Keywords: caveolin-1 scaffolding domain peptide, chronic obstructive pulmonary disease, cigarette smoke exposure, tobacco smoke exposure
Abstract
Chronic obstructive pulmonary disease (COPD) is a debilitating lung disease with no effective treatment that can reduce mortality or slow the disease progression. COPD is the third leading cause of global death and is characterized by airflow limitations due to chronic bronchitis and alveolar damage/emphysema. Chronic cigarette smoke (CS) exposure damages airway and alveolar epithelium and remains a major risk factor for the pathogenesis of COPD. We found that the expression of caveolin-1, a tumor suppressor protein; p53; and plasminogen activator inhibitor-1 (PAI-1), one of the downstream targets of p53, was markedly increased in airway epithelial cells (AECs) as well as in type II alveolar epithelial (AT2) cells from the lungs of patients with COPD or wild-type mice with CS-induced lung injury (CS-LI). Moreover, p53- and PAI-1-deficient mice resisted CS-LI. Furthermore, treatment of AECs, AT2 cells, or lung tissue slices from patients with COPD or mice with CS-LI with a seven amino acid caveolin-1 scaffolding domain peptide (CSP7) reduced mucus hypersecretion in AECs and improved AT2 cell viability. Notably, induction of PAI-1 expression via increased caveolin-1 and p53 contributed to mucous cell metaplasia and mucus hypersecretion in AECs, and reduced AT2 viability, due to increased senescence and apoptosis, which was abrogated by CSP7. In addition, treatment of wild-type mice having CS-LI with CSP7 by intraperitoneal injection or nebulization via airways attenuated mucus hypersecretion, alveolar injury, and significantly improved lung function. This study validates the potential therapeutic role of CSP7 for treating CS-LI and COPD.
NEW & NOTEWORTHY Chronic cigarette smoke (CS) exposure remains a major risk factor for the pathogenesis of COPD, a debilitating disease with no effective treatment. Increased caveolin-1 mediated induction of p53 and downstream plasminogen activator inhibitor-1 (PAI-1) expression contributes to CS-induced airway mucus hypersecretion and alveolar wall damage. This is reversed by caveolin-1 scaffolding domain peptide (CSP7) in preclinical models, suggesting the therapeutic potential of CSP7 for treating CS-induced lung injury (CS-LI) and COPD.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects up to 24 million people and is the third leading cause of death in the United States. Acute exacerbations of COPD are the second leading cause of hospital stays and incur costs of more than 18 billion dollars annually in the United States. Chronic exposure to cigarette smoke (CS) due to active cigarette smoking or passive CS exposure is the most common risk factor for COPD, with cigarette smokers known to have greater COPD-related mortality than nonsmokers (1–4). Despite considerable progress in the past decade, molecular pathogenesis of COPD remains poorly understood. There are currently no interventions to reverse the progression of COPD-related lung injury. In COPD, chronic lung inflammation leads to narrowing of small airways, airway obstruction, and alveolar wall destruction. Airway epithelial cells (AECs) and alveolar epithelial cells are common targets for damage from CS and mediators and cytokines released from inflammatory cells (5–7). The pathogenesis of COPD is directly associated with AEC metaplasia and airway inflammation leading to mucus hypersecretion (MH) as well as loss of alveolar structure due to replicative senescence and apoptosis of alveolar epithelial cells, including progenitor type II alveolar epithelial (AT2) cells (8–11). These cognate events consequently result in airway obstruction and emphysema, respectively, and contribute significantly to morbidity and mortality (1–2).
Chronic bronchitis (CB) from longtime smoking is known to involve aberrant cellular and inflammatory responses of the airways. This results in the disruption of the AEC function and has often been attributed to a reduction in AEC cilia length and death. These cellular changes are followed by reepithelialization by goblet cells and excessive mucus production, leading to impaired mucociliary clearance (MCC). Clinically, mucolytic drugs have been shown to reduce COPD exacerbation and improve the quality of life in patients (2–4), thus underscoring the importance of targeting MH in COPD therapy. Among the 21 genes that are ascribed to encode mucins in the human genome, mucin 5AC (MUC5AC) is highly expressed in the airways (12). Mucus may alter the normal structure and status of goblet cells after failing to incorporate with MUC5AC. Without the normal reaction between MUC5AC and mucus, airway viscoelasticity becomes susceptible to plugging (13). Goblet cell differentiation is dictated by a large network of genes in which transcription factors sterile alpha motif-pointed domain-containing E26 transformation-specific like factor (SPDEF) and forkhead box protein A2 (FOXA2) are two key regulators. SPDEF is required for goblet cell differentiation and excessive mucus production, including secreted airway mucin, MUC5AC (14, 15), whereas FOXA2 is a potent inhibitor of goblet cell differentiation in the lung (16, 17). Forkhead box protein A3 (FOXA3) is highly expressed in airway goblet cells and induces SPDEF, MUC5AC, and AGR2 genes that are required for goblet cell metaplasia in the airway epithelium. The observed effects of FOXA3 on mucus-related gene expression are likely mediated, at least in part, by its ability to induce SPDEF (18).
