TO THE EDITOR:
Since May 2022, when the multinational mpox (formerly known as monkeypox) clade IIb virus outbreak was first reported, more than 30,000 cases have been identified in the United States.1 In one study involving more than 1900 patients with mpox, more than 35% of the patients also had human immunodeficiency virus (HIV) infection.2
We report a death due to mpox in a patient in the United States. A 33-year-old man with HIV infection (CD4+ T-cell count, <35 per cubic millimeter) and recently treated syphilis became infected with mpox virus (MPXV) (clade IIb). He received two courses of oral tecovirimat (from Aug. 6 through Aug. 20, 2022, and from Aug. 21 through Sept. 4, 2022) and died on hospital day 27.
The patient had not received a vaccine for orthopoxvirus and reported no known exposure to persons with mpox. He had a prodrome of fever and chills, followed by skin lesions on the face, mouth, trunk, arms, legs, genitals, and perianal area that began to appear 4 days later. On day 15 of his illness, mpox was diagnosed; oral tecovirimat was prescribed 6 days later. On day 25 of his illness, he was admitted to the hospital for severe MPXV infection, dehydration, and difficulty with swallowing oral medication. He received intravenous fluids, pain medication, a second course of oral tecovirimat, and broad-spectrum antibiotic agents (see Section S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). He had severe proctitis that led to large-bowel obstruction, sepsis, anasarca, and an exudative pleural effusion on the right side. On hospital day 25, hypoxic respiratory failure, septic shock, and renal failure developed. He was transitioned to comfort care and died on hospital day 27.
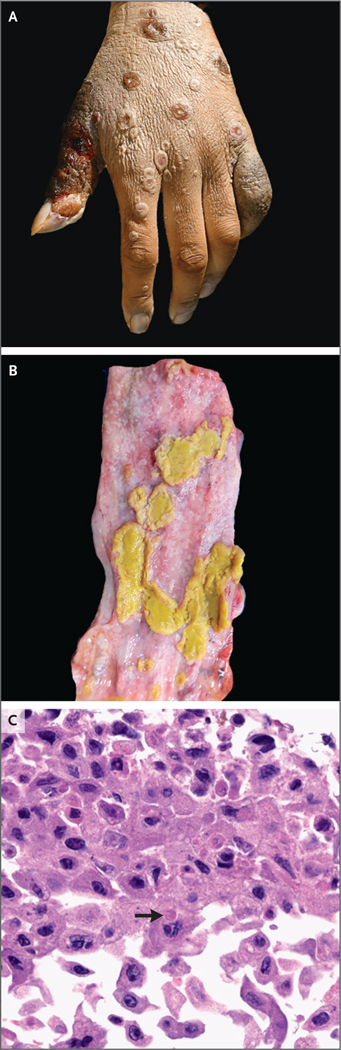
An autopsy identified disseminated mpox (Fig. 1). Polymerase-chain-reaction tests of swabs of skin lesions and of tissue specimens of the brain, bone marrow, and testicles were positive for nonvariola orthopoxvirus (see Section S1). Histologic examination of a bone marrow specimen revealed evidence of hemophagocytic lymphohistiocytosis. Histologic specimens did not show evidence of cancer or of other infectious processes.
Figure 1. Lesions and Cytopathic Effects.
Photographs show lesions on the left hand (Panel A) and in the esophagus (Panel B). Histologic examination of the esophageal mucosa (Panel C; hematoxylin and eosin stain) shows an epithelial cell with an intracellular eosinophilic inclusion that is consistent with a Guarnieri body (arrow).
Whole-genome sequencing of samples obtained during autopsy identified six known mutations linked with high-level resistance to tecovirimat in vaccinia virus encoding the VP37 protein.3,4 Virus was cultured, and tecovirimat resistance was confirmed by phenotypic testing in 12 of 15 samples (Table S1). The cause of death was determined to be disseminated mpox.
In hospitalized patients with severe mpox, it is important to consider treatment with intravenous tecovirimat.5 Second-line therapies including cidofovir, brincidofovir, and vaccinia immune globulin may also be considered. If progressive or persistent lesions are present after 14 days of treatment with tecovirimat, pharmacokinetic testing of tecovirimat and testing of lesion specimens for antiviral resistance are warranted.5 Patients with low CD4+ T-cell counts who become infected with MPXV should be monitored closely, given the potential risk of more severe illness.
Supplementary Material
Acknowledgments
Supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases cooperative agreement of the Centers for Disease Control and Prevention (grant 6 NU50CK000498).
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Jemma Alarcón, Centers for Disease Control and Prevention Atlanta, GA
Moon Kim, Los Angeles County Department of Public Health Los Angeles, CA
Dawn Terashita, Los Angeles County Department of Public Health Los Angeles, CA
Kusha Davar, Los Angeles County–University of Southern California Medical Center Los Angeles, CA
Jacob M. Garrigues, Los Angeles County Department of Public Health Los Angeles, CA
Jack P. Guccione, Los Angeles County Department of Medical Examiner–Coroner Los Angeles, CA
Mark G. Evans, Caris Life Sciences Phoenix, AZ
Peera Hemarajata, Los Angeles County Department of Public Health Los Angeles, CA
Noah Wald-Dickler, Los Angeles County–University of Southern California Medical Center Los Angeles, CA
Paul Holtom, Los Angeles County–University of Southern California Medical Center Los Angeles, CA
Rodrigo Garcia Tome, Los Angeles County–University of Southern California Medical Center Los Angeles, CA
Lovelyn Anyanwu, Los Angeles County Department of Public Health Los Angeles, CA
Naman K. Shah, Los Angeles County Department of Public Health Los Angeles, CA
Matthew Miller, Los Angeles County Department of Medical Examiner–Coroner Los Angeles, CA
Todd Smith, Centers for Disease Control and Prevention Atlanta, GA
Audrey Matheny, Centers for Disease Control and Prevention Atlanta, GA
Whitni Davidson, Centers for Disease Control and Prevention Atlanta, GA
Christina L. Hutson, Centers for Disease Control and Prevention Atlanta, GA
Jonathan Lucas, Los Angeles County Department of Medical Examiner–Coroner Los Angeles, CA
Odey C. Ukpo, Los Angeles County Department of Medical Examiner–Coroner Los Angeles, CA
Nicole M. Green, Los Angeles County Department of Public Health Los Angeles, CA
Sharon E. Balter, Los Angeles County Department of Public Health Los Angeles, CA
References
- 1.Centers for Disease Control and Prevention. Mpox: 2022 outbreak cases and data. February 15, 2023. (https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html).
- 2.Curran KG, Eberly K, Russell OO, et al. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duraffour S, Lorenzo MM, Zöller G, et al. ST-246 is a key antiviral to inhibit the viral F13L phospholipase, one of the essential proteins for orthopoxvirus wrapping. J Antimicrob Che-mother 2015;70:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. FDA Mpox response. February 22, 2023. (https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/fda-monkeypox-response).
- 5.Centers for Disease Control and Prevention. Update on managing monkeypox in patients receiving therapeutics. November 17, 2022. (https://emergency.cdc.gov/han/2022/han00481.asp).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.