Abstract
Epstein-Barr virus (EBV) is a prevalent virus that can be detected in the vast majority of the population. Most people are asymptomatic and remain chronically infected throughout their lifetimes. However, in some populations, EBV has been linked to a variety of B-cell lymphoproliferative disorders (LPDs), such as Burkitt lymphoma, classic Hodgkin lymphoma, and other LPDs. T-cell LPDs have been linked to EBV in part of peripheral T-cell lymphomas, angioimmunoblastic T-cell lymphomas, extranodal nasal natural killer/T-cell lymphomas, and other uncommon histotypes. This article summarizes the current evidence for EBV-associated LPDs in light of the upcoming World Health Organization classification and the 2022 ICC classification.
Keywords: EBV, Lymphoproliferative diseases
Introduction
Epstein-Barr virus-related lymphoproliferative diseases (EBV+ LPD) constitute a diverse group of diseases characterized by the presence of the Epstein–Barr virus (EBV) in one or more lymphoid cell types, resulting in uncontrolled proliferation of infected cells. This phenomenon is intricately linked to the development of a spectrum of lymphoproliferative diseases (LPDs), ranging from non-cancerous to precancerous and malignant conditions (Figure 1).1–7
Figure 1.
EBV-latency immunogenicity in different EBV-related lymphoproliferative diseases.
Approximately 95% of the population is seropositive for EBV. Initial Infection with the virus may cause infectious mononucleosis, present with minimal non-specific symptoms, or remain entirely asymptomatic. Subsequently, the virus establishes latency within its host, rendering the affected person asymptomatic for the duration of their life. However, a small subset of carriers—particularly those with immunodeficiency-develop an EBV+ LPD weeks, months, years, or even decades after the primary Infection. Globally, approximately 1% of all malignancies are attributed to EBV infection, with LPDs constituting the great majority of these malignancies.1,8,9 Global health is greatly impacted by the non-malignant, premalignant, and malignant types of EBV+ LPD.1
This review adheres to the categorization and nomenclature outlined by the World Health Organization’s 2016 modifications and the International Collaboration on Cancer Reporting’s 2022 refined criteria to elucidate the complex landscape of EBV+ LPDs.10–11 By exploring these classifications comprehensively, we aim to enhance our understanding of the diverse clinical entities within the realm of EBV-associated LPD.
Pathophysiology
The germinal center model describing the normal maturation of B cells within lymph nodes and other lymphoid tissues delineates the progression of naive B cells entering these structures into lymphoblasts, centroblasts, centrocytes, memory B cells, and plasma cells, ultimately acquiring the capability to produce functional antibodies. Throughout this maturation process, B cells undergo rearrangement of their immunoglobulin genes at various loci.9 Notably, naïve B cells serve as the primary target for EBV invasion.
Upon infiltrating naïve B cells, EBV orchestrates the expression of genes that regulate cell maturation, compelling the cells to undergo forced maturation, diminishing the infected cell’s recognition by the host’s immune system, and triggering uncontrolled proliferation, culminating in the development of a B cell-related LPD. The virus may also spread from the initially infected B cells to T or NK cells, instigating subsequent rounds of multiplication and the potential of LPD formation.12
Natural killer T cells (NK), Gamma delta T cells, cytotoxic T cells (CTL), helper T cells (Th), and follicular helper T cells (TFH) are susceptible to EBV infection.13 The mechanism by which EBV infects dendritic-histiocytic cells (i.e. follicular dendritic cells) adds further complexity to the understanding of the viral dissemination. Although follicular dendritic cells are connective tissue cells rather than lymphoid cells, their expression of the surface membrane receptor CD21 serves as an entry point for EBV. Through this CD21 entrance mechanism, EBV may potentially escape from infected B cells and establish Infection within follicular dendritic cells.14
It is crucial to emphasize that in the majority of cases (>95%) EBV infections result in non-complicated or asymptomatic outcomes. Host responses, particularly involving T memory cells and CD8+ T cells, play a pivotal role in controlling the outgrowth of infected B cells, confining the EBV genome to episomes during the latency phase. However, in some cases, a prevailing lytic phase can lead to a persisting EBV infection and a chronic viral state influenced by diverse genetic and environmental factors.
This influence is exemplified by the impact of ethnic and geographical diversity on the occurrence of EBV-related malignancies. Notably, specific Asian populations exhibit distinct genetic predispositions, presumably linked to particular human leukocyte antigen (HLA) types, influencing their response to primary EBV infection. For instance, certain Asian ethnic groups with a heightened prevalence of HLA A11, a type associated with an EBNA-4 mutation that hinders cytotoxic T-cell recognition of EBV, may experience an aberrant response to the virus. In contrast, A11 is uncommon among Europeans, and cytotoxic T cells recognizing the EBNA-4 peptide dominate the immunological response to EBV in Europeans. These variations in the HLA phenotype offer a plausible explanation for the higher prevalence of EBV-positive malignancies, particularly nasal T/NK cell lymphomas, among Asian populations.9
Furthermore, observations indicate that some immunocompetent hosts have the capacity to eliminate EBV viral particles through oropharyngeal secretions intermittently and asymptomatically over their lifetime. On the other hand, immunodeficient hosts exhibit an increased frequency and quantity of viral particles in oropharyngeal secretions, potentially leading to increased EBV transmission.
EBV-Associated Reactive Lymphoid Proliferations
EBV-associated reactive lymphoid proliferations represent a group of conditions characterized by the growth of B cells or NK/T cells in response to EBV infection. Typically, these conditions are self-limiting and non-malignant, posing a low risk of progressing to malignant LPD.1
Epstein–Barr virus-positive infectious mononucleosis
Approximately 90% of cases of infectious mononucleosis (IM) are attributed to EBV. Beyond EBV, IM-like infections can result from human cytomegalovirus, adenovirus, or other pathogens.15 acute EBV infection is frequently asymptomatic or mild in children under the age of 5 years, while 25–75% of adolescents and adults develop overt IM following Infection.11 Within weeks of EBV infection, signs and symptoms of IM appear. The majority of cases show self-limiting flu-like symptoms such as fever, sore throat, enlarged, painful lymph nodes in the head and neck, and splenomegaly.
These symptoms normally resolve within six weeks. In more severe cases, symptoms may persist longer and may be associated with rare but serious complications such as hepatitis, anemia, thrombocytopenia, hemophagocytosis, meningoencephalitis, myocarditis, pericarditis, pneumonitis, parotitis, pancreatitis, and, in extremely severe cases, life-threatening complications such as spleen rupture or progression to other LPD such as hemophagocytic lymphohistiocytosis.16–17
During the acute phase of EBV infection, individuals have high amounts of infective EBV in their oral/nasal secretions, as well as high blood levels of EBV, atypical lymphocytes, CD8+ T cells, and memory B cells (up to 50% of the latter cells being EBV+). Tonsils and cervical lymph nodes display hyperplasia, featuring a mix of normal-appearing lymphocytes, activated lymphocytes, plasma cells, and Reed-Sternberg-like cells.15 Notably, many of these normal-appearing and activated B cells, along with a small fraction of the tissue’s T and NK cells, are EBV+, with the virus predominantly being in the lytic phase rather than latent.1 Mild IM cases are often overlooked or diagnosed based on clinical and routine laboratory findings.
Diagnosis of EBV-associated IM is conclusive when EBV, IgM antibody to EBV viral-capsid antigen (VCA-IgM), IgG antibody to VCA (IgG-VCA), and IgG antibody to EBV viral-capsid antigen (EBNA1-IgG) are detected in the blood during the initial period of Infection and/or EBV is detected in the oral or nasal secretions.11 There are no randomized controlled trials for the treatment of uncomplicated EBV+ IM. Patients experiencing airway obstruction, autoimmune reactions (e.g., autoimmune anemia or thrombocytopenia), or other disease consequences are commonly prescribed short-term corticosteroid regimes.17 Treatment of severe IM cases often entails regimens that are tailored to the unique characteristics of each type of complication.11
Epstein–Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH)
EBV-HLH presents as a rare condition characterized by a systemic inflammatory response or, in severe cases, as an overwhelming cytokine storm. The virus impairs the capacity of cytotoxic T cells to eliminate other EBV-infected cells, leading to excessive production of pro-inflammatory cytokines, such as TNF-alpha, IL1beta, and CXCL9, by activated histiocytes.1
Two forms of HLH exist. Primary HLH (also known as genetic or familial HLH) is caused by loss of function (i.e. inactivating) mutations in genes crucial for cytotoxic T and/or NK cells to eliminate EBV-infected cells. These mutations encompass UNC13D, STX11, RAB27A, STXBP2, LYST, PFP, SH2D1A, BIRC4, ITK1, CD27, and MAGT1 genes.18
Secondary HLH is associated with and potentially aggravated by malignant and non-malignant illnesses that, like primary HLH, impair the immune system’s ability to target EBV-infected cells. Hematological malignancies, autoimmune disorders,18 immunodeficiency disorders,19–21 and infections are linked to secondary HLH.
The typical manifestations of HLH include fever, decreased circulating white blood cells and/or platelets, enlarged liver and/or spleen, clinical signs of hepatitis, and/or central nervous system disorders21 such as irritability, decreased levels of consciousness, seizures, meningitis, impaired cranial nerve function, hemiplegia, and ataxia.18
Histological examination reveals infiltration of small EBV+ T cells, scattered EBV+ B cells, reactive histiocytes, reactive macrophages, and, in approximately 70% of cases, hemophagocytosis—the ingestion of erythrocytes, leukocytes, platelets, and/or their precursor cells by histiocytes and macrophages—in various tissues such including lymphatic, bone marrow, liver, and neuronal tissues. It is essential to note that the presence of hemophagocytosis does not necessarily mean that HLH has been diagnosed. Rather than being in a latent phase, the EBV-infected lymphocytes are in their lytic cycle.1
The combination of etoposide and dexamethasone is currently recommended for the treatment of EBV+ HLH. Allogenic hematopoietic stem cell transplantation is selectively employed after induction therapy, especially in cases with primary HLH.21 However, the success rate in managing EBV+ HLH remains lower than that of other secondary HLH causes.11 The use of anti-thymocyte globulin, the DEP regimen (liposomal doxorubicin, etoposide, methylprednisolone), an anti-interferon gamma monoclonal antibody, and especially rituximab are explored as novel approaches to HLH, especially in cases of refractory or recurrent disease.11, 23
EBV-Positive T and NK Cell Lymphoproliferative Disorders of Childhood
Childhood EBV+T and NK LPDs are a rare group of disorders that primarily affect children, with sporadic occurrence in adults. The 2022 ICC has implemented substantial revisions to this category of diseases.11 The prior 2017 WHO classification identified two categories: systemic EBV+ T cell lymphoma of childhood and chronic active EBV (CAEBV) infection with both cutaneous and systemic forms.10 The ICC 2022 now recognizes four distinct disorders: severe mosquito bite allergy, hydroa vacciniforme (HV) LPD, systemic EBV-positive T cell lymphoma of childhood, and systemic chronic active EBV (CAEBV) disease. These recognitions are a result of new findings and a deeper understanding of these disorders.10
Chronic active Epstein–Barr virus disease
The term CAEBV disease is favored over CAEBV infection since most adults harbor latent EBV infections displaying a persistent viral presence, yet only a fraction develops the clinical condition.11,23 Characterized by a prolonged duration exceeding three months, CAEBV disease progresses in the absence of known immunodeficiency, manifesting as significantly elevated blood levels of EBV DNA and organ involvement by EBV-infected cells.