The breakdown of ciliated cells also contributes to the dysfunction of MCC. AECs exposed to CS show more than a 70% decrease in the number of ciliated cells as well as shortening of the cilia. One of the mechanisms under investigation involves autophagy that is dependent on histone deacetylase 6 (HDAC6). HDAC6 is upregulated in AECs of patients with COPD where it targets damaged and misfolded proteins for proteasomal degradation. In the case of ciliary shortening, HDAC6 was found to colocalize with acetylated alpha-tubulin. The HDAC6 then associates with LC3 which is a well-known essential molecule for autophagy. Notably, LC3 exists in two molecular forms; LC3-I and LC3-II, conjugation of cytosolic LC3-I to phosphatidylethanolamine (PE) forms LC3-II during induction of autophagy and tethered via PE to both inner and outer surface of growing phagophore (19, 20). The level of LC3-II directly relates to the number of autophagosomes indicative of autophagy. This is consistent with a recent report implicating an increased expression of autophagy markers in the development of COPD (21, 22). The pathogenesis of COPD involves progressive loss of alveolar epithelial regeneration due to AT2 cell dysfunction and apoptosis leading to alveolar wall damage and injury. AT2 cells play a vital role in alveolar epithelial regeneration by dividing and differentiating into type I alveolar epithelial cells besides secretion of surfactant proteins. As in aging, CS limits the proliferative recovery of alveolar epithelium, especially AT2 cells, which leads to premature cellular aging and promotes senescence and apoptosis in AT2 cells.
Caveolin‐1 (CAV1) is the structural protein component of caveolae which are vesicular invaginations of the plasma membrane. CAV1 participates in signal transduction by acting as a scaffolding protein that concentrates and functionally regulates signaling molecules within caveolar membranes. CAV1 expression is prominently increased in AECs and AT2 cells during lung injury, which in turn is intricately connected with induction of p53 and PAI-1 expression (23, 24). The effects are clinically relevant and occur in patients with COPD. Our data and recent publications using AECs and AT2 cells, or lung sections of patients with COPD and a mouse model of chronic CS-induced lung injury (CS-LI) link these findings (8, 11, 14, 16). These studies show that p53-mediated induction of PAI-1 augments lung inflammation, MH by AECs, and senescence and apoptosis in AT2 cells (25). These changes predispose to respiratory infection and airway obstruction, alveolar wall damage, and loss of elastic recoil, which often occur in COPD. Furthermore, a deficiency in p53 or PAI-1 expression leaves mice resistant to CS-LI (25). These studies, combined with the close association between lung inflammation, MUC5AC secretion, loss of alveolar structure, and reduced AT2 cells viability, led us to hypothesize that induction of p53 and PAI-1 due to increased CAV1 may be involved in excess production of MUC5AC in AECs and increased AT2 cell senescence and apoptosis. We further investigated whether the blockade of p53 and PAI-1 by targeting CAV1 signaling using a seven amino acid peptide derived from the CAV1 scaffolding domain, CSP7, may mitigate airway MH and distal lung damage. Our study suggests concurrently targeting increased CAV1 signaling in AECs and AT2 cells using CSP7 as an effective therapeutic strategy to alleviate CB and emphysema associated in the pathogenesis of COPD. Augmented CAV1 promotes airway MH and alveolar damage during CS-LI. This report for the first time demonstrates that CSP7 markedly suppresses MH, cilia dysfunction, and accumulation of inflammatory cells besides improving AT2 cell viability, suggesting the therapeutic potential of CSP7 for the treatment of CS-LI and COPD. Also, we note that Phase 1 clinical trial of CSP7 has been successfully completed; Phase 1 b in patients with COPD will be initiated in short order.
MATERIALS AND METHODS
Cell Culture
Primary human AECs from histologically normal and COPD lungs were purchased from American Type Culture Collection (ATCC). These cells were cultured in AEC basal medium with glutamine, extract P, human serum albumin, lenoleic acid and lecithin (HLL supplement), and AEC supplement and 1% penicillin-streptomycin at 37°C and 5% CO2. AT2 cells were isolated from C57BL/6 mice following the method of Corti and colleagues with minor modifications as we described elsewhere (25). The cells were plated on plastic culture dishes precoated with anti-CD-32 and anti-CD-45 antibodies for 2 h at 37°C. Nonadherent cells were collected, and the purity of AT2 cell preparations was assessed by lithium carbonate staining for inclusion bodies.
Preparation of Cigarette Smoke Extract for the Treatment of AECs In Vitro
Research cigarettes 2R4F were purchased from the Tobacco Health Research, University of Kentucky (Lexington, KY). Cigarette smoke extract (CSE) was prepared by burning research cigarettes in a sidearm flask, and the smoke generated was bubbled into phosphate-buffered saline (PBS) at room temperature through an attached peristaltic pump as we described earlier (25). An absorbance of 1.0 at 230 nm is considered 100%. CSE was filter sterilized by passing it through a 0.2-µm filter.
Peptide Preparation
CSP7 and CP were dissolved in DMSO and diluted in HBSS as we described elsewhere (26). These peptides were used to treat AECs in vitro or intraperitoneal (IP) injection of CS-exposed mice. For airway delivery by liquid nebulization, CSP7 (0.579 mg/mL) was added to PBS containing lactose monohydrate (15.456 mg/mL). The pH was adjusted to 8.4 to give a clear solution, filtered through a 0.2 µm syringe filter as we described earlier (26).
Therapeutic Effect of CSP7 in Mice with CS-LI
All experiments involving animals were performed in compliance with ARRIVE guidelines and in accordance with other relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee (IACUC) of The University of Texas Health Science Center at Tyler (UTHSCT). Wild-type (WT) C57BL/6 J, p53−/−, and PAI-1−/− mice of 6–8 wk old (weighing 20–25 g) were purchased from The Jackson Laboratory (Bar Harbor, ME). These mice (n = 10/group) were exposed to passive CS from 40 research cigarettes over a 2-h period twice a day, 5 days/wk for 20 wk (∼90 mg/m3 total solid particulates) using a mechanical smoking chamber (Teague Enterprises, Davis, CA). Control mice were kept in ambient air. Sixteen weeks later, mice with CS-LI were intraperitoneally injected with CSP7 or CP (1.5 mg/kg), or CSP7 was delivered via airways by nebulization, as we described once a day 5 days a week for 4 wk along with exposure to CS (25–27). All mice were euthanized, and lungs were used for further analyses of 20 wk post-CS-LI.
