Abstract
Background:
Cefuroxime has played a crucial role in the prevention and treatment of bacterial infections. However, the differences in adverse events across formulations and routes remain unclear.
Objectives:
This study aimed to investigate the post-marketing safety of cefuroxime, particularly concerning formulations and routes.
Design:
A retrospective pharmacovigilance study of cefuroxime was conducted using the data from Food and Drug Administration Adverse Event Reporting System database.
Methods:
The clinical characteristics and concomitant drugs reported with cefuroxime were investigated. Adverse event signals of cefuroxime were identified based on four disproportionality algorithms. The signal differences of cefuroxime across formulations and routes were further examined.
Results:
A total of 1810 adverse event reports associated with cefuroxime were identified, and 181 cefuroxime-associated signals were detected. Compared with tablets, injections were more likely to cause preferred terms ‘blood pressure decreased’ and ‘anaphylactic shock’. In addition, system organ class ‘eye disorders’ significantly increased when cefuroxime was administered intraocularly, underscoring the importance of exercising caution regarding ocular toxicity.
Conclusion:
The adverse events associated with cefuroxime were significantly different across formulations and routes, which deserve special attention in clinical use.
Keywords: adverse event, cefuroxime, eye disorder, FDA Adverse Event Reporting System (FAERS), formulation, route
Plain language summary
Post-marketing safety concerns with cefuroxime
Background:
Cefuroxime is a commonly used antibiotic. This study investigated the safety of cefuroxime using Food and Drug Administration Adverse Event Reporting System database.
Research design and methods:
We analyzed the clinical characteristics and concomitant drugs reported with cefuroxime. Then, we detected the signals of cefuroxime. We further examined the signal differences of cefuroxime across formulations and routes.
Results:
We retrieved 1810 reports and identified 181 signals associated with cefuroxime. In comparison to tablets, injections had a higher likelihood of causing decreased blood pressure and anaphylactic shock. Furthermore, the administration of cefuroxime intraocularly increased the possibility of experiencing eye disorders.
Conclusion:
The signals associated with cefuroxime were significantly different across formulations and routes, which deserve special attention in clinical use.
Introduction
Cefuroxime, a second-generation cephalosporin antibiotic, demonstrates broad-spectrum effectiveness against a wide range of both Gram-positive and Gram-negative bacteria. 1 It functions by inhibiting bacterial cell wall synthesis, thereby impeding cell division and growth, ultimately resulting in cell lysis and death. 1 Since receiving approval from the United States Food and Drug Administration (FDA) in 1988, cefuroxime has been extensively utilized for the treatment of respiratory, digestive, urinary, musculoskeletal, skin, and soft tissue infections caused by susceptible bacteria.2,3 In addition, cefuroxime can effectively prevent surgical site infections in various procedures, including cardiac, pulmonary, vascular, esophageal, joint replacement, spine, and caesarean sections surgeries. 4 Till now, cefuroxime has played a crucial role in the treatment and prevention of bacterial infections globally.
However, cefuroxime is available in various formulations such as injections, tablets, and oral suspensions, offering multiple administration routes. Although the adverse events associated with cefuroxime are relatively well-documented, the differences across formulations and routes remain unclear. Moreover, cefuroxime has the potential to cause severe adverse events such as acute coronary syndrome, pseudomembranous colitis, and anaphylactic shock, which can even be life-threatening.5,6 Considering the widespread use of cefuroxime and the gravity of certain adverse events, a more comprehensive pharmacovigilance study of cefuroxime is necessary to analyze its post-marketing safety.
Spontaneous reporting system is the cornerstone of pharmacovigilance for suspected adverse events. 7 The United States FDA Adverse Event Reporting System (FAERS) is a publicly accessible database that collects adverse event reports. 8 In recent years, the FAERS database has been widely utilized for post-marketing safety evaluation of drugs like semaglutide, nirmatrelvir/ritonavir, and secukinumab.9–11 This study investigated the adverse events of cefuroxime using FAERS database to provide a comprehensive real-world assessment of post-marketing safety concerns with cefuroxime.
Methods
Data source
The data were downloaded from the FAERS database in ASCII format using the free access link https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. Six datasets including the patient demographic and administrative information (DEMO), therapy start dates and end dates for reported drugs (THER), drug information (DRUG), coded for the adverse events (REAC), patient outcomes for the event (OUTC), and indications for use/diagnosis (INDI) were utilized. 12 The period of this study covered from the first quarter of 2019 to the second quarter of 2023.
