Abstract
The bovine herpesvirus 1 (BHV-1) UL3.5 gene encodes a 126-amino-acid tegument protein. Homologs of UL3.5 are present in some alphaherpesviruses and have 20 to 30% overall amino acid homology that is concentrated in the N-terminal 50 amino acids. Mutant pseudorabies virus lacking UL3.5 is deficient in viral egress but can be complemented by BHV-1 UL3.5 (W. Fuchs, H. Granzow, and T. C. Mettenleiter, J. Virol. 71:8886–8892, 1997). The function of BHV-1 UL3.5 in BHV-1 replication is not known. To get a better understanding of its function, we sought to identify the proteins that interact with the BHV-1 UL3.5 protein. By using an in vitro pull-down assay and matrix-assisted laser desorption ionization mass spectrometry analysis, we identified BHV-1 α-transinducing factor (αBTIF) as a BHV-1 UL3.5-interacting protein. The interaction was verified by coimmunoprecipitation from virus-infected cells using an antibody to either protein, by indirect immunofluorescence colocalization in both virus-infected and transfected cells, and by the binding of in vitro-translated proteins. In virus-infected cells, UL3.5 and αBTIF colocalized in a Golgi-like subcellular compartment late in infection. In transfected cells, they colocalized in the nucleus. Deletion of 20 amino acids from the N terminus of UL3.5, but not 40 amino acids from the C terminus, abolished the UL3.5-αBTIF interaction both in vitro and in vivo. The interaction between UL3.5 and αBTIF may be important for BHV-1 maturation and regulation of αBTIF transactivation activity.
Virions of alphaherpesviruses are structurally composed of membrane, tegument, nucleocapsid, and core (24). The tegument is an amorphous structure between the nucleocapsid and membrane. In addition to being essential virion structural components, tegument proteins are important for releasing viral genomic DNA early in infection, nucleocapsid formation, viral DNA packaging, and regulation of viral gene expression (24). However, the process of tegument assembly and the precise functions of most tegument proteins are still unclear.
Analysis of the genome of Bovine herpesvirus 1 (BHV-1), an alphaherpesvirus, reveals that there are at least 16 proteins known or presumed to be present in the tegument. A short open reading frame (ORF) in the BHV-1 genome designated the UL3.5 gene encodes a 13-kDa tegument protein expressed late in infection (23). Unlike most alphaherpesviral proteins, UL3.5 is not conserved throughout the alphaherpesvirus family. Homologs have been found only in Pseudorabies virus (PrV) (6), Varicella zoster virus (VZV) (5), Equine herpes virus 1 (EHV-1) (25), and Infectious laryngotracheitis virus (11). Herpes simplex virus type 1 (HSV-1) and HSV-2 do not have UL3.5 homologs (18, 19). Moreover, homologs of UL3.5 differ in size (from 71 amino acids [aa] for VZV to 220 aa for PrV) (5, 6, 13) and have overall 20 to 30% amino acid sequence homology that is restricted mostly to the N-terminal 50 aa (13). The roles of UL3.5 homologs in virus replication are apparently different. PrV UL3.5 is required for virus egress. A PrV mutant lacking UL3.5 replicates very poorly in the one-step replication and plaque assays (10). On the other hand, VZV lacking gene ORF57, the homolog of the UL3.5 gene, grows in cell culture at a same rate as wild-type virus (3). Neither the need for nor the function of UL3.5 in BHV-1 replication has been determined. However, BHV-1 UL3.5 rescued a PrV UL3.5 deletion mutant (9) implying that BHV-1 UL3.5 may also participate in virus egress.
BHV-1 α-transinducing factor (αBTIF), encoded by the UL48 gene, is a virion component that transactivates immediate-early gene promoters during viral lytic infection (20). Homologs are present in HSV-1, EHV-1, and VZV (1, 5, 15, 22) and probably all other Alphaherpesviruses. In addition to being a transcription factor, the HSV homolog, αTIF, also known as VP16 or Vwm65, is a tegument protein indispensable for virion assembly (27). No structural function of other αTIF homologs has been reported.
One approach to understanding tegument assembly and the function of tegument proteins is to identify and characterize proteins that associate with previously characterized tegument proteins. In this study, we sought to identify proteins that interact with BHV-1 UL3.5. Five methods showed a specific interaction between BHV-1 UL3.5 and αBTIF. (i) αBTIF specifically attached to His-tagged BHV-1 UL3.5 in an in vitro pull-down assay. (ii) A complex containing UL3.5 and αBTIF immunoprecipitated from BHV-1-infected cell lysates with either anti-UL3.5 or anti-αBTIF polyclonal antibodies. (iii) Confocal microscopy showed that BHV-1 UL3.5 and αBTIF colocalized in a Golgi-like subcellular compartment late in infection. (iv) In a transient expression system, UL3.5 and αBTIF colocalized in the nucleus. (v) Deletion of the N-terminal 20 aa, but not the C-terminal 40 aa, of UL3.5 abolished the binding of αBTIF in the pull-down assay and the colocalization in the transient expression system. The interaction between UL3.5 and αBTIF may have roles in virion assembly and regulation of αBTIF transactivation activity.
MATERIALS AND METHODS
Virus, cells, and media.
BHV-1 (Cooper strain; ATCC VR-864) was replicated in Madin-Darby bovine kidney (MDBK; ATCC CCL22) cells in minimum essential medium (MEM) (Gibco Laboratories, Life Technologies, Inc.) supplemented with 5% fetal bovine serum (FBS) (Hyclone) at 35°C in a 5% CO2 humidified atmosphere. Vero cells were cultured in MEM supplemented with 10% FBS at 35°C in a 5% CO2 humidified atmosphere.
Escherichia coli JM109 (Promega) was used for plasmid maintenance and transformation, E. coli BL21(DE3)pLysS (Novagen) was used for His-tagged fusion protein expression, and E. coli BL21(Novagen) was used for glutathione S-transferase (GST) fusion protein expression.
Plasmids.
The UL3.5 complete ORF was amplified by PCR with Pfu polymerase by using the N-terminal primer CGGGATCCGCCATGGCCCGCGTGCGCGCCG and the C-terminal primer GTGAATTCTTATTGGAACGTGCGGTAATTG (viral sequences underlined) from plasmid pSD72 (17). The PCR product was digested with BamHI and EcoRI and cloned into both the His-tagged fusion protein expression vector pRSET (Invitrogen) and the eukaryotic expression vector pcDNA3 (Invitrogen). The resulting plasmids were called pUL3.5SET and pUL3.5cDNA3, respectively.
