classification was not validated using other cohorts or prospectively investigated. Third, a substantial amount of echocardiographic data during follow-up were missing. Fourth, the theory of the staging classification infers a causal association between DMR and the advancement of cardiac damage.8 Although other concomitant comorbidities (eg, coronary artery disease, chronic lung disease) may coexist, and the observed structural changes might be rather due to those factors, the consistency of the findings in several sensitivity analyses suggests that the cardiac-oriented staging classification is useful for risk stratification in clinical practices. Also, although we defined severe TR as a result of right heart damage, concomitant TR might be potentially due to the consequence of tricuspid valve leaflet prolapse, especially in patients with mitral valve prolapse.29
Conclusions
Advanced cardiac damage is associated with an increased risk of mortality in patients with DMR treated with TEER. The staging classification of cardiac damage offers prognostic implications for clinical outcomes after TEER. TEER may mitigate the risk of mortality rehospitalization and improve their symptomatic status, irrespective of the extent of cardiac damage. Nonetheless, a higher incidence of the recurrence of MR and repeat mitral valve treatment during the follow-up was observed in patients with advanced cardiac damage, which may be associated with long-term clinical outcomes. Based on the findings, it is also possible to conclude that the timing of MR treatment is critical in patients with DMR so that patients may need Heart team evaluation earlier in the disease process.
ARTICLE INFORMATION
Acknowledgments
The authors thank all investigators participating in this registry.
Sources of Funding
The Optimized Catheter Valvular Intervention (OCEAN)-Mitral registry is part of the OCEAN-SHD registry and is supported by Edwards Lifesciences, Medtronic Japan, Boston Scientific, Abbott Medical Japan, and Daiichi-Sankyo Company.
Disclosures
Dr Sugiura has received research grant from Edwards and honoraria for lectures from Edwards Lifesciences and Abbott Medical, outside the submitted work. Drs Kubo, Saji, Izumo, Watanabe, and Amaki are clinical proctors of transcatheter edge-to-edge repair for Abbott Medical, and have received consultant fee from Abbott Medical. Drs Asami and Kodama have received speaker fees from Abbott Medical. Drs Yamamoto and Nakajima are clinical proctors of transcatheter edge-to-edge repair for Abbott Medical and have received lecture fees from Abbott Medical. Dr Yamaguchi is clinical proctor of transcatheter edge-to-edge repair for Abbott Medical and has received a lecture fee and a scholarship donation from Abbott Medical. Dr Ohno has received consultant, advisor, and speaker fees from Abbott Medical. Drs Enta, Shirai, Mizuno, and Bota are clinical proctors of transcatheter edge-to-edge repair for Abbott Medical. Dr Nickenig has received research grants and speaker honoraria from Abbott, outside the submitted work. The other authors report no conflicts.
Supplemental Material
Tables S1–S3
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- DMR
- degenerative mitral regurgitation
- LA
- left atrium
- LV
- left ventricle
- MR
- mitral regurgitation
- NYHA
- New York Heart Association
- TEER
- transcatheter edge-to-edge repair
- TR
- tricuspid regurgitation
A. Sugiura and M. Yamamoto contributed equally.
For Sources of Funding and Disclosures, see pages 507–508.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.123.013794.
Contributor Information
Masanori Yamamoto, Email: masa-nori@nms.ac.jp.
Mike Saji, Email: mikesaji8@gmail.com.
Masahiko Asami, Email: asami560725@yahoo.co.jp.
Yusuke Enta, Email: yusuke0831@gmail.com.
Masaki Nakashima, Email: m2izumo@marianna-u.ac.jp.
Shinichi Shirai, Email: shirai440130@gmail.com.
Masaki Izumo, Email: m2izumo@marianna-u.ac.jp.
Shingo Mizuno, Email: shingo.waterfield@gmail.com.
Yusuke Watanabe, Email: yusuke0831@gmail.com.
Makoto Amaki, Email: amakimako@hotmail.com.
Kazuhisa Kodama, Email: kazuhisa-kodama@saiseikaikumamoto.jp.
Junichi Yamaguchi, Email: j.yamaguchi0110@gmail.com.
Yoshifumi Nakajima, Email: nyoshifumi@hotmail.com.
Toru Naganuma, Email: torunaganuma@gmail.com.
Hiroki Bota, Email: hiroki_bouta@yahoo.co.jp.
Yohei Ohno, Email: yohei_ohno@hotmail.com.
Masahiro Yamawaki, Email: m_yamawaki@tobu.saiseikai.or.jp.
Hiroshi Ueno, Email: hiroshi.ueno.md@gmail.com.
Kazuki Mizutani, Email: ikki1127@gmail.com.
Yuya Adachi, Email: y.adachino@gmail.com.
Toshiaki Otsuka, Email: otsuka@nms.ac.jp.
Shunsuke Kubo, Email: sk12750@kchnet.or.jp.
Georg Nickenig, Email: georg.nickenig@ukb.uni-bonn.de.
