Abstract
Existing data demonstrate reduced delta power during sleep in patients with depression and chronic pain. However, there has been little examination of the relationship between delta power and pain-reports, or pain-catastrophizing. We recruited female participants (n=111) with insomnia and temporomandibular disorder, and measured nocturnal and daytime measures of pain and pain catastrophizing, and calculated relative nocturnal delta (0.5–4 Hz) power during sleep. We fit linear regression models, and further examined the moderating effect of depressive symptom severity. Lower relative delta power across the whole night was significantly associated with greater nocturnal pain (B = −20.276, p = 0.025, R2 = 0.214). Lower relative delta power during the first-third of the night, was associated with greater nocturnal pain (B = −17.807, p = 0.019, R2 = 0.217), next-day pain (B = 13.876, p = 0.039, R2 = 0.195), and next-morning pain (B = −15.751, p = 0.022, R2 = 0.198). Lower relative delta power during the final-third of the night was significantly associated with greater nocturnal (B = −17.602, p = 0.029, R2 = 0.207) and next-morning pain (3rd: B = −14.943, p = 0.042, R2 = 0.187). Depressive symptom severity did not moderate these relationships. Delta power was not significantly associated with nocturnal or daytime pain catastrophizing. These findings demonstrate that greater relative delta power during sleep is associated with lower nocturnal and next-day pain in patients with temporomandibular disorder. This data may guide the use of sleep interventions in clinical pain populations, with the aim of improving pain outcomes.
Keywords: Temporomandibular Joint Disorders, Polysomnography, Insomnia Disorder, Chronic Pain, Pain Catastrophizing
Introduction
Numerous lines of evidence support the notion of tri-directional relationships among depression, insomnia and chronic pain23. A high proportion of patients with temporomandibular joint disorder (TMD), an idiopathic pain syndrome affecting 12%31 of the population comprising of masseter and temporomandibular joint pain, endorse depressive symptoms (ranging from 3039 to 40%)8 and insomnia (ranging from 36 to 72%29, 53, 58). Longitudinal data also gives credence to the hypothesis that depression exacerbates both insomnia and pain symptoms, as the incidence of chronic musculoskeletal pain in insomnia patients at six-years, is increased by the presence of depressive symptoms at baseline24.
The connection between chronic pain and depression is of particular pertinence because pain has both sensory and emotional consequences, in particular being linked to a number of maladaptive cognitive-emotional strategies. Catastrophizing is a central concept in theoretical models of depression and pain, which has enjoyed increasing salience due to its robust relationship with both pain (e.g., severity and interference, increased opioid-medication use41) and mental health outcomes (e.g., depressive symptoms20, suicidal ideation10, and affective distress4, 30, 35, 36, 60). Furthermore, whilst depression and catastrophizing are associated with worse outcomes in isolation, their co-occurrence results in synergistic increase in pain and disability38, and contributions to disorder chronicity in TMD59. Data from a three-path mediation model in fibromyalgia, a frequently co-occurring idiopathic chronic pain condition, also demonstrated that previous night’s sleep quality predicted morning pain catastrophizing, which subsequently predicted afternoon pain severity and evening pain interference44. Nevertheless, the role of nocturnal pain catastrophizing, occurring during periods of disturbed sleep, on next-day pain remains largely unexplored. Although, a study from our group in knee osteoarthritis previously revealed significant reductions in nocturnal pain catastrophizing following treatment with cognitive behavioral therapy for insomnia32 the neurophysiological mechanisms of this effect were not explored.
Slow wave sleep (SWS) represents a promising candidate for a putative neurophysiological substrate which may modulate pain catastrophizing, given that SWS disruption has been linked to both increased pain sensitivity48 and impaired cognitive functioning in healthy participants26, 55. Reduced SWS has been observed in individuals with chronic pain9, and has been further implicated as a predictor of poor executive control and attentional impairment in patients with insomnia37. Taken together, these data support the premise for a theoretical model whereby SWS disruption results in executive dysfunction, which subsequently elevates propensity to engage in maladaptive cognitive-emotional strategies in response to pain. However, the predominate line of enquiry has focused on sleep macro-architecture (sleep stages), rather than analyses of its spectral component waveforms. SWS (otherwise referred to as N3 or stage-3 sleep) is a stage of sleep characterized by dominance (>20% per 30s epoch) of waveforms in the delta power band (typically defined as 0.5– 4 Hz), which is proposed to play a crucial role in regulation of physiological40 and cognitive processes55. However, delta activity can occur throughout the night, in stages other than SWS. Therefore, focusing on SWS alone may render analyses less sensitive to detect the effects of delta activity on next day functioning when confined to macro-architecture (sleep stages) alone.
To our knowledge, no study has investigated whether the amount of SWS or delta power present during sleep predicts the intensity of next-day pain or pain catastrophizing. Nighttime pain during sleep is one of the most frequent complaints in chronic pain disorders, which has potential to impact on sleep by preventing both sleep initiation and maintenance51, 52. Therefore, extending previous work32 we also aimed to investigate how the experience of nocturnal pain and catastrophizing is influenced by delta activity during sleep. Given the existing evidence, which suggests that depressive symptoms contribute to worsening of pain symptoms, and the known link to SWS dysregulation in depression42, 63, it is reasonable to postulate that depressive symptoms may alter the relationship between delta-activity during sleep and next-day pain intensity.
Therefore, we aimed to investigate the relationship of delta-activity during sleep with nocturnal and daytime reports of pain and pain catastrophizing in TMD, both with and without comorbid depressive symptoms. We hypothesized based on precedent literature that: A) lower relative delta power would be associated with greater nocturnal and next-day pain as well as higher nocturnal and next-day pain catastrophizing; and B) depressive symptom severity would be a significant moderator of the relationship between relative delta power, pain, and pain catastrophizing (i.e., both nocturnal and next day), such that greater depressive symptom severity would increase their associations.
Methods
Design & Participants
We report here a secondary analysis from a clinical trial dataset. Data were collected as part of the parent study (ClinicalTrials.gov Identifier: NCT01794624), during which females with TMD and high pain catastrophizing, comorbid with insomnia disorder were randomized to receive one of three behavioral interventions. We used polysomnography and sleep diary data from the pre-randomization dataset to address the relationship between nocturnal delta power (from PSG) and subjective reports of pain (from diaries). The main outcome paper is currently in preparation, the aims of which do not overlap with those in the current study.
As TMD is a disorder that primarily impacts women, we recruited a female only sample to limit heterogeneity which may have impeded statistical power of the parent project. Participants aged 18–60 years were recruited according to criteria to establish the presence of TMD, symptoms of insomnia (≥8 ISI3) and pain catastrophizing (≥8 Pain Catastrophizing Scale54). Participants were excluded on the basis of exhibiting severe or unstable psychiatric or medical conditions, suicidal ideation, current drug use, or the presence of clinically significant sleep apnea. A full breakdown of inclusion and exclusion criteria are contained in table 1. Recruitment took place during the period 2013–2018, during which 496 participants were deemed eligible out of 1642 potential participants screened via the initial telephone screening. 144 participants met full study criteria, and completed polysomnography (PSG) at baseline. However, diary data were only available for 111 participants the day following the PSG, therefore our analysis dataset was limited to these participants. Approval for this study was granted by the institutional review boards at both Johns Hopkins School of Medicine and University of Maryland School of Dentistry. All participants provided informed consent prior to participating in the study.
