Abstract
Background and Purpose:
Metabolites are reliable biomarkers for many diseases. However, their role in acute ischemic stroke (AIS) pathogenesis is not well understood. The objective of this systematic review is to evaluate the current literature on the presence of metabolites in thrombi retrieved by mechanical thrombectomy from AIS patients.
Methods:
Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines, we searched OVID Medline, PubMed, OVID Embase, Scopus, and Web of Science until July 13, 2022. Metabolites lists were extracted, and pathway analysis was performed in MetaboAnalyst database.
Results:
Four articles listing metabolites were included in this systematic review. D-Glucose, diacylglycerol, phytosphingosine, galabiosylceramide, glucosylceramide and 4-hydroxynonenal were reported to be associated with clots. Metabolomics data analysis showed that glycolysis, lactose, and sphingolipid metabolism pathways were enriched.
Conclusion:
Results of the present study show that the thrombi niche has a glycolytic phenotype. Future studies should work to better understand the metabolic properties of AIS thrombi.
Keywords: Stroke, metabolite, signature, systematic review
Introduction
Effective interventions for the treatment of acute ischemic stroke (AIS) require the identification of potential therapeutic targets that can improve the response to thrombolysis and mechanical thrombectomy (MT). In this context, the use of metabolomics may help to determine the etiology of stroke and design specific treatment strategies.
Conventional histological and immunohistochemical approaches have provided valuable insights into the cellular components of stroke clots. However, our current understanding of the metabolic composition of the clots retrieved by MT from AIS patients remains limited. Although several key biomarkers of stroke have been investigated [1], recent evidence suggest that changes in the total omics profile remodel the clots [2] and alterations in metabolites can influence thrombus formation and indicate cellular activity [3].
This systematic review aimed to evaluate the existing literature on the presence of metabolites in AIS thrombi that serve as key signatures for stroke etiology and pathogenesis.
Methods
Systematic Review
Preferred Reporting Items for Systematic Reviews and meta-Analysis (PRISMA) 2020 guidelines were followed to carry out this systematic review. A search was performed in the databases on reports of clots or thrombi retrieved from patients with large vessel occlusion.
Literature Search
The literature search was performed by an experienced research librarian on July 11–13, 2022, in Medline, PubMed, Embase, Scopus, and Web of Science databases using Medical Subject Headings (MeSH) and keywords such as “blood coagulation”, “stroke”, “metaboli, thrombus”, “clot”, “emboli”, and “clot composition”. No date limits or other limitations were applied.
Study Selection Criteria
The current study considered original research articles, reports, cohorts, and progressive studies reporting metabolites from the stroke clot or thrombi. Liquid chromatography with mass spectrometry method was found to be relevant and included. Review articles and articles from animal studies or in vitro clot analogs were excluded from the study.
Data Extraction and Synthesis
Information such as author name, publication year, type of study, types of clots, sample size, quantitative and qualitative information on clot composition were retrieved. Due to heterogeneity in the outcome, conducting a meta-analysis was not feasible. Therefore, a systematic review and narration with synthesized metabolites is summarized and presented.
After data extraction, enrichment analysis was performed using MetaboAnalyst to predict possible pathways.
Results
Search Outcome and Study Summary
The study selection and its outcome are shown in the flow chart (Figure 1). A total of 1214 results were retrieved, and the final total after deduplication was 1013 results. Following screening, four studies which reported the metabolites in clots retrieved by MT were included. The sample size and the metabolomics technique used in each study are presented in Table 1. The results section is divided in two parts, including the summary of the main findings of the studies included in the present review and the pathway analysis that was performed in MetaboAnalyst.
Figure 1.