About half of CAEBV patients exhibit symptoms similar to infectious mononucleosis, such as lymphadenopathy, hepatosplenomegaly, and fever.24 Additional symptoms include severe mosquito bite allergy, a pronounced rash, hepatitis/hepatic failure, diarrhea, uveitis, myocarditis, and interstitial pneumonia.25 While the clinical course varies, it tends to be protracted, with some patients exhibiting prolonged stability., Notably, patients with EBV-infected T cells have worse survival rates, more pronounced systemic symptoms, and elevated blood EBV DNA titers compared to those with NK cell involvement. Conversely, severe allergies to mosquito bites and elevated blood IgE levels are common in patients with NK cell-related cases. In 2022, the ICC designated these cases as CAEBV disease due to their aggressive clinical course and absence of typical hydroa vacciniforme (HV) lesions.11,26 Currently, hematopoietic stem cell transplantation remains the sole curative treatment. Histopathological examination27 reveals infiltrating cells in affected organs without malignant lymphoproliferation. A biopsy of the liver or lymph nodes is usually conducted for diagnosis. Liver biopsy typically shows portal and sinusoidal inflammation, indicative of viral hepatitis, while lymph nodes may exhibit small granulomas, localized necrosis, or follicular or paracortical hyperplasia. EBV infections affect 40% of NK cells and 60% of T cells. The majority of the invading T cells are CD4+, with a small percentage being CD8+. Epstein-Barr virus-encoded small RNA (EBER) is generally positive.24 While the pathophysiology remains uncertain, genetic predisposition may play a role.28 TCR gene rearrangement may be monoclonal, oligoclonal, or polyclonal. Recent genetic studies have identified somatic mutations in DDX3D and KMT2D, particularly in cases of NK cell involvement, suggesting a premalignant nature of the disease. Frequent intragenic deletions in the EBV genome highlight the pivotal role of these mutations in the pathogenesis of the disease.29
Severe mosquito bite allergy
Severe mosquito bite allergy (SMBA) is a rare condition that primarily affects young East Asians with a median age of onset at 6.7 years. In most cases, SMBA is a symptom of CAEBV disease of the EBV+ NK cell type, with approximately 33% of all CAEBV patients developing this allergic response. SMBA has also been reported in rare cases of other EBV EBV-related conditions such as Hodgkin lymphoma, hydroa vacciniforme, aggressive NK cell lymphoma/leukemia), and extranodal NK/T-cell lymphoma, nasal type. Additionally, it has been observed in EBV-negative LPD, such as chronic lymphocytic leukemia and mantle cell lymphoma.30
In the context of CAEBV, SMBA is characterized by skin redness, swelling, ulceration, necrosis, and/or scarring at the site of the bite. Concurrently, patients may experience fever and malaise, enlarged lymph nodes, liver, and/or spleen, liver dysfunction, hematuria, and proteinuria.30 Severe cases may involve hemophagocytosis, NK/T-cell lymphoma, or aggressive NK cell leukemia.31 Pathologically, the skin lesions exhibit invading NK cells in the epidermis and subcutaneous tissue, with a minor fraction of these cells being EBV+, indicating that the virus is in its latency II phase. A high density of EBV+ NK cells in these lesions may suggest that the disease has progressed to NK/T cell lymphoma or NK cell leukemia.1 The cause of SMBA is not fully understood, but it is suspected that mosquito salivary gland allergenic proteins cause EBV reactivation in latently infected NK cells. This reactivation, facilitated by EBV genes, such as LMP1, can lead to immortalization, proliferation, and, in rare cases, malignancy of EBV-reactivated NK cells.30 The optimum treatment for SMBA remains uncertain. Mild and uncomplicated cases can be managed conservatively. In contrast, cases showing evidence of substantial CAEBV disease, such as hemophagocytosis, NK/T cell lymphoma, or aggressive NK cell lymphoma, may require chemotherapeutic regimens.30 Cases of EBV+ SMBA with concurrent aggressive CAEBV have been treated with success using a three-step CAEBV treatment regimen (immunotherapy, cytoreduction, reconstruction).32 Rare cases of SMBA have been observed in individuals initially lacking an obvious underlying condition but later developing CAEBV, highlighting the need for thorough evaluation and follow-up in such cases.30,33
Hydroa vacciniforme-like lymphoproliferative disease
Hydroa vacciniforme presents as an uncommon photodermatitis reaction characterized by pruritic skin papules and vesicles that progress to crusting and scarring upon exposure to sunshine—predominantly appearing on sun-exposed facial and dorsal hand areas. This EBV+ condition primarily affects children, demonstrating a variable course with remissions and exacerbations before resolving in early adulthood. However, adults can also be susceptible to this illness. In some instances, the condition can progress to create severe, extensive, and disfiguring skin lesions that are unrelated to sun exposure. Additional manifestations include facial edema and systemic symptoms, such as fever, weight loss, lymphadenopathy hepatomegaly, and/or splenomegaly. Notably, EBV+ LPD, including T cell lymphoma, T cell leukemia, B cell lymphoma, or B cell leukemia may arise.34 The milder and more aggressive forms of hydroa vacciniforme were initially categorized as classic hydroa vacciniforme and hydroa vacciniforme-like lymphoma, respectively. The extensive overlap between the two disease types prompted the World Health Organization in 2016 to reclassify them as Hydroa vacciniforme-like LPD, falling under the umbrella of CAEBV. Histological examination of skin lesions reveals infiltrating lymphocytes, predominantly T cells with a minority of NK- or B-cells.34 EBV is commonly detected in T cells and, to a lesser extent, in NK cells of skin lesions.31 Marker assays show that EBV is in latency phase II in these cells.10
Non-aggressive cases of hydroa vacciniforme-like LPD typically respond to well to conventional dermatological approaches suitable for non-malignant diseases. Immunotherapeutic drugs, such as prednisone, interferon, chloroquine, and thalidomide, can provide temporary remissions and improvements in malignant cases of the disease. Standard chemotherapy and radiotherapy protocols, commonly effective for lymphoma and leukemia have shown only transient benefits in this context often accompanied by severe toxicities.34 The three-step CAEBV treatment regimen has been employed with reasonable efficacy in cases of EBV+ hydroa vacciniforme-like LPD where there is clear evidence of concomitant CAEBV.35
Systemic Epstein–Barr virus-positive T cell lymphoma of childhood
Systemic EBV-positive T cell lymphoma of childhood (TCLC) is a rare and severe T cell lymphoma predominantly affecting children, adolescents, and young adults, with a higher prevalence in Asian and Latin American populations. The disease typically arises as a complication or progression of either EBV-positive infectious mononucleosis (EBV+ IM) or CAEBV disease.1 The manifestations of TCLC occur as a deterioration of signs and symptoms, either three weeks after the onset of an EBV+ IM-like disease or at any point during CAEBV. Clinically, TCLC presents with progressive hepatosplenomegaly, worsening liver dysfunction, new skin rashes, pancytopenia, hemophagocytosis in the bone marrow and spleen, coagulopathy, sepsis, and potential organ failures. Notably, patients with TCLC show low or undetectable levels of circulating IgM antibody but measurable amounts of IgG antibody targeting EBV capsular antigens, distinguishing it from the immunological profile seen in IM. Rapid proliferation of small or, less commonly, large lymphoid cells in affected organs characterizes the disease. These cells are EBV-positive cytotoxic T cells that express CD8, CD3, CD2, TIA1, and granzyme, but not CD56. In the context of CAEBV disease, these cells are usually CD4+ T cells or a mix of CD4+ and CD8+ T cells. The condition is usually lethal within a few weeks after diagnosis. However, a subset of patients responded to the HLH-2004 chemotherapy program involving agents such as etoposide, dexamethasone, cyclosporine A, or, in certain cases, corticosteroids and intrathecal methotrexate, with or without subsequent hematopoietic stem cell transplantation.31
EBV+ NK/T Cell Lymphoproliferative Diseases
While EBV primarily targets B cells, it can infect various immune cells, including CD4+ T cells (T helper cells), CD8+ cells (cytotoxic T cells), and natural killer (NK) cells, The mechanism by which EBV spreads to these other cell types remains unclear, but it is postulated that direct contact with virus-infected B cells facilitates such infections.1
Extranodal NK/T cell lymphoma, nasal type, peripheral T cell lymphoma, not otherwise specified (PTL, NOS), and angioimmunoblastic T-cell lymphoma (AITL) are among the NK-cell or T-cell malignancies that fall under the category of peripheral T cell lymphomas (PTCL).
Extranodal NK/T cell lymphoma, nasal type
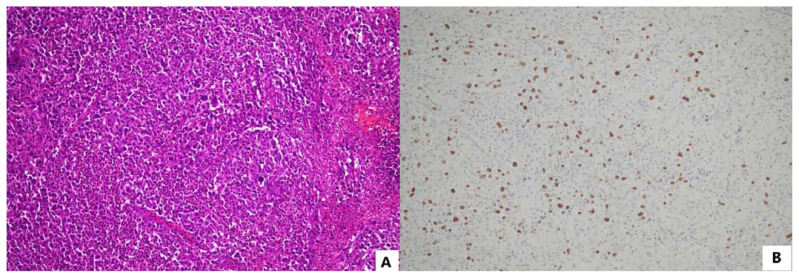
Extranodal NK/T cell lymphoma, nasal type (ENKTL), is a type of NK or, less commonly, T cell lymphoma that typically affects individuals of Asian descent and indigenous populations in Mexico, Central America, and South America. This malignancy typically manifests as tumors in the nasal cavities, paranasal sinuses, palate, tonsils, nasopharynx, hypopharynx, and/or larynx, with approximately 20% of cases exhibiting tumors in the skin, soft tissues, gastrointestinal (GI) tract, testes, and/or central nervous system (CNS). Affected individuals are typically middle-aged and present with noticeable tumors, hemoptysis, ulcerating skin nodules, upper airway obstruction, and/or obstructions/bleeding in the lower GI tract, particularly in the colon. Lymph node involvement is atypical and usually arises from tumor dissemination from their initial locations.1 Approximately 70% of ENLTL cases are diagnosed with stage I or II diseases, indicating tumors that are limited to a specific site or region of the body, with the remaining cases having widespread stage III or IV disease.36 ENKTL is characterized by destructive, ulcerating, and necrotic lesions at all stages. Histologically, the tumors comprise small, medium-sized, or large malignant lymphoid cells, which are frequently accompanied by a mix of benign inflammatory cells. Malignant cells express NK and/or T cell markers (e.g., CD2, CD56, CD38), granzyme B, perforin, TIA1, and, in the case of T cells, T-cell receptor gamma and delta chains.31 TP53 and/or PD-L1 are often overexpressed.13 In nearly all instances, the lymphoma cells are EBER+ and have a latency II pattern of EBV infection (Figure 2).1
Figure 2.