Data collection
After matching the DRUG and DEMO datasets from the FAERS database, reports in which cefuroxime was the primary suspected (PS) drug were extracted by searching for generic name of the DRUG dataset (CEFUROXIME, CEFUROXIME AXETIL, or CEFUROXIME SODIUM in the prod_ai column). In reports where cefuroxime was identified as a PS drug, other drugs labeled as ‘secondary suspect’, ‘concomitant’, or ‘interacting’ were considered concomitant drugs. 12 The adverse events associated with cefuroxime were extracted from the REAC dataset. All the preferred terms (PTs) in the REAC dataset were classified to the corresponding primary system organ class (SOC) according to the standardized Medical Dictionary for Regulatory Activities (MedDRA) version 25.1, which can be downloaded from the link https://www.meddra.org/.13,14
Statistical analysis
The clinical characteristics including reporter country, report season, reporter type, onset time, formulation, sex, age, weight, indication, and outcome of cefuroxime-associated reports were examined after removing missing data.12–14 The dose and frequency of different formulations across countries were further examined. To standardize the dose units for different formulations, every 5 mL of oral suspension was equivalent to 125 mg of cefuroxime. Subsequently, the concomitant drugs reported with cefuroxime were analyzed. 12
The signals of cefuroxime were explored at the SOC and PT levels.12–14 Four disproportionality algorithms were employed, including reporting odds ratio (ROR),15,16 proportional reporting ratio,13,17 Bayesian confidence propagation neural network,13,14,17,and the multi-item gamma Poisson shrinker.13,14,17 A signal of cefuroxime at PT level was detected when it conformed to the four algorithm criteria simultaneously. Differences of cefuroxime signals concerning formulations and routes were further analyzed using the ROR algorithm and Fisher’s exact test. 12 All data were processed using Python 3 programming language in Jupyter Notebook version 6.4.12.
Results
Clinical characteristics
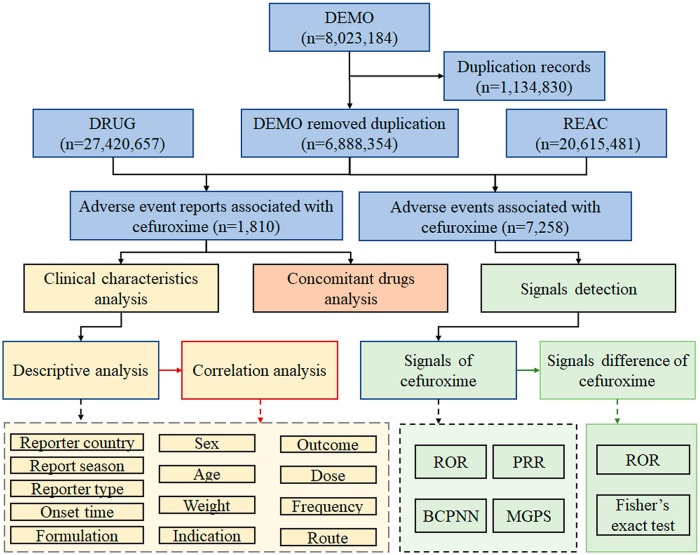
8,023,184 adverse event reports were obtained from the DEMO dataset, and 1,134,830 duplicates were removed, resulting in a reduction in the number of reports to 6,888,354. Ultimately, 1810 adverse event reports and 7258 adverse events associated with cefuroxime were identified. Data collection and analysis flow chart of cefuroxime-associated adverse events is shown in Figure 1.
Figure 1.
Data collection and analysis flow chart of cefuroxime-associated adverse events.