To generate UL3.5 deletion mutants, a modified inverse PCR mutagenesis method described by Fisher and Pei (8) was used. Briefly, a pair of primers flanking the gene region to be deleted were used to amplify the plasmid pUL3.5cDNA3 by PCR with Pfu polymerase. PCR products that contained the mutated UL3.5 gene and the whole vector sequence were treated with DpnI to remove the plasmid templates and then self-ligated and transformed into E. coli JM109. Primer UL3.5N00 (CATGGCGGATCCGAGCTCGGTACCAAGCTT) and primer UL3.5N10 (GGGGAGGCCCGGGTGGCCACGGTGGCGGAC) were used to produce pN10UL3.5cDNA3, which encoded the entire UL3.5 protein except the N-terminal 10 aa. Primer UL3.5N00 and primer UL3.5N20 (TACACGCAGTTTCTCGCGGCCAACCGCGCC) were used to produce pN20UL3.5cDNA3. Primer UL3.5C00 (TAAGAATTCTGCAGATATCCATCACACTGG) and primer UL3.5C30 (GGGACTGGCGGCCGCGTAGAGGCGCGCGGC) were used to produce pC30UL3.5cDNA3. Primer UL3.5C00 and primer UL3.5C40 (CCGGGCCTCCGCGGGCGGCAGGCGCTCTTC) were used to produce pC40UL3.5cDNA3. To create His-tagged UL3.5 deletion mutants, all mutant UL3.5 gene fragments were digested from pcDNA3 with BamHI and EcoRI and cloned into pRSET.
To clone the αBTIF gene, primers CGGGATCCGTTGTCTTTGGGATGAGCGGGCGCA and CCGAATTCTAGAAGTCCAGCAGCTGGTTGAGGC (viral sequences underlined) were used to amplify the complete αBTIF ORF by PCR with Pfu polymerase. Since the two ends of the αBTIF ORF were present in two different BHV-1 HindIII clones, the PCR template for amplification of αBTIF was generated by ligation of BHV-1 HindIII fragments J and M, which were gel purified from pSD57 (17) and pSD62 (17), respectively. The PCR product was digested with BamHI and EcoRI and cloned into pGEX-KG (12) in frame with the GST coding sequence and pcDNA3. The plasmids were designated pGST-αBTIF and pαBTIFcDNA3, respectively.
All the inserts were confirmed by DNA sequence analysis.
Antibodies.
Production of rabbit anti-UL3.5 polyclonal antibody was previously described (23). To produce anti-αBTIF, a bacterially expressed GST-αBTIF fusion protein was produced as an antigen. The plasmid pGST-αBTIF was transformed into E. coli BL21, and GST-αBTIF was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM for 7 h with gentle shaking at 26°C. The cells were suspended in phosphate-buffered saline (PBS) containing 0.25% Tween 20, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1 mM chymostatin and lysed by sonication. The lysate was centrifuged, and GST-αBTIF was collected from the supernatant with glutathione-Sepharose 4B (Pharmacia) according to the manufacturer's instructions. The fusion protein was eluted in buffer (10 mM glutathione, 50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.2% Triton X-100). The purified GST-αBTIF was emulsified in Freund's complete adjuvant and injected intraperitoneally into BALB/c mice. Mice were boosted twice at 3-week intervals with GST-αBTIF emulsified with Freund's incomplete adjuvant. Sera were sampled 2 weeks following each boost.
Radioimmunoprecipitation.
Radiolabeled uninfected and BHV-1-infected MDBK cells were prepared as described by Marshall et al. (16). MDBK cells were infected at a multiplicity of infection (MOI) of 10 and labeled with [3H]leucine (ICN Pharmaceuticals Inc.) from 6 to 18 h after infection. The labeled cells were lysed in NET buffer (150 mM NaCl, 5 mM EDTA, 50 mM Tris [pH 8]) containing 1 mM PMSF and 0.5% Triton X-100. Immunoprecipitations were done with 10 μl of antiserum for each 106 cells. Proteins were immunoprecipitated from in vitro translation reaction mixtures or from lysates of BHV-1-infected and uninfected MDBK cells on protein A-Sepharose (Sigma) coated with rabbit anti-UL3.5 or mouse anti-αBTIF polyclonal antibodies.
Purification of His-tagged fusion proteins and in vitro pull-down assay.
Cells from a 2-ml overnight culture of E. coli BL21(DE3)pLysS containing the His-tagged fusion protein vector pUL3.5SET or deletion mutants of this same plasmid were collected by centrifugation and resuspended with an equal volume of Luria broth (LB) and inoculated 1:100 into LB containing 100 μg of ampicillin per ml. The culture was incubated at 37°C with shaking until the optical density at 600 nm reached 1.0, IPTG was added to a final concentration of 0.5 mM, and the culture was incubated for an additional 2 h at 37°C with shaking. The cells were suspended with lysis buffer (50 mM Tris-HCl [pH 8.0], 500 mM NaCl, 1 mM imidazole, 1 mM PMSF) and lysed by sonication. A 50% slurry of Ni-nitrilotriacetic acid (NTA) agarose (Qiagen) was added to the supernatant of the bacterial lysate, and the mixture was incubated with gentle agitation at 4°C for 1 h. The Ni-NTA agarose pellet was washed two times with lysis buffer containing 20 mM imidazole and two times with lysis buffer containing 40 mM imidazole and was finally equilibrated in TN buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM PMSF) before being applied to a pull-down assay. To prepare a control pRSET/Ni-NTA agarose, exactly the same protocol was used, except for starting with a culture of E. coli containing vector pRSET.
To prepare cell lysates for the in vitro pull-down assay, BHV-1-infected (at an MOI of 10) or mock-infected MDBK cells were labeled with [35S]methionine and [35S]cysteine (ICN Pharmaceuticals Inc.) from 6 to 18 h postinfection (hpi), harvested at 18 hpi, and lysed with TN buffer containing 0.5% Triton X-100. The lysate was precleaned by incubation with Ni-NTA agarose for 1 h at 4°C. The His-tagged UL3.5 bound to the Ni-NTA agarose was incubated with either precleaned cell lysate or in vitro-translated αBTIF at 4°C with gentle agitation for 3 h. The agarose was then washed two times with TN buffer containing 20 mM imidazole. Proteins bound to the Ni-NTA agarose were eluted by two 15-min treatments with TN buffer containing 100 mM imidazole at room temperature. The eluates were combined and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For each pull-down assay, about 4 μg of His-tagged UL3.5 was used for 5 × 106 cells and 1 μg of His-tagged UL3.5 was used for one-fourth of an in vitro translation reaction mixture.
Mass spectrometry protein identification.
