Limb ischaemia is classified on the basis of onset and severity. Complete acute ischaemia will lead to extensive tissue necrosis within six hours unless the limb is surgically revascularised. Incomplete acute ischaemia can usually be treated medically in the first instance. Patients with irreversible ischaemia require urgent amputation unless it is too extensive or the patient too ill to survive.
Classification of limb ischaemia
| Terminology | Definition or comment |
| Onset: | |
| Acute | Ischaemia <14 days |
| Acute on chronic | Worsening symptoms and signs (<14 days) |
| Chronic | Ischaemia stable for >14 days |
| Severity (acute, acute on chronic): | |
| Incomplete | Limb not threatened |
| Complete | Limb threatened |
| Irreversible | Limb non-viable |
Clinical features
Apart from paralysis (inability to wiggle toes or fingers) and anaesthesia (loss of light touch over the dorsum of the foot or hand), the symptoms and signs of acute ischaemia are non-specific or inconsistently related to its completeness. Pain on squeezing the calf indicates muscle infarction and impending irreversible ischaemia.
Symptoms and signs of acute limb ischaemia
| Symptoms or signs | Comment |
| Pain | Occasionally absent in complete ischaemia |
| Pallor | Also present in chronic ischaemia |
| Pulseless | Also present in chronic ischaemia |
| Perishing cold | Unreliable as ischaemic limb takes on ambient temperature |
| Paraesthesia* | Leading to anaesthesia (unable to feel touch on foot or hand) |
| Paralysis* | Unable to wiggle toes or fingers |
*Anaesthesia and paralysis are the key to diagnosing complete ischaemia that requires emergency surgical treatment
Acute arterial occlusion is associated with intense spasm in the distal arterial tree, and initially the limb will appear “marble” white. Over the next few hours, the spasm relaxes and the skin fills with deoxygenated blood leading to mottling that is light blue or purple, has a fine reticular pattern, and blanches on pressure. At this stage the limb is still salvageable. However, as ischaemia progresses, stagnant blood coagulates leading to mottling that is darker in colour, coarser in pattern, and does not blanch. Finally, large patches of fixed staining progress to blistering and liquefaction. Attempts to revascularise such a limb are futile and will lead to life threatening reperfusion injury. In cases of real doubt the muscle can be examined at surgery through a small fasciotomy incision. It is usually obvious when the muscle is dead.
Differentiation of embolus and acute arterial thrombosis (thrombosis in situ)
| Clinical features | Embolus | Thrombosis |
| Severity | Complete (no collaterals) | Incomplete (collaterals) |
| Onset | Seconds or minutes | Hours or days |
| Limb affected | Leg 3:1 arm | Leg 10:1 arm |
| Multiple sites | Up to 15% | Rare |
| Embolic source | Present (usually atrial fibrillation) | Absent |
| Previous claudication | Absent | Present |
| Palpation of artery | Soft, tender | Hard, calcified |
| Bruits | Absent | Present |
| Contralateral leg pulses | Present | Absent |
| Diagnosis | Clinical | Angiography |
| Treatment | Embolectomy, warfarin | Medical, bypass, thrombolysis |
Aetiology
Acute limb ischaemia is most commonly caused by acute thrombotic occlusion of a pre-existing stenotic arterial segment (60% of cases) or by embolus (30%). Distinguishing these two conditions is important because treatment and prognosis are different. Other causes are trauma, iatrogenic injury, popliteal aneurysm, and aortic dissection.
More than 80% of peripheral emboli arise from the left atrial appendage in association with atrial fibrillation. They may also arise from the left ventricle, heart valves, prosthetic bypass grafts, aneurysmal disease, paradoxical embolism, and atrial myxoma (rare). In 15% of cases the source of embolus is obscure. Thrombosis in situ may arise from acute plaque rupture, hypovolaemia, or pump failure (see below).
Management
General measures
When a patient is suspected to have an acutely ischaemic limb the case must be discussed immediately with a vascular surgeon. A few hours can make the difference between death or amputation and complete recovery of limb function. If there are no contraindications (acute aortic dissection or multiple trauma, particularly serious head injury) give an intravenous bolus of heparin to limit propagation of thrombus and protect the collateral circulation.