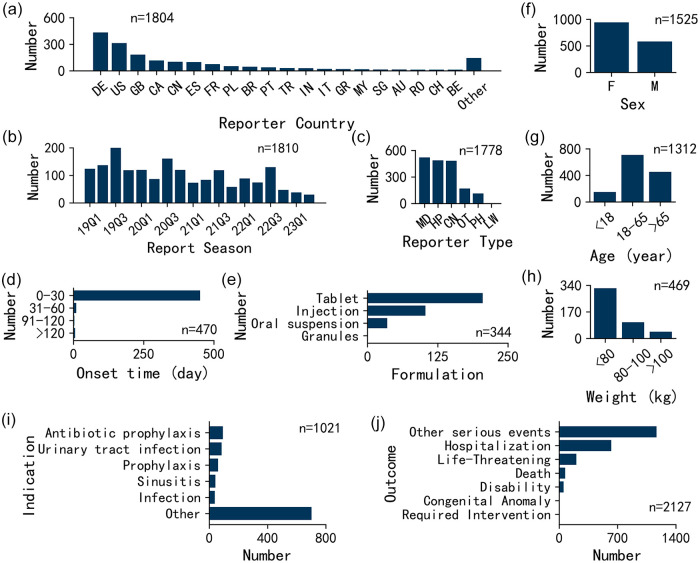
The clinical characteristics of the 1810 cefuroxime-associated reports are shown in Figure 2. The adverse event reports for cefuroxime were submitted by 52 nations, indicating the global utilization of cefuroxime. Figure 2(a) displays the top 20 countries ranked by the number of reports. The number of reports and abbreviations of 52 countries can be found in Supplemental Table S1. Regarding report season, the number of reports ranged from 30 to 200 with an average of 101 reports per quarter. The highest number of reports was observed during the third quarter of each year, possibly due to the increased prevalence of infectious diseases during that season. The most common reporter type was physicians (29.2%, n = 520). The onset of symptoms occurred within the initial 0–30 days for the majority of patients, encompassing 96.0% of the cases (n = 451). Cefuroxime-associated adverse event reports were classified into four formulations, including tablets at 59.6% (n = 205), injections at 29.9% (n = 103), oral suspensions at 10.2% (n = 35), and granules at 0.3% (n = 1). Females made up the majority of reports (61.8%, n = 943). The reported ages in the study varied widely, spanning from 1 month to 98 years, with an average age of 53 years. The predominant age group was 18–65 years, constituting 54.0% of the patients (n = 709). In addition, the majority of patients weighed less than 80 kg, accounting for 68.7% of the cases (n = 322), and the average weight was 69 kg. The most common indication was ‘antibiotic prophylaxis’ (9.2%, n = 94) and the most common outcome was ‘other serious’ events (54.9%, n = 1167).
Figure 2.
Clinical characteristics of cefuroxime-associated adverse events: (a) reporter country, (b) report season, (c) reporter type, (d) onset time, (e) formulation, (f) sex, (g) age, (h) weight, (i) indication, and (j) outcome.
CN, consumer; F, female; HP, health-professional; LW, lawyer; M, male; MD, physician; OT, other health-professional; PH, pharmacist; abbreviations of countries can be found in Supplemental Table S1.
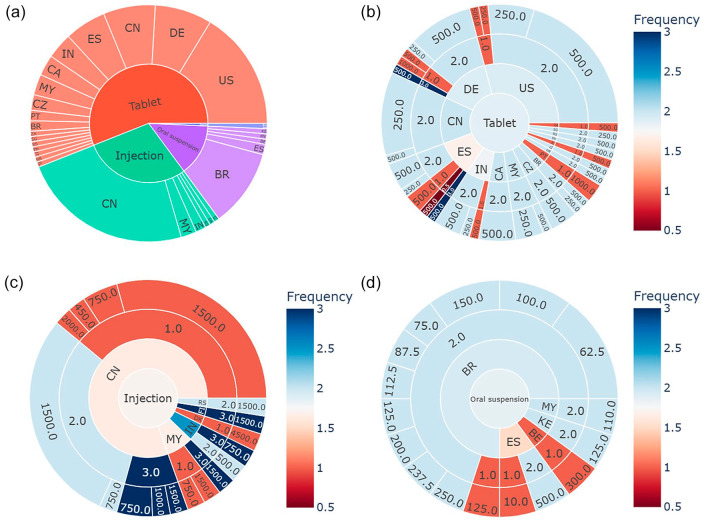
The distribution of dose and frequency for formulations across different countries was further investigated, as shown in Figure 3. A total of 141 cases recorded the formulation, reporter country, dose, and frequency simultaneously. Sunburst plot showing reporter countries for different formulations is presented in Figure 3(a). Figure 3(b) to (d) illustrate sunburst plots depicting the dose and frequency distribution for tablets, injections, and oral suspensions in different countries. Among the reports indicating a once-a-day frequency for tablets, the most commonly prescribed doses were 500 and 1000 mg. These instances were primarily observed in the United States, Germany, and Spain. Concerning injections, the problem of low frequency was more prominent compared to other formulations. Cases reporting once-daily frequency were mainly reported in China, Malaysia, and Denmark. The issue of once-daily frequency for oral suspensions was predominantly observed in Brazil, Spain, and Belgium.
Figure 3.
Sunburst plots of dose and frequency for formulations across different countries: (a) formulations across different countries; (b) dose and frequency for tablets across different countries; (c) dose and frequency for injections across different countries; and (d) dose and frequency for oral suspensions across different countries.
Abbreviations of countries can be found in Supplemental Table S1.