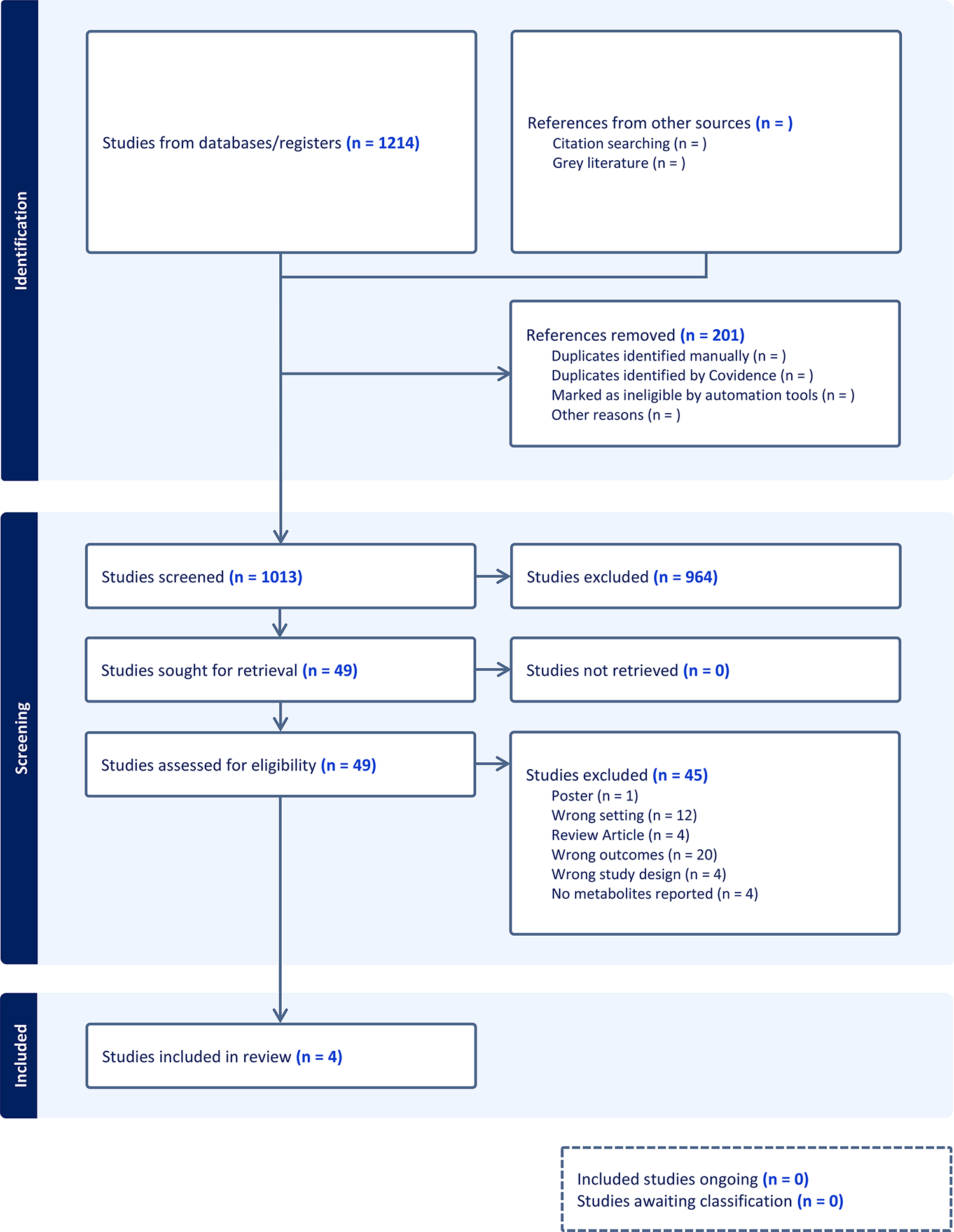
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Table 1.
Summary of the studies included in the present systematic review.
| STUDY | SAMPLE SIZE | METHODOLOGY |
|---|---|---|
|
| ||
| DOCHE ET AL. 2022 | 69 | LC-MS |
| LI ET AL. 2022 | 48 | UHPLC/Q-TOF-MS |
| SUISSA ET AL. 2021 | 41 | LC-MS |
| OSAKADA ET AL. 2021 | 52 | IHC |
LC-MS: Liquid chromatography-mass spectrometry; UHPLC/Q-TOF-MS: ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry; IHC: Immunohistochemistry.