Table 1:
Criteria for inclusion and Exclusion
| Inclusion | Exclusion |
|---|---|
| Meeting research diagnostic criteria for TMD based upon an exam performed by an experienced research dental hygienist trained to criterion in the RDC TMD dental examine and supervised by board certified orofacial pain dentist. | BMI ≥35 |
| reporting facial pain for at least 3 months | resting systolic blood pressure > 140 mm Hg and diastolic blood pressure > 90 mm Hg |
| reporting facial pain for at least 10 days out of the past 30 days | history of TMJ surgery or TMJ growth disturbances, neoplasm or injury to the TMJ area in the past 6 months |
| average pain severity score over the past week of ≥ 3 on a 0–10 numerical rating scale | surgery scheduled for TMD during study period |
| ≥ 8 Insomnia Severity Index (ISI) | history of major medical conditions influencing sleep, the central nervous system (e.g., COPD, seizure disorder, cancer), or peripheral neuropathy |
| reporting difficulties initiating and/or maintaining sleep regularly (≥ 3 days/week) for at least 1 month | diagnosis of Raynaud’s Syndrome |
| ≥ 8 Pain Catastrophizing Scale | history of unstable major psychiatric disorder (e.g., psychotic disorder, bipolar disorder |
| on the same treatment regimen for the last 30 days, if using non-opioid medication for pain treatment | Recent alcohol abuse (past 6 months) |
| willing to undergo a 4-week washout period if using an opioids for pain, AND/OR benzodiazepine/benzodiazepine receptor agonists, sedating tricyclic antidepressant (e.g., trazadone, amitriptyline, doxepin) for sleep > 3 days/week | been regularly (≥ 3x/week) using opioids, benzodiazepines, or sedating tricyclic antidepressants |
| agree to use contraception throughout the study, if of childbearing potential | stable preferred sleep phase between 10 am and 10 pm (e.g., night workers) or self-reported significant variability in sleep due to changes in work shifts (e.g., nurses) |
| menopausal for at least 12 consecutive months prior to screening, if post-menopausal | Score ≥ 27 on CES-D scale or current suicidal ideation |
| able to understand and willing to comply with all study procedures and is available for the duration of the study | positive urine toxicology screen test (i.e., barbiturates, THC, alcohol, cocaine, and other recreational drugs of abuse) result |
| positive urine pregnancy test result | |
| respiratory disturbance index ≥ 15 as determined by baseline PSG; or periodic limb movement index with arousals ≥ 15 as determined by baseline PSG |
Abbreviations: TMD = Temporomandibular Joint Disorder, BMI = Body Mass Index, RDC = Research Diagnostic Criteria, ISI = Insomnia Severity Index, TMJ = Temporomandibular Joint, COPD = Chronic Obstructive Pulmonary Disease, THC = Tetrahydrocannabinol, PSG = Polysomnography, CES-D = Center for Epidemiologic Studies Depression (CES-D).
Procedure
Participants underwent an initial telephone screening survey, followed by an in-person screening visit to establish eligibility in accordance with the list of inclusion/exclusion criteria (see table 1). During this visit, insomnia diagnosis was confirmed using a structured clinical interview for sleep disorders 56. A specifically trained dental hygienist performed a clinical examination to verify research diagnostic criteria (RDC) for TMD47. Eligible participants then completed a baseline study session comprising: self-report measurements and a structured clinical interview for sleep disorders. During this visit, participants were trained by research staff on the use of an interactive voice response (IVR) system diary which they completed for 14 days. Participants were subsequently fitted with an ambulatory polysomnography (PSG) montage which was used to collect data for one night in the home environment. Participants completed the IVR diary by telephoning a dedicated number and providing their responses on the telephone keypad. The diary was completed in the morning and evening following the PSG, containing standardized measures of relating to sleep and the daytime and nocturnal (concurrent with PSG) pain. In the present study, we only used data collected from diaries the morning and daytime following PSG.
Polysomnography (PSG) Procedures
Overnight PSG data were collected using at-home ambulatory PSG devices which was fitted to the participant by trained registered polysomnography technicians (RPSGTs) at Center for Interdisciplinary Sleep Research and Education (CISRE). The standardized montage was setup according to the American Academy of Sleep Medicine (AASM) standards6, 7, consisting of six Electroencephalography (EEG) electrodes (F3, F4, C3, C4, O1, O2), two electro-oculography (EOG) electrodes (EOG1, EOG2), two submental electromyography (EMG) electrodes, and two electrocardiography (ECG) electrodes (to facilitate artefact detection). Signals were recorded using an Embla N7000 device (Natus, Iceland). Electrode sites were measured using the international 10–20 EEG system28 and positioned according to AASM guidelines7. EEG channels were recorded with a sampling rate of 512 Hz using Cz as online reference and FpZ as the ground electrode. For analysis, signals were re-referenced to the contra-lateral mastoid (A1 and A2). The following sleep variables were calculated: sleep onset latency (SOL), wake-time after sleep onset (WASO), total sleep time (TST), and the duration and percentage of wake, N1, N2, N3, and REM. To assess respiratory and muscular events, we further gathered EMG data from bilateral anterior tibilalis muscles, thoracic and abdominal effort bands, a nasal thermistor and canula, pulse oximeter. The clinical diagnostic sensor montage was applied in the late evening in the lab and subjects were sent home to sleep ad libitum at their habitual bed and wake times. PSG data were scored according to AASM guidelines7 by registered polysomnography technicians at the CISRE, who achieved > 80% inter-rater concordance and subsequently reviewed by a board registered sleep medicine specialist. Participants demonstrating clinically significant criteria for sleep apnea (respiratory disturbance index > 15) or periodic limb movements (>15 PLMS with arousals/hour) were subsequently excluded from the study following the PSG night and were not included in the analyses. All 111 participants included in the analyses met these inclusion criteria.
Spectral Analysis
We analyzed data from the six EEG scalp electrodes. EEG data were pre-processed to remove motion and EMG artifacts by sequentially applying median based and Savitisky-Golay filters50. We then applied a bandpass filter ranging from .5–150 Hz. Finally, we applied a third order Finite Impulse Response (FIR) filter (0.3–35 Hz) with a 60 Hz notch filter to remove electrical artifact. We conducted power spectral analysis on the frontal and central electrodes (F3, F4, C3, C4) in MATLAB to decompose the EEG waveforms from all epochs scored as sleep to quantify average relative power spectra (μV2/Hz/total power) for their constituent frequencies via fast Fourier transforms. Channels O1 and O2 were removed as delta activity occurs primarily in frontal and central regions61, and these electrodes are more prone to movement artefact61. We deployed two artifact rejection procedures commonly used for spectral analysis of sleep EEG, including a multi-tapering method11, 57, removing data points >95th or < 5th percentiles of power. After artifact rejection the mean duration of recordings included In the spectral analysis was 455.508mins (SD = 89.595, range = 219–695mins), According to commonly observed procedure in analyzing sleep-EEG49, relative power over absolute was utilized in order to mitigate any inter-individual differences in overall absolute EEG power observed in women2, 18 and minimize outlier behavior its influence on interpretations made about delta power. However, as a supplementary analysis, and for the reporting of complete data, we also analyzed absolute delta power across the entire night and its relationship our with primary outcome variables. We deployed two artifact rejection procedures commonly used for spectral analysis of sleep EEG, including a multi-tapering method11, 57, removing data points >95th or < 5th percentiles of power. EEG features were normalized by their means and SD. In accordance with our core hypotheses surrounding relative delta power, we only included data from the delta power band (0.5–4 Hz) in our primary statistical models. However, to provide a comprehensive account of the relationship between power in the remaining spectral bands and pain reports, we further analysed both absolute and relative power in the theta (4–8hz), alpha (8–12Hz), sigma (12–15Hz) and beta (15–35hz) power bands. Nevertheless, it is important to note that we did not allocate a priori hypotheses on these secondary analyses, nor did we correct for multiple comparisons as our a priori focus was on delta power. Full findings from these analyses are reported in supplemental table 1. In order to further reduce alpha inflation and type 1 error, we limited our independent variable to the average relative delta power derived across all four electrodes. As delta power is homeostatically regulated, and typically strongest during the first third of the night, declining throughout the night, with each successive NREM sleep cycle16, we decomposed values for relative delta power into the first, second and final-third of the night, and also calculated an average value across the whole night, a common procedure in studies where delta power is the primary outcome of focus45, 65. This approach enabled us to assess whether any effects on pain were specific to early homeostatic sleep dissipation (first third), or delta power more generally across the night (whole night and subsequent thirds).