To prepare the sample for protein identification, a preparative pull-down was performed using a lysate of 8 × 107 unlabeled BHV-1-infected MDBK cells and about 100 μg of His-tagged UL3.5. To monitor the assay, a parallel experiment using [35S]methionine- and [35S]cysteine-labeled virus-infected cell lysate was done. After SDS-PAGE, the gel was stained with 0.1% Coomassie blue and destained in 10% acetic acid–50% methanol–40% H2O. A strip of the gel containing the radiolabeled sample was dried and autoradiographed to identify the band of interest. A prominent band at about 60 kDa containing about 1 μg of protein was excised, frozen at −80°C, and sent for matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) analysis. MALDI-MS was performed by the W. M. Keck Facility, Yale University.
In vitro transcription and translation of UL3.5 and αBTIF.
In vitro transcriptions were carried out according to the AmpliScribe protocol (Epicentre Technologies). Briefly, 1 μg of pUL3.5cDNA3 or pαBTIFcDNA3 was linearized with XhoI and mixed with 2 μl of 10× T7 reaction buffer, 1.5 μl of 100 mM (each) ATP, CTP, GTP, and UTP, 2 μl of 100 mM dithiothreitol, and 2 μl of AmpliScribe T7 enzyme. The reaction mixture was incubated at 37°C for 2 h. Transcripts were translated using Red Nova reticulocyte lysate kit (Novagen) as described by the manufacturer. To optimize the translation efficiency, final salt concentrations in the reaction mixture were adjusted to 50 mM for potassium acetate and 0.25 mM for magnesium acetate. The UL3.5 protein was labeled with [3H]leucine, and αBTIF was labeled with [35S]methionine and [35S]cysteine.
Western blotting.
Western blotting was carried out as described previously (28). Proteins were separated by SDS-PAGE and transferred to nitrocellulose paper (Bio-Rad). The nitrocellulose was blocked for 30 min with blocking buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20, 5% powdered skim milk), incubated with appropriate primary antibodies diluted in blocking buffer, and then reacted with peroxidase-labeled anti-rabbit or anti-mouse immunoglobulin (Ig) antibody (1:3,000; Amersham). The results were visualized by an enhanced chemiluminescence reaction (Amersham).
Transfections and confocal microscopy.
The transfections were performed with Lipofectamine Plus reagent (Gibco) as described by the manufacturer. The day before transfection, Vero cells (4 × 105) were plated into six-well plates containing one coverslip per well. Each well of cells was transfected with 2 μg of expression plasmid made up to 5 μg with pcDNA3 DNA. Forty hours after transfection, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized with PBS containing 0.5% Triton X-100 for 15 min. For immunofluorescence analysis of virus-infected cells, MDBK cells were grown to 75% confluency on coverslips and infected with BHV-1 at an MOI of 10. At different times after infection, virus-infected cells were fixed and permeabilized as described above. For both transfected cells and BHV-1-infected cells, the monolayers were blocked with PBS containing 5% bovine serum albumin (BSA) at room temperature for 30 min and then incubated with rabbit anti-UL3.5 (1:150) and mouse anti-αBTIF(1:300) polyclonal antibodies diluted in PBS containing 5% BSA at room temperature for 1 h. The monolayers were washed extensively with PBS containing 0.2% Tween 20 and incubated with Texas Red-conjugated anti-rabbit IgG (Molecular Probes) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (Gibco), both at a final concentration of 10 μg/ml, in PBS containing 5% BSA at room temperature for 45 min. Again, the monolayers were extensively washed with PBS containing 0.2% Tween 20. Coverslips were mounted onto slides with mounting medium (10% 10× PBS, 90% glycerol, 1 mg of p-phenylenediamine/ml), sealed with fingernail polish, and kept in the dark at 4°C until examination. The mounted coverslips were examined in two channels with a Bio-Rad MRC-1024 confocal microscope.
RESULTS
BHV-1 UL3.5 interacts with αBTIF.
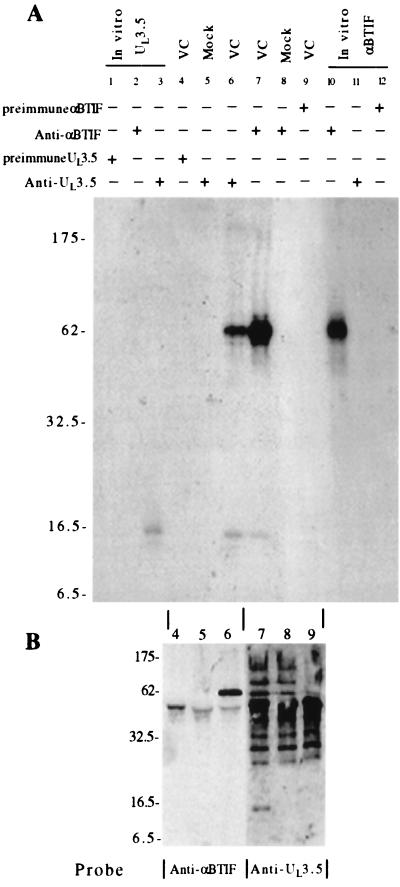
To investigate the role of BHV-1 UL3.5 in virus replication, we decided to first identify the proteins that associate with UL3.5. Previously, Schikora et al. developed a polyclonal antibody that specifically recognizes the BHV-1 UL3.5 protein (23). In the present experiment, that same anti-UL3.5 polyclonal antibody immunoprecipitated UL3.5 from both BHV-1-infected MDBK cells and partially purified BHV-1 virions but not from mock-infected MDBK cells (data not shown and Fig. 2, lanes 4 to 6). Strikingly, a 60-kDa protein also was immunoprecipitated by UL3.5 antiserum, but not preimmune serum, from virus-infected MDBK cells and BHV-1 virions.
FIG. 2.
Coimmunoprecipitation of UL3.5 and αBTIF from virus-infected cells. (A) Cells were infected with BHV-1 at an MOI of 10 or were mock infected and labeled with [3H]leucine for 12 h beginning at 6 h pi. Proteins from BHV-1-infected (VC) and mock-infected (Mock) cells were precipitated with anti-UL3.5 antibodies (lanes 5 and 6) or anti-αBTIF antibodies (lanes 7 and 8). Proteins from BHV-1-infected cells were also precipitated by UL3.5 and αBTIF preimmune sera (lanes 4 and 9). The UL3.5 protein was synthesized in vitro in the presence of [3H]leucine and was immunoprecipitated with anti-UL3.5, anti-αBTIF, and UL3.5 preimmune sera (lanes 3, 2, and 1, respectively). [35S]Met- and [35S]Cys-labeled in vitro-synthesized αBTIF was immunoprecipitated with anti-αBTIF, anti-UL3.5, and αBTIF preimmune serum (lanes 10 to 12, respectively). The precipitated proteins were analyzed by SDS-PAGE on a 10 to 20% gradient gel and fluorographed. (B) One-fifth of samples 4 through 9 from panel A were separated by SDS-PAGE on a 13% gel. The proteins were transferred onto a nitrocellulose membrane and probed with either anti-UL3.5 or anti-αBTIF antibodies as indicated. The bound antibodies were detected by enhanced chemiluminescence.