Extranodal NK/T cell lymphoma, nasal-type showing a diffuse infiltrate of lymphoid cell of various sizes with angiotropism, destruction of vessels, and necrosis (A, H/E, 100x). The neoplastic cells are diffusely and strongly positive for EBER (B, EBER cISH, 100x).
They harbor numerous somatic gene mutations, including commonly mutated genes such as beta-catenin and ECSIT, involved in cell development and survival.31 Other genes, such as JAK3, STAT3, and STAT5B, associated with pro-malignant pathways, are altered in significantly fewer cases. Furthermore, epigenetic regulators (BCOR, KMT2D, ARID1A, EP300), tumor suppressor genes (TP53, MGA) and RNA helicase DDX3X are implicated.37–40 A comprehensive investigation has identified seven genetic clusters associated with various clinical outcomes.41 However, the relationship between EBV infection, these gene modifications, and the development of ENKTL remains unknown.31
Epstein–Barr virus–related peripheral T cell lymphoma, not otherwise specified
Peripheral T cell lymphoma, not otherwise specified (PTCL, NOS), is an unfavourable, heterogeneous category of T cell neoplasia defined by characteristics that do not align with the diagnostic criteria for other forms of PTCL.10 PTCL, NOS accounts for 30–40% of all PTCL cases. This lymphoma most typically affects men with a median age of 60 years—the majority of cases present in advanced stage III or IV disease (70%). T-cell infiltration leads to widespread lymph node swelling and concurrent bone marrow, liver, spleen, and/or skin involvement.42 Patients frequently exhibit B symptoms, including fever, night sweats, and weight loss.43 Histologically, the involved tissues reveal mature T cells expressing CD4.44 Despite efforts to establish diagnostic criteria for PTCL and NOS based on histology and immunophenotyping, these criteria have not been widely implemented in clinical practice.45 Various fusion rearrangements of VAV1 or TBX21 genes, as well as fusion rearrangements of the ITK gene with the SYK, FER, or ERBB4 genes, have been associated with PTLC and NOS. Two distinct profiles of gene expression have been identified: malignant cells may overexpress genes such as GATA3, MYC, mTOR, and β-catenin genes, or TBX21, interferon-, and NF-B genes. Individuals with GATA3 gene expression in their malignant cells have a lower overall five-year survival rate compared to those with TBX2 gene expression.42 In approximately 30% of PTCL, NOS cases are infected with EBV, with the virus being in its latency II phase. Significant EBER expression in malignant T cells is observed in only a few of these cases. EBER expression is typically restricted to the small and large benign B cells within the background of the disease’s lesions. Consequently the role of EBV in the initiation and progression of PTCL, NOS remains unclear.1
Angioimmunoblastic T-cell lymphoma
Angioimmunoblastic T cell lymphoma (AITL) is a systemic lymphoma characterized by the presence of mature follicular helper T cells (TFH cells).1 Two other nodal T-cell lymphomas, follicular T-cell lymphoma (FTCL) and nodal peripheral T-cell lymphoma with the TFH cell phenotype (PTCL-TFH) have been described, both having different morphologic features than AITL but sharing a TFH cell immunophenotype as well as genetic and molecular characteristics. Additionally, cutaneous T-cell proliferations with the TFH cell phenotype have been reported.
AITL typically presents shortly after antibiotic use, Infection, or allergic reactions. Common manifestations include generalized lymph node swelling, enlarged liver and spleen, skin lesions such as rash or, less usually, nodules, plaques, purpura, and urticaria, bone marrow involvement, and B symptoms such as fever, weight loss, and night sweats. Additional manifestations may encompass arthralgias, arthritis, pleural effusions, ascites, lung lesions, as well as neurological and gastrointestinal symptoms. Common laboratory findings include immune-mediated hemolytic anemia, elevated blood levels of eosinophils, gamma globulins, and LDH, a high erythrocyte sedimentation rate (ESR) and positive blood tests for autoantibodies like rheumatoid factor, anti-nuclear antibody, and anti-smooth muscle antibody, suggesting an underlying immune system problem. Histologically, involved tissues exhibit small lymphoid cells surrounding venules against a background of TFH cells, activated lymphocytes, follicular dendritic cells, epithelioid cells, plasma cells, and eosinophils. Among these, only the TFH cells are malignant, constituting 5–30% of all cells in the lesions. These malignant TFH cells express specific markers, such as CD3, CD4, CD10, PD-1, and also the B lymphocyte chemoattractant, CXCL13.46 The recent identification of TET2 and DNMT3A mutations in TFH lymphomas motivated research into the link between two seemingly unrelated types of hematologic malignancies. Interestingly, investigations have revealed that TFH lymphomas can originate from divergent clonal evolution from TET2 and DNMT3A-mutant progenitor cells after acquiring RHO and/or IDH2 mutations. Notably, almost all cases show a dispersion of EBV+ B cells, indicating that the virus is possibly in a confined latency II phase. However, EBV is absent in the aggressive TFH cells themselves. The EBV+ B cells carry non-malignant alterations, exhibit excessive proliferation, and can potentially transform into EBV+ B cell lymphomas.1 Despite the implication of EBV in the development and transition of these B cells, the precise role of the virus in AITL and its association with the aggressive TFH cells remain unknown.
Follicular T cell lymphoma
The World Health Organization (2016) reclassified follicular T cell lymphoma (FTCL), previously considered a variant of peripheral T cell lymphomas, within the category of angioimmunoblastic T cell lymphoma (AITL) and other nodal TFH cell lymphomas. This rare disorder shares similarities with AITL as a lymph node-based malignancy or TFH cells. However, it differs from AITL by allowing a diagnosis at an early, limited, and comparatively less aggressive stage, with tissue lesions lacking AITL-specific features, such as vascular proliferation.1 FTCL is predominantly observed in the elderly; however, it has been documented in individuals as young as 27 years old. Common clinical presentations involve advanced stage III or IV disease, characterized by lymphadenopathy, hepato- and splenomegaly, and malignant cell infiltrations in the bone marrow or, less commonly, tonsils, salivary glands, and/or hard palate. B symptoms occur in a third of cases. A positive Coombs test with or without autoimmune hemolytic anemia, high blood LDH, and high gamma globulins are examples of laboratory abnormalities.47 Histopathologically, a follicular lymphoma-like pattern is observed, in which malignant TFH cells form nodules, and that is interpreted as a progressive transformation of a germinal centre-like pattern, in which malignant TFH cells form irregularly-shaped nodules surrounded by IgD-positive mantle cells. These lesions may also contain large immunoblasts and, on rare occasions, Reed-Sternberg cell-like B cells. In 50–60% of FTCL, one or more of these B cell types, but not the malignant TFH cells, are infected with EBV.1 FTCL diagnosis relies on clinical and laboratory findings, histopathology, and the presence of TFH cells in lymph nodes, skin, or other lesions confirmed by expression of relevant markers like PD-1, ICOS, CXCL13, CXCR5, and TOX.
Epstein–Barr virus-associated aggressive NK cell leukemia
EBV-associated aggressive NK cell leukemia (EBV+ ANKL) is a rare NK cell cancer that primarily affects Asians and young to middle-aged individuals. It can also arise directly from other NK cell proliferative diseases, such as chronic active EBV infection (CAEBV), which is more common among the younger population.1 A study in China observed that almost all patients had B symptoms (weight loss, fever, night sweats) and an enlarged liver and/or spleen but no lymph node involvement. Laboratory studies revealed pancytopenia in almost all cases, with small increases in the levels of circulating large granular lymphocytes suspected to be malignant NK cells in 50% of cases. Increased numbers of NK cells were found in the bone marrow in all cases, accompanied by highly elevated blood levels of lactic acid dehydrogenase and beta2 microglobulin. Liver damage was evident with increased blood levels of enzymes, total bilirubin, and indirect total bilirubin. All cases showed the presence of EBV+ cells in bone marrow and tissue infiltrates, with circulating EBV+ lymphocytes in a few cases.48 Other studies reported EBV+ NK cells in 85–100% of patients.1 Histological examination of affected tissues revealed infiltrates of large granular EBV+ NK cells mixed with benign inflammatory cells often localized around small vessels, accompanied by tissue necrosis. With EBV in latency II, the EBV+ NK cells express the CD56 antigen and are malignant.49 The LMP1 viral protein is expressed at relatively high levels in NK cells, potentially activating the NF-kB cell signaling system and stimulating the proliferation of EBV-infected cells.1 These findings are identified in 84% of individuals who have “classic ANKL.” “Sub-acute ANKL”, affecting 16% of individuals, manifests symptoms resembling infectious mononucleosis for 3–15 months before progressing to the aggressive course indicative of classic ANKL.50
Intravascular NK/T-cell lymphomas
Intravascular NK-cell lymphoma and intravascular T-cell lymphoma represent two exceedingly rare forms of intravascular lymphomas caused by EBV infection of NK- and cytotoxic T-cells, respectively. Afflicting primarily young individuals, these conditions manifest with skin lesions and signs of CNS involvement. In a minority of cases, there is additional involvement in bone marrow, liver, kidneys, ovaries, and/or cervix.51 Affected individuals display signs and symptoms of disseminated disease, such as fever, weight loss, night sweats, arthralgias, jaundice, cytopenias, and multiple organ involvement.52 In general, both intravascular lymphomas exhibit aggressive and rapid progression, with patients typically responding poorly to treatment and having short life spans.53–56
Immunodeficiency - Associated Lymphoproliferative Disorders
Posttransplant LPD (PTLD) and distinct LPD emerging in patients receiving immunosuppressive therapy, including those associated with several chemotherapeutic treatments, are examples of iatrogenic immunodeficiency-associated LPD. They are included in this review because a significant subset of them is EBV+; PTLDs were identified more than 50 years ago and are recognized separately from lymphomas and other iatrogenic immunodeficiency - related lymphoproliferative disorders in the 2001, 2008, and 2016/2017 WHO classifications, as well as in the 2022 ICC.57–59 The 2022 ICC recommends a similar classification for the latter entities. These LPDs, like PTLDs, can be EBV+ or EBV-negative. It remains uncertain whether current clinical guidelines for treating PTLD are applicable to patients with other iatrogenic LPDs,58–59 and some general recommendations may be inappropriate in a non-transplant setting. This holds true for biologic studies of PTLD, including investigations into molecular or tumor microenvironmental characteristics.60–62