Overview of Metabolites Associated with Clots
The list of metabolites identified in the clots is shown in Table 2. The metabolites from this table were further used for downstream analysis. D-glucose is a carbohydrate involved in glycolysis providing energy needed for the cell. Diacylglycerol (DG) is a fatty acid with glycerol moiety involved in triglycerol metabolic pathways and cell signalling. Phytosphingosine is a sphingolipid metabolite involved in various cellular signalling and metabolic pathways of fatty acid synthesis. Galabiosylceramide is a type of sphingolipid with carbohydrate moieties a cellular component present in epithelial and neuronal cell types. 4-hydroxynonenal (4-HNE) is a cytotoxic product resulted from the peroxidation of lipids.
Table 2.
Metabolites identified in acute ischemic stroke thrombi.
| Match | Human Metabolome Database (HMDB) | PubChem | KEGG | SMILES | Match | References |
|---|---|---|---|---|---|---|
|
| ||||||
| D-GLUCOSE | HMDB0000122 | 5793 | C00221 | C([C@@H]1[C@H]([C@@H]([C@H](C(O1)O)O)O)O)O | D-Glucose | Doche et al. 2022 (“ESOC 2022 Abstract Book,” 2022) |
| DIACYLGLYCEROL DG(14:0/14:0/0:0) | HMDB0007008 | 10369168 | C16667 | CCCCCCCCCCCCCC(=O)OC[C@H](CO)OC(=O)CCCCCCCCCCCCC | DG(14:0/14:0/0:0) | (Wei et al., 2022) |
| PHYTOSPHINGOSINE | HMDB0004610 | 122121 | C12144 | CCCCCCCCCCCCCC[C@H]([C@H]([C@H](CO)N)O)O | Phytosphingosine | (Wei et al., 2022) |
| GALABIOSYLCERAMIDE (d18:1/9Z-18:1) | HMDB0004832 | 20057273 | C06126 | CCCCCCCCCCCCC/C=C\C(C(CO[C@H]1[C@@H]([C@H]([C@H]([C@H](O1)CO)O[C@@H]2[C@@H]([C@H]([C@H]([C@H](O2)CO)O)O)O)O)O)NC(=O)CCCCCCC/C=C\CCCCCCCC)O | Galabiosylceramide (d18:1/9Z-18:1) | (Wei et al., 2022) |
| GLUCOSYLCERAMIDE | HMDB0000140 | 53477677 | C01190 | CCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@@H](CO[C@H]1[C@@H]([C@H](C([C@H](O1)CO)O)O)O)[C@@H](/C=C/CCCCCCCCCCCCC)O | Glucosylceramide | (Wei et al., 2022) |
| 4-HYDROXYNONENAL | HMDB0004362 | 5283344 | NA | CCCCCC(/C=C/C=O)O | 4-Hydroxynonenal | (Osakada et al., 2021) |
Doche et al. performed liquid chromatography-mass spectrometry (LC-MS) to study glycolytic metabolites in AIS thrombi. The authors showed that high glucose levels in the clots were associated with excellent prognosis whereas the admission hyperglycemia had poor prognosis. This finding suggests that entrapped glucose in the core may provide ATP which is beneficial for the stroke outcome. On contrary, the supply of glucose by collaterals in the penumbra can be deleterious [4].
Li et al. analyzed 48 AIS thrombi using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS) and found increased levels of 88 and 55 metabolites in large artery atherosclerosis (LAA) and cardioembolic (CE) clots, respectively. Furthermore, different metabolic pathways were enriched in the two types of thrombi. Six metabolites could clearly differentiate between the two etiologies: diglyceride (DG) (18:3/24:0), DG (22:0/24:0), phytosphingosine and galabiosylceramide (18:1/24:1) for LAA origin; triglyceride (TG) (15:0/16:1/o-18:0) and glucosylceramide (18:1/24:0) for CE origin [5].