Microcomputed Tomography Scanning and Measurements of Lung Volume and Pulmonary Function
Ambient air kept control mice as well as mice with CS-LI treated with or without CSP7 or control peptide of scrambled sequence (CP) were subjected to microcomputed tomography (µCT) scanning as we described (26). Lung volumes were calculated from lung renditions collected at full inspiration using Microview software. Pulmonary function tests were performed immediately before µCT imaging and before mice were killed. Briefly, anesthetized mice were intubated by inserting a sterile, 20-gauge intravenous cannula through the vocal cords into the trachea. Elastance, compliance, and total lung resistance were measured using the SCIREQ flexiVent system (Tempe, AZ) with a tidal volume set to 30 mL/kg at a frequency of 150 breaths/min against 2–3 cmH2O positive end-expiratory pressure, according to the manufacturer’s specifications.
Hematoxylin and Eosin, Immunohistochemical, and Immunofluorescence Staining of Lung Sections and AECs
Deparaffinized lung sections were stained with hematoxylin and eosin (H&E). For immunofluorescence staining, AECs were plated on sterile coverslips and, subjected to designated treatments, subsequently, the cells were washed with PBS three times, fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 (Biosharp) for 20 min, blocked with 3% bovine serum albumin for 1 h, and then incubated overnight with primary antibody stained for MUC5AC (1:100, Abcam), HDAC6 (1:100, NOVUS Bio), LC3B (1:100, Abcam), and acetylated Tubulin (1:100, Santa Cruz) proteins as described in Table 1. The cells were later stained with FITC-conjugated secondary antibody (Alexa Fluor) and DAPI for staining nucleus. Fluorescent images of cells were captured using a confocal microscope (Carl Zeiss, Göttingen, Germany) and the Zeiss LSM program.
Table 1.
Antibody sources
| Antibody | Source | Cat. No. | Western Blot | If/IHC | |
|---|---|---|---|---|---|
| 1 | Mucin 5AC (WB) | Abcam | ab24071 | 1:1,000 | 1:200 |
| 2 | Mucin 5AC (IHC) | Abcam | ab3649 | 1:1,000 | 1:200 |
| 3 | p53 | Cell Signaling | 9282 | 1:1,000 | 1:200 |
| 4 | Caveolin-1 | Cell Signaling | 3238 | 1:1,000 | 1:200 |
| 5 | FOXA2 | Abcam | ab60721 | 1:1,000 | |
| 6 | FOXA3 | Invitrogen | PA1-813 | 1:500 | |
| 7 | HDAC6 | Novus Biologicals | NBP1-69127 | 1:1,000 | 1:200 |
| 8 | PAI-1 | Cell Signaling | D9C4 | 1:1,000 | |
| 9 | SPDEF | Novus Biologicals | NBP1-74237 | 1:1,000 | |
| 10 | PP2A C | Cell Signaling | 2038 | 1:1,000 | |
| 11 | Acetylated α Tubulin | Santa Cruz Biotechnology | sc-23950 | 1:1,000 | 1:200 |
| 12 | LC3B | Abcam | ab48394 | 1:1,000 | 1:200 |
| 13 | Erk1/2 | Cell Signaling | 9102 | 1:1,000 | |
| 14 | MMP1 | Santa Cruz Biotechnology | SC-39086 | 1:1,000 | |
| 15 | Cleaved caspase3 | Cell Signaling | 9661 | 1:1,000 | |
| 16 | Caspase3 | Cell Signaling | 9662 | 1:1,000 | |
| 17 | SPC | Santa Cruz Biotechnology | SC-7705 | 1:1,000 | |
| 18 | p53AC | Cell signaling | 2527 | 1:1,000 | |
| 19 | p53S15 | Cell signaling | 9284 | 1:1,000 | |
| 20 | β-gal | Cell signaling | 27198 | 1:1,000 | |
| 21 | β-Actin | Cell signaling | 8457 | 1:1,000 |
COPD, chronic obstructive pulmonary disease; CSP7, caveolin-1 scaffolding domain peptide; HDAC6, histone deacetylase 6; PAI-1, plasminogen activator inhibitor-1; WT, wild type.
Periodic Acid Schiff Staining
The formalin-fixed slides were deparaffinized, treated with a 0.5% periodic acid solution for 5 min, rinsed in distilled water, and incubated with Periodic Acid Schiff (PAS) reagent from mucin staining kit (Abcam) for 15 min following the instruction of the manufacturer.
Determination of Protein Phosphatase 2 A Activity
Protein phosphatase 2 A (PP2A) activity was determined in isolated AECs and lung homogenates using the PP2A activity assay kit (Millipore; 28).