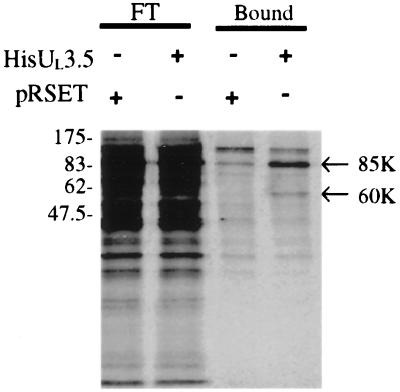
An in vitro pull-down assay was used to establish that the 60-kDa protein reacted directly with BHV-1 UL3.5. The BHV-1 UL3.5 ORF was amplified by PCR and cloned into bacterial His-tagged fusion protein expression vector pRSET. His-tagged UL3.5 expressed in E. coli was purified and immobilized onto Ni-NTA agarose (HisUL3.5/Ni-NTA agarose). The HisUL3.5/Ni-NTA agarose was then incubated with metabolically labeled BHV-1-infected or mock-infected MDBK cell lysates. As a control, a pRSET/Ni-NTA agarose generated from a lysate of bacteria containing the empty vector preparation was also incubated with a virus-infected cell lysate. The radiolabeled proteins binding to unlabeled His-tagged UL3.5 were eluted along with the His-tagged UL3.5 with imidazole and analyzed by SDS-PAGE and radioautography. A 60-kDa protein and an 85-kDa protein from BHV-1-infected cell lysate bound specifically to HisUL3.5/Ni-NTA agarose (Fig. 1). Mock-infected cell lysates did not have these two proteins (data not shown), suggesting that they might be viral proteins. The 60-kDa protein from the pull-down assay comigrated in SDS-PAGE with the one immunoprecipitated by anti-UL3.5 antiserum (data not shown).
FIG. 1.
Analysis of proteins that bind to His-tagged UL3.5. MDBK cells were infected with BHV-1 at an MOI of 10, labeled with [35S]Met and [35]Cys for 12 h beginning 6 h after infection, and lysed with Triton X-100. HisUL3.5/Ni-NTA agarose and the control pRSET/Ni-NTA agarose were prepared as described in Materials and Methods. The agarose was incubated with the infected cell lysate and washed. Unbound flowthrough (FT) and bound proteins were analyzed by SDS-PAGE on a 13% gel and autoradiographed. Arrows, 60- and 85-kDa proteins.
MALDI-MS was used to identify the 60-kDa protein. The pull-down assay was scaled up to prepare enough protein, the eluted proteins were separated by SDS-PAGE on an 8 to 15% gradient gel, and the area containing the 60-kDa protein was excised and sent for MALDI-MS analysis. Sixteen out of 70 measured peptides derived from in-gel trypsin digestion of the 60-kDa band matched αBTIF (EMBL accession no. Z11610) (20) and covered 30% of the αBTIF sequence. Thus the 60-kDa protein was identified unambiguously as αBTIF. The 85-kDa protein was not pursued further in this study.
UL3.5 and αBTIF coimmunoprecipitate.
To biochemically verify the MALDI-MS result, a coimmunoprecipitation study was performed. Anti-αBTIF polyclonal antibody was generated by immunizing mice with GST-αBTIF. BHV-1-infected MDBK cell lysates were immunoprecipitated by either anti-UL3.5 or anti-αBTIF polyclonal antibodies. To test the specificity of both polyclonal antibodies, immunoprecipitation of in vitro-translated UL3.5 and αBTIF was also performed. Figure 2A shows the immunoprecipitation results. Anti-UL3.5 and anti-αBTIF, but not their preimmune sera, specifically recognized in vitro-translated UL3.5 and αBTIF, respectively (lanes 1, 3, 10, and 12). Moreover, anti-UL3.5 did not precipitate in vitro-translated αBTIF and vice versa (lanes 2 and 11). Two bands, 60 and 13 kDa, were precipitated by anti-αBTIF specifically from BHV-1-infected MDBK cells (lane 7) but not from mock-infected cells (lane 8). The 60-kDa protein precipitated from virus-infected cells by anti-αBTIF comigrated in SDS-PAGE gel with in vitro-translated αBTIF (lane 10). Again, the anti-UL3.5 antibody precipitated two proteins, 13 and 60 kDa, from BHV-1-infected cells (lane 6) but not from mock-infected cells (lane 5). The slight difference in migration rates between the in vitro-synthesized UL3.5 and the native UL3.5 may be due to posttranslational modification of UL3.5 in infected cells. The 13-kDa proteins, as well as the 60-kDa proteins, which were precipitated from virus-infected cells by either anti-UL3.5 or anti-αBTIF antibodies, comigrated in SDS-PAGE gel (lanes 6 and 7), suggesting that they were identical.
To further demonstrate that UL3.5 and αBTIF coimmunoprecipitated with each other, Western blotting was done on samples 4 through 9 from Fig. 2A, which were separated on another gel. The 60-kDa αBTIF was specifically detected in virus-infected cells immunoprecipitated with anti-UL3.5 (Fig. 2B, lane 6), and the 13-kDa UL3.5 was detected in the αBTIF immunoprecipitate (Fig. 2B, lane 7). In summary, UL3.5 and αBTIF formed a complex that can be precipitated by either anti-UL3.5 or anti-αBTIF antibodies.
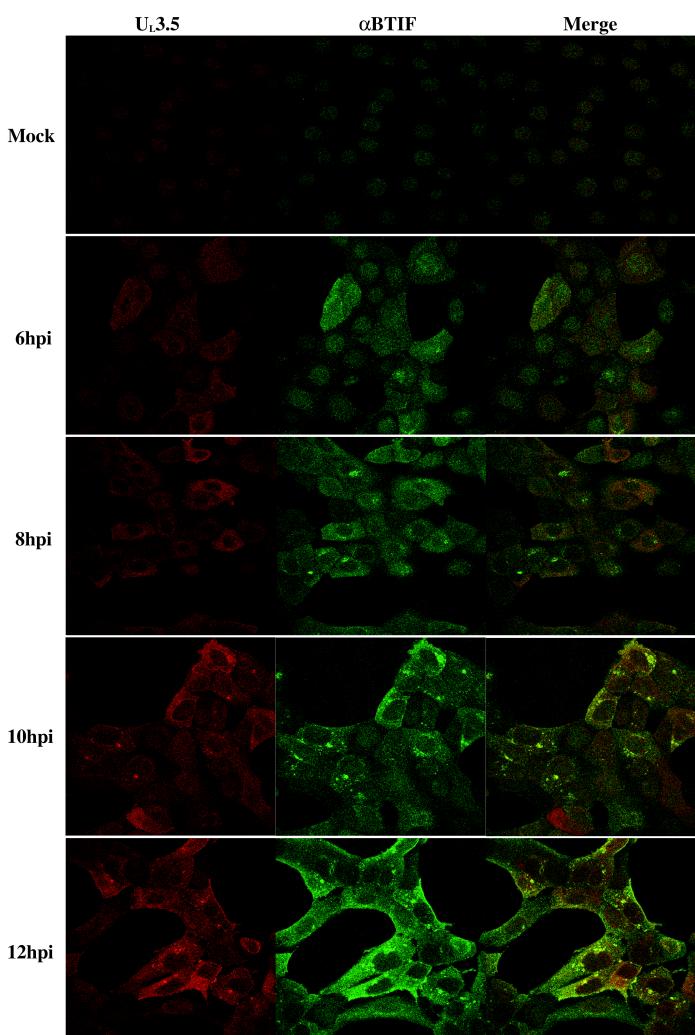
UL3.5 and αBTIF colocalize in virus-infected cells.