Suissa et al. identified 2456 proteins and 5019 molecular profiles in 41 thrombi (34 CE and 7 LAA) analyzed by untargeted proteomics and metabolomic techniques, respectively. The untargeted proteomic approach revealed numerous proteins associated with the immune cells: CKLF-like MARVEL transmembrane domain-containing protein 5 (basophils, neutrophils), azurocidin (neutrophils, monocytes), epoxide hydrolase 1 (monocytes) isoform 2 of cell surface glycoprotein MUC18 (mucosa-associated invariant T - MAIT cells) and guanine nucleotide-binding protein subunit α-11 (neutrophils). This underlines the important role of inflammatory cells in thrombus formation. Additionally, the authors quantitatively assessed by targeted proteomics several protein biomarkers previously identified by immunohistochemistry (IHC) and found a higher abundance of glycophorin A (a marker of red blood cells, RBCs) and fibrinogen α, β, γ in the CE thrombi compared to LAA clots suggesting a RBCs/fibrin-rich composition of the formers. Furthermore, the combined untargeted proteomics and metabolomic analysis showed different omic signatures for CE vs LAA thrombi: ubiquitin-conjugating enzyme E2D3, azurocidin and ubiquitin-like-conjugating enzyme ATG3 for CE origin; aspartate aminotransferase, collagen type IV α, cell surface glycoprotein MUC18, CD99 antigen and epoxide hydrolase 1 for LAA origin. Therefore, the combination of proteomics and metabolomics may help to identify the etiology of AIS [6].
4-HNE is involved in angiogenesis and platelet aggregation in the atherosclerotic lesions. Osakada et al. explored the expression of 4-HNE by IHC in 52 AIS thrombi. LAA clots displayed a localized expression corresponding to the core of the plaque [7].
The studies included in this systematic review highlights the use of advanced technologies such as MS-based metabolomics in addition to conventional histology and IHC for the detection of novel biomarkers of AIS etiology and pathogenesis.