Questionnaires
Participants completed daily dairies in the morning (reflecting morning and nocturnal pain measures) and evening (reflecting daytime pain measures). Participants completed their morning diaries on average at 10:40 AM (SD = 3 hours), and evening diaries at 10:15 PM (SD = 1.1 hours). Our analyses centered around the diary data collected the morning and evening immediately following the polysomnography night. Diary completion was high for both evening (88.32%) and morning (94.01%) timepoints.
Nocturnal Pain Catastrophizing:
In the morning following the PSG, participants rated the degree to which they experienced certain thoughts and feelings relating to pain they experienced the previous night. We elected to collect retrospective reports of nocturnal pain in the morning, as opposed to reports completed during the night, to prevent artificial disruption of sleep as a result of survey completion. Three items related to: 1) ‘I felt like I couldn’t stand the pain’ 2) ‘I felt like the pain was never going to get any better’ 3) ‘I couldn’t stop thinking about how much It hurt’. Participants rated on a scale 0 (Not at All) to 10 (Very Much) for each item32. Score was indicated by the total of the three items, with higher scores indicative of higher pain catastrophizing.
Daytime Pain Catastrophizing:
In the evening, participants responded to the following items relating to pain experienced during that daytime: 1) ‘ When I had pain today, I felt like I couldn’t stand the pain’ 2) ‘When I had pain today, I felt like the pain was never going to get any better’ 3) ‘When I had pain today, I couldn’t stop thinking about how much it hurt’. Participants responded on a scale 0 (Not At All) to 10 (Extremely)12. Score was indicated by the total of the three items, with higher scores indicative of higher pain catastrophizing.
Based on previous studies from our group21, 32, 33, items for nocturnal (section above) and daytime pain catastrophizing were adapted from the Pain Catastrophizing Scale54 by selecting the items with the strongest factor loading for each of the scale’s factors (Rumination, Magnification, Helplessness).
Self-Report Daily Pain Intensity:
At morning and evening timepoints, participants completed measures of the intensity of pain experienced the previous night, and throughout the day following the PSG, respectively. Morning measures included morning pain intensity (‘What is your current level of pain right now’) and average nocturnal pain intensity (‘What was your average level of pain last night while trying to sleep’) both where 0 is “No Pain” and 10 is “Worst Pain Imaginable.” Evening measures included average daily pain intensity (‘What was your average or typical level of pain today, where 0 is not at all and 10 is worst pain imaginable?’)
Depressive Symptom Severity:
Depressive symptom severity was measured at the baseline prior to PSG, using the Center for Epidemiologic Studies-Depression scale34. The scale comprises 20 items (scored 0–3) and has good internal consistency (α = 0.63–0.93), as well as test-retest reliability (0.61)17. We used total score as out outcome, totaling 0–60, where increased score is indicative of more symptomology.
Data Handling and Analysis
Our primary analyses were centered around relative delta power as a predictor of nocturnal pain severity and catastrophizing, as well as next-day pain intensity and catastrophizing. We fit a series of multiple linear regression models to all outcome variables with relative delta power as the main predictor. We systematically tested the moderating effect of depressive severity (measured in CES-D scores) on the relationship between relative delta power and outcomes. Statistical analyses were conducted in R using the ‘lm’ function. Outcomes used in the regression models underwent visual inspection using histograms, and skewness and kurtosis testing to assess normality of distribution. All data were normally distributed (Skewness <2 kurtosis <7)62. Due to instances of moderate missing data (up to 36%) in some outcomes measures, we handled missing data using multiple imputation (MI). MI is superior to other default missing data handling methods (e.g., listwise deletion and pairwise deletion22) and robust to degrees of missingness up to 50%25. MI was performed using the ‘MICE’ package in R using predictive mean matching with 50 imputed datasets with a random seed, to reduce sampling variability from the imputation process27. Linear regression models from each permuted dataset were then pooled to create an overall estimate of the regression parameters and effect sizes. Missingness was not associated with socio-demographic variables (i.e., race, income, education) other than age, and was also associated with anxiety and depressive symptom severity. To increase the precision of MI estimation, age, anxiety and depression which were associated with missingness were included as auxiliary variables for imputation64. To control for confounding effects observed in the previous literature, we further included total sleep time (TST), BMI, age, and anxiety symptoms as continuous covariates across all models. Due to high incidence of bruxism in the sample, which may influence morning jaw pain ratings, we included the presence of self or bed-partner reported nocturnal bruxism as an additional categorical covariate (Bruxism/No-Bruxism). Secondary analyses included testing the effects of absolute delta power, as well as the percent of SWS (instead of relative delta power) as a predictor in the above-mentioned regression models. Further secondary analyses were conducted to characterize the relationships among sleep duration and continuity and our outcomes in the sample (see supplementary table 2). Note that when planning the analyses, we opted not to not adjust for multiple comparisons, given the early-stage exploratory nature of the present research, and recommendations to prioritize reducing the probability of Type II versus Type 1 error at this stage5, 14. Furthermore, as our study follows hypothesis driven research questions, supported by previous mechanistic findings, we elected to follow recommendations to avoid multiple comparison correction1. We hence note throughout the manuscript that these analyses should be considered exploratory, hypothesis-generating, and should be confirmed in future studies.
Results
Participants
The mean age was 36 years (SD = 11.1), with the majority of the participants (74%) selfi-dentifying as White, and 18% as Black/African American. A large proportion of participants (77%) had obtained a college degree or above, and 71% had an annual household income of ≤$50,000. Close to half (46.6%) of the participants were either married or living with a partner. Socio-demographic characteristics of the current sample are represented in Table 2. A total of 76 participants (68.5%) met threshold for depressive symptoms (CESD ≥16), typically interpreted as ‘at-risk’ for depression. The mean pain severity of participants, as determined by the Brief Pain Inventory15 (BPI) was 4.25 (SD =1.67) out of a maximum of 10, indicating mild to moderate pain intensity.
Table 2:
Baseline Demographics
| Sex, Female (%)* | Mean | SD |
|---|---|---|
| Sex, Female (%)* | 100 | |
| Age (years) | 36.02 | 11.10 |
| Depressive symptoms (mean) | 18.35 | 7.77 |
| Pain Severity (BPI) (mean) | 4.25 | 1.67 |
| Average Nocturnal Pain┐ | 2.97 | 0.80 |
| Average Morning Pain┐ | 3.85 | 1.87 |
| Average Daytime Pain | 0.66 | 0.64 |
| Nocturnal Pain Catastrophizing | 2.19 | 2.42 |
| Daytime Pain Catastrophizing | 2.96 | 2.38 |
| Pain Catastrophizing Scale (PCS) | 20.37 | 11.15 |
| Insomnia Severity (ISI) (mean) | 15.69 | 3.98 |
| Total Sleep Time (mean mins) | 431.32 | 94.00 |
| SOL (mean mins) | 28.15 | 35.57 |
| WASO (mean mins) | 28.44 | 5.41 |
| SE (%) | 84.98 | 12.21 |
| Race (%) | ||
| Asian | 4.5 | - |
| Black or African-American | 18.2 | - |
| More than one race | 0.9 | - |
| Other | 2.7 | - |
| White | 73.6 | - |
| Education (%) | - | |
| Post-graduate | 28.7 | - |
| College graduate | 41.7 | - |
| Some college | 19.4 | - |
| High school/Tech. School/GED | 10.7 | - |
Data are mean (SD) or %, BPI = Brief Pain Inventory, ISI = Insomnia Severity Index, SD = Standard Deviation, GED = General Educational Development (GED)
only female participants were recruited.
SOL = Sleep Onset Latency, WASO = Wake After Sleep Onset, SE = Sleep Efficiency. SOL, WASO and SE were defined by Polysomnography.