Is angiography required?
If ischaemia is complete, the patient must be taken directly to the operating theatre because angiography will introduce delay, thrombolysis is not an option, and lack of collateral flow will prevent visualisation of the distal vasculature. If ischaemia is incomplete the patient should have preoperative angiography since simple embolectomy or thrombectomy is unlikely to be successful, thrombolysis may be an option, and the surgeon requires a “road map” for distal bypass.
Factors predisposing to acute thrombosis
| Cause | Comment |
| Dehydration | Hot weather, diabetes, infection, gastroenteritis |
| Hypotension | Myocardial infarction, arrhythmia, heart failure, gastrointestinal haemorrhage, septic shock, multiple organ failure |
| Unusual posture or activity | Prolonged sitting, kneeling |
| Malignancy | Solid and haematological |
| Hyperviscosity | Polycythaemia, thrombocytosis |
| Thrombophilia | Protein C or S and antithrombin III deficiencies; activated protein C resistance; factor V Leiden; antiphospholipid syndrome |
Acute embolus
Embolic occlusion of the brachial artery is not usually limb threatening, and in elderly people non-operative treatment is reasonable. Younger patients should have embolectomy to prevent subsequent claudication, especially if the dominant arm is affected.
A leg affected by embolus is nearly always threatened and requires immediate surgical revascularisation. Emboli usually lodge at the common femoral bifurcation or, less commonly, the popliteal trifurcation. Femoral embolus is associated with profound ischaemia to the level of the upper thigh because the deep femoral artery is also affected. A femoral pulse does not exclude the diagnosis. Embolectomy can be done under local, regional, or general anaesthetic.
The adequacy of embolectomy should be confirmed by angiography while the patient is on the operating table. On-table thrombolysis should be considered if mechanical clearance has been unsuccessful. If the embolus has occurred in an area of longstanding atherosclerotic disease, surgical bypass may be necessary.
Postoperatively the patient should continue to receive heparin to prevent formation of further emboli. Many surgeons postpone heparin for six hours after surgery to reduce the risk of a haematoma forming. Warfarin reduces the risk of recurrent embolism, and unless contraindicated, should be prescribed to all patients long term. Patients should not be given warfarin without first being on heparin for 48 hours since warfarin can produce a transient procoagulant state due to inhibition of the vitamin K dependent anticoagulant proteins C and S.
Opinions differ about how thorough you should be in establishing the source of emboli. Transthoracic echocardiography is poor at detecting a thrombus in patients with atrial fibrillation, and a negative result does not exclude the diagnosis. Transoesophageal echocardiography provides excellent views of the left atrium but is moderately invasive and not universally available. In patients with suspected paroxysmal tachyarrhythmias, 24 hour electrocardiographic monitoring should be considered. Even if no source of embolism is found, anticoagulation should continue long term.
Although immediate loss of a limb after correctly managed acute embolus is unusual, many series report a 10-20% in-hospital mortality from heart failure or recurrent embolism, particularly stroke.
Saddle embolus
Patients with acute embolic occlusion of the aortic bifurcation have femoral pulses and appear marble white or mottled to the waist. They may also present with paraplegia due to ischaemia of the cauda equina, which can be irreversible. Immediate bilateral embolectomy restores lower limb perfusion, but many patients subsequently die from reperfusion injury.
Popliteal aneurysm
A popliteal aneurysm can initiate acute ischaemia by forming a thrombus or acting as a source of emboli. Thrombolysis is often the best treatment as simple embolectomy or thrombectomy usually leads to early rethrombosis and surgical bypass is often precluded by obliteration of the distal run-off. Once the circulation is restored, a bypass should be performed to exclude the aneurysm.