Metabolite Analysis
Metabolite Set Enrichment Analysis (MSEA) was performed using the MetaboAnalyst 5.0. The metabolites extracted from the four articles were fed into the system and the results are shown in Figure 2. Interestingly, both the sphingolipid metabolism and glycolysis/gluconeogenesis were revealed together with other relevant pathways such as lactose degradation and synthesis, and the Warburg effect, a marker of ischemia. Further connecting these pathways reveals three distinct pathways: glycolysis metabolism, lactose metabolism and sphingolipid metabolism. These pathways are not connected, and there are missing nodes that should be identified in future studies.
Figure 2.
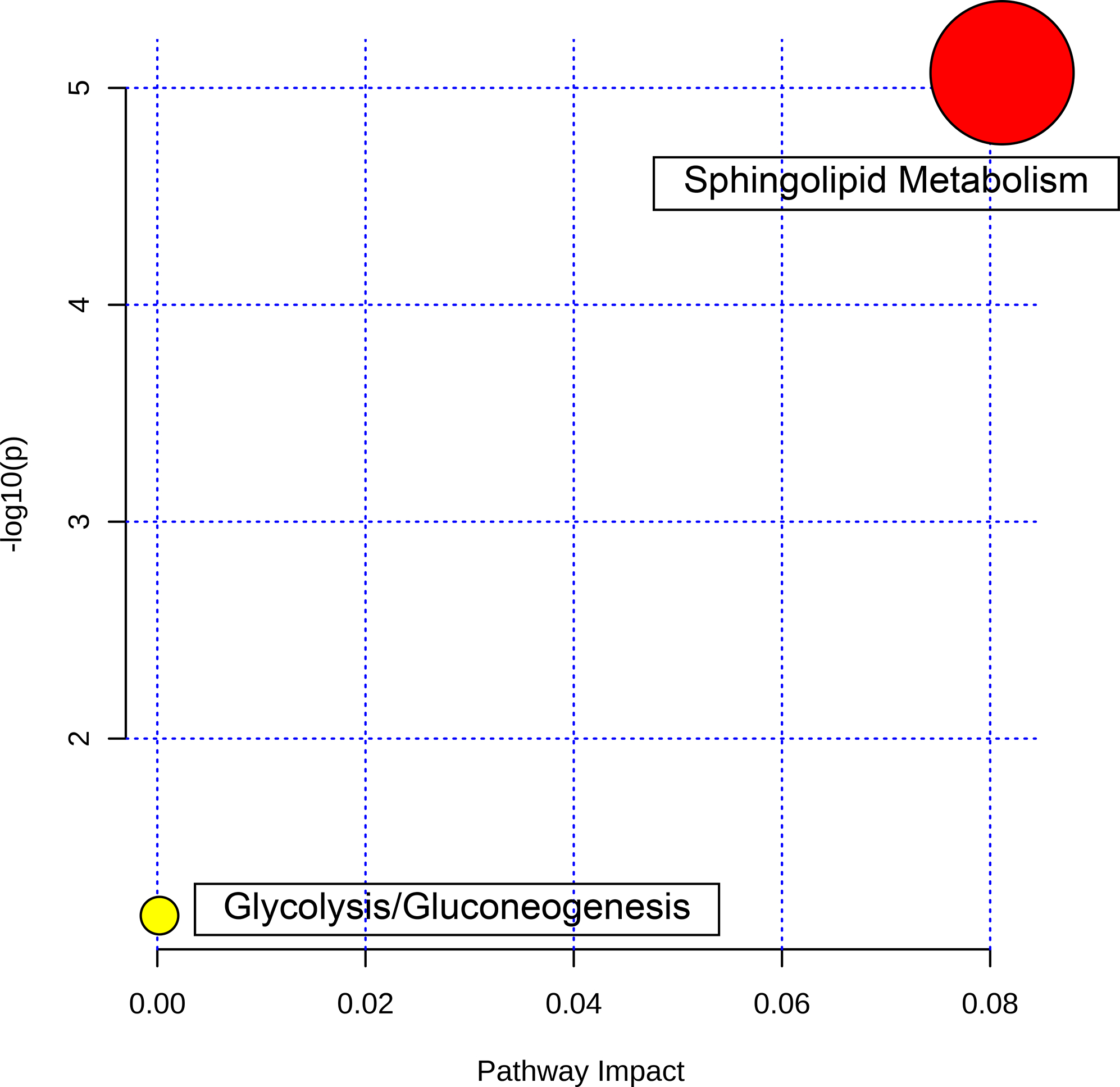
Pathway Analysis of the metabolites showing two enriched pathways: glycolysis/gluconeogenesis (yellow) and sphingolipid metabolism (red).
Sphingolipid Metabolism is Enriched in Stoke Clots
Further pathway analysis showed that sphingolipid metabolism pathway had the highest log(p) value (p <0.001) and impact value with a false discovery rate of 0.001772 which implies that sphingolipid metabolism is a key contributor to thrombus formation (Figure 2). In addition, glycolysis and gluconeogenesis pathway exhibited with a lower impact and a p value of 0.081.
Metabolite enrichment analysis
The overview of enriched metabolite set is shown in Figure 3. Sphingolipid metabolism emerged as the top candidate confirming the results from the pathway analysis. The glycolysis metabolism was also predicted in the metabolite enrichment overview. In addition, lactose degradation, glucose-alanine cycle, lactose synthesis, acetyl group transfer to mitochondria, gluconeogenesis galactose metabolism and the Warburg effect featured in the enriched list.
Figure 3.

Overview of the metabolite sets enrichment.
The three distinct pathways
Network view of the associated pathways further confirmed three distinct pathways associated with the metabolites (Figure 4).
Figure 4.
