Many mysteries remain about the eukaryotic nucleus. In the past decade, much has been discovered anew about the three-dimensional (3D) organization of the nucleus and the dynamic interactions therein that influence cellular function. Studies have uncovered the critical roles that topologic structure, histone modifications, and DNA modifications play in regulating transcription. By contrast, the understanding of interactions among proteins, RNA, and chromatin in macromolecular assemblies is less developed. These condensates are the molecular basis for discrete nuclear spatial organization of active and repressive chromatin as well as distinct nuclear structures such as the nucleolus (1, 2). On page 1386 of this issue, Klein et al. (3) begin to dissect the functional relevance of nuclear condensates in regulating the distribution and activity of fluorescent-labeled antineoplastic agents with specific nuclear localization profiles. This finding could have wide-ranging implications for the understanding of pharmacologic mechanisms and has substantive consequences for therapeutic development, drug delivery, and target engagement.
Recent technologies have allowed for appreciation of hierarchical 3D structures within the nucleus that have both known and unknown functional importance. Most widely accepted is the principle of spatial separation of chromatin into regions of active transcription (euchromatin). These regions share the property of deoxyribonuclease (DNase) or transposase accessibility and the presence of transcriptional coactivators, including the mediator (MED) complex. These genomic regions are spatially separate from regions of transcriptional inactivity (heterochromatin), densely packed histone-bound chromatin, often demarcated by repressive histone methylation marks.
Beyond euchromatin and heterochromatin, other segregated nuclear subcompartments have been described as self-organizing condensates. Through careful assessment of nuclear condensates (4, 5), Klein et al. observed that fluorescent-labeled small molecules had distinct and specific spatial aggregation in different nuclear compartments. Fluorescent-labeled cisplatin, a chemotherapy drug that damages and cross-links DNA, was observed to aggregate in mediator of RNA polymerase II transcription subunit 1 (MED1) transcriptional condensates while diffusing freely through heterochromatin protein 1 (HP1)–demarcated heterochromatin. Upon locus-specific assessment of cisplatin-induced DNA damage (platination) across the genome, only the DNA contained in MED1 condensates had high amounts of cisplatin incorporation. This finding suggests specificity of the pharmacologic effect of cisplatin to a distinct type of condensate. Additionally, the differential effect on platinum incorporation was lost with bromodomain-containing protein 4 (BRD4) inhibition, which disrupts MED1 condensates.
The authors also assessed the localization of tamoxifen and found that this targeted endocrine chemotherapy aggregated in MED1 condensates, competing with and evicting its target, estrogen receptor α (ERα), from these intranuclear structures. In human breast cancer cells, they found that ERα interacts with the MYC locus in MED1 condensates; this association was abrogated by tamoxifen and condensate accumulation. Notably, patient-derived ERα mutations that induce tamoxifen resistance led to their retention in MED1 condensates, rendering tamoxifen unable to outcompete oncogenic ERα.
Klein et al. demonstrate that a subset of small-molecule therapeutics can have differential enrichment in nuclear compartments (see the figure). Moreover, functional studies have demonstrated that redistribution of high-density regions of histone 3 lysine 27 acetylation (H3K27ac), consistent with transcription-activating super-enhancer function, within condensates may contribute to platinum resistance in human ovarian cancer cell lines (6). These two observations suggest an important, dynamic link between transcriptional units and epigenetic marks in nuclear condensates with pharmacologic agents possessing condensate-specific proclivities. These studies further suggest that the modulation of condensate structure and function has critical roles in gene regulation and therapeutic response. This raises the question of whether the localization and efficacy of cisplatin and of other small-molecule therapeutics are driven by transcriptional and epigenetic regulation, by the structure of nuclear condensates, or by these two dynamic processes acting in concert.
Pharmacophore-specific condensates.