Atheroembolism
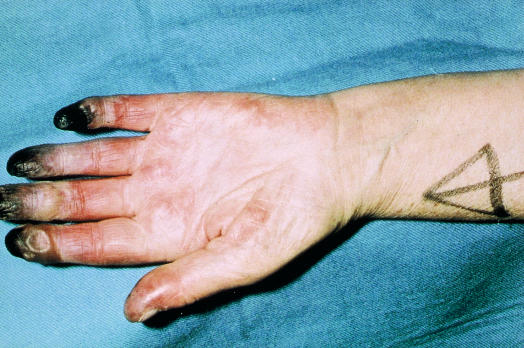
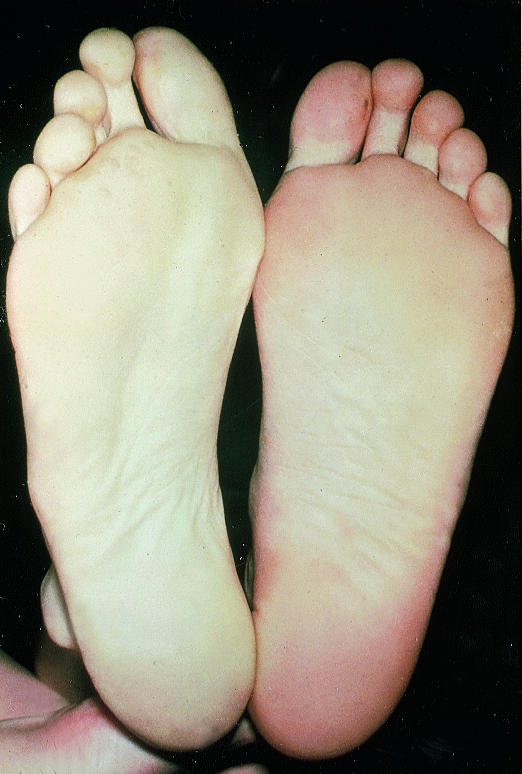
Cholesterol emboli are shed from a complex, often acutely ruptured, atherosclerotic plaque. Distal pulses are usually present. The patient characteristically presents with the blue toe (finger) syndrome, which may mimic Raynaud's phenomenon. If the blue toe syndrome is not recognised patients may deteriorate rapidly and require amputation.
Thrombosis in situ
Limbs affected by stable chronic ischaemia do not usually suddenly deteriorate without a reason—for example, silent myocardial infarction or underlying, hitherto asymptomatic, malignancy. Septicaemia, particularly pneumococcal and meningococcal, may be associated with widespread thrombosis.
Initial management of acute limb ischaemia
Sensation and movement absent
Intravenous heparin
Rapid resuscitation to best medical condition
Intravenous fluids, catheter, and good urine output
Urgent surgery—embolectomy or bypass
Sensation and movement present
Optimise patient to best medical condition
Admit to hospital
Intravenous heparin
Observe limb for signs of deterioration (and act if it occurs)
Arteriogram when convenient
Trauma
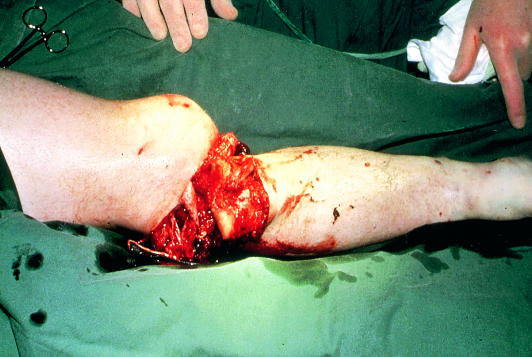
The commonest causes of non-iatrogenic injury are limb fractures and dislocations (supracondylar fractures of the humerus in children, tibial fractures in adults), blunt injuries occurring in road traffic accidents, and stab wounds. In the United Kingdom, acute traumatic limb ischaemia is often iatrogenic, being caused by arterial cannulation (coronary angioplasty, aortic balloon pump), vascular and orthopaedic procedures on the limb (especially if exsanguinating tourniquets are used), or pelvic surgery (cystectomy, anterior resection) in patients with subclinical aortoiliac disease in whom the ligated pelvic collaterals form the main blood supply to the legs. Postoperative assessment of lower limb ischaemia may be confused by the presence of epidural or spinal anaesthesia.
The presence of distal pulses does not exclude serious arterial injury. Pulse oximetry, Doppler signals, and measurement of the ankle brachial pressure index may be helpful, but in cases of doubt, proceed to angiography.