To determine whether UL3.5 and αBTIF interact with each other in vivo, indirect immunofluorescence and confocal microscopy were performed to detect the colocalization of the two proteins in virus-infected cells. MDBK cells were infected with BHV-1 at an MOI of 10. At 6, 8, 10, and 12 hpi, cells were fixed and stained for UL3.5 and αBTIF (Fig. 3). Newly synthesized UL3.5 was detected in the cytoplasm by 6 hpi and was distributed predominantly in the cytoplasm from 8 through 12 hpi. Starting at 10 hpi, a small portion of UL3.5 also appeared in specific perinuclear structures. αBTIF was mainly in the nucleus at 4 hpi (data not shown), was localized throughout the cells from 6 to 8 hpi, and was concentrated in the cytoplasm from 10 to 12 hpi. Localization of αBTIF in perinuclear structures was also observed by 8 hpi. Although both the UL3.5 and the αBTIF were expressed by 6 hpi, colocalization of the proteins was not observed until 10 hpi, was restricted to the perinuclear structures, and increased dramatically up to 12 hpi, the last time point observed. The perinuclear fluorescent dots observed at 10 and 12 hpi resembled those observed in BHV-1-infected MDBK cells stained for gD (4) and in PrV-infected MDBK cells stained for gE (26), suggesting that they might represent part of the Golgi apparatus or virion assembly sites.
FIG. 3.
Colocalization of UL3.5 and αBTIF in BHV-1-infected MDBK cells. MDBK cells were mock infected or infected with BHV-1 at an MOI of 10. At 6, 8, 10, and 12 hpi, the cells were fixed and incubated with both rabbit anti-UL3.5 and mouse anti-αBTIF polyclonal antibodies. Cells were further incubated with Texas Red-conjugated anti-rabbit IgG and FITC-conjugated anti-mouse IgG and examined with a dual-channel confocal microscope.
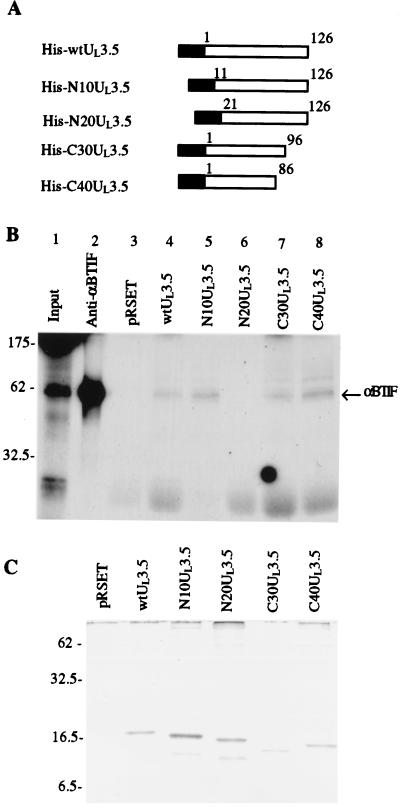
Interaction of UL3.5 and αBTIF in vitro requires only aa 10 to 86 of UL3.5.
The in vitro pull-down assay was performed to demonstrate the direct interaction between UL3.5 and αBTIF. αBTIF was synthesized in an in vitro transcription-translation system in which it was labeled with [35S]methionine and [35S]cysteine. In vitro-synthesized αBTIF was incubated with HisUL3.5/Ni-NTA agarose or pRSET/Ni-NTA agarose. Proteins bound to the agarose after extensive washing were eluted with imidazole and analyzed by SDS-PAGE and autoradiography. The in vitro translation reaction mixture immunoprecipitated with anti-αBTIF was also run on the gel as a control (Fig. 4B, lane 2). Figure 4B shows that in vitro-synthesized αBTIF bound to HisUL3.5/Ni-NTA agarose but not to pRSET/Ni-NTA agarose (lanes 4 and 3), demonstrating the specific in vitro interaction between UL3.5 and αBTIF.
FIG. 4.
An in vitro pull-down assay shows that only aa 10 to 86 of UL3.5 were required for UL3.5-αBTIF interaction. (A) Diagrammatic description of UL3.5 mutants used in the in vitro pull-down assay. The black box represents the fusion partner containing the His tag encoded by the pRSET vector. The white box represents the UL3.5 amino acid sequence. wt, wild type. (B) The αBTIF protein was translated in vitro in the presence of [35S]Met and [35S]Cys. In vitro-synthesized αBTIF (lane 1) was incubated with anti-αBTIF bound to protein A-Sepharose (lane 2), control pRSET/Ni-NTA agarose (lane 3), full-length UL3.5 (lane 4), and truncated UL3.5 (lanes 5 to 8) bound to Ni-NTA agarose. The bound proteins were analyzed by SDS-PAGE and autoradiography. (C) A Coomassie blue-stained gel was used to show that about equal amounts of recombinant His-tagged UL3.5 proteins were loaded in the lanes shown in panel B.
To examine which part of UL3.5 is required for the UL3.5-αBTIF interaction, a series of UL3.5 deletion mutants were made (Fig. 4A) and tested in the pull-down assay. A Coomassie blue-stained gel was used to show that approximately equal amounts of recombinant UL3.5 proteins were used (Fig. 4C). Deletion of 10 aa from the N terminus of UL3.5 did not influence UL3.5's ability to interact with αBTIF (Fig. 4B, lane 5). However, deletion of 20 aa from the N terminus totally abolished the interaction (Fig. 4B, lane 6). In contrast, mutants with the deletion of up to 40 aa from the C terminus (C30 and C40 mutants) still interacted with in vitro-synthesized αBTIF (Fig. 4B, lanes 7 and 8). It is not clear why the His-tagged C30 mutant migrated faster than the C40 mutants in SDS-PAGE gel (Fig. 4C). Since the plasmid construct encoding the C30 mutant was confirmed by DNA sequencing, it is likely that the C30 mutant suffered degradation during expression in E. coli. However, the His tag was fused to the N terminus of UL3.5. That the C30 mutant can be purified by Ni-NTA agarose demonstrated that the N-terminal part of the mutant was intact. So the smaller size of the C30 mutant did not influence our conclusion. In conclusion, only UL3.5 aa 11 to 86 were required for the binding of UL3.5 to αBTIF.