In the context of identifying lymphoid proliferations in patients following solid organ or stem cell transplantation, the 2022 ICC recommends as a first step to determine whether the condition represents a PTLD or whether another explanation exists, such as a specific infection, a non-specific process, or, in the cases involving the transplanted organ, whether it reflects rejection. In some instances, both rejection and a PTLD may coexist. While a majority of PTLD are EBV+, 20–40% are not, and there is an increasing number of late EBV-negative PTLD.58 EBV+ patients typically exhibit latency pattern III or, less commonly, pattern II. Notably, in immunocompetent hosts, the presence of a limited number of EBV+ cells do not necessarily imply the PTLD development, as scattered EBV+ cells can be found in various lymphoid proliferations. After Following PTLD confirmation, the next priority is subclassification. Excisional biopsy is recommended for diagnosis, and re-biopsy of recurrent lesions is advisable when feasible to rule out evolution or alternative causes beyond PTLD.58–59
Non-destructive PTLDs
Non-destructive PTLDs have a preserved underlying architecture characterized by a small lymphocytic proliferation along with hyperplastic and polytypic plasma cells. This includes lymphoplasmacytic proliferation with prominent immunoblasts reminiscent of IM in an immune-competent host, or the presence of a marked follicular hyperplasia. As these histological patterns are non-specific reactive proliferations, their detection is often reliant on substantial EBV positivity, best visualized using EBER in situ hybridization, or, less commonly, due to their conspicuous mass-forming nature. Some individuals manifesting non-destructive PTLD may develop overt PTLD at other sites either synchronously or metachronously. Notably, non-destructive PTLD can harbor clonal populations with cytogenetic and mutational abnormalities, although the majority do not display such characteristics.60–61,63
Polymorphic PTLD
Polymorphic PTLD represents the most prevalent yet challenging subtype of PTLD, characterized by the architectural effacement of underlying tissues. it is distinguished by the presence of variably sized and shaped lymphoid cells, plasma cells, and immunoblasts some of which may bear resemblance to Reed-Sternberg cells. In specific locations, angioinvasion and necrosis may be observed. Notably, polymorphic PTLDs are not expected to meet the criteria for lymphoma in an immunocompetent host. These entities typically harbor clonal B cell populations, with the clone size often being modest, and may exhibit mutations, albeit not as frequently as observed in monomorphic PTLDs.64 The literature remains divided on whether patients with polymorphic PTLD experience a more favorable prognosis compared to those with monomorphic PTLD.65
Monomorphic PTLDs
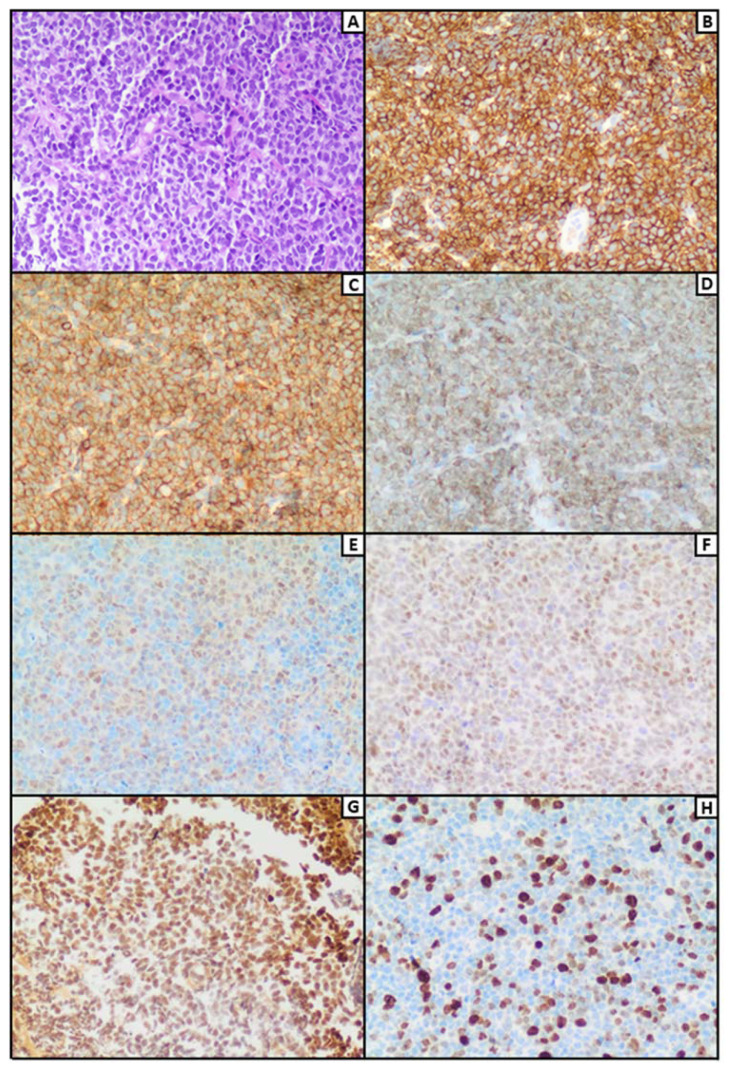
Monomorphic PTLDs bear resemblance to various lymphomas observed in immune-competent hosts; however, not all cases are composed solely of sheets of large cells, challenging the conventional understanding of the term “monomorphic”. Presently, the term encompasses cases, that may involve proliferations of monomorphic large cells or immunoblasts, with some displaying a polymorphic appearance. B-cell monomorphic PTLD, for instance, may exhibit a high concentration of reactive T cells or plasmacytic differentiation, while other cases may consist primarily of plasma cells. A substantial number of monomorphic T cell PTLD do not manifest as sheets of large transformed cells. Furthermore, EBV+ MALT lymphomas were included as a form of PTLD in the 2016–2017 WHO review (Figure 3).66
Figure 3.
H&E staining (A, 200x) of multiple fragments from a submandibular lymph-node extensively replaced by a blastoid lymphoid population consisting of medium to large sized elements with thick irregular nuclei and prominent nucleoli, showing immunoreactivity for CD20 (B, 200x), CD5 (C, 200x), BCL-2 (D, 200x), cyclin D1 (E, 200x), SOX-11 (F, 200X) and concrete expression of EBER (G, 200x). Mitotic activity of this post-transplant blastoid mantle cell lymphoma is dispersed with a proliferative index (ki67) of 35–40% (H, 200x).
The most prevalent monomorphic PTLDs resemble DLBCL, often falling it in the non-germinal center B cell subtype; particularly in EBV+ patients. Studies highlight both striking differences and similarities in mutational and gene expression patterns between monomorphic PTLD of DLBCL-type and related lymphomas in immune-competent hosts, in particularly in EBV-negative patients.64,67 Noteworthy molecular and chromosomal changes seen in T/NKPTLD, as opposed to B cell PTLDs, bear resemblance to those described for peripheral T cell lymphomas in immunocompetent hosts.68 Burkitt lymphoma-type monomorphic B cell PTLD is a significant diagnosis, as individuals with this condition may require immediate, intensive therapy.69–70 It is imperative to distinguish monomorphic PTLD resembling plasma cell neoplasms from more aggressive cases resembling multiple myeloma. Those resembling plasmacytoma-like presentations may recover without the necessity for intensive therapy.
Classic Hodgkin lymphoma PTLD
Some PTLD are nearly always EBV+ and resemble classical Hodgkin lymphoma (CHL), however they are uncommon in the posttransplant scenario. Although it is advisable to proceed cautiously because many other PTLD cases have cells that resemble Reed-Sternberg cells, this is a specific diagnosis that must be made because it is a different type of PTLD that is usually treated right away with conventional CHL-therapy. Because the survival rates of monomorphic and polymorphic PTLDs varies greatly, immunohistology tests are essential for ruling out these conditions.68 This is not the case for other iatrogenic LPDs in patients with rheumatic disorders, where Hodgkin-type LPD had a similar overall survival rate to DLBCL-type LPD patients, but the PFS was worse in the Hodgkin cases.71
B-Cell Lymphoproliferative Diseases
Epstein–Barr virus-positive mucocutaneous ulcer
EBV+ mucocutaneous ulcer is a rare lymphoproliferative condition characterized by isolated, well-defined ulcers in mucous membranes and skin caused by invading B lymphocytes.1 Affected individuals typically exhibit a compromised immune systems a due to factors such as advanced age, immunosuppressive disorders (e.g., HIV/AIDS), immunosuppressive medication, or allogeneic hematopoietic stem cell transplantation. Immunomodulatory drugs implicated in ulcer formation include methotrexate, cyclosporin A, azathioprine, mycophenolate, TNF inhibitors, tacrolimus, and topical steroids. The diminished immune surveillance associated with certain predisposing diseases or therapies is still sufficient to maintain systemic EBV latency except where EBV+ B cells are prominent, such as in affected mucous membranes and skin. Consequently, EBV+ cells in these locations undergo unchecked multiplication, leading to tissue destruction and the formation of ulcerative lesions.72
These ulcers predominantly affect the elderly, typically presenting as isolated lesions in the oral mucosa, and less frequently in the skin or gastrointestinal tract mucosa. Individuals with EBV+ mucocutaneous ulcer are usually asymptomatic and lack signs of lymphadenopathy or involvement in other tissues, B symptoms, aside from localized pain at the ulcer site and potential severe tissue degradation). However, gastrointestinal ulcers can cause a variety of abdominal symptoms, including perforations representing an acute emergency. Unlike most other types of EBV+LPD, EBV-related mucocutaneous ulcers are not associated with detectable EBV levels in the blood.72 Microscopically, these ulcers are composed of lymphocytes, including EBV+ B cells and occasionally various other EBV+ lymphoid cell types. In addition, histiocytes, plasma cells, eosinophils, and scattered giant immunoblasts resembling, but distinct from the Reed-Sternberg cells found in Hodgkin lymphoma are observed.31 These Reed-Sternberg-like cells are identified as EBV+ B cells expressing the cell surface membrane protein, CD30, the B cell surface membrane protein, CD20,72 and EBV replication cycle latency II or III proteins.1
Epstein–Barr virus-positive Burkitt lymphoma
Burkitt lymphoma is classified into three distinct types. Endemic Burkitt lymphoma (eBL) is frequent in regions such as Africa, the Middle East, Brazil, Papua New Guinea, and other malaria-endemic areas. Typically, presenting in children aged 4 to 7 years, eBL is consistently associated with EBV infection.73 Sporadic Burkitt lymphoma (sBL) is extremely uncommon, affecting primarily children and, less frequently, adults over the age of 60.31 with its main occurrence in Northern and Eastern Europe, East Asia, and North America.74 In the United States, an estimated 1,200 cases are reported annually.30 Only about 10–15% of sBL cases are linked to EBV infection.75 The immunodeficiency-related form of Burkitt lymphoma (iBL) afflicts 30–40% of individuals with HIV-induced AIDS31 and, in rare cases, individuals who have undergone a bone marrow or organ transplant. In the latter cases, individuals have consistently received immunomodulatory drugs and are thus immunocompromised.73 Approximately 30% of iBL cases are EBV-associated.76
A jaw mass, periorbital swelling resulting from an orbital tumor, or an abdominal mass attributed to a tumor in the retroperitoneum, kidney, or ovary are all frequently observed in eBL. Additionally, eBL may present with the less common manifestation of a sudden onset of paraplegia or urine incontinence indicating tumor penetration into neural tissue. sBL is characterized by abdominal discomfort, nausea, vomiting, and/or gastrointestinal bleeding, all stemming from the growth of an abdominal tumor, a head or neck tumor involving lymph nodes, tonsils, nose, sinuses, and/or oropharynx or substantial bone marrow infiltration by malignant tumor cells.73 Fever, along with other constitutional symptoms, and the presence of malignant disease in the gastrointestinal tract, bone marrow, liver, lung, and central nervous system are common indicators of iBL.77 Histologic examination of BL-involved tissues reveals infiltrations by a uniform population of rapidly proliferating lymphocytes with a high mitotic index approaching 100%, creating intermittent clear spaces resembling a “starry sky” pattern due to macrophages containing ingested dead cells. The predominant lymphocytes are B cells expressing CD20 and CD10 antigens, with a background of few T cells.73 These B cells predominantly derived from germinal center B cells, exhibit EBV in latency I, and express substantial quantities of EBNA1 and EBER viral proteins. Under certain circumstances, EBNA and LMP2A products are also expressed.1 The protein EBNA1 and EBER may contribute to the development and/or progression of BL by blocking apoptosis in the infected cells, while the product of LMP2A may stimulate the PI3K cell signaling pathway, boosting cell proliferation.