Intra-arterial drug administration
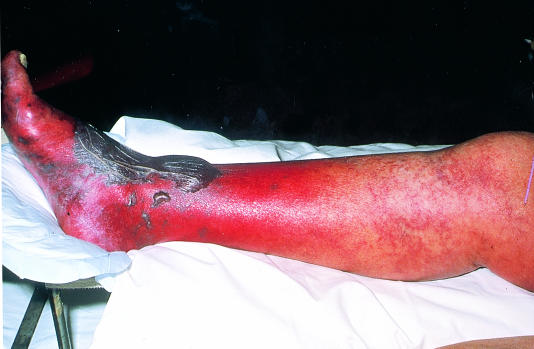
Intra-arterial drug administration leads to intense spasm and microvascular thrombosis. The leg is mottled and digital gangrene is common, but pedal pulses are usually palpable. The mainstay of treatment is supportive care, hydration to minimise renal failure secondary to rhabdomyolysis, and full heparinisation. Vascular reconstruction is almost never indicated, but fasciotomy may be required to prevent a compartment syndrome.
Venous gangrene
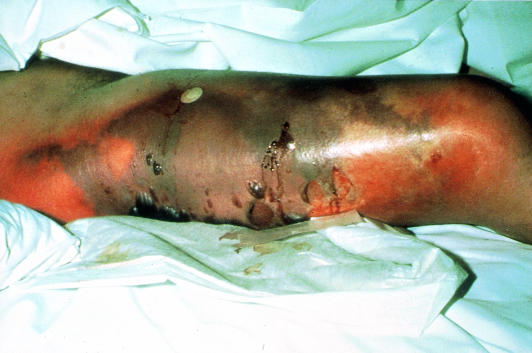
Venous gangrene can be mistaken for acute limb ischaemia. However, the leg is invariably swollen and the superficial veins full. Oedema may make it impossible to palpate pedal pulses, but Doppler examination will show normal distal waveforms and pressures. Management includes elevation, heparinisation, thrombolysis, and treatment of the underlying cause (usually pelvic or abdominal malignancy).
Aortic dissection
This may cause upper and lower limb ischaemia due to pinching of the ostia of the relevant arteries by the false lumen.
Thoracic outlet syndrome
Pressure on the subclavian artery from a cervical rib or abnormal soft tissue band may lead to a post-stenotic dilatation lined with thrombus, which predisposes to occlusion or embolisation. The distal circulation may be chronically obliterated and digital ischaemia advanced before the thoracic outlet syndrome is diagnosed. The diagnosis is based on the results of duplex ultrasonography or angiography, or both. Treatment options include thrombolysis, thrombectomy or embolectomy, excision of the cervical rib, and repair of the aneurysmal segment.
Thrombolysis
In thrombolysis a cannula is embedded into the distal extent of the thrombus and streptokinase or, preferably, recombinant tissue plasminogen activator is infused. The technique cannot be used in patients with complete ischaemia because thrombus dissolution takes several hours. It is also relatively ineffective against the organised thrombus present in most peripheral emboli and is associated with an appreciable minor (20%, mainly groin haematoma) and major (5%, serious haemorrhage and stroke) complication rate. Thrombolysis should be undertaken only in an environment where experienced nursing and medical staff can closely monitor the patient.
Post-ischaemic syndromes
Reperfusion injury
The reintroduction of oxygenated blood after a period of ischaemia causes more damage than the ischaemia alone. Generation of highly reactive, oxygen free radicals is greatly increased, and these activate neutrophils which migrate into the reperfused tissue causing injury. For vascular injury to occur neutrophils must be present and must adhere to the endothelium. The damaged endothelial cells become more permeable.
Effects of reperfusion syndrome
Local—Limb swelling due to increased capillary permeability causes a compartment syndrome, impaired muscle function due to ischaemia, and subsequent muscle contracture if the muscle infarcts.
General—Acidosis and hyperkalaemia occur due to leakage from the damaged cells, causing cardiac arrhythmias and myoglobinaemia, which can result in acute tubular necrosis. Acute respiratory distress syndrome may also develop, and gastrointestinal endothelial oedema may lead to increased gastrointestinal vascular permeability and endotoxic shock.