Isolation of Mouse Tracheobronchial Epithelial Cells
Mouse tracheobronchial epithelial cells (TECs) were isolated by overnight digestion of minced tracheal tissue of mice in 0.15% pronase solution at 4°C. Later, the tracheal tissues were removed from the pronase solution, and the solution was set aside on ice. Tracheal tissues were transferred into a tube containing Ham’s F12 media, mixed 12 times, and the process was repeated twice. The Pronase solution was combined with the F12 media supernatants and centrifuged at 1,400 rpm for 10 min at 4°C. The pellet was resuspended in 1 mL DNAse solution and kept on ice for 5 min. The solution was then centrifuged at 1,400 rpm for 5 min at 4°C. The cell pellet was resuspended in an 8 mL TEC medium containing 10% FBS, plated in culture plates, and incubated at 37°C in an atmosphere of 95% air, 5% CO2 for 5 h. The suspended cells from plates were collected, and an aliquot was subjected to cytospin, trypan blue vital staining, and cell counting as described (29).
Treatment of Human Lung Tissues with CSP7 Ex Vivo
For ex vivo studies, deidentified human COPD lung tissues and histologically “normal” lung (nL) tissues were obtained from transplant recipients and from the Gift of Life Donor Program (www.donors1.org) from Temple University, Philadelphia, PA, USA. The demographic data for patients with COPD are provided in Table 2. The Gift of Life Donor Program does not provide demographics for tissues from “normal” lung (nL) as these donors typically suffered fatal trauma as a cause of death. Ex vivo studies using human lung tissues without patient identity were approved by the Institutional (UTHSCT) Review Board (IRB) through exempt protocol No. 12-003. nL and COPD tissues (n = 4–5) were treated with or without CSP7 for 72 h ex vivo, as we described previously (26). Total protein and RNA extracted from the lung tissues were analyzed by Western blotting and qPCR for MUC5AC protein and mRNA, respectively. In separate experiments, AT2 cells isolated from nL and COPD lungs were treated with CSP7 or CP and immunoblotted for changes in senescence or apoptosis, p53, PAI-1, and SP-C expression. Similarly, nL and COPD tissues were treated with or without CSP7 or CP for 72 h ex vivo, and AT2 cells isolated from these tissues were analyzed for senescence or apoptosis, p53, PAI-1, and SP-C.
Table 2.
Human demographic data
| Age | Sex | Race | Tissue | Grade |
|---|---|---|---|---|
| 70 | F | C | COPD | Gold 4 |
| 63 | M | C | COPD | Gold 4 |
| 68 | F | C | COPD | Gold 4 |
| 71 | F | C | COPD | Gold 4 |
| 67 | M | AA | COPD | Gold 4 |
| 68 | F | C | COPD | Gold 4 |
| 49 | M | C | NL | |
| 48 | F | C | NL | |
| 62 | F | C | NL | |
| 70 | M | C | NL | |
| 67 | M | C | NL |
COPD, chronic obstructive pulmonary disease.
Expression of CAV1, p53, or PAI-1 in AECs
AECs isolated from nL were separately transduced with Ad-CAV1 or Ad-p53 or Ad-PAI-1. AECs transduced with Ad-Ev were used as controls. In a separate experiment, AECs from nL in culture dishes were treated with Lv-p53 or Lv-PAI-1 shRNA to suppress its baseline expression before treating these cells with CSE. Naïve AECs and AECs exposed to nonspecific control shRNA were used as controls.
siRNA Transfection
AECs cultured in 60 mm petri plate were exposed to CAV1 or nonspecific control siRNA using Oligofectamine reagent (Invitrogen) following the manufacturer’s instructions. After 48 h of transfection, these cells were treated with CSE. MH and cilia disassembly were studied.
Western Blotting of Cell Lysate and Lung Homogenate
AEC and AT2 cell lysates were prepared using radioimmunoprecipitation assay (RIPA) lysis buffer (Pierce) containing a protease inhibitor cocktail (Roche, Germany) and phosphatase inhibitor cocktail (Sigma-Aldrich). AEC and AT2 cell lysates were subjected to Western blotting using ERK, MUC5AC, HDAC6, SPDEF, FOXA2, FOXA3, LC3B(I and II), p53, PAI-1, Acp53, S15p53, cleaved caspase-3, β-galactosidase, and β-actin antibodies at 1:500-1:1,000 dilution (Table 1). The membranes were stripped and subjected to Western blotting using an antibody to assess loading equality. Whole lung homogenates and isolated cell lysates were separated on SDS-PAGE and electroblotted onto a nitrocellulose membrane. After blocking with 1% bovine serum albumin (BSA) for 1 h, membranes were incubated with primary antibodies at respective dilutions at 4°C overnight, followed by washing and incubation with donkey anti-rabbit/Mouse/Goat horseradish peroxidase-conjugated secondary antibody at 1:2,000 dilution for 1 h at room temperature in 5% milk buffer. After washing, the protein was visualized using an enhanced chemiluminescence detection method. The list of antibodies and dilutions used is provided in Table 1. Quantification was done using ImageJ (NIH, Bethesda, MD).
RNA Isolation and Quantitative Real-Time PCR
Total RNA extracted from lung homogenates and cell lysates was reverse transcribed and subjected to quantitative real-time PCR (qPCR) for MUC5AC, HDAC6, FOXA2, FOXA3, and CAV1 transcripts. qPCR primers were purchased from Bio-Rad (Hercules, CA).
Statistical Analysis
All data are presented as means ± SD of at least two independent experiments. The Student’s t test was used to evaluate statistical differences between two groups. Nonparametric tests for statistical analysis among three or more groups were performed by one-way ANOVA Kruskal–Wallis test, with Dunn’s multiple group comparison tests as appropriate. A *P value < 0.05 or less was considered statistically significant.
Study Approval
Animals were housed in pathogen-free conditions according to protocols approved by the IACUC of the University of Texas Health Science Center at Tyler.