UL3.5 and αBTIF colocalize in transfected cells.
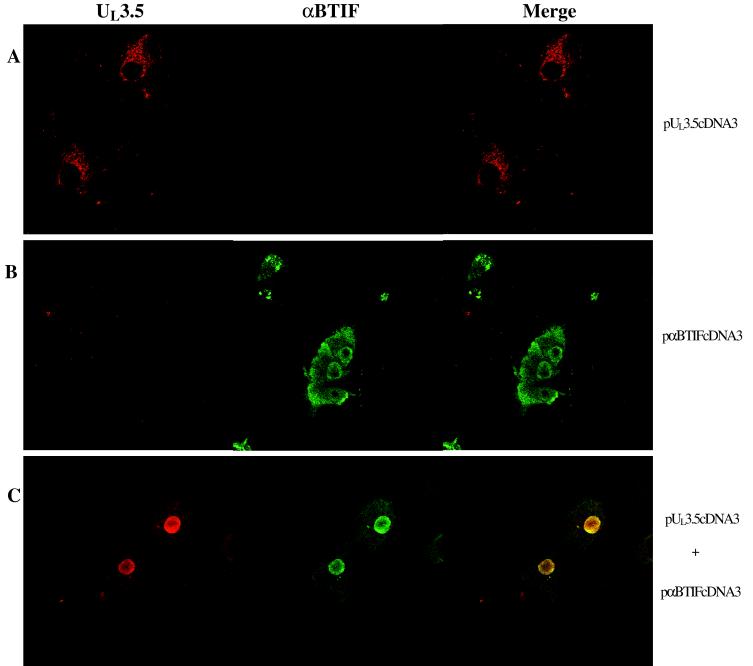
To show that BHV-1 UL3.5 and αBTIF can interact in vivo in the absence of other viral proteins, their colocalization was tested in a transient coexpression assay. Coding sequences for UL3.5, its mutants, and αBTIF were cloned into the expression vector pcDNA3. The plasmid DNAs were transfected into Vero cells. The cells were stained for αBTIF and UL3.5 40 h after transfection. When transiently expressed alone, αBTIF was distributed homogeneously in the nucleus and the cytoplasm of most of the cells (Fig. 5B), whereas the wild-type UL3.5 was distributed exclusively in the cytoplasm in a net-like pattern resembling those for the endoplasmic reticulum and Golgi apparatus (Fig. 5A). Because there was no green fluorescence detected in cells transfected with only pUL3.5cDNA3 and no red fluorescence in cells transfected with only pαBTIFcDNA3, the signals detected for UL3.5 and αBTIF were specific and not due to the cross-reactivity of the two antibodies. When wild-type UL3.5 and αBTIF were coexpressed, the two proteins colocalized in the nucleus in almost every cell analyzed (Fig. 5C). Colocalization was concentrated in the region near the inner surface of the nuclear membrane. Besides being detected in the nucleus, colocalization was detected in the cytoplasm in some cells (data not shown). However, in cells expressing less UL3.5 or αBTIF, colocalization was exclusively observed in the nucleus, suggesting that colocalization in the cytoplasm in the transient coexpression assay was a result of an excess of both proteins.
FIG. 5.
Colocalization of UL3.5 and αBTIF in transfected cells. Vero cells were transfected with pUL3.5cDNA3 (A), pαBTIFcDNA3 (B), and pUL3.5cDNA3 plus pαBTIFcDNA3 (C). The cells were fixed 40 h after transfection, incubated with both anti-UL3.5 and anti-αBTIF antibodies, stained with Texas Red- and FITC-conjugated secondary antibodies, and examined with a dual-channel confocal microscope.
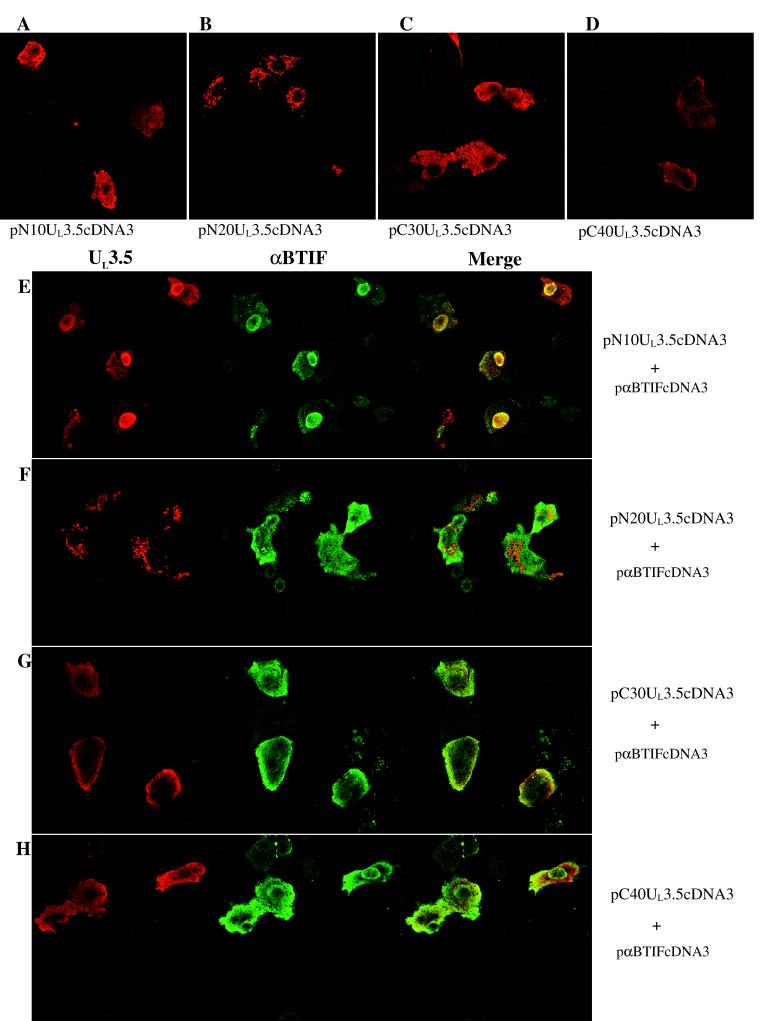
To confirm the dispensability of the UL3.5 termini for the interaction with αBTIF in an in vivo system, the colocalization of the UL3.5 mutants with αBTIF was also examined in the transient-expression assay. In this assay, all UL3.5 mutants expressed from the pcDNA3 vector could be recognized by the polyclonal anti-UL3.5 antibody but not by the polyclonal anti-αBTIF antibody (data not shown). Each of the UL3.5 mutants had a different subcellular localization (Fig. 6A to D). Only mutant N20UL3.5 was similar to the wild type (Fig. 5A). Mutant N10UL3.5 was distributed homogeneously in the cytoplasm and the nucleus, whereas the C30UL3.5 and C40UL3.5 mutants stayed mostly in the cytoplasm. When coexpressed with αBTIF, only N10UL3.5 colocalized to the nucleus (Fig. 6E) similarly to the wild type (Fig. 5C). Consistent with the in vitro results, N20UL3.5 did not colocalize with αBTIF (Fig. 6F). For C30UL3.5 and C40UL3.5, colocalization was predominantly detected in the cytoplasm, primarily near the cell membrane (Fig. 6G and H). In conclusion, results of the study from transfected cells showed that the direct UL3.5-αBTIF interaction occurred in living cells in the absence of other viral proteins and also strongly supported the requirement for aa 11 to 86 for the UL3.5-αBTIF interaction.