Compartment syndrome
Increased capillary permeability and oedema on reperfusion in the calf, where muscles are confined within tight fascial boundaries, causes an increase in interstitial pressure leading to muscle necrosis despite apparently adequate inflow— compartment syndrome. There is swelling and pain on squeezing the calf muscle or moving the ankle. Palpable pedal pulses do not exclude the syndrome. The key to management is prevention through expeditious revascularisation and a low threshold for fasciotomy. (If in doubt—do it.)
Chronic pain syndromes
Acute complete ischaemia can lead to peripheral nerve injury that manifests as the chronic pain syndrome, also referred to as causalgia, reflex sympathetic dystrophy, and many other terms. If the syndrome is recognised and treated early then many patients gain prolonged relief from drugs or chemical or surgical sympathectomy.
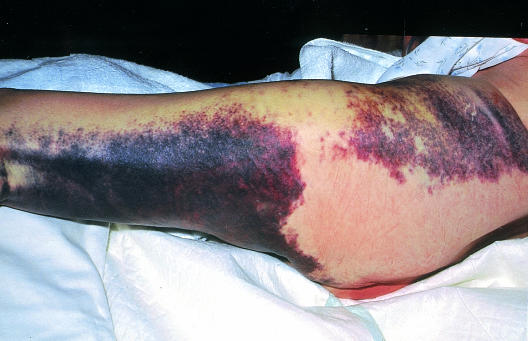
Figure.
Marble white foot (left of picture) in patient with acute ischaemia
Figure.
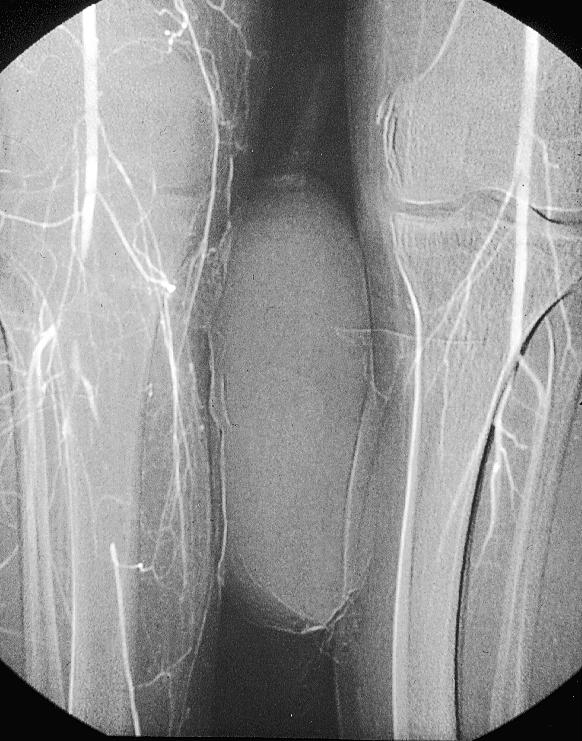
Embolus at popliteal trifurcation
Figure.
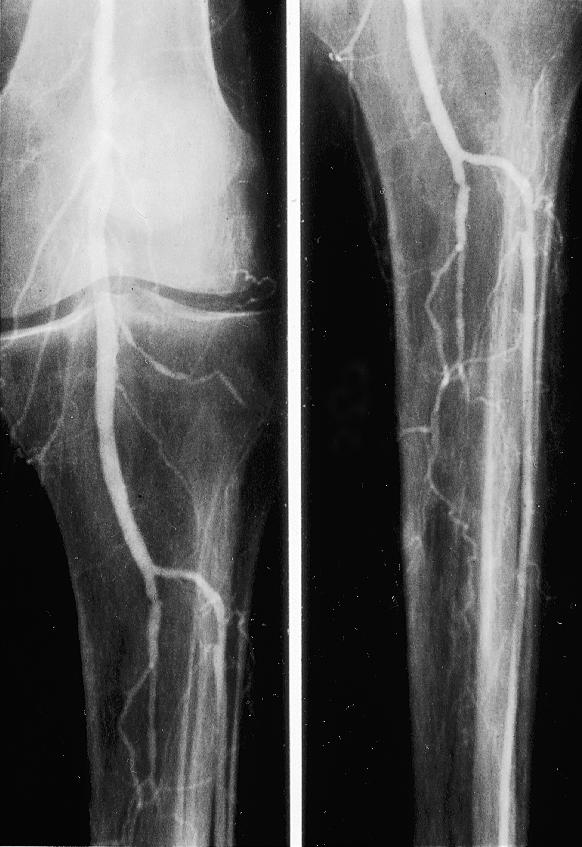
On-table angiograms showing incomplete clearance of embolus
Figure.