Kentaro Hayashida, Email: khayashidamd@gmail.com.
REFERENCES
- 1.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. doi: 10.1016/S0140-6736(09)60692-9 [DOI] [PubMed] [Google Scholar]
- 2.Dziadzko V, Clavel M-A, Dziadzko M, Medina-Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez-Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet. 2018;391:960–969. doi: 10.1016/S0140-6736(18)30473-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e82–e227. doi: [DOI] [PubMed] [Google Scholar]
- 4.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, et al. ; ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 5.Feldman T, Foster E, Glower DD, Glower DG, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: [DOI] [PubMed] [Google Scholar]
- 6.Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. 2017;38:3351–3358. doi: 10.1093/eurheartj/ehx381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamekhi J, Sugiura A, Spieker M, Iliadis C, Weber M, Öztürk C, Becher MU, Tiyerili V, Zimmer S, Horn P, et al. A staging classification of right heart remodelling for patients undergoing transcatheter edge-to-edge mitral valve repair. EuroIntervention. 2022;18:43–49. doi: 10.4244/EIJ-D-21-00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butcher SC, Essayagh B, Steyerberg EW, Benfari G, Antoine C, Grigioni F, Le Tourneau T, Roussel J-C, van Wijngaarden A, Marsan NA, et al. Factors influencing post-surgical survival in degenerative mitral regurgitation. Eur Heart J. 2023;44:871–881. doi: 10.1093/eurheartj/ehad004 [DOI] [PubMed] [Google Scholar]
- 9.Bernard J, Altes A, Dupuis M, Toubal O, Mahjoub H, Tastet L, Côté N, Clavel M-A, Dumortier H, Tartar J, et al. Cardiac damage staging classification in asymptomatic moderate or severe primary mitral regurgitation. Struct Heart. 2022;6:100004. doi: 10.1016/j.shj.2022.100004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saji M, Yamamoto M, Kubo S, Asami M, Enta Y, Shirai S, Izumo M, Mizuno S, Watanabe Y, Amaki M, et al. ; OCEAN-Mitral Investigators. Short-Term outcomes following transcatheter edge-to-edge repair: insights from the OCEAN-Mitral registry. JACC Asia. 2023;3:766–773. doi: 10.1016/j.jacasi.2023.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlotter F, Miura M, Kresoja K-P, Alushi B, Alessandrini H, Attinger-Toller A, Besler C, Biasco L, Braun D, Brochet E, et al. Outcomes of transcatheter tricuspid valve intervention by right ventricular function: a multicentre propensity-matched analysis. EuroIntervention. 2021;17:e343–e352. doi: 10.4244/EIJ-D-21-00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prins KW, Rose L, Archer SL, Pritzker M, Weir EK, Olson MD, Thenappan T. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic Lung disease. J Am Heart Assoc. 2019;8:e011464. doi: 10.1161/JAHA.118.011464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiura A, Weber M, Tabata N, Goto T, Grube E, Treede H, Werner N, Nickenig G, Sinning J-M. Association of heart failure duration with clinical outcomes after transcatheter mitral valve repair for functional mitral regurgitation. Catheter Cardiovasc Interv. 2021;98:E412–E419. doi: 10.1002/ccd.29390 [DOI] [PubMed] [Google Scholar]
- 14.Shamekhi J, Weber M, Sugiura A, Öztürk C, Treede H, Grube E, Werner N, Nickenig G, Sinning J-M. Impact of coronary artery disease on outcomes in patients undergoing percutaneous edge-to-edge repair. JACC Cardiovasc Interv. 2020;13:2137–2145. doi: 10.1016/j.jcin.2020.05.031 [DOI] [PubMed] [Google Scholar]
- 15.Safiriyu I, Nagraj S, Otulana R, Saralidze T, Kokkinidis DG, Faillace R. Prognostic impact of pre- and post-procedural renal dysfunction on late all-cause mortality outcome following transcatheter edge-to-edge repair of the mitral valve: a systematic review and meta-analysis. Cardiovasc Revascularization Med. 2022;42:6–14. doi: 10.1016/j.carrev.2022.03.023 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Sugiura A, Öztürk C, Vogelhuber J, Tabata N, Wilde N, Zimmer S, Nickenig G, Weber M. Transcatheter edge-to-edge repair for atrial secondary mitral regurgitation. JACC Cardiovasc Interv. 2022;15:1731–1740. doi: 10.1016/j.jcin.2022.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Benfari G, Sorajja P, Pedrazzini G, Taramasso M, Gavazzoni M, Biasco L, Essayagh B, Grigioni F, Bae R, Tribouilloy C, et al. Association of transcatheter edge-to-edge repair with improved survival in older patients with severe, symptomatic degenerative mitral regurgitation. Eur Heart J. 2022;43:1626–1635. doi: 10.1093/eurheartj/ehab910 [DOI] [PubMed] [Google Scholar]