Correlation coefficient between nocturnal and morning pain = 0.79.
Characteristics of relative delta power in the sample
Data indicated that relative delta power across the entire night did not differ between participants with and without elevated depressive symptoms (Non-depressed = 0.198 [SD 0.024], depressed = 0.197 [SD = 0.024]). A one-way ANOVA indicated a significant effect of time, indicating relative delta power differed between thirds of the night. (One-way ANOVA: F (2) = 18.84, p = <0.001, delta power: 1st = 0.210 [SD 0.032], 2nd = 0.185 [SD 0.028], 3rd = 0.199 [SD 0.030]. Tukey Honest Significant Difference (HSD) tests indicated that all three terciles differed from each other significantly (first vs third: p = 0.0265, second vs third: p = 0.001, first vs second: p = <0.001). See figure 1 below for a summaries and visualizations of spectral characteristics within the sample. For further spectral parameters, see supplementary figures 1 and 2 for further characterizations of spectral parameters
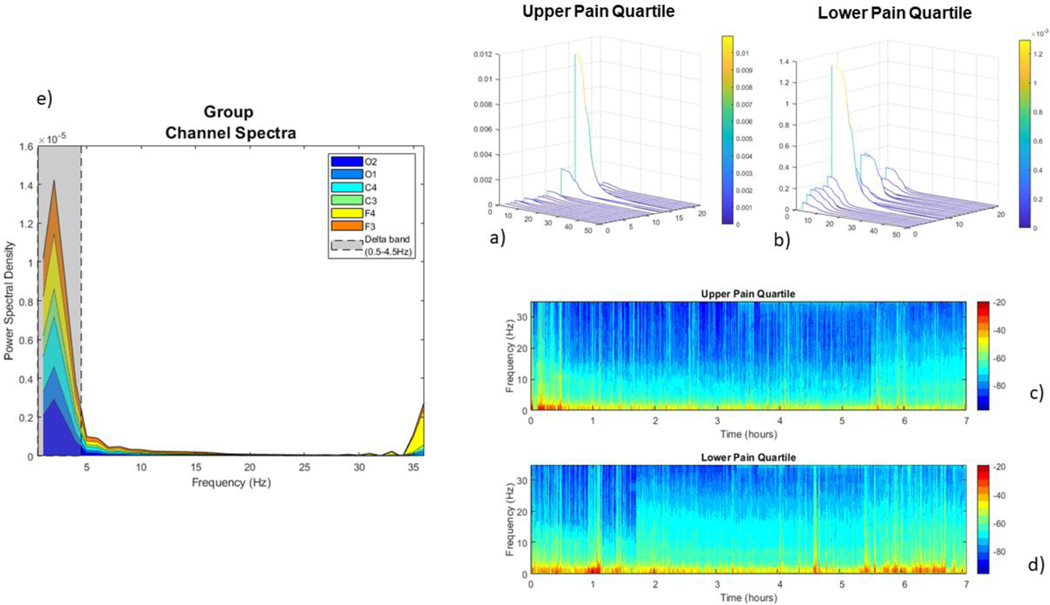
Figure 1:
representation of group average and pain quartile spectra. Panel a) and panel b): Waterfall plot representing the channel spectra (0.3–35Hz) of participants classified into upper (a) and lower (b) quartiles, according to Morning Pain Values. Individual spectra are plotted for each participant on independent axes. Simplified average plots are displayed in supplementary figure 2. Panel c) and d) represent the whole night spectrogram for participants in the upper and lower pain quartiles according to morning pain scores. Data are plotted according to the mean sleep duration (in hrs) of the participants. Relative power is represented by the color gradient, with blue indicating low relative power, and red indicating high relative power. Panel e) represents the average spectra (0.3–35Hz) of all participants in the cohort at each channel.
Primary Analyses: Delta Power
Nocturnal pain, next-morning pain, and daytime pain as outcomes
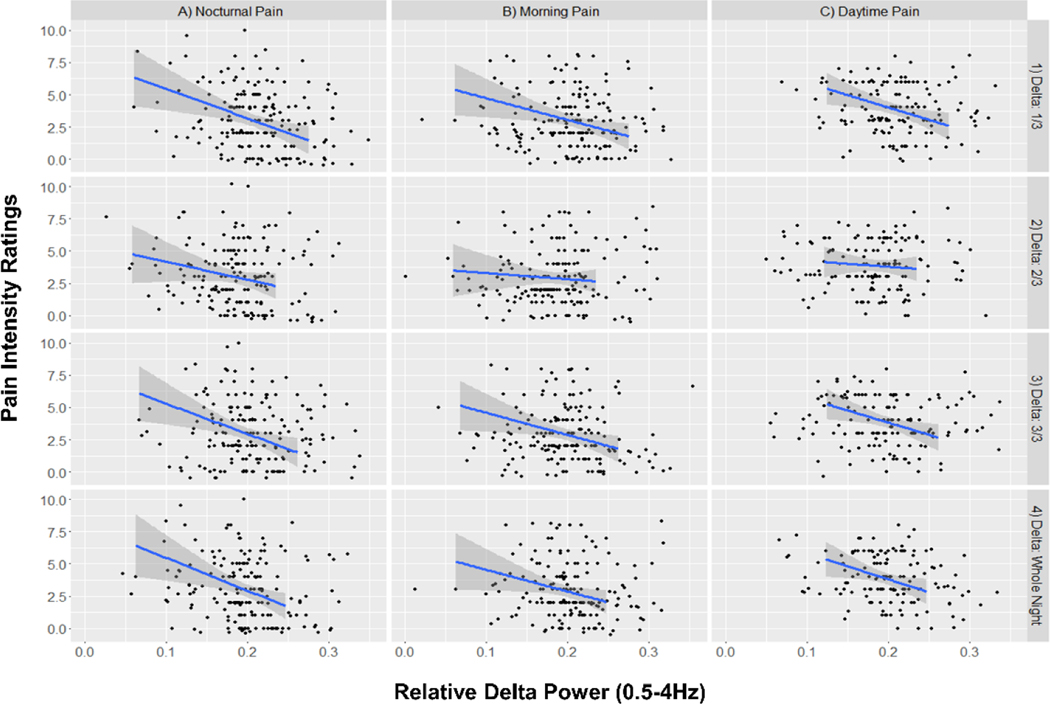
Controlling for age, TST, BMI, self-reported bruxism and anxiety symptoms, greater relative delta power across the whole night was significantly associated with lower nocturnal pain (B = −20.276, p = 0.025, R2 = 0.214) but not next-morning pain (B = −15.806, p = 0.053, R2 = 0.186) or average daytime pain (B = −14.518, p = 0.065, R2 = 0.184), despite similar effect sizes. Greater relative delta power during the first and third portions of the night were significantly associated with nocturnal pain (1st:: B = −17.807, p = 0.019, R2 = 0.217| 3rd: B = −17.602, p = 0.029, R2 = 0.207) and next-morning pain (1st: B = −15.751, p = 0.022, R2 = 0.198 | 3rd: B = −14.943, p = 0.043, R2 = 0.187) (see table 3 and figure 2) . During the first third of the night, relative delta power was significantly associated with average daytime pain (B = 13.876, p = 0.039, R2 = 0.195). Moderation analyses indicated depressive symptom severity did not moderate the relationships between relative delta power and outcome variables (i.e., next-morning pain, average nocturnal pain, and average daytime pain) across any third of the night (see table 3).