Concomitant drugs
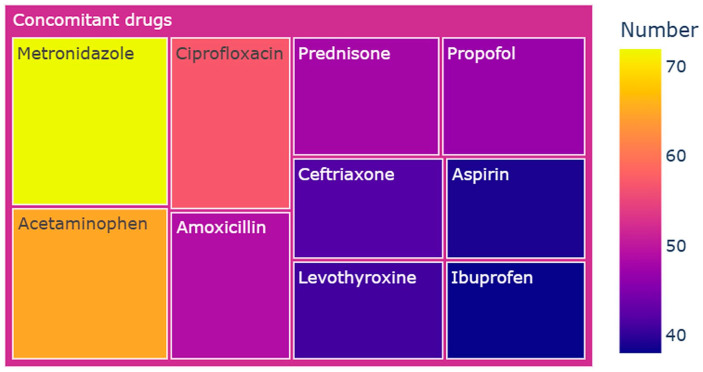
The concomitant drugs associated with 1810 reports of cefuroxime involved 870 different drugs. In summary, the concomitant drugs of cefuroxime mainly consisted of antimicrobials, non-steroidal anti-inflammatory drugs, and glucocorticoids. Figure 4 presents the top 10 ranked concomitant drugs reported with cefuroxime. Metronidazole emerged as the most commonly used concomitant medication, accounting for 4.0% (n = 72) of the 1810 cases. Cefuroxime was also frequently administered alongside non-steroidal anti-inflammatory drugs, including acetaminophen at 3.6% (n = 65), aspirin at 2.2% (n = 39), and ibuprofen at 2.1% (n = 38). These findings indicate a potential strategy for addressing both symptomatic relief and the underlying cause.
Figure 4.
Top 10 ranked concomitant drugs reported with cefuroxime.
Signals detection
The signal strengths of reports of cefuroxime at the SOC level are shown in Supplemental Table S2. Statistically, cefuroxime-associated adverse events involved 27 SOCs. The SOCs ‘immune system disorders’, ‘eye disorders’, and ‘pregnancy, puerperium and perinatal conditions’ simultaneously met the four criteria of four disproportionality algorithms. At the PT level, 203 signals of cefuroxime were detected. Among these 203 signals, 22 cefuroxime-unrelated signals such as 2 signals of SOC ‘product issues’, 1 signal of SOC ‘surgical and medical procedures’, and 5 signals consistent with indications were found. The signal strengths of reports of 22 cefuroxime-unrelated signals at the PT level are listed in Supplemental Table S3. After excluding the 22 cefuroxime-unrelated signals, the signal strengths of reports of 181 cefuroxime-associated signals at the PT level are shown in Supplemental Table S4. Most signals of cefuroxime were consistent with findings from label and clinical trials. Interestingly, 31 signals of SOC ‘eye disorders’ and 9 signals of SOC ‘pregnancy, puerperium and perinatal conditions’ emerged as new significant adverse event signals, which were uncovered in the label of cefuroxime.
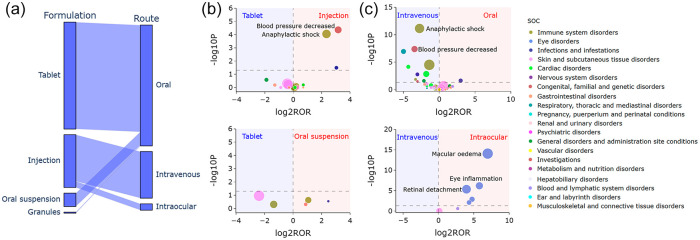
The relationship between formulations and routes is further illustrated in Figure 5(a). Tablets, oral suspensions, and granules were primarily used for oral administration, while injections were used not only for intravenous administration but also for intraocular applications. The volcano plots for difference detection of cefuroxime signals among formulations and routes are shown in Figure 5(b) and (c). Notably, injections were more likely to cause PT ‘blood pressure decreased’ (Injections versus Tablets: p < 0.001, ROR: 8.96, 95% CI: 2.60–30.90) and ‘anaphylactic shock’ (Injections versus Tablets: p < 0.001, ROR: 5.07, 95% CI: 2.14–12.01), compared with tablets. In addition, the possibility of SOC ‘eye disorders’ such as PTs ‘macular edema’ (Intraocular versus Intravenous: p < 0.001, ROR: 124.55, 95% CI: 16.45–942.96), ‘eye inflammation’ (Intraocular versus Intravenous: p < 0.001, ROR: 55.16, 95% CI: 6.85–444.12), and ‘retinal detachment’ (Intraocular versus Intravenous: p < 0.001, ROR: 15.57, 95% CI: 4.74–51.19) were significantly high when cefuroxime was administered intraocularly.