RESULTS
CSP7 Mitigates Mucus Hypersecretion and Cilia Shortening in AECs from COPD Lungs
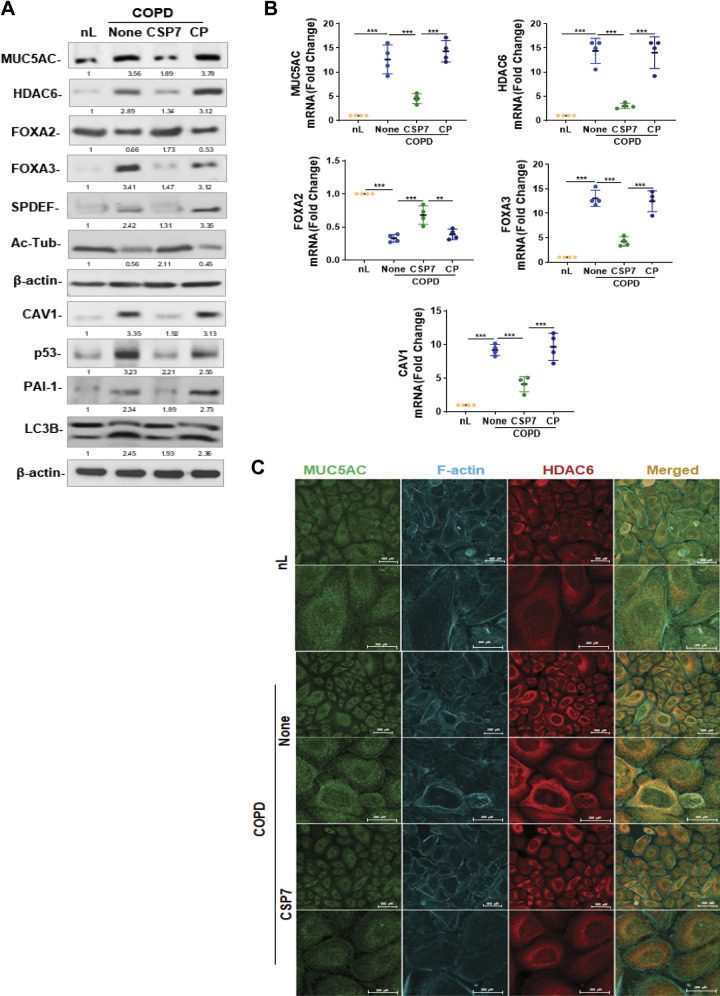
To understand the mechanism of airway secreted-polymeric mucin expression, we analyzed the expression of MUC5AC and FOXA3 in AECs isolated from the lungs of patients with COPD and control subjects. We found a surge in MUC5AC and FOXA3 expression in AECs from the lungs of patients with COPD compared with AECs from nL of control subjects (Fig. 1A). Consistent with increased MUC5AC and FOXA3, we found elevated levels of SPDEF and HDAC6 in AECs from COPD lungs. As shown in Fig. 1A, treatment of AECs isolated from COPD lungs with CSP7 reduced the expression of MUC5AC, HDAC6, FOXA3, SPDEF, and, LC3BII, which are otherwise increased in COPD AECs. These changes were associated with the restoration of FOXA2 and acetylated alpha-tubulin, and inhibition of CAV1, p53, and PAI-1 in AECs of COPD lungs exposed to CSP7. Analysis of AECs obtained from COPD lungs demonstrated augmented MUC5AC, HDAC6, and FOXA3 mRNA expression, with a parallel increase in CAV1 mRNA levels. These changes were significantly suppressed with a concurrent elevation of baseline FOXA2 mRNA expression in AECs from COPD lungs treated with CSP7 (Fig. 1B). This suggests that increased p53 and PAI-1, due to the induction of CAV1, contributes to enhanced MUC5AC expression. The AECs from COPD lungs exposed to CP or left untreated continued to exhibit elevated levels of MUC5AC, HDAC6, FOXA3, and CAV1 mRNA with low FOXA2 mRNA, compared with AECs from nL. These findings were independently confirmed by immunofluorescence staining analysis and colocalization for MUC5AC, F-actin, and HDAC6 (Fig. 1C).
Figure 1.
CSP7 mitigates MH and cilia shortening in AECs from COPD lungs. AECs isolated from nL and COPD lungs were treated with or without CSP7 or CP in vitro for 48 h. A: AEC lysates were immunoblotted for MUC5AC, HDAC6, FOXA2, FOXA3, SPDEF, acetylated tubulin (Ac-Tub), CAV1, p53, PAI-1, and LC3BII. Same samples were analyzed for β-actin to assess equal loading. Representative image from two independent experiments is shown. B: total RNA from AECs (n = 4) treated as in A were analyzed for MUC5AC, HDAC6, FOXA2, FOXA3, and CAV1 mRNA by qPCR. C: immunofluorescence staining (scale bar 500 µM and 200 µM) and colocalization of MUC5AC and HDAC6 in AECs from nL, and COPD lungs treated with or without CSP7. Representative image from two experiments is shown. Each experiment was repeated at least two to three times, and data are presented as means + SD and **P < 0.01 and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. AECs, airway epithelial cells; CAV1, caveolin‐1; COPD, chronic obstructive pulmonary disease; CSP7, caveolin-1 scaffolding domain peptide; FOXA2, forkhead box protein A2; HDAC6, histone deacetylase 6; MH, mucus hypersecretion; MUCAC, mucin 5AC; nL, “normal” lung; PAI-1, plasminogen activator inhibitor-1; SPDEF, domain-containing E26 transformation-specific like factor.