Table 3:
Outcome Values and Linear Regression Models
| Outcome | Mean (SD) | Predictor | Unstandardized B | STD error | t value | P Value | Confidence Interval | Effect Size | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5% | 97.5% | R2 | 5% | 95% | ||||||||
| Average Nocturnal Pain | 3.161 (1.860) | Delta Power 1/3 | −17.806 | 7.451 | −2.389 | 0.019 | −32.600 | −3.013 | 0.217 | 0.084 | 0.374 | |
| Depression* Delta Power 1/3 | 1.186 | 1.026 | 1.156 | 0.251 | −0.853 | 3.226 | 0.229 | 0.094 | 0.386 | |||
| Delta Power 2/3 | −11.836 | 8.327 | −1.421 | 0.1 59 | −28.386 | 4.713 | 0.189 | 0.063 | 0.345 | |||
| Depression* Delta Power 2/3 | −0.555 | 1.053 | −0.526 | 0.5 99 | −2.650 | 1.540 | 0.163 | 0.046 | 0.319 | |||
| Delta Power 3/3 | −17.602 | 7.925 | −2.221 | 0.0 29 | −33.340 | −1.865 | 0.206 | 0.077 | 0.362 | |||
| Depression* Delta Power 3/3 | 1.442 | 1.088 | 1.326 | 0.187 | −0.718 | 3.603 | 0.192 | 0.068 | 0.345 | |||
| Delta Power (Whole Night) | −20.276 | 8.895 | −2.280 | 0.025 | −37.938 | −2.614 | 0.214 | 0.081 | 0.371 | |||
| Depression* Delta Power (Whole Night) | 0.676 | 1.191 | 0.567 | 0.571 | −1.691 | 3.044 | 0.183 | 0.061 | 0.337 | |||
| Average Daytime Pain | 3.482 (1.586) | Delta Power 1/3 | −13.875 | 6.618 | −2.097 | 0.040 | −27.070 | −0.682 | 0.194 | 0.064 | 0.357 | |
| Depression* Delta Power 1/3 | −0.458 | 0.818 | −0.560 | 0.576 | −2.084 | 1.167 | 0.190 | 0.059 | 0.354 | |||
| Delta Power 2/3 | −6.427 | 6.830 | −0.941 | 0.349 | −20.019 | 7.163 | 0.153 | 0.036 | 0.315 | |||
| Depression* Delta Power 2/3 | −0.707 | 0.834 | −0.847 | 0.398 | −2.366 | 0.951 | 0.159 | 0.040 | 0.320 | |||
| Delta Power 3/3 | −12.645 | 6.872 | −1.839 | 0.070 | −26.330 | 1.040 | 0.178 | 0.051 | 0.342 | |||
| Depression* Delta Power 3/3 | −0.412 | 0.891 | −0.463 | 0.645 | −2.185 | 1.360 | 0.179 | 0.052 | 0.344 | |||
| Delta Power (Whole Night) | −14.518 | 7.758 | −1.871 | 0.065 | −29.973 | 0.936 | 0.185 | 0.056 | 0.347 | |||
| Depression* Delta Power (Whole Night) | −0.765 | 0.951 | −0.805 | 0.423 | −2.657 | 1.126 | 0.183 | 0.054 | 0.347 | |||
| Next Morning Pain | 3.507(1.845) | Delta Power 1/3 | −15.751 | 6.766 | −2.328 | 0.022 | −29.184 | −2.318 | 0.198 | 0.072 | 0.352 | |
| Depression* Delta Power 1/3 | 0.897 | 0.917 | 0.977 | 0.331 | −0.927 | 2.722 | 0.191 | 0.068 | 0.344 | |||
| Delta Power 2/3 | −5.236 | 7.306 | −0.716 | 0.475 | −19.747 | 9.270 | 0.155 | 0.043 | 0.302 | |||
| Depression* Delta Power 2/3 | 0.103 | 0.926 | 0.112 | 0.911 | −1.738 | 1.944 | 0.144 | 0.037 | 0.291 | |||
| Delta Power 3/3 | −14.943 | 7.265 | −2.057 | 0.0 43 | −29.374 | −0.511 | 0.187 | 0.064 | 0.340 | |||
| Depression* Delta Power 3/3 | 1.180 | 0.983 | 1.198 | 0.233 | −0.775 | 3.131 | 0.188 | 0.065 | 0.341 | |||
| Delta Power (Whole Night) | −15.806 | 8.096 | −1.952 | 0.053 | −31.882 | 0.269 | 0.185 | 0.063 | 0.338 | |||
| Depression* Delta Power (Whole Night) | 0.820 | 1.070 | 0.766 | 0.446 | −1.307 | 2.948 | 0.176 | 0.057 | 0.327 | |||
| Nocturnal Pain Catastrophizing | 2.202 (1.923) | Delta Power 1/3 | −12.824 | 7.304 | −1.755 | 0.082 | −27.329 | 1.679 | 0.201 | 0.068 | 0.362 | |
| Depression* Delta Power 1/3 | 1.516 | 1.031 | 1.469 | 0.145 | −0.535 | 3.567 | 0.213 | 0.079 | 0.372 | |||
| Delta Power 2/3 | −5.491 | 8.144 | −0.674 | 0.501 | −21.670 | 10.688 | 0.173 | 0.051 | 0.26 | |||
| Depression* Delta Power 2/3 | 0.249 | 1.029 | 0.242 | 0.809 | −1.798 | 2.297 | 0.175 | 0.052 | 0.333 | |||
| Delta Power 3/3 | −12.460 | 8.012 | −1.555 | 0.123 | −28.368 | 3.448 | 0.189 | 0.061 | 0.349 | |||
| Depression* Delta Power 3/3 | 1.608 | 1.099 | 1.462 | 0.147 | −0.578 | 3.795 | 0.209 | 0.077 | 0.368 | |||
| Delta Power (Whole Night) | −11.966 | 8.704 | −1.375 | 0.172 | −29.24 | 5.310 | 0.136 | 0.030 | 0.288 | |||
| Depression* Delta Power (Whole Night) | 1.313 | 1.199 | 1.095 | 0.276 | −1.072 | 3.670 | 0.200 | 0.070 | 0.359 | |||
| Daytime Pain Catastrophizing | 2.541(1.915) | Delta Power 1/3 | 3.086 | 8.806 | 0.350 | 66.325 | 0.727 | −14.494 | 0.147 | 0.034 | 0.304 | |
| Depression* Delta Power 1/3 | −0.051 | 1.086 | −0.047 | 0.962 | −2.212 | 2.109 | 0.138 | 0.032 | 0.287 | |||
| Delta Power 2/3 | 2.305 | 9.172 | 0.251 | 0.802 | −15.984 | 20.594 | 0.146 | 0.033 | 0.303 | |||
| Depression* Delta Power 2/3 | −2.197 | 1.056 | −2.080 | 0.041 | −4.298 | −0.095 | 0.178 | 0.057 | 0.332 | |||
| Delta Power 3/3 | 6.188 | 9.235 | 0.670 | 0.505 | −12.223 | 24.599 | 0.150 | 0.036 | 0.307 | |||
| Depression* Delta Power 3/3 | 0.215 | 1.156 | 0.185 | 0.853 | −2.087 | 2.516 | 0.133 | 0.030 | 0.281 | |||
| Delta Power (Whole Night) | 4.882 | 10.348 | 0.471 | 0.638 | −15.769 | 25.533 | 0.148 | 0.035 | 0.305 | |||
| Depression* Delta Power (Whole Night) | −0.795 | 1.249 | −0.636 | 0.526 | −3.281 | 1.691 | 0.134 | 0.029 | 0.284 | |||
Data are values from pooled multiple imputation models, displayed across thirds of the night and the whole night. Delta Power 1/3, 2/3, 3/3: Delta Power during the 1st 2nd & 3rd third of the night, respectively. Interaction terms are displayed as the formula: x*y. SD= Standard Deviation.
Figure 2: Relationship between delta power subjective pain reports.
Power 1/3, 2/3, 3/3 = Relative Delta Power (0.5–4hz) during the 1st 2nd & 3rd third of the night, respectively. Data are results from uncorrected linear regression models. Blue lines indicate the model line of best fit. Gray shaded areas indicate the 95% Confidence Intervals of the model.