FIG. 6.
Interaction of UL3.5 mutants with αBTIF in transfected Vero cells. Vero cells were transfected with pN10UL3.5cDNA3 (A), pN20UL3.5cDNA3 (B), pC30UL3.5cDNA3 (C), pC40UL3.5cDNA3 (D), pN10UL3.5cDNA3 and pαBTIFcDNA3(E), pN20UL3.5cDNA3 and pαBTIFcDNA3(F), pC30UL3.5cDNA3 and paBTIFcDNA3(G), or pC40UL3.5cDNA3 and pαBTIFcDNA3(H). The cells were stained and examined as described for Fig. 5.
DISCUSSION
This research strongly suggests that BHV-1 UL3.5 binds directly to αBTIF both in vitro and in vivo through a domain located between aa 11 and 86 in UL3.5. Since UL3.5 does not have a cysteine, the interaction between UL3.5 and αBTIF is likely noncovalent. In our study, we cannot completely rule out the possibility that an intermediary is required for the interaction. However, the absence of stoichiometric amounts of other proteins in complexes immunoprecipitated by antibodies against either UL3.5 or αBTIF suggests that no intermediary is required. Nevertheless, it is possible that the intermediary was not efficiently radiolabeled by [3H]leucine and remained undetected. Because all the assays were done in the presence of mammalian cells or cell lysates, an intermediary, if any, must be common to MDBK cells, African green monkey kidney cells, and rabbit red blood cells.
The sites of UL3.5 and αBTIF colocalization for virus-infected and transfected cells are dramatically different. In virus-infected cells, newly synthesized UL3.5 and αBTIF colocalized at specific perinuclear structures (Fig. 3), whereas in transfected cells they colocalized in the nucleus (Fig. 5). The reason(s) for the difference is not clear. Possibly, colocalization of UL3.5 and αBTIF at the proper intracellular compartment in virus-infected cells requires ongoing synthesis of other viral proteins. Alternatively, downregulation or upregulation of a specific cellular protein(s) resulting from viral infection might account for the difference. Also note that when either UL3.5 or αBTIF was transiently expressed alone, it accumulated in a different pattern than when the two were transiently expressed together or when expressed during viral infection, indicating that both binding together and some other effect in virus-infected cells determined where the proteins localized. The C terminus of UL3.5 seems to have an effect on the site of colocalization since UL3.5 C-terminal deletions (Fig. 6G and H), but not the N-terminal 10-aa deletion (Fig. 6E), resulted in colocalization concentrated in the cytoplasm of transfected cells. A mutant BHV-1 containing a UL3.5 C-terminal deletion would be helpful in answering the question of whether the C terminus of UL3.5 is required to direct colocalization to the proper site during viral infection.
Although newly synthesized UL3.5 and αBTIF can be detected throughout the cytoplasm of virus-infected cells at 6 hpi, colocalization restricted to Golgi-like regions was not observed until 10 hpi, suggesting that the interaction may be specifically regulated. What regulates the interaction? Phosphorylation may be a good candidate. Phosphorylation has been recognized as an important regulatory mechanism for other herpesviral tegument proteins. For example, phosphorylation catalyzes herpesvirus tegument disassembly (21). Also, the nonphosphorylated, but not the phosphorylated, form of HSV-1 VP22 is incorporated into the virion (7). Our unpublished results suggest that both UL3.5 and αBTIF are phosphorylated in virus-infected cells. Detailed study of the relationship between UL3.5 and αBTIF interaction and phosphorylation may answer this question.
What is the function of the UL3.5-αBTIF interaction? Both UL3.5 and αBTIF are brought into cells upon virus infection since they are both virion components (20, 23). As shown for the transfected cells (Fig. 5), coexpression of UL3.5 and αBTIF resulted in localization of both proteins to the nucleus. The interaction with UL3.5 might allow αBTIF to enter the nucleus more efficiently at the beginning of infection, thus enhancing intermediate-early gene transactivation. Indeed, our preliminary results (data not shown) obtained with a transient transfection assay did show that cotransfection of UL3.5 with αBTIF enhanced transactivation of BHV-1 immediate-early promoter 1 by αBTIF.
Alternatively, an interaction between UL3.5 and αBTIF may be important for viral assembly and egress. Indeed, colocalization of two proteins at probable sites of virion assembly became obvious 10 h after infection, just before infectious particles are released (4, 14). It has been shown that homologs of both UL3.5 and αBTIF have important functions in herpesviral assembly. In cells infected with a PrV mutant lacking UL3.5, naked nucleocapsids accumulate near the trans-Golgi region (10), implying that PrV UL3.5 is a key component for virus egress. Although the exact function of BHV-1 UL3.5 in BHV-1 replication is still unknown, BHV-1 UL3.5 is able to rescue a PrV UL3.5-negative virus (9), suggesting that BHV-1 UL3.5 might play a similar role in BHV-1 viral egress. HSV-1 VP16 is essential for virion assembly (27). Like its HSV counterpart, αBTIF is synthesized at the late stage of virus infection (20). Given the shared transcriptional-activation functions of αBTIF and VP16, one might speculate that αBTIF, like VP16, also is essential in virus assembly. Results from the UL3.5 deletion mutagenesis study may suggest that the UL3.5-αBTIF interaction is required for virus assembly. The PrV UL3.5 domain required for viral assembly and egress is apparently located in the N terminus because disruption of the C terminus does not dramatically impair viral growth in cell culture (10). Since the most conserved region between PrV and BHV-1 UL3.5 proteins is limited to the N-terminal 50 aa (13), it is reasonable to postulate that the N-terminal part of BHV-1 UL3.5 involved in αBTIF binding also contains the essential functional domain required for rescuing the PrV mutant. Thus, the successful rescuing of the PrV UL3.5-null virus by BHV-1 UL3.5 might be due to the interaction between the BHV-1 UL3.5 protein and the PrV homolog of αBTIF.