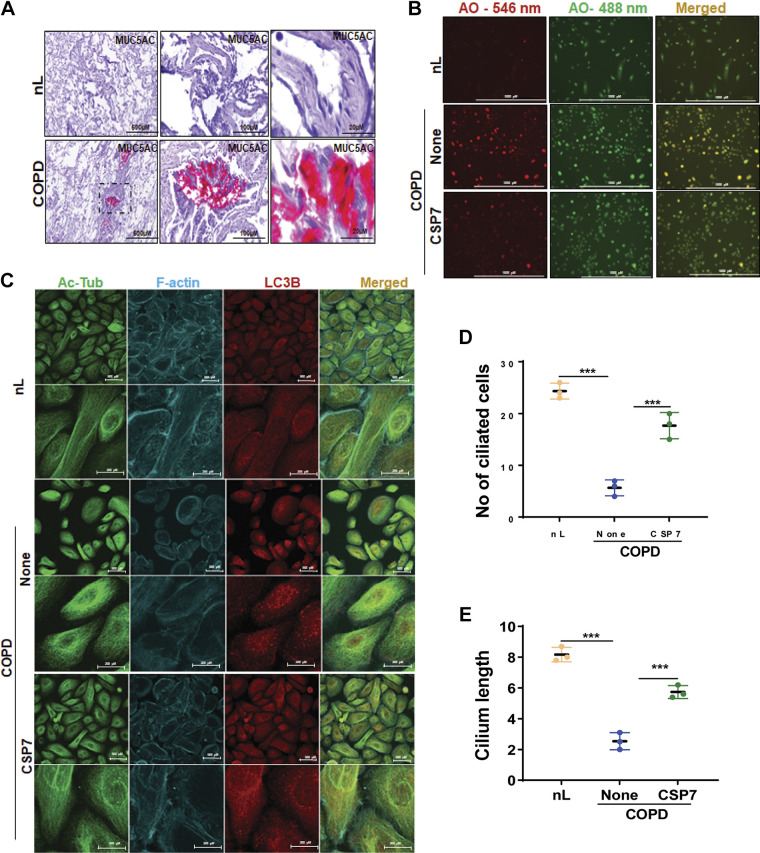
Furthermore, immunohistochemical (IHC) analysis of COPD lung sections showed increased MUC5AC staining compared with their intensity in nL sections (Fig. 2A). We found reduced acetylated alpha-tubulin, and elevated HDAC6 levels in AECs of patients with COPD. HDAC6 has been shown to regulate primary cilia resorption in response to extracellular stress (30) has been demonstrated to regulate autophagy through autophagosome-lysosome fusion (31). Ciliophagy, an HDAC6-dependent autophagic pathway is critical to cilia homeostasis and airway cilia motility in response to CS and COPD pathogenesis. Evaluation after acridine orange staining, a lysosomotropic agent used for tracking acidic vesicles, showed an increase in red fluorescence intensity (Fig. 2B) in AECs from COPD lung, indicative of increased late autophagic vacuoles. Acridine orange staining revealed a marked reduction in red fluorescence in AECs from COPD lungs treated with CSP7. We also examined the changes in the expression of endogenous LC3BII in AECs and found that rapid accumulation of LC3BII (corresponding to characteristic lipidation of this protein during autophagosome formation) in AECs from COPD lungs was reversed with CSP7. Interestingly, immunofluorescence staining revealed increased colocalization of MUC5AC and HDAC6 (as shown in Fig. 1C), and acetylated alpha-tubulin and LC3B (Fig. 2C) in AECs of COPD lungs. COPD caused a significant reduction in the number of ciliated AECs (Fig. 2D) as well as cilia length in individual AECs (Fig. 2E). These ciliary defects were restored following treatment of AECs from COPD lungs with CSP7. The cilia lengths were measured in AECS by evaluating the positive staining of acetylated alpha-tubulin, with 20 µm used as the standard for measurement. The mean value of the cilia length was measured using image J software and calculated from 20 measurements per area.
Figure 2.
CSP7 alleviates MH and cilia shortening in AECs from COPD lungs. A: IHC images showing increased MUC5AC staining in the lung sections of patients with COPD (scale bar 500 µM, 100 µM, and 20 µM). B: acridine orange (AO) staining of AECs from nL and COPD. AECs from COPD lungs were left untreated or treated with CSP7 in vitro for 6 h, stained with acridine orange (acidic vesicles), and subjected to fluorescence microscopy (scale bar 1,000 µm). C: immunofluorescence staining and colocalization of Ac-Tub/LC3B in AECs of nL, and COPD lungs treated as in Fig. 1D (scale bar 500 µM and 200 µM). Image represents the findings of two independent experiments. Bar graph showing the number of ciliated cells (D) and cilia length (E) in AECs from nL, and COPD lungs treated with or without CSP7 or CP. Each experiment was repeated at least two to three times, and data are presented as means + SD, and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. Ac-Tub, acetylated tubulin; AECs, airway epithelial cells; COPD, chronic obstructive pulmonary disease; CSP7, caveolin-1 scaffolding domain peptide; MH, mucus hypersecretion; MUCAC, mucin 5AC; nL, “normal” lung; AO, acridine orange.