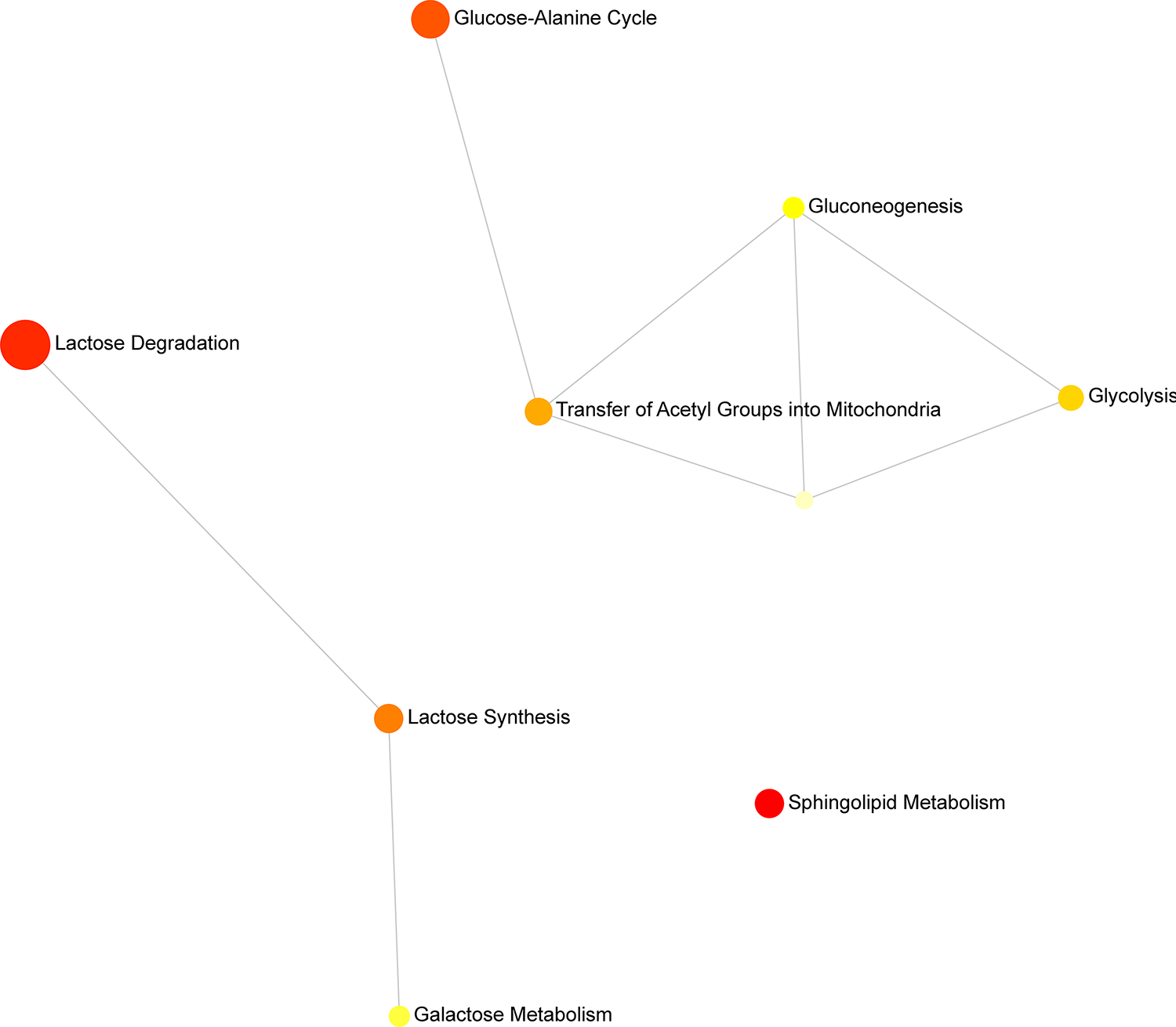
Metabolite sets enrichment network map showing three distinct networks: 1) glycolysis 2) lactose degradation and 3) sphingolipid metabolism,
Glycolysis: This pathway prediction connects gluconeogenesis and Warburg effect with further nodes leading to acetyl group transfer to mitochondria and glucose-alanine cycle. The presence of D-glucose may explain the association of all 3 nodes.
Lactose degradation: Lactose degradation could possibly lead to lactose synthesis and galactose metabolism as shown from the nodes.
Sphingolipid metabolism: The association of D-Glucose with glucosylceramide leads to a separate pathway involving sphingolipid metabolism. This is a standalone pathway and does not have any further nodes.
Discussion
Recently, multi-omics approaches, including metabolomics, have emerged as valuable tools for reliable detection of disease biomarkers. In this systematic review, we report the presence of several metabolites in AIS thrombi that may represent specific metabolomic signatures related to stroke etiology and prognosis. Our analysis based on these metabolites revealed the glycolytic phenotype of the clot and identified three major enriched pathways: glycolysis, lactose, and sphingolipid metabolism.
Recent studies have investigated the potential role of glycolysis, lactose and sphingolipid metabolism in AIS. [8–11]. Glycolysis metabolites, such as lactate and pyruvate, have been found to be elevated in both plasma and cerebrospinal fluid (CSF) of AIS patients, suggesting presence of anaerobic glycolysis [12]. Changes in plasma ceramide levels were also associated with AIS, indicating the involvement of sphingolipid metabolism in the AIS pathogenesis [13]. Moreover, lactosylceramide, a sphingolipid metabolite, has been identified as a potential biomarker for AIS [14]. These findings highlight the potential of metabolomic analysis to provide more accurate and reliable biomarkers for AIS diagnosis and prognosis.