Figure 5.
Difference detection of cefuroxime signals among formulations and routes. (a) Parallel categories plot of formulations and routes. (b) Volcano plots among formulations. (c) Volcano plots among routes. In the volcano plots, the x-axis is the logarithm of the ROR value (log2ROR) based on ROR algorithm, and the y-axis is the negative logarithm of the p value calculated using Fisher’s exact test (−log10P). The colors of each point represent different SOCs. The sizes of each point represent the number of reports of each PT induced by cefuroxime. The larger values in y-direction represented a strongly significant difference and the bigger size represented a high frequency of each signal at PT level. Signals within 181 significant disproportionality PTs of cefuroxime are shown.
PT, preferred term; ROR, reporting odds ratio; SOC, system organ class.
Discussion
This study evaluated the post-marketing safety concerns with cefuroxime. Since the spectra of cefuroxime and metronidazole include most Enterobacterales and anaerobes, the combination is commonly recommended for surgical site infection prophylaxis. 18 In this study, metronidazole was the most frequently co-administered drug. Other commonly utilized antimicrobials included ciprofloxacin, amoxicillin, and ceftriaxone. This suggests that the treatment of infectious diseases often involves a strategy of combining multiple antimicrobials. However, it is not recommended to use cefuroxime in conjunction with penicillin or other cephalosporin antibiotics, as it may potentially increase the risk of adverse events. In addition, inappropriate or excessive use of antimicrobials contributes to the emergence and spread of antimicrobial resistance. Cefuroxime should be prescribed in combination with other antimicrobials to provide broad-spectrum coverage, based on careful consideration of the local epidemiology, susceptibility patterns, and documented clinical efficacy. It is essential to avoid unnecessary use of multiple antimicrobials that have similar mechanisms of action.
Cefuroxime is a time-dependent antibiotic, meaning its effectiveness is associated with maintaining a sufficient concentration in the plasma for a certain duration. 19 Therefore, multiple administrations are required to ensure the maintenance of an effective therapeutic concentration. The recommended dosing regimens for cefuroxime vary depending on formulations. For tablets, the standard dose ranges from 250 to 500 mg or 10 to 15 mg/kg, administered twice daily. Injections are typically given at dosages of 750–3000 mg, 2–4 times daily. Oral suspensions are commonly prescribed at doses of 125 to 500 mg or 20 mg/kg, taken twice daily. However, the results of dose and frequency highlight these three formulations exhibited suboptimal frequency, with injections displaying a notably higher density of suboptimal frequency. In countries like China, Malaysia, and Denmark, there were even reports exceeding half of the cases that utilized frequencies lower than what is recommended in the label of cefuroxime injection. These findings emphasize the need for particular attention to the appropriate frequency of cefuroxime. It should be noted that the correlation analysis only represented the adverse event reports where the formulation, reporter country, dose, and frequency were simultaneously recorded and could not represent all cases in which cefuroxime was used.
Approximately one in four pregnant women will be prescribed antibiotics during pregnancy, which accounts for nearly 80% of all prescribed medications. 20 Cefuroxime can penetrate the placenta during late pregnancy or delivery, 20 but no experimental evidence has suggested that cefuroxime can cause embryonic diseases. In this study, the SOC ‘pregnancy, puerperium and perinatal conditions’ was identified as significant. Nine-related signals such as ‘premature baby’, ‘premature delivery’, and ‘fetal distress syndrome’ emerged. The number of reports was relatively low, which could be the main reason why they were not observed in clinical trials. These findings indicate that despite cefuroxime being classified as class B for use during pregnancy, caution should still be exercised when cefuroxime is administered to pregnant women.
This study analyzed the differences in 181 significant disproportionality signals across formulations. Injections were found to be more prone to adverse events, possibly due to their unique in vivo processes. Specifically, injections were more likely to cause PTs like ‘blood pressure decreased’ and ‘anaphylactic shock’. As a result, it is crucial to enhance monitoring for severe adverse events when administering injections.
In the clinic trial of cefuroxime, no adverse events affecting the eyes have been reported. However, this study found significant disproportionality of SOC ‘eye disorders’ as well as 31 significant-related PTs. In contrast to SOC ‘pregnancy, puerperium and perinatal conditions’, the number of reports of the 31 PTs was relatively higher. Obviously, the occurrence cannot be solely attributed to chance factor. The disparity between this finding and those from clinic trial has sparked our significant interest.