CSP7 Inhibits MH and Cilia Dysfunction in AECs Exposed to CS Extract
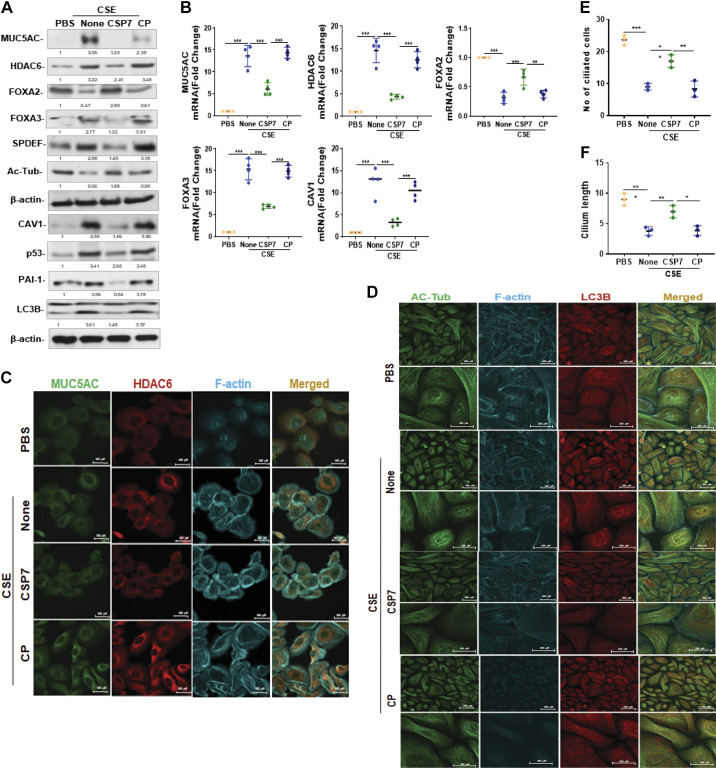
To further confirm the earlier findings, AECs isolated from human nL were exposed to CSE in vitro for 48 h and analyzed for MUC5AC, HDAC6, FOXA2, FOXA3, SPDEF, acetylated alpha-tubulin, p53, PAI-1, and LC3BII. As shown in Fig. 3A, treatment of AECs from nL with CSE increased MUC5AC, HDAC6, FOXA3, SPDEF, CAV1, p53, PAI-1, and LC3BII, whereas reducing FOXA2 and acetylated alpha-tubulin expression, which were reversed following the treatment with CSP7. The changes at protein level allied with corresponding changes at the mRNA levels (Fig. 3B). Furthermore, immunofluorescence staining of AECs exposed to CSE revealed increased colocalization of MUC5AC and HDAC6 (Fig. 3C), and acetylated alpha-tubulin and LC3B (Fig. 3D) versus diffused staining in control AECs from nL. CSE-induced colocalization of MUC5AC and HDAC6, and acetylated alpha-tubulin and LC3B were inhibited following treatment with CSP7. Immunofluorescence staining further revealed a decrease in acetylated alpha-tubulin levels in CSE-treated AECs that was improved with CSP7 treatment. Treatment of AECs with CSE also caused a significant (P < 0.01) reduction in the total number of ciliated cells (Fig. 3E), as well as cilia length (Fig. 3F) in individual AECs. These changes were significantly reversed in AECs exposed to CSE and treated with CSP7, while CP-treated control cells failed to respond.
Figure 3.
Effect of CSP7 on CSE-induced MH and cilia dysfunction. A: Western blot images showing expression of MUC5AC, HDAC6, FOXA2, FOXA3, SPDEF, acetylated tubulin (Ac-Tub), CAV1, p53, PAI-1, and LC3BII in lysates of naïve AECs from human nL or nL AECs exposed to CSE left untreated (None) or treated with CSP7 or CP for 48 h in vitro. Same samples were analyzed for β-actin to assess equal loading. B: total RNA from nL AECs (n = 4) treated as in A tested for MUC5AC, HDAC6, FOXA2, FOXA3, and CAV1 mRNA expression by qPCR. Immunofluorescence staining of AECs from nL treated as Fig. 2A for colocalization of MUC5AC and HDAC6 (scale bar 500 µM; C) or Ac-Tub and LC3B (scale bar 500 µM and 200 µM; D). Representative image from two independent experiments was shown. Bar graph depicting number of ciliated cell (E) and cilia length (F) in AECs (n = 3) from nL treated as in Fig. 2. Each experiment was repeated at least two to three times, and data are presented as means + SD, and *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. AECs, airway epithelial cells; CAV1, caveolin‐1; CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; FOXA2, forkhead box protein A2; HDAC6, histone deacetylase 6; MH, mucus hypersecretion; MUCAC, mucin 5AC; nL, “normal” lung; PAI-1, plasminogen activator inhibitor-1; SPDEF, domain-containing E26 transformation-specific like factor.