Malignant B cells in all three variants of BL frequently exhibit chromosomal translocations affecting the MYC gene. MYC situated on the long arm of human chromosome 8 (8q24), is a proto-oncogene that can induce cancer when properly mutated or overexpressed. (). The translocations involve MYC relocated to the IGH (immunoglobulin heavy chain) gene locus at 14q32, the IGK (immunoglobulin kappa light chain) gene locus at 2p12, or the IGL (immunoglobulin lambda light chain) gene locus at 22q11. These translocations place MYC under the transcriptional control of these antibody-forming loci, leading to the overexpression of the MYC product, enabling the cells to undergo uncontrolled multiplication. Concurrently, other genes within the BL cells may be undergo alterations; for instance, approximately, 30% of BL cases exhibit changes in the TP53 gene, which may improve cell survival.31 Due to these alternative pathways to malignancy, some of which may be EBV-independent, and considering that not all BL cases involve EBV, many cases of EBV+ BL are probably not solely caused or promoted by EBV. While the ubiquitous virus is likely the cause of almost all cases of eBL, it may act as an innocent passenger virus in numerous instances of sBL and iBL.1 EBV infection is usually associated with increased mutation load, with type 1 EBV having a larger mutational burden than type 2. Although sporadic and immunodeficiency-associated BLs revealed comparable genetic profiles, endemic BLs had more mutations in BCL7A and BCL6, but less in DNMT1, SNTB2, and CTCF. Silencing mutations in ID3 were seen in all three BL subtypes. In vitro mass spectrometry-based proteomics revealed that the ID3 protein predominantly binds to TCF3 and TCF4. In vivo deletion of ID3 enhanced the effects of MYC, resulting in fast carcinogenesis and tumor characteristics similar to those seen in human illness.
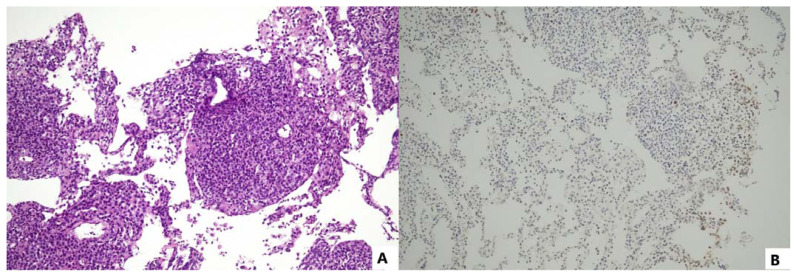
Epstein–Barr virus-positive lymphomatoid granulomatosis
EBV+ lymphomatoid granulomatosis (EBV+ LG) is an uncommon condition characterized by the coexistence of malignant B cells and reactive, non-malignant T cells, almost invariably associated EBV+.1 This LPD primarily affects middle-aged males with a male-to-female ratio of 2:1. Clinically, EBV+ LG typically presents as a lung manifestation with coughing, hemoptysis, shortness of breath, and chest X-rays revealing multiple nodular lesions at the base of both lungs observe in approximately 90% of cases. Additionally, manifestations may extend to nodular or infiltrative lesions in the skin, central nervous system, kidney, liver,1 and/or peripheral nervous system. Notably, lymph nodes are uninvolved at initial presentation1 and in certain instances, lung involvement may be absent.78 EBV+ LG lesions are characterized by the presence of sporadic large, atypical B cells79 amidst a background of abundant reactive CD4+ Helper T cells, plasma cells, macrophages, and a variable number of giant atypical lymphoid cells resembling immunoblasts, plasmablasts, or Reed-Sternberg cells. The lesions frequently center on and exhibit damage of small blood vessels but they lack well-formed granulomas.78 Within the lesions, only lymphoid B cells are positive for EBV, expressing the viral proteins LMP1 and EBNA2 indicative of latency III phase (Figure 4).1
Figure 4.
Lymphomatoid granulomatosis shows a polymorphous infiltrate of T-lymphocytes, macrophages, and plasma cells that displays an angiocentric and angiodestructive pattern, admixed with EBER+ Reed-Sternberg-like B-lymphoid cells (A: H/E, 100x; B: EBER cISH, 100x).
Individuals afflicted by EBV+ LG may have compromised immune function due to subtle reductions in immune activity1 or, as indicated by individual case reports, underlying immunodeficiency diseases.78 Case studies also suggest a potential association with inflammatory or autoimmune disorders.80 In skin manifestations of the disease, non-malignant or malignant lymphoid proliferations may progress to or be complicated by EBV+ LG.81 The pathogenesis of EBV+ LG involves in part the virus infecting B cells, which generate chemokines that attract and activate T lymphocytes to cause tissue damage, particularly affecting blood vessels. Impaired host immune activity, coupled with the infected cells’ failure to produce viral proteins recognized by cytotoxic T cells, facilitates the evasion of detection and proliferation of EBV+ B cells.78
LG is stratified into three grades based on the histological characteristics of biopsied tissues: grade I (<5 EBV+ cells per high power microscopic field (hpf), no atypical cells/hpf, and minimal necrosis); grade II (5–20 EBV+ cells/hpf, occasional atypical cells/hpf, and moderate necrosis); and grade III (>20 EBV+ cells/hpf, predominance of atypical cells/hpf, and extensive necrosis).
Epstein–Barr virus-positive Hodgkin lymphoma
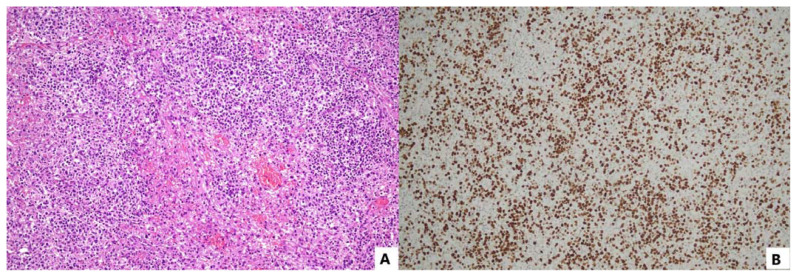
Hodgkin lymphoma (HL) is classified into two histologic subtypes: nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) and classical Hodgkin lymphoma (cHL), with cHL subtypes including nodular sclerosis (NSHD), mixed cellularity (MCHL), lymphocyte rich (LRHL), and lymphocyte depleted (LDHL). EBV is detected in 30% to 50% of cHL cases, but only in 10% cases of LRHL, or NLPHL cases. The infiltrating cells in HL encompass T cells, B cells, macrophages, eosinophils, fibroblasts, surrounding the Reed-Sternberg cells (HRS cells). HRS cells which are large mono- or multinuclear cells derived from lymph node and/or spleen germinal center B cells are the sole malignant cells in HL. In approximately 30–50% of HL cases, these cells may harbor EBV and express viral products indicative of stage II latency (Figure 5).40
Figure 5.
EBV-positive Hodgkin lymphoma comprises a polymorphous infiltrate of T- and B-cells, eosinophils, macrophages, and fibroblasts surrounding HRS cells (A: H/E, 100x). Neoplastic cells are strongly EBV-positive (B: EBER cISH, 100x).
The role of EBV in the pathogenesis of EBV+ HL involves overexpression of the virus’s LMP1 gene in HRS cells. LMP1 mimics activated human TNF receptors sustaining continuous stimulation of NF-kB, PI3K, and JAK-STAT signaling pathways. This continuous activation promotes cell proliferation, survival, and the production of cytokines that may suppress the EBV’s lytic cycle, thus maintaining the viability of HRS cells.40 HRS cells also express the virus’s LMP2A gene product, which mimics the human BCR gene product, aiding in cell survival.1 Additionally, the presence of EBV in HRS cells associates with crippling mutations in the rearranged immunoglobulin (Ig) genes, inhibiting Ig expression and prompting HRS cells to secret cytokines and chemokines. These mediators recruit various cell types into the pathogenic infiltrates of EBV+HL, creating a local milieu that enables HRS cells to evade the immune system and proliferate.82
EBV+ HL is more prevalent in children and young adults, but it can also develop in individuals at older age, possibly due to age-related decline in immune system function, infectious illnesses, or malnutrition.1 The incidence of EBV+ HLs in HIV/AIDS patients is substantially higher, approximately 10-fold, compared to the normal population, although the specific causes remain unknown.40 Symptoms of EBV+ HL are comparable to EBV-negative HL, including fever, night sweats, weight loss in the presence of swollen lymph nodes, and indications of tumor invasion into other organs.