- 18.Gucuk Ipek E, Singh S, Viloria E, Feldman T, Grayburn P, Foster E, Qasim A. Impact of the MitraClip procedure on left atrial strain and strain rate. Circ Cardiovasc Imaging. 2018;11:e006553. doi: 10.1161/CIRCIMAGING.117.006553 [DOI] [PubMed] [Google Scholar]
- 19.Lurz P, Serpytis R, Blazek S, Seeburger J, Mangner N, Noack T, Ender J, Mohr FW, Linke A, Schuler G, et al. Assessment of acute changes in ventricular volumes, function, and strain after interventional edge-to-edge repair of mitral regurgitation using cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1399–1404. doi: 10.1093/ehjci/jev115 [DOI] [PubMed] [Google Scholar]
- 20.Kataoka A, Watanabe Y; OCEAN-SHD Family. MitraClip: a review of its current status and future perspectives. Cardiovasc Interv Ther. 2023;38:28–38. doi: 10.1007/s12928-022-00898-4 [DOI] [PubMed] [Google Scholar]
- 21.Gammie JS, Chu MWA, Falk V, Overbey JR, Moskowitz AJ, Gillinov M, Mack MJ, Voisine P, Krane M, Yerokun B, et al. ; CTSN Investigators. Concomitant tricuspid repair in patients with degenerative mitral regurgitation. N Engl J Med. 2022;386:327–339. doi: 10.1056/NEJMoa2115961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kar S, Von Bardeleben RS, Rottbauer W, Mahoney P, Price MJ, Grasso C, Williams M, Lurz P, Ahmed M, Hausleiter J, et al. Contemporary outcomes following transcatheter edge-to-edge repair. JACC Cardiovasc Interv. 2023;16:589–602. doi: 10.1016/j.jcin.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 23.von Bardeleben RS, Rogers JH, Mahoney P, Price MJ, Denti P, Maisano F, Rinaldi M, Rollefson WA, De Marco F, Chehab B, et al. Real-World outcomes of fourth-generation mitral transcatheter repair: 30-Day results from EXPAND G4. JACC Cardiovasc Interv. 2023;16:1463–1473. doi: 10.1016/j.jcin.2023.05.013 [DOI] [PubMed] [Google Scholar]
- 24.Sugiura A, Kavsur R, Spieker M, Iliadis C, Goto T, Öztürk C, Weber M, Tabata N, Zimmer S, Sinning J-M, et al. Recurrent mitral regurgitation after MitraClip: predictive factors, morphology, and clinical implication. Circ Cardiovasc Interv. 2022;15:e010895. doi: 10.1161/CIRCINTERVENTIONS.121.010895 [DOI] [PubMed] [Google Scholar]
- 25.Marcoff L, Koulogiannis K, Aldaia L, Mediratta A, Chadderdon SM, Makar MM, Ruf TF, Gößler T, Zaroff JG, Leung GK, et al. ; CLASP IID Pivotal Trial Investigators. Echocardiographic outcomes with transcatheter edge-to-edge repair for degenerative mitral regurgitation in prohibitive surgical risk patients. JACC Cardiovasc Imaging. 2023;S1936–878X(23)00443. doi: 10.1016/j.jcmg.2023.09.015 [Google Scholar]
- 26.Smith RL, Lim DS, Gillam LD, Zahr F, Chadderdon S, Rassi AN, Makkar R, Goldman S, Rudolph V, Hermiller J, et al. ; CLASP IID Pivotal Trial Investigators. 1-Year outcomes of transcatheter edge-to-edge repair in anatomically complex degenerative mitral regurgitation patients. JACC Cardiovasc Interv. 2023;16:2820–2832. doi: 10.1016/j.jcin.2023.10.020 [DOI] [PubMed] [Google Scholar]
- 27.Lim DS, Smith RL, Gillam LD, Zahr F, Chadderdon S, Makkar R, Stephan von Bardeleben R, Kipperman RM, Rassi AN, Szerlip M, et al. Randomized comparison of transcatheter edge-to-edge repair for degenerative mitral regurgitation in prohibitive surgical risk patients. JACC Cardiovasc Interv. 2022;15:2523–2536. doi: 10.1016/j.jcin.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Lowenstern AM, Vekstein AM, Grau-Sepulveda M, Badhwar V, Thourani VH, Cohen DJ, Sorajja P, Goel K, Barker CM, Lindman BR, et al. Impact of transcatheter mitral valve repair availability on volume and outcomes of surgical repair. J Am Coll Cardiol. 2023;81:521–532. doi: 10.1016/j.jacc.2022.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guta AC, El-Tallawi KC, Nguyen DT, Qamar F, Nguyen T, Zoghbi WA, Lawrie G, Graviss EA, Shah DJ. Prevalence and clinical implications of tricuspid valve prolapse based on magnetic resonance diagnostic criteria. J Am Coll Cardiol. 2023;81:882–893. doi: 10.1016/j.jacc.2022.11.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.