The observed association of thrombi with glycolysis, lactose, and sphingolipid metabolism, which is in agreement with previous studies reporting high levels of metabolites such as lactate in serum, plasma or CSF of AIS patients[15]. Moreover, the Warburg effect emerged as one of the predicted pathways, confirming the ischemic nature of the niche and potentially serving as a key signature. Further, a recent study has provided evidence demonstrating the existence of an outer shell layer composed of dense fibrin, von Willebrand factor, and aggregated platelets that covers the AIS clot, impeding the process of fibrinolysis [16]. The outer shell surrounding the clot can impact the flow of blood and other metabolites to and from the inner region of the clot. The modified metabolic pathways within the clot may affect the post-translational modifications of key protein components within the clot. These modifications could potentially contribute to the clot’s resistance to thrombolysis.
Despite the rigorous methodology employed in our systematic review, there were limitations. Firstly, we identified a limited number of studies that specifically investigated metabolites in blood clots of AIS patients. This highlights a need for more research in this area to better understand the metabolic changes occurring in stroke pathophysiology. Additionally, the pathways involved in clot formation and dissolution are complex and not yet fully elucidated, further complicating the identification of clot-specific metabolites. Secondly, due to the technical challenge of isolating clot-specific metabolites, we were unable to exclude plasma metabolites entirely, which may have influenced our results. Future studies employing more sensitive and specific techniques for metabolite isolation could provide greater insights into clot-specific metabolic changes in AIS.
Metabolomic signatures are valuable tools in stroke management, offering unique insights into the intricate metabolic alterations that accompany stroke. The identification of specific metabolites as potential biomarkers holds promise for stroke diagnosis, prognosis, and treatment monitoring. Moreover, metabolomic profiling enables the prediction of stroke outcomes, elucidates underlying mechanisms, facilitates personalized treatment approaches, and contributes to the discovery of novel therapeutic targets. Integrating metabolomics into stroke research has the potential to revolutionize diagnostic and prognostic practices, optimize treatment strategies, and ultimately enhance patient outcomes.
Conclusion
The systematic review provides a compressive overview of metabolite composition of AIS thrombi and the possible pathways associated with stroke. Our findings underscore the importance of conducting detailed studies on the metabolic alterations underlying AIS pathophysiology with the ultimate goal of developing targeted interventions to improve outcomes for stroke patients.
Acknowledgement
Research reported in this publication was in part supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number NS105853. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interests
The authors declare no conflict of interest.
References
- 1.Deng W, Beecher C, Burant C, De Jong F, Lopez M, Wickham T, Elia M, Feeney K, McMullin D, Buonanno F, Lo E, Ning M. Metabolomic analysis reveals novel small molecule plasma markers of hyperacute ischemic stroke (S30.001). 2015; 84: S30.001. [Google Scholar]
- 2.Montaner J, Ramiro L, Simats A, Tiedt S, Makris K, Jickling GC, Debette S, Sanchez J-C, Bustamante A. Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke. Nature Reviews Neurology. 2020; 16: 247–64. 10.1038/s41582-020-0350-6. [DOI] [PubMed] [Google Scholar]
- 3.Maekawa K, Sugita C, Yamashita A, Moriguchi-Goto S, Furukoji E, Sakae T, Gi T, Hirai T, Asada Y. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thrombosis research. 2019; 177: 136–44. 10.1016/j.thromres.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 4.ESOC 2022 Abstract Book. European Stroke Journal. 2022; 7: 3–545. 10.1177/23969873221087559. [DOI] [Google Scholar]