Perioperative antibiotic prophylaxis plays a crucial role in reducing the risk of postoperative bacterial infection for specific surgical procedures.21,22 In 2006, the European Society of Cataract and Refractive Surgeons (ESCRS) Endophthalmitis Study Group first reported the benefits of intraocular administration of cefuroxime in reducing the incidence of postoperative endophthalmitis. 23 Subsequently, a multicenter study conducted by the ESCRS in 2007 demonstrated that the absence of a prophylactic regimen involving intracameral cefuroxime was associated with a 4.92-fold increase in the risk of total postoperative endophthalmitis. 24 Afterwards, more evidences supporting the use of intraocular cefuroxime for endophthalmitis prophylaxis have been presented.25–27 However, it is important to note that there have been reports of severe visual complications, such as hemorrhagic occlusive retinal vasculitis. 28 Sun et al. 29 identified the intraoperative use of 1 mg/mL cefuroxime as a significant risk factor for macular edema on the first day after cataract surgery (p < 0.05). Miyake et al. 28 suggested that cefuroxime induces pronounced inflammatory effects on vascular endothelial cells, leading to retinal toxicity that extends to the inner nuclear layers. In this study, it was discovered that the proportions of SOC ‘eye disorders’, especially PTs ‘macular edema’, ‘eye inflammation’, and ‘retinal detachment’, were significantly higher when cefuroxime was administered intraocularly. These results suggest the need to be cautious of the ocular toxicity when cefuroxime is used intraocularly.
In recent years, there have been investigations into the influential factors contributing to ocular toxicity caused by intraocular cefuroxime. Raharja et al. 30 suggested that cefuroxime toxicity resulting from accidental scleral penetration is the likely cause. Therefore, it is crucial to prevent cefuroxime from entering the vitreous cavity to avoid irreversible damage. 28 Other factors that may increase the risk of toxicity include high dosage, elevated intraocular pressure, sclerotomy leaks, and the use of intraocular tamponade.31,32 A survey conducted among 250 ophthalmic surgeons across Europe highlighted the current concerns in this field. The lack of an approved commercial preparation and related anxieties regarding the risk of dilution errors and contamination were identified as the most significant issues. 33 More than 90% of the respondents expressed their willingness to use cefuroxime if an approved single-unit dose product were commercially available. 33 These results emphasize the importance of developing a single-unit dose cefuroxime product specifically for intraocular administration.
Limitations
There were several limitations in this study that should be acknowledged. First, apart from factors like formulations and routes, there are still various influencing factors for cefuroxime-related adverse events, such as sex, age, weight, indication, race, and concomitant medication. For instance, cefuroxime in conjunction with other cephalosporin antibiotics may potentially influence the occurrence of adverse events. Additionally, traditional Chinese medicines have also been proven to possess antibacterial activities.34,35 However, this study did not conduct an in-depth analysis of the potential impact of these factors on the occurrence of adverse events. Second, while this study focused on analyzing the observed phenomena, it did not delve into the underlying causes of these phenomena. For instance, although significant differences were found in adverse events across formulations and routes, no additional experiments were employed to identify the critical quality attributes of products, as well as to systematically investigate the sensitizing mechanisms of impurities. In future research endeavors, further investigations should be conducted to obtain a more comprehensive understanding of the factors that influence the occurrence of adverse events associated with cefuroxime.
Conclusion
The adverse events associated with cefuroxime were significantly different across formulations and routes, which deserve special attention in clinical use.
Supplemental Material
Supplemental material, sj-docx-1-taw-10.1177_20420986241258049 for A retrospective pharmacovigilance study of post-marketing safety concerns with cefuroxime by Cheng Jiang, Xiaoxiao Zheng, Ping Li, Jiancheng Qian and Qin Li in Therapeutic Advances in Drug Safety
Acknowledgments
The authors thank all the contributors of the FAERS database.
Appendix
List of abbreviations
FDA Food and Drug Administration
FAERS Food and Drug Administration Adverse Event Reporting System
PS Primary suspected
PT Preferred term
MedDRA Medical Dictionary for Regulatory Activities
SOC System organ class
ROR Reporting odds ratio
PRR Proportional reporting ratio
BCPNN Bayesian confidence propagation neural network
MGPS Multi-item gamma Poisson shrinker
ESCRS European Society of Cataract and Refractive Surgeons
Footnotes
ORCID iD: Cheng Jiang  https://orcid.org/0000-0001-8454-749X
https://orcid.org/0000-0001-8454-749X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Cheng Jiang, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, China; School of Pharmacy, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Xiaoxiao Zheng, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, China.