Epstein–Barr virus-positive diffuse large B cell lymphoma, NOS
Diffuse large B-cell lymphoma (DLBCL) stands as the most prevalent form of lymphoma, primarily affecting elderly people, with comparatively lower incidence in younger individuals and infrequent occurrences in children. In addition to swollen lymph nodes, elderly individuals often present with symptoms arising from malignant cell infiltrations into the upper gastrointestinal tract, lungs, upper airways, and/or other organs. In contrast, younger patients typically exhibit enlarged lymph nodes but rarely have B symptoms or involvement of extranodal tissues. The disease tends to be more aggressive in the elderly.31 Traditionally, DLBCL was classified based on the cell types in tissue infiltrates into three patterns: the anaplastic variant (3% of cases) characterized by Reed-Sternberg-like cells83 embedded in a background of histiocytes and lymphocytes; the immunoblastic variant (8–10% of cases) dominated by 90% immunoblasts; and the centroblastic variant (80% of cases) characterized by a prevalence of centroblasts.83 These histological characteristics are usually accompanied by the invasion and destruction of small blood vessels. The current classification is based on the cell of origin of the disease, resulting in germinal center B cell DLBCL (GCB-DLBCL) and activated B cell DLBCL (ABC-DLBCL). Recent breakthroughs in genetic technologies, including next-generation sequencing, have uncovered multiple recurrent genetic anomalies in DLBCL. Two separate groups have presented large-scale genomic studies with clinical data. These investigations developed new molecular classifications based on various genetic anomalies associated with DLBCL prognosis. A multi-omics investigation of over 300 DLBCL cases was conducted, finding that DLBCL may be classified into five groups (C1, C2, C3, C4, and C5) based on the combination of recurrent genetic anomalies. This enabled risk classification for DLBCL patients and promoted focused therapy. For example, C5 cases typically exhibit CD79B and MYD88-L265P mutations, ABC-DLBCL cases have poor outcomes, and sensitivity to BTK inhibitors or lenalidomide has been found. DLBCL is less reliant on its microenvironment, which is consistent with a total breakdown of normal lymphoid structure. However, like with other B-cell lymphomas, recent data suggests that the immune system is critical to the development and outcome of DLBCL. In DLBCL, disturbed cross-talk between lymphoma cells and the microenvironment contributes to lymphoma cells’ ability to evade immune monitoring by the host. Immune escape methods include concealing from the immune system by deleting or lowering recognition molecules (B2M, MHCI, MHC-II), dampening antitumor immune activity (i.e. CREBBP, EZH2), and generating a microenvironment that promotes lymphoma growth. Altering immune recognition, in particular, plays a significant role in DLBCL tumor formation and progression, and its molecular basis is being studied extensively.31 Uncommonly, DLBCL can arise through a Richter transition of chronic lymphocytic leukemia (CLL) to an exceedingly aggressive type of DLBCL. This transition is particularly observed in EBV-associated CLL cases, constituting 10–15% of all CLL cases. However, it should be noted that a significant number of Richter syndromes is not EBV-related, since aberrant TP53 mutations are present with subclonal selection, in particular following chemoimmunotherapy.84 Patients with EBV-positivity account for around 10–15% of DLBCL cases. EBV+ DLBCL, not otherwise defined (EBV+ DLBCL) is more common in East Asia and Mexico. Distinguishing features of EBV+ DLBCL include the expression of EBV genes typical for the virus’s latency III (common in the elderly) or II (common in younger individuals) by nearly all large B cells.75 These centroblastic B-cells express EBER,31 LMP1, EBNA1, EBNA2, and other viral proteins1 along with traditional B cell antigenic markers such CD20, BCL6, and CD19 in more than 50% of patients. The viral proteins are postulated to activate signaling pathways such as NF-kB, STAT/JAK, NOD-like receptor, and TLR in infected cells, potentially enhancing cell proliferation and survival.1
EBV+ DLBCL is notably prevalent in immunocompromised individuals, and its occurrence in the elderly is hypothesized to be linked to immunosenescence. Immunosenescence includes an age-related decline in specific types of CD4+ and CD8+ lymphocytes that function to suppress EBV+ cell development.1 Additionally, EBV+ DLBCL can arise in immunocompromised individuals due to conditions such as HIV/AIDS, where 33% of patients are EBV+ or as a consequence of anti-rejection drug therapy post-solid organ transplantation, where 30% to 70% of these cases are EBV+.83 Similarly, the Richter transition of EBV+ CLL to EBV+ DLBCL is observed in CLL cases treated with immunosuppressive medications, indicating a correlation with immunosuppression-related reactivation of latent EBV infection of these CLL cells.84
Epstein–Barr virus-associated diffuse large B cell lymphoma associated with chronic inflammation
DLBCL associated with chronic inflammation (DLBCL-CI) is an exceptionally rare subtype of EBV-positive DLBCL.1 typically presenting as a tumor in areas characterized by chronic inflammation, often occurring within bodily cavities or confined spaces.85 The majority of reported cases of DLBCL-CI involve pyothorax-associated lymphoma (PAL). PAL develops years after pneumothorax is intentionally induced for therapeutic purposes, such as collapsing a lobe or complete lung around a cavity85 or to manage pleurisy86 resulting from an uncontrollable underlying condition, most commonly pulmonary tuberculosis. This distinctive lymphoma subtype has been predominantly observed in older Japanese males. DLBCL-CI seldom occurs in conjunction with other chronic inflammatory disorders such as osteomyelitis, medical insertion of a foreign body (such as intrauterine contraceptive devices, metallic implants, surgical mesh), as well as skin ulcers and venous ulcers. The clinical manifestations of DLBCL-CI represent the destructive consequences of the cancer in the affected areas. Infiltrative lesions comprise a combination of benign, EBV-negative chronic inflammatory white blood cells and diffuse large EBV+ B cells in latency III. EBV+ large B cells in these lesions frequently exhibit reduced expression of the CD20 antigen and genetic abnormalities such as TP53 mutations, MYC overexpression, and TNFAIP3 deletion, at variance from alterations found in other EBV+ DLBCL NOS. Research indicates that the condition is initiated by the EBV-driven proliferation of large, activated B cells in a confined anatomical area, thereby isolating them from immune surveillance.15 Additionally, the EBV-induced release of anti-inflammatory cytokines, such as Interleukin 6 and Interleukin 10, may further facilitate infected cells in evading the immune system.1
Fibrin-associated diffuse large B cell lymphoma
The World Health Organization classified fibrin-associated diffuse large B cell lymphoma (FA-DLBCL) as a form of DLBCL-CI in 2016. This exceedingly rare disease exclusively affects immunologically competent individuals.1 FA-DLBCL is characterized by large B cells infiltrating into long-standing, avascular fibrin-based masses which develop in or around various structures, such as long-standing hamartomas, pseudocysts, cardiac myxomas, prosthetic heart valves,1 thrombus-laden endovascular grafts, hematomas,31 hydroceles, and hip prosthetic implants.87 The infiltrations consist of sheets, bands, or clusters of proliferating large B cells within avascular tissue covered with or containing abundant fibrin. Notably, there is a scarcity or absence of other types of inflammatory cells that comprise the infiltrations.87 Furthermore, rare B-cell lymphomas related to breast implants were observed and were either classified as DLBCL-CI or FA-DLBCL. These B-cell forms are often EBER+.88 The large B cells are infected with EBV, which is present In latency III, expressing the virus’s EBER, EBNA2, and LMP-1 genes.31 Importantly, these infiltrations rarely extend beyond the initial sites, with no involvement of lymph nodes, spleen, or other tissues. FA-DLBCL represents a benign expansion of EBV+ large B cells. Similar to DLBCL-CI, FA-DLDCL localized immune suppression at the sites of origin may contribute to the development of FA-DLBCL. However, in contrast to DLBCL-CI, the large B cells in FA-DLBCL appear to be unable to proliferate and survive long-term outside of the sequestered areas. FA-DLBCL does not exhibit a highly malignant nature.31 The large EBV+ B cells in FA-DLBCL, unlike those in DLBCL-CI, do not overexpress the MYC gene and have few karyotype chromosomal abnormalities.87
Patients with FA-DLBCL present with signs and symptoms corresponding to the location of the infiltrative lesion. Cardiovascular symptoms, such as stroke, may occur when lesions affect the heart or vessels, such as myxoma or prosthetic valves. Besides these cardiovascular consequences, the disease typically progresses slowly and remains localized to the site of origin. The curative approach often involves the surgical removal of affected tissues and any associated foreign implant.
Epstein–Barr virus-positive plasmablastic lymphoma
Plasmablastic lymphoma (PBL) is a rare lymphoma that predominantly affects immunocompromised individuals, particularly those with HIV/AIDS, marking it as an AIDS-defining clinical condition.31 Additionally, individuals who have undergone an organ transplant, received chemotherapy, or are subject to age-related immunological senescence are susceptible to PBL.89 Chronic autoimmune or inflammatory disorders, such as rheumatoid arthritis, Graves’ disease, giant-cell arteritis, sarcoidosis, or severe psoriasis, have been implicated in the development of PBL.90 This condition can affect people of all ages, with a male-to-female ratio of 4:1. PBL typically manifests as a tumor of the head and neck region, gastrointestinal system, skin, or other tissues. Histologically, PBL is classified into two types: monomorphic PBL, primarily composed of immunoblastic cells, and plasmacytic PBL, which consists primarily of plasma cells at various stages of development. Despite their B cell origin, these cells display plasma cell markers such as CD79a, IR4, BLIMP1, CD38, and CD138, while typically showing a CD20 negativity.31 Approximately 70% of PBL cases are EBV-positive, with the majority of lymphoma cells expressing EBV genes, indicating that the virus is in latency phase 0 or I.1 The disease appears to develop and progress as a result of the actions of both EBV and the human immunodeficiency virus (i.e. HIV). PBL, in particular the EBV-positive form, is associated with the overexpression of the MYC gene, emphasizing the role of the Myc protein in driving the disease. However, the precise contribution of EBV in MYC gene overexpression, as well as its role in the initiation and progression of EBV-positive PBL, remains unclear.