Absolute delta power was significantly associated with next-morning pain, but was not significantly associated with daytime pain or nocturnal pain, despite similar effect sizes between measures (Next-morning pain R2= 0.039, p = 0.037, Average Nocturnal Pain R2= 0.028, p = 0.087, Average Daytime Pain R2= 0.016, p = 0.175). Depression did not moderate the relationship between absolute delta power and Average Nocturnal Pain (p = 0.128, R2 = 0.187) Next Morning Pain (p = 0.087, R2 = 0.028) (See supplemental table 3).
Results on Pain Catastrophizing
Nocturnal Pain Catastrophizing
Controlling for age, TST, BMI, self-report bruxism and anxiety symptoms, relative delta power was not significantly associated with nocturnal pain catastrophizing across the whole night (B = 11.966, p = 0.172, R2 = 0.136), nor the first (B = −12.825, p = 0.082, R2 = 0.201) second (B = −5.49, p = 0.502, R2 = 0.173) or third (B = −12.460, p = 0.123, R2 = 0.189) portion or the night (see table 3 and figure 2). Moderation analyses indicated that depressive symptom severity did not moderate the relationship between relative delta power and pain catastrophizing across the whole night (B = −11.966, p = 0.172, R2 = 0.136), during the first third of the night (B = 1.516, p = 0.145, R2 = 0.214), second, (B = 0.249, p = 0.809, R2 = 0.175) or final third of the night (B = 1.608, p = 0.147, R2 = 0.209). Absolute delta power was significantly associated with nocturnal pain catastrophizing (B =11.394, p = 0.015, R2 = 0.065). Depression moderated the relationship between absolute delta power across the whole night and Nocturnal Pain Catastrophizing (B = 11.394 p = p = 0.011, R2 = 0.263) (see supplementary table 3).
Daytime Pain Catastrophizing
Controlling for age, TST, BMI, self-report bruxism, and anxiety symptoms, relative nocturnal delta power was not associated with daytime pain catastrophizing, across the whole night (B = 4.882, p = 0.639, R2 = 0.148), nor during the first (B = 3.086, p = 0.727, R2 = 0.147), second (B = 2.305, p = 0.802, R2 = 0.146) or third (B = 6.187, p = 0.505, R2 = 0.150) portion of the night (see table 3 and figure 2). Depressive symptom severity did not significantly moderate the relationship between relative nocturnal delta power and daytime catastrophizing across the whole night (B = −0.795, p = 0.526, R2 = 0.134), nor the first (B = −0.051, p = 0.962, R2 = 0.138) or third portion of the night. Depressive symptom severity did moderate the relationship between relative nocturnal delta power during the second third of the night and daytime pain catastrophizing (B = −2.197, p = 0.041, R2 = 0.179), however Johnson-Neyman intervals indicated that the slope of relative delta power x daytime pain catastrophizing was not significant at any value. Absolute delta power was significantly associated with daytime pain catastrophizing (B =5.098, p = 0.272, R2 = 0.013). Depression marginally moderated the relationship between absolute delta power across the whole night and Daytime Pain Catastrophizing (B = −2.444, p = 0.051, R2 = 0.179) (see supplemental table 3).
Post-hoc Analyses: Percent SWS as a Predictor
SWS% was not significantly associated with average nocturnal pain (B = −0.029, p = 0.368, R2 = 0.185), next-morning pain (B −0.032, p = 0.291, R2 = 0.172), or average daytime pain (B = 0.003, p = 0.925, R2 = 0.139), nor was it associated with nocturnal pain catastrophizing or (B = −0.019, p = 0.562, R2 = 0.183) or daytime pain catastrophizing (B = 0.036, p = 0.297, R2 = 0.156). Moderation analyses indicated that depressive symptom severity did not moderate the relationship between SWS% and nocturnal pain (B = 0.0001, p = 0.978, R2 = 0.188), daytime pain (B = −0.003, p = 0.180, R2 = 0.153), or next-morning pain (B = 0.001, p = 0.649, R2 = 0.161). Similarly depressive symptom severity did not moderate the relationship between nocturnal (B = 0.003, p = 0.380, R2 = 0.190) or daytime pain catastrophizing (B = −0.002, p = 0.510, R2 = 0.151). See supplemental table 1 for analyses on sleep duration and continuity.
Secondary Analyses
Effects of remaining spectral bands on next-day pain
To provide a complete account of the relationship between spectral power and next-day pain, we further analyzed activity in the alpha, beta, theta and sigma power bands as secondary outcomes. After controlling for age, TST, BMI, self-report bruxism, and anxiety symptoms, relative power in the alpha, beta, theta and sigma power bands was not significantly associated with next-morning pain, average daytime pain, average nocturnal pain, nocturnal pain catastrophizing or daytime pain catastrophizing. Similarly, after controlling for age, TST, BMI, self-report bruxism, and anxiety symptoms, absolute power in the alpha, theta, and sigma power bands was not significantly associated with next-morning pain, average nocturnal pain, average daytime pain, nocturnal pain catastrophizing, or daytime pain catastrophizing. Absolute power in the beta power band was significantly associated with daytime pain catastrophizing (B = 73329.911, p = 0.009, R2 = 0.159). However, beta power was not associated with any of the remaining pain variables (see supplemental table 3 for full results).
Association between nocturnal delta power and trait pain catastrophizing
Participants demonstrated a mean score on the Pain Catastrophizing Scale of 20.37 (SD = . Pain catastrophizing was not significantly associated with relative delta power across the whole night (B = 20.68, P = 0.647, R2 = 0.002).
Discussion
This study investigated the role of nocturnal delta activity in nocturnal and daytime pain and pain catastrophizing. We hypothesized that higher relative delta power would be associated with lower subjective reports of pain, and pain catastrophizing. Further, we hypothesized that the relationship between relative delta power, pain and pain catastrophizing, would be moderated by depressive symptom severity. Here we present findings demonstrating that relative delta power is significantly associated with subjective reports of pain experienced during nocturnal, morning and daytime periods, in patients with comorbid insomnia and TMJD.
The principal finding of this study was that relative delta power was significantly associated with self-reported pain intensity but not catastrophizing during both nocturnal (concurrent with PSG night) and following daytime periods across a variety of timepoints including nocturnal, next-morning, and daytime pain. To our knowledge, this is the first study to examine the relationship between relative or absolute delta power during sleep and next day reports of pain in patients with TMD. Similarly, reports of the relationship between delta power and next-day pain in other clinical pain populations are rare. Only one early study43 in a small sample (N=8) of patients with fibromyalgia undergoing treatment with chlorpromazine and L-tryptophan reported that the higher percentage of delta power per minute was associated with a higher overnight decrease in subjective pain reports. Our study provides further support for these findings in a larger sample of women with insomnia and TMD, suggesting that delta activity during sleep may play a role in pain regulation in chronic pain disorders. Future studies should investigate whether similar findings are observed in chronic pain patients who do not report insomnia symptoms, or in those with sub-clinical insomnia. When considering our findings, it is pertinent that our reports of nocturnal pain were collected retrospectively, therefore it is possible that participants reports were influenced by their current level of pain. Nevertheless, this procedure remains necessary to avoid completion of surveys during the night from artificially disrupting sleep.
Contrary to our hypotheses, relative delta power was not associated with nocturnal or next-day pain catastrophizing. As we examined state, and not trait-like catastrophizing, it is possible that results may differ if trait-like measures were considered. However, data from our group highlights the importance of utilizing state-catastrophizing measures, as this construct has been shown to be altered by CBTi interventions32, and predicted pain ratings and pain tolerance during cold-pressor testing, whereas trait-like catastrophizing did not21. Further contrary to our hypotheses, depressive symptom severity did not significantly moderate the relationship between relative delta power and next-day pain or pain catastrophizing. Previous evidence demonstrates that delta dysregulation is common in depression, with patients demonstrating reduced delta power across the whole night42, 63, as well as a reduced delta-sleep-ratio (the proportion of delta power between the first and second NREM cycle)46. Hence, we hypothesized that this dysregulation may contribute further to the exacerbation of next-day pain. Despite these previous findings, our data suggest that depressive symptom severity do not alter the observed relationship between nocturnal delta power and next day pain functioning. One possibility for this discrepancy is that the mean depressive symptom severity of our participants was in the low range (mean CES-D score = 18.35), with very few participants (n=14) meeting criteria for severe depressive symptoms (CES-D > 27), whilst many of the historical reports of SWS dysregulation were observed in severely depressed populations42, 63. To this note, values for delta power, did not significantly differ between participants with and without elevated depressive symptoms. To this end, future studies should seek to investigate potential moderating effects in more severely depressed participants.