We should note that there are substantial differences among homologs of UL3.5 and αBTIF, so no definite conclusion about the function of the UL3.5-αBTIF interaction is possible from this study. For example, VZV ORF57 (homolog of the UL3.5 gene) is not required for VZV replication (3). In PrV, there is no virion component that transactivates immediate-early genes (2). Future study on BHV-1 containing different UL3.5 or αBTIF mutants will be helpful to understand the roles of the UL3.5-αBTIF interaction in BHV-1 replication.
ACKNOWLEDGMENTS
This project was supported by USDA Animal Health Formula Funds and a grant from the University of Wisconsin Graduate School.
We are grateful to Chad Johnson and Shixuan Wu for valuable discussions and help with cell culture.
REFERENCES
- 1.Campbell M E, Palfreyman J W, Preston C M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 2.Campbell M E, Preston C M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987;157:307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- 3.Cox E, Reddy S, Iofin I, Cohen J I. Varicella-zoster virus ORF57, unlike its pseudorabies virus UL3.5 homolog, is dispensable for viral replication in cell culture. Virology. 1998;250:205–209. doi: 10.1006/viro.1998.9349. [DOI] [PubMed] [Google Scholar]
- 4.Dasika G K, Letchworth G J. Cellular expression of bovine herpesvirus 1 gD inhibits cell-to-cell spread of two closely related viruses without blocking their primary infection. Virology. 1999;254:24–36. doi: 10.1006/viro.1998.9553. [DOI] [PubMed] [Google Scholar]
- 5.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 6.Dean H J, Cheung A K. A 3′ coterminal gene cluster in pseudorabies virus contains herpes simplex virus UL1, UL2, and UL3 gene homologs and a unique UL3.5 open reading frame. J Virol. 1993;67:5955–5961. doi: 10.1128/jvi.67.10.5955-5961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott G, O'Reilly D, O'Hare P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology. 1996;226:140–145. doi: 10.1006/viro.1996.0638. [DOI] [PubMed] [Google Scholar]
- 8.Fisher C L, Pei G K. Modification of a PCR-based site-directed mutagenesis method. Biotechniques. 1997;23:570–571. doi: 10.2144/97234bm01. , 574. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs W, Granzow H, Mettenleiter T C. Functional complementation of UL3.5-negative pseudorabies virus by the bovine herpesvirus 1 UL3.5 homolog. J Virol. 1997;71:8886–8892. doi: 10.1128/jvi.71.11.8886-8892.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs W, Klupp B G, Granzow H, Rziha H J, Mettenleiter T C. Identification and characterization of the pseudorabies virus UL3.5 protein, which is involved in virus egress. J Virol. 1996;70:3517–3527. doi: 10.1128/jvi.70.6.3517-3527.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs W, Mettenleiter T C. DNA sequence and transcriptional analysis of the UL1 to UL5 gene cluster of infectious laryngotracheitis virus. J Gen Virol. 1996;77:2221–2229. doi: 10.1099/0022-1317-77-9-2221. [DOI] [PubMed] [Google Scholar]
- 12.Guan K L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 13.Khattar S K, van Drunen Littel-van den Hurk S, Babiuk L A, Tikoo S K. Identification and transcriptional analysis of a 3′-coterminal gene cluster containing UL1, UL2, UL3, and UL3.5 open reading frames of bovine herpesvirus-1. Virology. 1995;213:28–37. doi: 10.1006/viro.1995.1543. [DOI] [PubMed] [Google Scholar]
- 14.Köppel R, Fraefel C, Vogt B, Bello L J, Lawrence W C, Schwyzer M. Recombinant bovine herpesvirus-1 (BHV-1) lacking transactivator protein BICPO entails lack of glycoprotein C and severely reduced infectivity. Biol Chem. 1996;377:787–795. doi: 10.1515/bchm3.1996.377.12.787. [DOI] [PubMed] [Google Scholar]
- 15.Lewis J B, Thompson Y G, Caughman G B. Transcriptional control of the equine herpesvirus 1 immediate early gene. Virology. 1993;197:788–792. doi: 10.1006/viro.1993.1658. [DOI] [PubMed] [Google Scholar]
- 16.Marshall R L, Rodriguez L L, Letchworth G J. Characterization of envelope proteins of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) by biochemical and immunological methods. J Virol. 1986;57:745–753. doi: 10.1128/jvi.57.3.745-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayfield J E, Good P J, VanOort H J, Campbell A R, Reed D E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain) J Virol. 1983;47:259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 19.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 20.Misra V, Bratanich A C, Carpenter D, O'Hare P. Protein and DNA elements involved in transactivation of the promoter of the bovine herpesvirus (BHV) 1 IE-1 transcription unit by the BHV α gene trans-inducing factor. J Virol. 1994;68:4898–4909. doi: 10.1128/jvi.68.8.4898-4909.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison E E, Wang Y F, Meredith D M. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J Virol. 1998;72:7108–7114. doi: 10.1128/jvi.72.9.7108-7114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pellett P E, McKnight J L, Jenkins F J, Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc Natl Acad Sci USA. 1985;82:5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schikora B, Lu Z, Kutish G F, Rock D, Magyar G, Letchworth G J. The bovine herpesvirus type 1 UL3.5 open reading frame encodes a virion structural protein. Virology. 1998;240:76–82. doi: 10.1006/viro.1997.8918. [DOI] [PubMed] [Google Scholar]
- 24.Steven A C, Spear P G. Herpesvirus capsid assembly and envelopment. In: Chiu W, Burnett R M, Garcea R L, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1997. pp. 312–351. [Google Scholar]
- 25.Telford E A, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 26.Tirabassi R S, Enquist L W. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J Virol. 1999;73:2717–2728. doi: 10.1128/jvi.73.4.2717-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinheimer S P, Boyd B A, Durham S K, Resnick J L, O'Boyle D R. Deletion of the VP16 open reading frame of herpes simplex virus type 1. J Virol. 1992;66:258–269. doi: 10.1128/jvi.66.1.258-269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu X, Wu S, Letchworth G J. Yeast-secreted bovine herpesvirus type 1 glycoprotein D has authentic conformational structure and immunogenicity. Vaccine. 1997;15:679–688. doi: 10.1016/s0264-410x(96)00234-4. [DOI] [PubMed] [Google Scholar]