Ping Li, School of Pharmacy, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Jiancheng Qian, Zhejiang Academy of Traditional Chinese Medicine, Tongde Hospital of Zhejiang Province, No. 234 Gucui Road, Hangzhou, Zhejiang 310012, China.
Qin Li, School of Pharmacy, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Declarations
Ethics approval and consent to participate: There was no trackable personal patient or reporter information from the FAERS database. The ethics approval for the conduct of this study was granted by the ethics committee of Tongde Hospital of Zhejiang Province.
Consent for publication: Not applicable.
Author contributions: Cheng Jiang: Formal analysis; Writing – original draft; Writing – review & editing.
Xiaoxiao Zheng: Formal analysis; Writing – original draft; Writing – review & editing.
Ping Li: Formal analysis; Writing – original draft.
Jiancheng Qian: Conceptualization; Supervision; Writing – review & editing.
Qin Li: Conceptualization; Supervision.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Public Service Technology Research Project of Zhejiang Province (LGF22H300010), National Natural Science Foundation of China (82174132), Medical Health Science and Technology Project of Zhejiang Province (2023RC141), and Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (2024ZL362).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Raw data for this article can be downloaded at https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html; further inquiries can be directed to the corresponding author.
References
- 1. Gold B, Rodriguez WJ. Cefuroxime: mechanisms of action, antimicrobial activity, pharmacokinetics, clinical applications, adverse reactions and therapeutic indications. Pharmacotherapy 1983; 3: 82–100. [PubMed] [Google Scholar]
- 2. Song Z, Fu W, Zhou L. Cefuroxime, levofloxacin, esomeprazole, and bismuth as first-line therapy for eradicating Helicobacter pylori in patients allergic to penicillin. BMC Gastroenterol 2019; 19: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu W, Song Z, Zhou L, et al. Randomized clinical trial: esomeprazole, bismuth, levofloxacin, and amoxicillin or cefuroxime as first-line eradication regimens for Helicobacter pylori infection. Dig Dis Sci 2017; 62: 1580–1589. [DOI] [PubMed] [Google Scholar]
- 4. Sommerstein R, Troillet N, Harbarth S, et al. Timing of cefuroxime surgical antimicrobial prophylaxis and its association with surgical site infections. JAMA Netw Open 2023; 6: e2317370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tiwari R, Singh M, Srivastava T, et al. Cefuroxime hypersensitivity leading to myocardial ischaemia. Indian J Anaesth 2022; 66: S178–S179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mahiat C, Robaye S, Levecq L, et al. Anaphylactic shock following cataract surgery: a documented intracameral cefuroxime allergy. J Investig Allergol Clin Immunol 2022; 32: 236–238. [DOI] [PubMed] [Google Scholar]
- 7. Egberts AC, Meyboom RH, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf 2002; 25: 453–458. [DOI] [PubMed] [Google Scholar]
- 8. Kong W, Mao W, Zhang L, et al. Disproportionality analysis of quinolone safety in children using data from the FDA Adverse Event Reporting System (FAERS). Front Pediatr 2022; 10: 1069504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shu Y, He X, Wu P, et al. Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA adverse event reporting system. Front Public Health 2022; 10: 996179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pannu V, Udongwo N, Imburgio S, et al. Adverse events of SARS-CoV-2 therapy: a pharmacovigilance study of the FAERS database. Ann Pharmacother 2024; 58: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shu Y, Ding Y, Liu Y, et al. Post-marketing safety concerns with secukinumab: a disproportionality analysis of the FDA Adverse Event Reporting System. Front Pharmacol 2022; 13: 862508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang C, Qian J, Jiang X, et al. Is pitolisant safe for clinical use? A retrospective pharmacovigilance study focus on the post-marketing safety. Pharmacol Res Perspect 2024; 12: e1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yin Y, Shu Y, Zhu J, et al. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci Rep 2022; 12: 19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Dong C, He X, et al. Post-marketing safety of vemurafenib: a real-world pharmacovigilance study of the FDA Adverse Event Reporting System. J Pharm Pharm Sci 2022; 25: 377–390. [DOI] [PubMed] [Google Scholar]