Although effects were also observed at the whole night level, the key findings were observed preferentially in regards to delta power measured during the first third of the night. This suggests that the association between delta wave activity and pain may be driven by homeostatic sleep drive that occurs during the early portion of the night. Under normal sleeping conditions, delta power has been shown to follow a curvilinear decline across NREM periods throughout the night, which signifies a normalization of homeostatic sleep drive16. However, this decline varies as a function of the spectral delta band and age of participants, with younger sleepers demonstrating steeper declines. Nevertheless, a decline in relative delta power (when defined as 0.3–4 Hz) of ~50% has been observed between the first and fourth NREM periods in both young and healthy adults16. Although our analyses indicated that relative delta power was significantly different across thirds of the night, the magnitude of decline was minimal (Delta power: 1st = 0.210 [SD 0.032], 2nd = 0.185 [SD 0.028], 3rd = 0.199 [SD 0.030]). This could suggest a blunted dissipation of homeostatic sleep drive, as evidenced by minimal decline in delta power across the night, observed in those with insomnia and TMD symptoms, which buffers the ability to derive restorative effects of delta activity on pain. However, this effect may be due to first night effects, indicating participants may have had greater sleeping difficulties the night of the PSG. Future studies should consider the use of an adaptation night, and further investigate how intra-nocturnal kinetics of delta activity relate to next-day pain reports.
This study has notable strengths. First, we analyzed PSG data by calculating spectral power, as opposed to relying on staged architectural sleep parameters, optimizing our sensitivity to detect a relationship between delta wave activity and pain. Illustrating this, many effects were selectively observed for delta power, and not SWS%. Ours is the largest study to do so, and therefore provides novel contribution to the literature. Second, we derived delta power across each third of the night, demonstrating how differences in homeostatic sleep drive across the night alter the relationship between delta activity to next-day pain. Finally, we examined the effects of how sleep maps onto pain measurements obtained the morning and daytime immediately after PSGs were obtained. Nevertheless, these strengths should be tempered against the study’s weaknesses. We did not use an adaptation night prior to collecting data from participants. Therefore, the sleep recorded in participants may not be fully reflective of the sleep one would obtain under normal circumstances. However, this is counterbalanced by the use of ambulatory PSG recordings in participants’ homes, resulting in measurements of higher ecological validity19, with a reduced likelihood of a first-night effect. Second, due to the inclusion criteria of the parent study, our sample was fixed and consisted entirely of females. Although this is a valid representation of a TMD population, this limits our capacity to translate these findings to other populations, due to the differences in sleep architecture and pain responses between males and females. It is also worth noting, that we observed a significant moderating effect of depressive symptom severity on the relationship between delta power in the second third of the night and daytime pain catastrophizing. Nevertheless, our post-hoc simple slope analyses indicated that the slope was not significant at any of the tested values for depressive symptom severity. Therefore, it is difficult to interpret this moderating effect, however it is possible that power limitations were responsible for this absence of effect, and that a larger sample may have been required to detect the values at which slopes were significant.
The consistent finding that higher delta power is associated with lower subjective reports of pain across different timepoints has potential relevance for the clinical treatment of chronic pain disorders. Evidence-based interventions for sleep such as CBTi have been shown to increase activity in the delta power band13, and increase the proportion of time spent in SWS, as well as reductions in pain catastrophizing32, interference and pain related disability32. Increasing access to such interventions may improve outcomes in patients with chronic pain by SWS mediated mechanisms. Therefore, further investigation of the causal mechanisms underpinning CBTi’s action on pain symptoms is warranted. Furthermore, continued investigation of how depression interacts with the relationship between sleep and pain among individuals with pain disorders may shed insights into our understanding of the clinical-triad of sleep, pain and depression.
Supplementary Material
Perspective:
This article presents data demonstrating an association between increased nocturnal delta power and reduced next-day pain. These findings may help promote interventions which aim to increase nocturnal delta power in clinical pain populations, with the goal of improving pain outcomes.
Highlights.
Previous evidence suggests that chronic pain patients have reduced delta EEG power during sleep
However, few studies have examined how delta power is linked to pain intensity in chronic pain
We used EEG spectral analysis to compute delta power across the night in 110 patients
Reduced delta power was associated with increased pain severity across multiple measures
Funding:
This research study was supported financially by the NIH Grant R01 DE019731 (Haythornthwaite, JA and Smith, MT). Darlynn M. Rojo-Wissar was supported by the National Institute of Mental Health’s Psychiatric Epidemiology Training Program (5T32MH014592-39; PI: Volk, Heather) and subsequently by the Eunice Kennedy Shriver National Institute of Child Health & Human Development’s Training Program in Childhood Stress, Trauma, & Resilience (T32HD101392; PI: Stroud, Laura).
Footnotes
Disclosure Statement: Financial Disclosure: PHF is on the Scientific Advisory Board of, and has equity interest in Ninnion. Non-financial Disclosure: none
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Althouse AD. Adjust for multiple comparisons? It’s not that simple. The Annals of thoracic surgery. 101:1644–1645, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Armitage R. The distribution of EEG frequencies in REM and NREM sleep stages in healthy young adults. Sleep. 18:334–341, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2:297–307, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Beck AT, Bredemeier K. A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science. 4:596–619, 2016 [Google Scholar]
- 5.Bender R, Lange S. Adjusting for multiple testing—when and how? Journal of clinical epidemiology. 54:343–349, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV: AASM scoring manual updates for 2017 (version 2.4), American Academy of Sleep Medicine, Journal of Clinical Sleep Medicine, 3:665–666, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry RB, Brooks R, Gamaldo CE, Harding SM, Marcus C, Vaughn BV. The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications, Darien, Illinois, American Academy of Sleep Medicine. 176:194, 2012 [Google Scholar]
- 8.Bertoli E, de Leeuw R. Prevalence of Suicidal Ideation, Depression, and Anxiety in Chronic Temporomandibular Disorder Patients. Journal of Oral & Facial Pain & Headache. 30:296–301 2016 [DOI] [PubMed] [Google Scholar]
- 9.Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: A review of controlled studies. Sleep Med Rev. 26:74–86, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LA, Lynch KG, Cheatle M. Pain catastrophizing as a predictor of suicidal ideation in chronic pain patients with an opiate prescription. Psychiatry research. 286:112893, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunner D, Vasko RC, Detka C, Monahan J, Reynolds C, Kupfer D. Muscle artifacts in the sleep EEG: Automated detection and effort on all-night EEG power spectra. J Sleep Res. 5:155–164, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L, Robinson M, Edwards RR, Smith MT. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis care & research. 67:1387–1396, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervena K, Dauvilliers Y, Espa F, Touchon J, Matousek M, Billiard M, Besset A. Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. Journal of sleep research. 13:385–393, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Chen S-Y, Feng Z, Yi X. A general introduction to adjustment for multiple comparisons. Journal of thoracic disease. 9:1725–1726, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Annals, academy of medicine, Singapore. 23:129–138, 1994 [PubMed] [Google Scholar]