Epstein–Barr virus-associated plasma cell myeloma
Plasma cell myeloma (PCM) is malignancy characterized by the infiltration of malignant plasma cells into the bone marrow or development of soft tissue masses known as plasmacytomas. EBV may be associated with this condition in rare cases, particularly in individuals with immune deficiencies (e.g., HIV/AIDS, history of organ donation) or chronic inflammation (e.g., rheumatoid arthritis). Notably, EBV positivity is more prevalent in plasmacytoma than in PCM with bone marrow infiltration.1 Tissues affected by EBV+ PCM often exhibit foci of EBV+ cells resembling immature or poorly differentiated anaplastic plasma cells with a high proliferation rate.1 The cells produce EBV gene products such as EBER,56 indicating that EBV is in a confined latency II phase.1 Although these cells are generated from B cells, they exhibit plasma cell markers rather than B cell markers. The specific role of EBV in the development and evolution of EBV+ PCM remains uncertain.15 In comparison to individuals with EBV-negative illness, patients with localized plasmacytoma and positive EBER are more likely to progress to the infiltrative (i.e. systemic) type of PCM.91
Conclusion
The new ICC and WHO classifications include numerous new entities and ideas for EBV-positive LPDs, reflecting advances in genetics and molecular virology. With a better understanding of LPD’s clinical and pathologic entities, we can perform EBV-ISH to detect clinically borderline Infection and neoplastic conditions, a history of recurrent inappropriate immune response, particularly in children, young adults or the elderly, and a pathologically polymorphous inflammatory background. The ultimate goal is to acquire accurate diagnosis and management of EBV-associated conditions, with some entities requiring a better understanding of histological and molecular features.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Rezk SA, Zhao X, Weiss LM. Epstein-Barr virus-associated lymphoid proliferations, a 2018 update. Human Pathology. 2018 June;79:18–41. doi: 10.1016/j.humpath.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022 January;375(6578):296–301. doi: 10.1126/science.abj8222. Bibcode:2022Sci...375.296B . [DOI] [PubMed] [Google Scholar]
- 3.Ascherio A, Munger KL. EBV and Autoimmunity”. Epstein Barr Virus Volume 1. Current Topics in Microbiology and Immunology. 2015;390:365–85. doi: 10.1007/978-3-319-22822-8_15. [DOI] [PubMed] [Google Scholar]
- 4.Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, Battaglin F, Soni S, McSkane M, Zhang W, Lenz HJ. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treatment Reviews. 2018 May;66:15–22. doi: 10.1016/j.ctrv.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss RA. Tumour-inducing viruses. British Journal of Hospital Medicine. 2016 October;77(10):565–568. doi: 10.12968/hmed.2016.77.10.565. [DOI] [PubMed] [Google Scholar]
- 6.Mastria G, Mancini V, Viganò A, Di Piero V. Alice in Wonderland Syndrome: A Clinical and Pathophysiological Review. BioMed Research International. 20162016 doi: 10.1155/2016/8243145. 8243145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussinovitch M, Prais D, Volovitz B, Shapiro R, Amir J. Post-infectious acute cerebellar ataxia in children. Clinical Pediatrics. 2003 September;42(7):581–4. doi: 10.1177/000992280304200702. [DOI] [PubMed] [Google Scholar]
- 8.Houldcroft CJ, Kellam P. Host genetics of Epstein-Barr virus infection, latency and disease. Reviews in Medical Virology. 2015 March;25(2):71–84. doi: 10.1002/rmv.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell PJ. Epstein-Barr Virus and Cancer. Annual Review of Pathology. 2018 August;14:29–53. doi: 10.1146/annurev-pathmechdis-012418-013023. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campo E, Jafe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140(11):1229–1253. doi: 10.1182/blood.2022015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Worth AJ, Houldcroft CJ, Booth C. Severe Epstein-Barr virus infection in primary immunodeficiency and the normal host. British Journal of Haematology. 2016 November;175(4):559–576. doi: 10.1111/bjh.14339. [DOI] [PubMed] [Google Scholar]
- 13.de Mel S, Soon GS, Mok Y, Chung TH, Jeyasekharan AD, Chng WJ, Ng SB. The Genomics and Molecular Biology of Natural Killer/T Cell Lymphoma: Opportunities for Translation. International Journal of Molecular Sciences. 2018 1931 June;19(7) doi: 10.3390/ijms19071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalia S, Shao H, Sagatys E, Cualing H, Sokol L. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Control. 2014 October;21(4):290–300. doi: 10.1177/107327481402100405. [DOI] [PubMed] [Google Scholar]
- 15.Kunitomi A, Hasegawa Y, Asano N, Kato S, Tokunaga T, Miyata Y, Iida H, Nagai H. EBV-positive Reactive Hyperplasia Progressed into EBV-positive Diffuse Large B cell Lymphoma of the Elderly over a 6-year Period. Internal Medicine (Tokyo, Japan) 2018 May;57(9):1287–1290. doi: 10.2169/internalmedicine.9112-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammas IN, Greenough A, Theodoridou M, Kramvis A, Christaki I, Koutsaftiki C, Koutsaki M, Portaliou DM, Kostagianni G, Panagopoulou P, Sourvinos G, Spandidos DA. Current views and advances on Paediatric Virology: An update for paediatric trainees. Experimental and Therapeutic Medicine. 2016 January;11(1):6–14. doi: 10.3892/etm.2015.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunmire SK, Verghese PS, Balfour HH. Primary Epstein-Barr virus infection. Journal of Clinical Virology. 2018 May;102:84–92. doi: 10.1016/j.jcv.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Wysocki CA. Comparing hemophagocytic lymphohistiocytosis in pediatric and adult patients. Current Opinion in Allergy and Clinical Immunology. 2017 December;17(6):405–413. doi: 10.1097/ACI.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 19.Bode SF, Ammann S, Al-Herz W, Bataneant M, Dvorak CC, Gehring S, Gennery A, Gilmour KC, Gonzalez-Granado LI, Groß-Wieltsch U, Ifversen M, Lingman-Framme J, Matthes-Martin S, Mesters R, Meyts I, van Montfrans JM, Pachlopnik Schmid J, Pai SY, Soler-Palacin P, Schuermann U, Schuster V, Seidel MG, Speckmann C, Stepensky P, Sykora KW, Tesi B, Vraetz T, Waruiru C, Bryceson YT, Moshous D, Lehmberg K, Jordan MB, Ehl S. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: implications for differential diagnosis and pathogenesis. Haematologica. 2015 July;100(7):978–88. doi: 10.3324/haematol.2014.121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daver N, McClain K, Allen CE, Parikh SA, Otrock Z, Rojas-Hernandez C, Blechacz B, Wang S, Minkov M, Jordan MB, La Rosée P, Kantarjian HM. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer. 2017 September;123(17):3229–3240. doi: 10.1002/cncr.30826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh RA. Epstein-Barr Virus and Hemophagocytic Lymphohistiocytosis. Frontiers in Immunology. 2017;1902;8 doi: 10.3389/fimmu.2017.01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Wang Z. Treatment of hemophagocytic lymphohistiocytosis. Current Opinion in Hematology. 2017 January;24(1):54–58. doi: 10.1097/MOH.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 23.Cohen JI, Jafe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;117(22):5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119(3):673–686. doi: 10.1182/blood-2011-10-381921. [DOI] [PubMed] [Google Scholar]
- 25.Okano M, Kawa K, Kimura H, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80(1):64–69. doi: 10.1002/ajh.20398. [DOI] [PubMed] [Google Scholar]
- 26.Plaza JA, Sangueza M. Hydroa vacciniforme-like lymphoma with primarily periorbital swelling: 7 cases of an atypical clinical manifestation of this rare cutaneous T-cell lymphoma. Am J Dermatopathol. 2015;37(1):20–25. doi: 10.1097/DAD.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 27.Bollard CM, Cohen JI. How I treat T-cell chronic active Epstein-Barr virus disease. Blood. 2018;131(26):2899–2905. doi: 10.1182/blood-2018-03-785931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen JI, Manoli I, Dowdell K, et al. Hydroa vacciniformelike lymphoproliferative disorder: an EBV disease with a low risk of systemic illness in whites. Blood. 2019;133(26):2753–2764. doi: 10.1182/blood.2018893750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuno Y, Murata T, Sato Y, et al. Defective Epstein-Barr virus in chronic active Infection and haematological malignancy. Nat Microbiol. 2019;4(3):404–413. doi: 10.1038/s41564-018-0334-0. [DOI] [PubMed] [Google Scholar]
- 30.Chiu TM, Lin YM, Wang SC, Tsai YG. Hypersensitivity to mosquito bites as the primary clinical manifestation of an Epstein-Barr virus infection. Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 2016 August;49(4):613–6. doi: 10.1016/j.jmii.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Ennishi D. The biology of the tumor microenvironment in DLBCL: Targeting the “don’t eat me” signal. J Clin Exp Hematop. 2021 Dec 22;61(4):210–215. doi: 10.3960/jslrt.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. International Journal of Hematology. 2017 April;105(4):406–418. doi: 10.1007/s12185-017-2192-6. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Ko YH. Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. The Journal of Dermatology. 2014 January;41(1):29–39. doi: 10.1111/1346-8138.12322. [DOI] [PubMed] [Google Scholar]
- 34.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintanilla-Martinez L, Ko YH, Kimura H, Jafe ES. EBVpositive T-cell and NK-cell lymphoproliferative diseases of childhood. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and lymphoid tissues. IARC; 2017. pp. 355–363. [Google Scholar]
- 36.Yamaguchi M, Miyazaki K. Current treatment approaches for NK/T-cell lymphoma. Journal of Clinical and Experimental Hematopathology. 2017 December;57(3):98–108. doi: 10.3960/jslrt.17018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montes-Mojarro IA, Chen BJ, Ramirez-Ibarguen AF, et al. Mutational profle and EBV strains of extranodal NK/T-cell lymphoma, nasal type in Latin America. Mod Pathol. 2020;33(5):781–791. doi: 10.1038/s41379-019-0415-5. [DOI] [PubMed] [Google Scholar]
- 38.Jiang L, Gu ZH, Yan ZX, et al. Exome sequencing identifes somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. 2015;47(9):1061–1066. doi: 10.1038/ng.3358. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Park HY, Kang SY, et al. Genetic alterations of JAK/STAT cascade and histone modifcation in extranodal NK/T-cell lymphoma nasal type. Oncotarget. 2015;6(19):17764–17776. doi: 10.18632/oncotarget.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong J, Cui BW, Wang N, et al. Genomic and transcriptomic characterization of natural killer T cell lymphoma. Cancer Cell. 2020;37(3)(e406):403–419. doi: 10.1016/j.ccell.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Dong G, Liu X, Wang L, et al. Genomic profling identifes distinct genetic subtypes in extranodal natural killer/T-cell lymphoma. Leukemia. 2022;36(8):2064–2075. doi: 10.1038/s41375-022-01623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broccoli A, Zinzani PL. Peripheral T-cell lymphoma, not otherwise specified. Blood. 2017 March;129(9):1103–1112. doi: 10.1182/blood-2016-08-692566. [DOI] [PubMed] [Google Scholar]
- 43.Nemani S, Korula A, Agrawal B, Kavitha ML, Manipadam MT, Sigamani E, George B, Srivastava A, Viswabandya A, Mathews V. Peripheral T cell lymphoma: Clinico-pathological characteristics & outcome from a tertiary care centre in south India. The Indian Journal of Medical Research. 2018 May;147(5):464–470. doi: 10.4103/ijmr.IJMR_1108_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan N, Ozkaya N, Moskowitz A, Dogan A, Horwitz S. Peripheral T-cell lymphoma-are we making progress? Best Practice & Research Clinical Haematology. 2018 September;31(3):306–314. doi: 10.1016/j.beha.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludvigsen M, Bjerregård Pedersen M, Lystlund Lauridsen K, Svenstrup Poulsen T, Hamilton-Dutoit SJ, Besenbacher S, Bendix K, Møller MB, Nørgaard P, d’Amore F, Honoré B. Proteomic profiling identifies outcome-predictive markers in patients with peripheral T-cell lymphoma, not otherwise specified. Blood Advances. 2018 October;2(19):2533–2542. doi: 10.1182/bloodadvances.2018019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosalpuria K, Bociek RG, Vose JM. Angioimmunoblastic T-cell lymphoma management. Seminars in Hematology. 2014 January;51(1):52–8. doi: 10.1053/j.seminhematol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Hu S, Young KH, Konoplev SN, Medeiros LJ. Follicular T-cell lymphoma: a member of an emerging family of follicular helper T-cell derived T-cell lymphomas. Human Pathology. 2012 November;43(11):1789–98. doi: 10.1016/j.humpath.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Meng Q, Yin W, Xu L, Lie L. Adult aggressive natural killer cell leukemia. The American Journal of the Medical Sciences. 2013 July;346(1):56–63. doi: 10.1097/MAJ.0b013e3182764b59. [DOI] [PubMed] [Google Scholar]