- 15. Altebainawi AF, Alfaraj LA, Alharbi AA, et al. Association between proton pump inhibitors and rhabdomyolysis risk: a post-marketing surveillance using FDA Adverse Event Reporting System (FAERS) database. Ther Adv Drug Saf 2023; 14: 1581606299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song Y, Xu YL, Lin Y, et al. Fractures due to aromatase inhibitor therapy for breast cancer: a real-world analysis of FAERS data in the past 15 years. Oncol Res Treat 2020; 43: 96–102. [DOI] [PubMed] [Google Scholar]
- 17. Guo M, Shu Y, Chen G, et al. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for niraparib. Sci Rep 2022; 12: 20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stavropoulou E, Atkinson A, Eisenring MC, et al. Association of antimicrobial perioperative prophylaxis with cefuroxime plus metronidazole or amoxicillin/clavulanic acid and surgical site infections in colorectal surgery. Antimicrob Resist infect Control 2023; 12: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaspersen AE, Hanberg P, Hvistendahl MA, et al. Evaluation of cefuroxime concentration in the intrathecal and extrathecal compartments of the lumbar spine: an experimental study in pigs. Br J Pharmacol 2023; 180: 1832–1842. [DOI] [PubMed] [Google Scholar]
- 20. Abduljalil K, Ning J, Pansari A, et al. Prediction of maternal and fetoplacental concentrations of cefazolin, cefuroxime, and amoxicillin during pregnancy using bottom-up physiologically based pharmacokinetic models. Drug Metab Dispos 2022; 50: 386–400. [DOI] [PubMed] [Google Scholar]
- 21. Hvistendahl MA, Hanberg P, Stilling M, et al. Subtherapeutic levels of cefuroxime inside a cannulated pedicle screw used in spine surgery: results from a porcine microdialysis study. Acta Orthop 2022; 93: 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmed NJ, Haseeb A, Alamer A, et al. Meta-analysis of clinical trials comparing cefazolin to cefuroxime, ceftriaxone, and cefamandole for surgical site infection prevention. Antibiotics (Basel) 2022; 11: 1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barry P, Seal DV, Gettinby G, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: preliminary report of principal results from a European multicenter study. J Cataract Refract Surg 2006; 32: 407–410. [DOI] [PubMed] [Google Scholar]
- 24. ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007; 33: 978–988. [DOI] [PubMed] [Google Scholar]
- 25. Conci L, Favarato AP, Pinheiro AG. Cost effectiveness of intracameral cefuroxime prophylaxis and its efficacy in preventing endophthalmitis after cataract surgery in a referral hospital. Arq Bras Oftalmol 2023; 86: 308–313. [DOI] [PubMed] [Google Scholar]
- 26. Wang M, Liu Y, Dong H. Effect of cefuroxime intracameral injection antibiotic prophylactic on postoperative endophthalmitis wound post-cataract: a meta-analysis. Int Wound J 2023; 20: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng AL, Tang WW, Li PS, et al. Intracameral cefuroxime in the prevention of postoperative endophthalmitis: an experience from Hong Kong. Graef Arch Clin Exp Ophthalmol 2016; 254: 1987–1992. [DOI] [PubMed] [Google Scholar]
- 28. Miyake H, Miyazaki D, Shimizu Y, et al. Toxicities of and inflammatory responses to moxifloxacin, cefuroxime, and vancomycin on retinal vascular cells. Sci Rep 2019; 9: 9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J, Wei Y, Li H, et al. Macular toxicity of low-concentration cefuroxime during cataract surgery in vitrectomized eyes. Ophthalmic Res 2022; 66: 116–123. [DOI] [PubMed] [Google Scholar]
- 30. Raharja A, Neffendorf JE, Williamson TH. Retinal toxicity secondary to subconjunctival cefuroxime following pars plana vitrectomy: a case report and literature review. Am J Ophthalmol Case Rep 2022; 26: 101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ku JY, Wong SW, Steeples LR, et al. High dose cefuroxime causing retinal toxicity in a patient undergoing trabeculectomy. Am J Ophthalmol Case Rep 2022; 25: 101343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jing LL, Ruan T, Li DF, et al. Toxicity to the macula after using small doses cefuroxime for phacoemulsification and vitrectomy combined surgery. Int J Ophthalmol 2022; 15: 2034–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg 2014; 40: 138–142. [DOI] [PubMed] [Google Scholar]
- 34. Tai B, Bai L, Ji R, et al. Phytochemical and pharmacological progress on Syringa oblata, a traditional Mongolian medicine. Chin Herb Med 2022; 14: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu X, Hao D, Xiao P. Research progress of Chinese herbal medicine compounds and their bioactivities: Fruitful 2020. Chin Herb Med 2022; 14: 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taw-10.1177_20420986241258049 for A retrospective pharmacovigilance study of post-marketing safety concerns with cefuroxime by Cheng Jiang, Xiaoxiao Zheng, Ping Li, Jiancheng Qian and Qin Li in Therapeutic Advances in Drug Safety