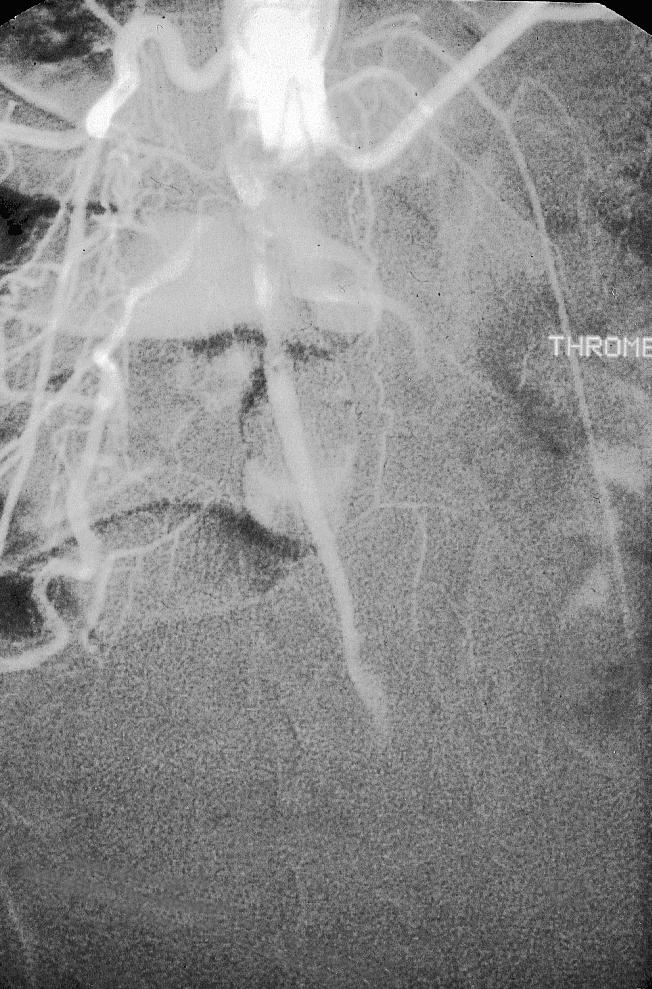
Aortic occlusion
Figure.
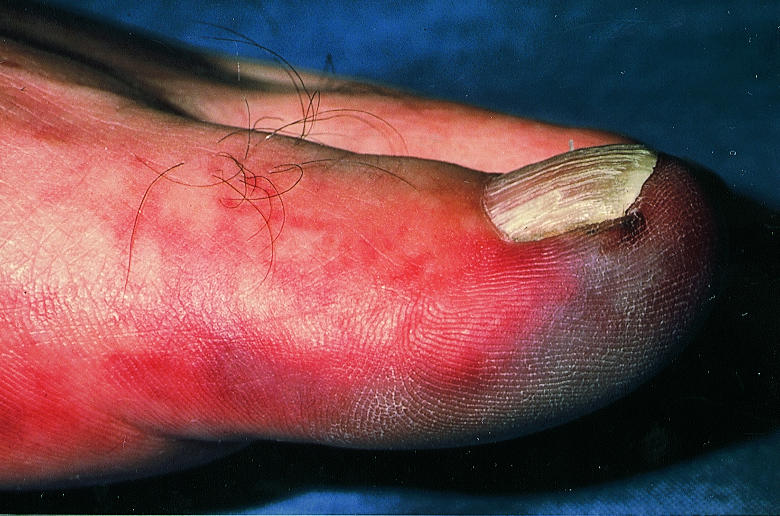
Blue toe syndrome must be promptly identified
Figure.