- 16.Darchia N, Campbell IG, Tan X, Feinberg I . Kinetics of NREM delta EEG power density across NREM periods depend on age and on delta-band designation. Sleep. 30:71–79, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devins GM, Orme CM, Costello CG, Binik YM, Frizzell B, Stam HJ, Pullin WM. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) scale. Psychology and Health. 2:139–156, 1988 [Google Scholar]
- 18.Dijk DJ, Beersma DG, Bloem GM. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 12:500–507, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Edinger JD, Fins AI, Sullivan RJ Jr, Marsh GR, Dailey DS, Hope TV, Young M, Shaw E, Carlson D, Vasilas D. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 20:1119–1126, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nature Reviews Rheumatology. 7:216–224, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. Journal of Pain. 6:338–339, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Enders CK: Applied missing data analysis, Guilford press,1:1–225, 2010. [Google Scholar]
- 23.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 17:173–183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Generaal E, Vogelzangs N, Penninx BW, Dekker J. Insomnia, Sleep Duration, Depressive Symptoms, and the Onset of Chronic Multisite Musculoskeletal Pain. Sleep. 40: 1–10, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Graham JW. Missing data analysis: Making it work in the real world. Annual review of psychology. 60:549–576, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Groeger JA, Stanley N, Deacon S, Dijk D-J. Dissociating effects of global SWS disruption and healthy aging on waking performance and daytime sleepiness. Sleep. 37:1127–1142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials–a practical guide with flowcharts. BMC medical research methodology. 17:1–10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 10:370–375, 1958 [PubMed] [Google Scholar]
- 29.Lee HJ, Kim ST. A questionnaire-based study of sleep-wake patterns and sleep quality in a TMJ and orofacial pain clinic. Cranio. 38:213–220, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of behavioral medicine. 30:77–94, 2007 [DOI] [PubMed] [Google Scholar]
- 31.LeResche L, Saunders K, Von Korff MR, Barlow W, Dworkin SF. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 69:153–160, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Lerman SF, Finan PH, Smith MT, Haythornthwaite JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. 158:2189–2195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman SF, Mun CJ, Hunt CA, Kunatharaju S, Buenaver LF, Finan PH, Campbell CM, Phillips J, Fernandez-Mendoza J, Haythornthwaite JA. Insomnia with Objective Short Sleep Duration in Women with Temporomandibular Joint Disorder: Quantitative Sensory Testing, Inflammation and Clinical Pain Profiles. Sleep Medicine, 90:26–35, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 12:277–278, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC psychiatry. 16:1–16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X, Gignac MA, Anis AH. Workplace, psychosocial factors, and depressive symptoms among working people with arthritis: a longitudinal study. The Journal of rheumatology. 33:1849–1855, 2006 [PubMed] [Google Scholar]
- 37.Li Y, Liu H, Weed JG, Ren R, Sun Y, Tan L, Tang X. Deficits in attention performance are associated with insufficiency of slow-wave sleep in insomnia. Sleep medicine. 24:124–130, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Linton SJ, Nicholas MK, MacDonald S, Boersma K, Bergbom S, Maher C, Refshauge K. The role of depression and catastrophizing in musculoskeletal pain. European Journal of Pain. 15:416–422, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Manfredini D, Borella L, Favero L, Ferronato G, Guarda-Nardini L. Chronic pain severity and depression/somatization levels in TMD patients. International Journal of Prosthodontics. 23:529–534 2010 [PubMed] [Google Scholar]
- 40.Maquet P, Degueldre C, Delfiore G, Aerts J, Péters J-M, Luxen A, Franck G. Functional neuroanatomy of human slow wave sleep. Journal of Neuroscience. 17:2807–2812, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martel M, Wasan A, Jamison R, Edwards RR. Catastrophic thinking and increased risk for prescription opioid misuse in patients with chronic pain. Drug and alcohol dependence. 132:335–341, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendelson WB, Sack DA, James SP, Martin JV, Wagner R, Garnett D, Milton J, Wehr TA. Frequency analysis of the sleep EEG in depression. Psychiatry Research. 21:89–94, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Moldofsky H, Lue F. The relationship of alpha and delta EEG frequencies to pain and mood in ‘fibrositis’ patients treated with chlorpromazine and L-tryptophan. Electroencephalography and clinical neurophysiology. 50:71–80, 1980 [DOI] [PubMed] [Google Scholar]
- 44.Mun CJ, Davis MC, Campbell CM, Finan PH, Tennen H. Linking nonrestorative sleep and activity interference through pain catastrophizing and pain severity: an intraday process model among individuals with fibromyalgia. The Journal of Pain. 21:546–556, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murck H, Frieboes R, Antonijevic I, Steiger A. Distinct temporal pattern of the effects of the combined serotonin-reuptake inhibitor and 5-HT1A agonist EMD 68843 on the sleep EEG in healthy men. Psychopharmacology. 155:187–192, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Nissen C, Feige B, König A, Voderholzer U, Berger M, Riemann D. Delta sleep ratio as a predictor of sleep deprivation response in major depression. Journal of psychiatric research. 35:155–163, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Ohrbach R, Turner JA, Sherman JJ, Mancl LA, Truelove EL, Schiffman EL, Dworkin SF. Research diagnostic criteria for temporomandibular disorders: evaluation of psychometric properties of the axis II measures. Journal of orofacial pain. 24:48–49, 2010 [PMC free article] [PubMed] [Google Scholar]
- 48.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. Journal of sleep research. 10:35–42, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Prerau MJ, Brown RE, Bianchi MT, Ellenbogen JM, Purdon PL. Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology. 32:60–92, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Press WH, Teukolsky SA. SavitzkyGolay smoothing filters. Computers in Physics. 4:669–672, 1990 [Google Scholar]
- 51.Smith M, Perlis M, Smith M, Giles D, Carmody T. Sleep quality and presleep arousal in chronic pain. Journal of behavioral medicine. 23:1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep medicine reviews. 8:119–132, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 32:779–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 7: 524–532, 1995 [Google Scholar]
- 55.Tassi P, Bonnefond A, Engasser O, Hoeft A, Eschenlauer R, Muzet A. EEG spectral power and cognitive performance during sleep inertia: the effect of normal sleep duration and partial sleep deprivation. Physiology & behavior. 87:177–184, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Taylor DJ, Wilkerson AK, Pruiksma KE, Williams JM, Ruggero CJ, Hale W, Mintz J, Organek KM, Nicholson KL, Litz BT. Reliability of the structured clinical interview for DSM-5 sleep disorders module. Journal of Clinical Sleep Medicine. 14:459–464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomson DJ. Spectrum estimation and harmonic analysis. Proceedings of the I.E.E.E. 70:41, 1982 [Google Scholar]
- 58.Veiga DM, Cunali R, Bonotto D, Cunali PA. Sleep quality in patients with temporomandibular disorder: a systematic review. Sleep Science. 6:120–124, 2013 [Google Scholar]
- 59.Velly AM, Look JO, Carlson C, Lenton PA, Kang W, Holcroft CA, Fricton JR. The effect of catastrophizing and depression on chronic pain–a prospective cohort study of temporomandibular muscle and joint pain disorders. PAIN. 152:2377–2383, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Vlaeyen JW, Linton SJ. Pain-related fear and its consequences in chronic musculoskeletal pain. 1: 83–104, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Werth E, Achermann P, BORBÉLY A. Frontooccipital EEG power gradients in human sleep. Journal of sleep research. 6:102–112, 1997 [DOI] [PubMed] [Google Scholar]
- 62.West SG, Finch JF, Curran PJ. Structural equation models with nonnormal variables: Problems and remedies. 1995 [Google Scholar]
- 63.Wichniak A, Wierzbicka A, Jernajczyk W. Sleep as a biomarker for depression. International review of psychiatry. 25:632–645, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Widaman KF. Best practices in quantitative methods for developmentalists: III. Missing data: What to do with or without them. Monographs of the Society for Research in Child Development. 71:42–64, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Yetton B, Whitehurst LN, Naji M, Mednick SC. The effect of zolpidem on memory consolidation over a night of sleep. Sleep. 43:zsaa084, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.