- 5.Wei L, Xuesong B, Jiheng H, Xin X, Feng L, Qunlong J, Chunguang D, Gaolei D, Fangda P, Meng Z, Yao F, Jiyue W, Xianyang C, Teng X, Xiaofan G, Zhaolin F, Wen-huo C, Liyong Z, Chaodong W, Liqun J. Thrombosis origin identification of cardioembolism and large artery atherosclerosis by distinct metabolites. Journal of NeuroInterventional Surgery. 2022: neurintsurg-2022–019047. 10.1136/neurintsurg-2022-019047. [DOI] [PubMed] [Google Scholar]
- 6.Suissa L, Guigonis J-M, Graslin F, Doche E, Osman O, Chau Y, Sedat J, Lindenthal S, Pourcher T. Metabolome of Cerebral Thrombi Reveals an Association between High Glycemia at Stroke Onset and Good Clinical Outcome. Metabolites. 2020; 10: 483. 10.3390/metabo10120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osakada Y, Yamashita T, Morihara R, Matsumoto N, Sasaki R, Tadokoro K, Nomura E, Kawahara Y, Omote Y, Hishikawa N, Takemoto M, Ohta Y, Suruga Y, Nagase T, Takasugi Y, Inoue S, Watanabe K, Deguchi K, Tokunaga K, Sasada S, Kobayashi K, Maeoka R, Fukutome K, Takahashi K, Ohnishi H, Kuga Y, Ohnishi H, Abe K. 4-Hydroxyl-2-Nonenal Localized Expression Pattern in Retrieved Clots is Associated with Large Artery Atherosclerosis in Stroke Patients. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2021; 30: 105583. 10.1016/j.jstrokecerebrovasdis.2020.105583. [DOI] [PubMed] [Google Scholar]
- 8.Tian H-p Qiu T-z, Zhao J Li L-x, Guo J. Sphingomyelinase-induced ceramide production stimulate calcium-independent JNK and PP2A activation following cerebral ischemia. Brain Injury. 2009; 23: 1073–80. 10.3109/02699050903379388. [DOI] [PubMed] [Google Scholar]
- 9.Mohamud Yusuf A, Hagemann N, Hermann DM. The Acid Sphingomyelinase/ Ceramide System as Target for Ischemic Stroke Therapies. Neuro-Signals. 2019; 27: 32–43. 10.33594/000000184. [DOI] [PubMed] [Google Scholar]
- 10.Sidorov EV, Xu C, Garcia-Ramiu J, Blair A, Ortiz-Garcia J, Gordon D, Chainakul J, Sanghera DK. Global Metabolomic Profiling Reveals Disrupted Lipid and Amino Acid Metabolism Between the Acute and Chronic Stages of Ischemic Stroke. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association. 2022; 31: 106320. 10.1016/j.jstrokecerebrovasdis.2022.106320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidorov EV, Rout M, Xu C, Larsen J, Fields E, Apple B, Smith K, Gordon D, Chainakul J, Sanghera D. Comparison of Acute and Chronic Stage Ischemic Stroke Metabolome with Controls. Research Square. 2023: rs.3.rs-2515376. 10.21203/rs.3.rs-2515376/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Bi X. Post-Stroke Cognitive Impairment: A Review Focusing on Molecular Biomarkers. Journal of molecular neuroscience: MN. 2020; 70: 1244–54. 10.1007/s12031-020-01533-8. [DOI] [PubMed] [Google Scholar]
- 13.Gong D Plasma ceramides and acute ischemic stroke patients. Journal of the Formosan Medical Association = Taiwan Yi Zhi. 2021; 120: 1660. 10.1016/j.jfma.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Peters L, Kuebler WM, Simmons S. Sphingolipids in Atherosclerosis: Chimeras in Structure and Function. International Journal of Molecular Sciences. 2022; 23: 11948. 10.3390/ijms231911948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouns R, Sheorajpanday R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP. Evaluation of lactate as a marker of metabolic stress and cause of secondary damage in acute ischemic stroke or TIA. Clinica chimica acta; international journal of clinical chemistry. 2008; 397: 27–31. 10.1016/j.cca.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Di Meglio L, Desilles JP, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, Loyau S, Olivot JM, Blanc R, Piotin M, Bouton MC, Michel JB, Jandrot-Perrus M, Ho-Tin-Noé B, Mazighi M. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019; 93: e1686–e98. 10.1212/wnl.0000000000008395. [DOI] [PMC free article] [PubMed] [Google Scholar]