Compound fracture of tibia with ischaemia
Figure.
Ischaemia after intra-arterial drug administration
Figure.
Venous gangrene
Figure.
Digital gangrene due to pressure on subclavian artery from cervical rib
Figure.
Haematoma due to thrombolysis
Figure.
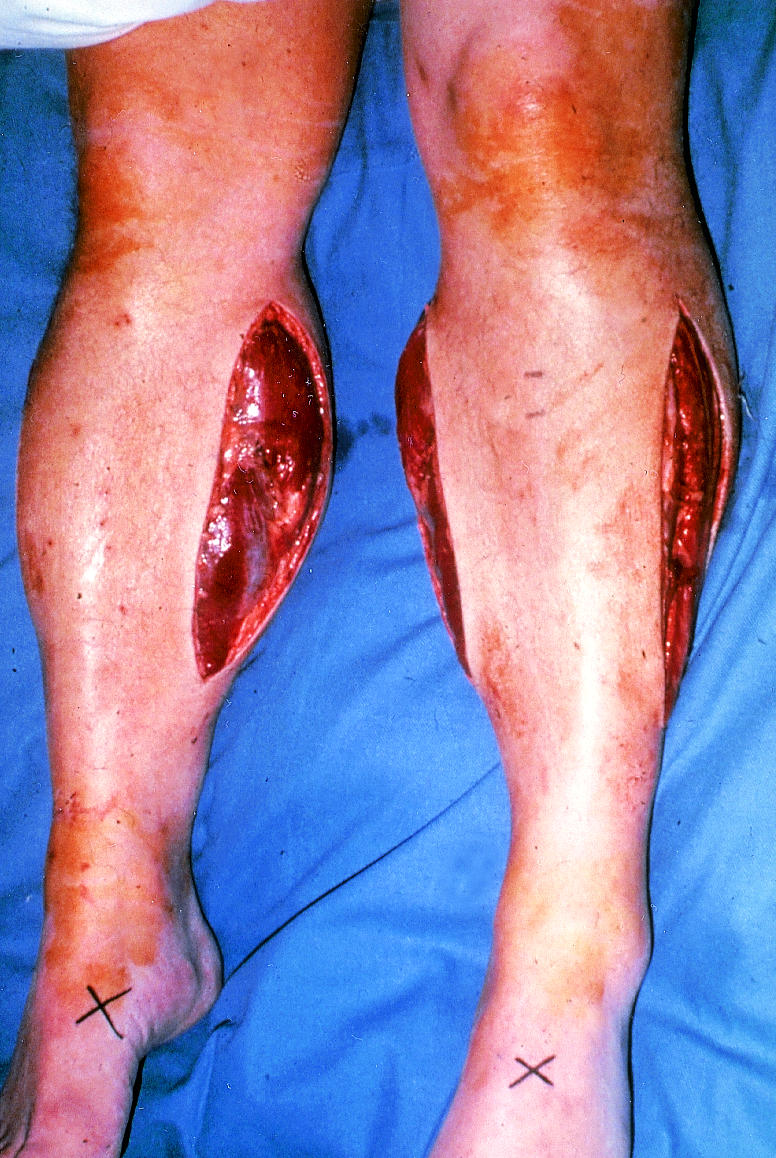
Fasciotomy
Footnotes
We thank Professor C V Ruckley, Mr A Jenkins, and Mr J A Murie for help with the illustrations.
Ken Callum is consultant surgeon, Derbyshire Royal Infirmary, Derby, and Andrew Bradbury is professor of surgery, University of Birmingham, Birmingham Heartlands Hospital, Birmingham.
The ABC of arterial and venous disease is edited by Richard Donnelly, professor of vascular medicine, University of Nottingham and Southern Derbyshire Acute Hospitals NHS Trust (richard.donnelly@nottingham.ac.uk) and Nick J M London, professor of surgery, University of Leicester, Leicester (sms16@leicester.ac.uk). It will be published as a book later this year.