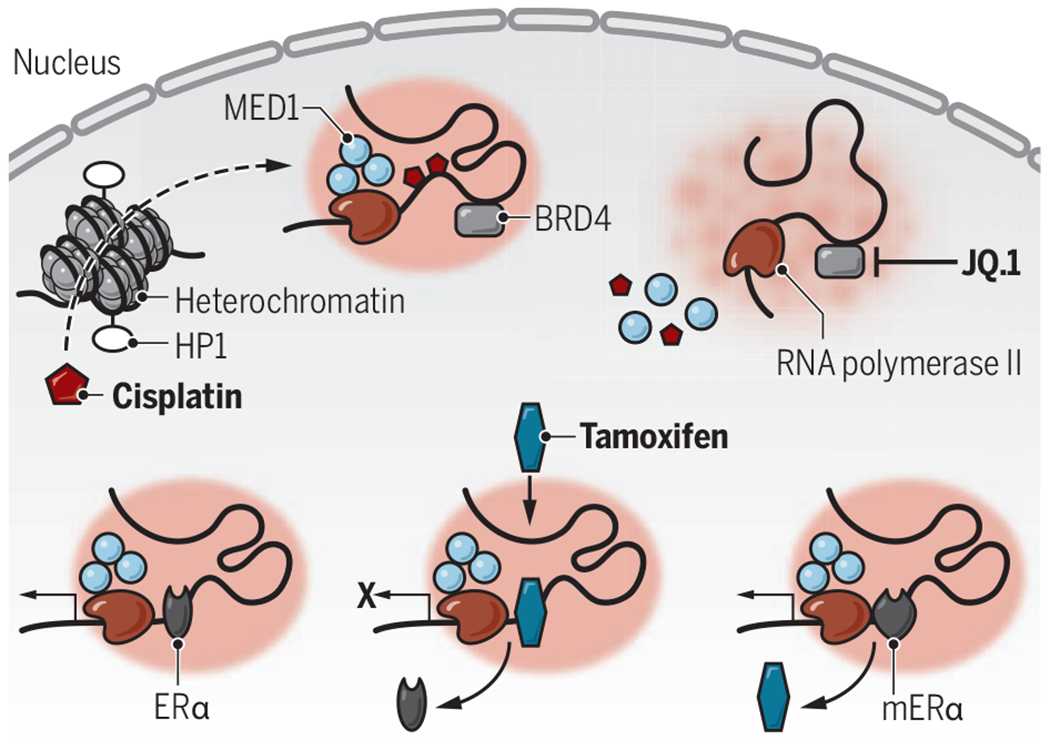
Cisplatin and tamoxifen aggregate in transcriptional condensates, affecting their pharmacokinetics. Cisplatin freely diffuses through HP1-labeled heterochromatin and accumulates in MED1 condensates. The BRD4 inhibitor JQ.1 abrogates MED1 condensates and attenuates drug-induced platination. Tamoxifen displaces ERα from target genes, but mutations that induce tamoxifen resistance (mERα) shift affinity as tamoxifen is evicted from transcriptional condensates.
BRD4, bromodomain-containing protein 4; ERα, estrogen receptor α; HP1, heterochromatin protein 1; MED1, mediator of RNA polymerase II transcription subunit 1.
Undoubtedly, there remain technical limitations to the ability to assess and elucidate the relationship between nuclear structure and specific perturbations, particularly in dynamic contexts, including malignant transformation and the response to specific therapies. As a cell responds to intracellular and extracellular signals, transcription factor activity is influenced by both chromatin conformation and other dynamic factors that have a critical role in these same biologic processes. Notably, a recent description of genetic alterations in transcription factors was shown to affect spatial localization to transcriptional condensates (7). Indeed, transcription-directed compartmentalization may have a critical role in regulating condensates, an observation best illustrated through the effects of herpes simplex virus type I in altering nuclear organization through highly accessible viral DNA binding sites and sequestration of RNA polymerase II (8).
Consistent with this hypothesis, intrinsically disordered regions within transcription factors can contribute to altered nuclear localization in human disease, as has been shown in syndactyly (fused fingers) driven by an intrinsically disordered region repeat expansion in homeobox protein 13 (HOXD13) (7). As was shown by Klein et al. with respect to pharmacophore-specific condensates, fluorescence recovery after photobleaching (FRAP)–based techniques were used to elucidate altered condensate formation in HOXD13 expansion syndactyly. Although FRAP cannot fully distinguish liquid diffusion from high-affinity protein structures (9), the relative contribution of liquid-liquid phase separation versus protein structured “hubs” remains an exciting and controversial question with respect to nuclear condensate formation and function.
These recent studies have dissected the inner workings of the nucleus, which are as beautiful and fascinating as much as they are still beyond complete understanding with respect to regulatory function and purpose. Enormous potential lies in the ability to manipulate these characteristics for therapeutic intervention. It is anticipated that further research will soon reveal the mechanistic importance of nuclear phase separation in cellular function, and the molecular consequences of linking spatial compartmentalization with nuclear function. ■
ACKNOWLEDGMENTS
Studies supported by Memorial Sloan Kettering core facilities were funded in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant P30 CA008748. A.D.V. is supported by National Cancer Institute career development grant K08 CA215317, the William Raveis Charitable Fund Fellowship of the Damon Runyon Cancer Research Foundation (DRG 117-15), and an EvansMDS Young Investigator grant from the Edward P. Evans Foundation. R.L.L. is on the supervisory board of Qiagen and is a scientific adviser to Loxo/Lilly, Zentalis, Imago, Mana Therapeutics, Auron, Ajax, Syndax, C4 Therapeutics, and Isoplexis. He receives research support from Prelude Therapeutics and has consulted for Celgene and Gilead.
REFERENCES AND NOTES
- 1.Brangwynne CP, Mitchison TJ, Hyman AA, Proc. Natl. Acad. Sci. U.S.A 108, 4334 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feric M et al. Cell 165, 1686 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein IA et al. 368, 1386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boija A et al. Cell 175, 1842 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabari BR et al. Science 361, eaar3958 (2018).29930091 [Google Scholar]
- 6.Ma Q et al. Cell Rep. 31, 107532 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Basu S et al. Cell 181, 1062 (2020).32386547 [Google Scholar]
- 8.McSwiggen DT et al. eLife 8, e47098 (2019).31038454 [Google Scholar]
- 9.McSwiggen DT et al. Genes Dev. 33, 1619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