- 49.Lima M. Extranodal NK/T cell lymphoma and aggressive NK cell leukaemia: evidence for their origin on CD56+bright CD16-/+dim NK cells. Pathology. 2015 October;47(6):503–14. doi: 10.1097/PAT.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 50.Tang YT, Wang D, Luo H, Xiao M, Zhou HS, Liu D, Ling SP, Wang N, Hu XL, Luo Y, Mao X, Ao QL, Huang J, Zhang W, Sheng LS, Zhu LJ, Shang Z, Gao LL, Zhang PL, Zhou M, Zhou KG, Qiu LG, Liu QF, Zhang HY, Li JY, Jin J, Fu L, Zhao WL, Chen JP, Du X, Huang G, Wang QF, Zhou JF, Huang L. Aggressive NK-cell leukemia: clinical subtypes, molecular features, and treatment outcomes. Blood Cancer Journal. 2017 December;7(12):660. doi: 10.1038/s41408-017-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi Y, Huo Z, Liang Z, Meng Y, Jia C, Shi X, Song L, Luo Y, Ling Q, Liu T. Intravascular NK-cell lymphoma: a case report and review of the literature. Diagnostic Pathology. 2015 July;10:84. doi: 10.1186/s13000-015-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan J, Zhang F, Luo D, Yao S, Chen Y, Xu F, Luo X, He J, Liu Y. Intravascular NK/T-cell lymphoma: a series of four cases. International Journal of Clinical and Experimental Pathology. 2017;10(9):9541–9550. [PMC free article] [PubMed] [Google Scholar]
- 53.Zanelli M, Mengoli MC, Del Sordo R, Cagini A, De Marco L, Simonetti E, Martino G, Zizzo M, Ascani S. Intravascular NK/T-cell lymphoma, Epstein-Barr virus positive with multiorgan involvement: a clinical dilemma. BMC Cancer. 2018 November;18(1):1115. doi: 10.1186/s12885-018-5001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gleason BC, Brinster NK, Granter SR, Pinkus GS, Lindeman NI, Miller DM. Intravascular cytotoxic T-cell lymphoma: A case report and review of the literature. Journal of the American Academy of Dermatology. 2008 February;58(2):290–4. doi: 10.1016/j.jaad.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Chen S, Ma H, Shi D, Huang C, Lu C, Gao T, Wang G. Intravascular NK/T-cell lymphoma: a report of five cases with cutaneous manifestation from China. Journal of Cutaneous Pathology. 2015 September;42(9):610–7. doi: 10.1111/cup.12515. [DOI] [PubMed] [Google Scholar]
- 56.Melchers RC, Willemze R, Jansen PM, Daniëls LA, Vermeer MH, Quint KD. A rare case of cutaneous Epstein-Barr virus-negative intravascular cytotoxic T-cell lymphoma. JAAD Case Reports. 2019 June;5(6):548–551. doi: 10.1016/j.jdcr.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campo E, Jafe ES, Cook JR, et al. The international consensus classifcation of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022;140(11):1229–1253. doi: 10.1182/blood.2022015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen UD, Preiksaitis JK. Practice ASTIDCo Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13652. doi: 10.1111/ctr.13652. [DOI] [PubMed] [Google Scholar]
- 59.Shah N, Eyre TA, Tucker D, et al. Front-line management of post-transplantation lymphoproliferative disorder in adult solid organ recipient patients - a British Society for Haematology Guideline. Br J Haematol. 2021;193(4):727–740. doi: 10.1111/bjh.17421. [DOI] [PubMed] [Google Scholar]
- 60.Vakiani E, Nandula SV, Subramaniyam S, et al. Cytogenetic analysis of B-cell posttransplant lymphoproliferations validates the World Health Organization classifcation and suggests inclusion of forid follicular hyperplasia as a precursor lesion. Hum Pathol. 2007;38(2):315–325. doi: 10.1016/j.humpath.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 61.Butzmann A, Sridhar K, Jangam D, et al. Mutations in JAK/STAT and NOTCH1 genes are enriched in post-transplant lymphoproliferative disorders. Front Oncol. 2021;11:790481. doi: 10.3389/fonc.2021.790481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcelis L, Tousseyn T. The tumor microenvironment in post-transplant lymphoproliferative disorders. Cancer Microenviron. 2019;12(1):3–16. doi: 10.1007/s12307-018-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson AA, Harrington AM, Kroft S, Dahar MA, Hamadani M, Dhakal B. Presentation and management of post-allogeneic transplantation EBV-positive mucocutaneous ulcer. Bone Marrow Transplant. 2016;51(2):300–302. doi: 10.1038/bmt.2015.245. [DOI] [PubMed] [Google Scholar]
- 64.Menter T, Juskevicius D, Alikian M, et al. Mutational landscape of B-cell post-transplant lymphoproliferative disorders. Br J Haematol. 2017;178(1):48–56. doi: 10.1111/bjh.14633. [DOI] [PubMed] [Google Scholar]
- 65.King RL, Khurana A, Mwangi R, et al. Clinicopathologic characteristics, treatment, and outcomes of post-transplant lymphoproliferative disorders: a single-institution experience using 2017 WHO diagnostic criteria. Hemasphere. 2021;5(10):e640. doi: 10.1097/HS9.0000000000000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galera P, Flavin R, Savage NM, et al. Epstein-Barr virusnegative marginal zone lymphoma as an uncommon form of monomorphic posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2020;44(10):1340–1352. doi: 10.1097/PAS.0000000000001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morscio J, Tousseyn T. Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders. World J Transplant. 2016;6(3):505–516. doi: 10.5500/wjt.v6.i3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Margolskee E, Jobanputra V, Jain P, et al. Genetic landscape of T-and NK-cell post-transplant lymphoproliferative disorders. Oncotarget. 2016;7(25):37636–37648. doi: 10.18632/oncotarget.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bobillo S, Abrisqueta P, Sanchez-Gonzalez B, et al. Posttransplant monomorphic Burkitt’s lymphoma: clinical characteristics and outcome of a multicenter series. Ann Hematol. 2018;97(12):2417–2424. doi: 10.1007/s00277-018-3473-8. [DOI] [PubMed] [Google Scholar]
- 70.Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defned Burkitt lymphomas are frequently MYCnegative and characterized by the 11q-gain/loss pattern. Haematologica. 2015;100(7):e275–279. doi: 10.3324/haematol.2015.124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaji D, Kusakabe M, Sakata-Yanagimoto M, et al. Retrospective analyses of other iatrogenic immunodeficiency-associated lymphoproliferative disorders in patients with rheumatic diseases. Br J Haematol. 2021;195(4):585–594. doi: 10.1111/bjh.17824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodlad JR. Epstein-Barr virus-associated Lymphoproliferative Disorders in the Skin. Surgical Pathology Clinics. 2017 June;10(2):429–453. doi: 10.1016/j.path.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Casulo C, Friedberg J. Treating Burkitt Lymphoma in Adults. Current Hematologic Malignancy Reports. 2015 September;10(3):266–71. doi: 10.1007/s11899-015-0263-4. [DOI] [PubMed] [Google Scholar]
- 74.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. Burkitt’s lymphoma” (PDF) Lancet. 2012 March;379(9822):1234–44. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 75.Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, Murray PG. The Epstein-Barr virus and the pathogenesis of lymphoma. The Journal of Pathology. 2015 January;235(2):312–22. doi: 10.1002/path.4459. [DOI] [PubMed] [Google Scholar]
- 76.Navari M, Etebari M, De Falco G, Ambrosio MR, Gibellini D, Leoncini L, Piccaluga PP. The presence of Epstein-Barr virus significantly impacts the transcriptional profile in immunodeficiency-associated Burkitt lymphoma. Frontiers in Microbiology. 2015;6:556. doi: 10.3389/fmicb.2015.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaplan LD. HIV-associated lymphoma. Best Practice & Research. Clinical Haematology. 2012 March;25(1):101–17. doi: 10.1016/j.beha.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Tang VK, Vijhani P, Cherian SV, Ambelil M, Estrada-Y-Martin RM. Primary pulmonary lymphoproliferative neoplasms. Lung India. 2018;35(3):220–230. doi: 10.4103/lungindia.lungindia_381_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chavez JC, Sandoval-Sus J, Horna P, Dalia S, Bello C, Chevernick P, Sotomayor EM, Sokol L, Shah B. Lymphomatoid Granulomatosis: A Single Institution Experience and Review of the Literature. Clinical Lymphoma, Myeloma & Leukemia. 2016 August;(16 Suppl):S170–4. doi: 10.1016/j.clml.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 80.Gangar P, Venkatarajan S. Granulomatous Lymphoproliferative Disorders: Granulomatous Slack Skin and Lymphomatoid Granulomatosis. Dermatologic Clinics. 2015 July;33(3):489–96. doi: 10.1016/j.det.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 81.Sigamani E, Chandramohan J, Nair S, Chacko G, Thomas M, Mathew LG, Pulimood S, Manipadam MT. Lymphomatoid granulomatosis: A case series from South India. Indian Journal of Pathology & Microbiology. 2018;61(2):228–232. doi: 10.4103/IJPM.IJPM_471_17. [DOI] [PubMed] [Google Scholar]
- 82.Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philosophical Transactions of the Royal Society of London Series B. Biological Sciences. 2017 October;372(1732) doi: 10.1098/rstb.2016.0271. 20160271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018 January;50(1):74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Jain N, Keating MJ. Richter transformation of CLL. Expert Review of Hematology. 2016 August;9(8):793–801. doi: 10.1080/17474086.2016.1199948. [DOI] [PubMed] [Google Scholar]
- 85.Grimm KE, O’Malley DP. Aggressive B cell lymphomas in the 2017 revised WHO classification of tumors of hematopoietic and lymphoid tissues. Annals of Diagnostic Pathology. 2018 October;38:6–10. doi: 10.1016/j.anndiagpath.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 86.Aozasa K. Pyothorax-associated lymphoma. Journal of Clinical and Experimental Hematopathology. 2006 March;46(1):5–10. doi: 10.3960/jslrt.46.5. [DOI] [PubMed] [Google Scholar]
- 87.Boyer DF, McKelvie PA, de Leval L, Edlefsen KL, Ko YH, Aberman ZA, Kovach AE, Masih A, Nishino HT, Weiss LM, Meeker AK, Nardi V, Palisoc M, Shao L, Pittaluga S, Ferry JA, Harris NL, Sohani AR. Fibrin-associated EBV-positive Large B-Cell Lymphoma: An Indolent Neoplasm With Features Distinct From Diffuse Large B-Cell Lymphoma Associated With Chronic Inflammation. The American Journal of Surgical Pathology. 2017 March;41(3):299–312. doi: 10.1097/PAS.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 88.Vets J, Marcelis L, Schepers C, et al. Breast implant associated EBV-positive Diffuse Large B-cell lymphoma: an underrecognized entity? Diagn Pathol. 2023;18:52. doi: 10.1186/s13000-023-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linke-Serinsöz E, Fend F, Quintanilla-Martinez L. Human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) related lymphomas, pathology viewpoint. Seminars in Diagnostic Pathology. 2017 July;34(4):352–363. doi: 10.1053/j.semdp.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Tchernonog E, Faurie P, Coppo P, Monjanel H, Bonnet A, Algarte Génin M, Mercier M, Dupuis J, Bijou F, Herbaux C, Delmer A, Fabiani B, Besson C, Le Gouill S, Gyan E, Laurent C, Ghesquieres H, Cartron G. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group. Annals of Oncology. 2017 April;28(4):843–848. doi: 10.1093/annonc/mdw684. [DOI] [PubMed] [Google Scholar]
- 91.Yan J, Wang J, Zhang W, Chen M, Chen J, Liu W. Solitary plasmacytoma associated with Epstein-Barr virus: a clinicopathologic, cytogenetic study and literature review. Annals of Diagnostic Pathology. 2017 April;27:1–6. doi: 10.1016/j.anndiagpath.2016.09.002. [DOI] [PubMed] [Google Scholar]