CSP7 Inhibits Chronic CS Exposure-Induced Lung Injury in Mice
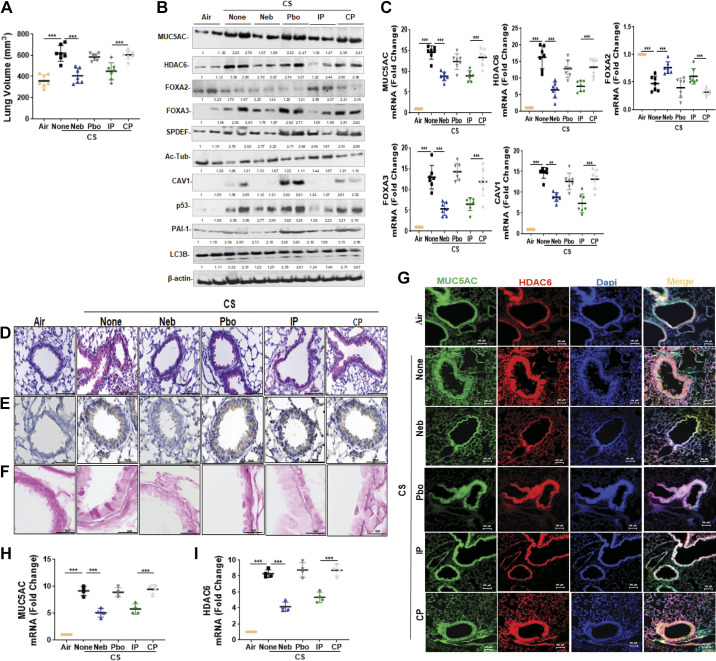
Local delivery of a drug often minimizes target dose requirements and lessens the chance for off-target effects associated with systemic administration. Local delivery can also be more convenient for patients with chronic diseases such as COPD. Therefore, we investigated whether CSP7 delivered via airways in liquid formulation mitigates chronic CS-induced lung injury. To test this mode of delivery, wild-type (WT) mice (n = 10/group) were exposed to CS for 4 h/day 5 days a week. After 16 wk, WT mice with CS-LI were left untreated or exposed to formulated CSP7 (5.8 mg) in 30 mL of PBS containing lactose monohydrate (154 mg) or placebo alone 2 h daily 5 days a week for 4 wk using a nebulization tower. WT mice with CS-LI were also treated with 1.5 mg/kg of CSP7 or CP by IP injection daily 5 days a week for 4 wk for comparison. Twenty weeks post-CS exposure, mice were subjected to quantitative chest µCT (not shown). Lung volumes were calculated from µCT renditions at full inspiration (Fig. 4A). Pulmonary function testing by SCIREQ suggested alveolar damage reflected by increased lung volume. These changes were significantly improved in mice with CS-LI and treated with CSP7. However, control CS-LI mice exposed to CP showed persistently high lung volumes, suggesting CS-induced lung damage.
Figure 4.
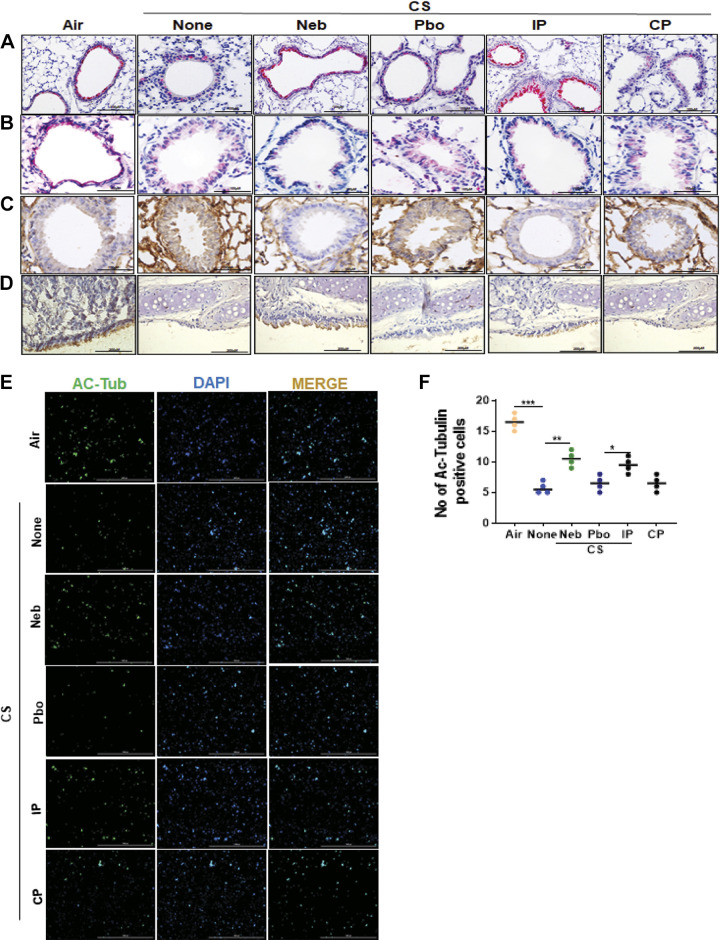
CSP7 mitigates CS-LI in WT mice. WT mice (n = 10/group) were kept in ambient Air or exposed to CS for 4 h/day 5 days a week as described in materials and methods. After 16 wk, WT mice exposed to CS were left untreated (None) or treated with CSP7 (5.8 mg) in 30 mL of PBS containing lactose monohydrate (154 mg) or placebo (Pbo) alone 2 h/day 5 days a week for 4 wk using a nebulization tower, or by IP injection of 1.5 mg/kg of CSP7 or CP/day 5 days a week for 4 wk. A: all mice were subjected to lung volume measurements 20 wk after CS exposure. Total lung homogenates were analyzed for MCM, autophagy marker, and β-actin proteins (B) and their mRNAs (C). Lung sections of above mice were subjected to IHC staining for MUC5AC (D) and HDAC6 (E) (scale bar 100 µM). F: lung sections were subjected Periodic acid-Schiff (PAS) staining to visualize goblet cells and scale bar 20 µM. G: immunofluorescence staining for MUC5AC and HDAC6 colocalization (scale bar 100 µM). Total RNA from tracheal epithelial cells isolated from mice (n = 4) treated as in A and was analyzed for MUC5AC (H) and HDAC6 (I) mRNA by qPCR. Experiments were performed at least two times, and data are presented as means + SD and **P < 0.01 and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; HDAC6, histone deacetylase 6; MUC5AC, mucin 5AC; WT, wild type.
We sought to understand the mechanisms by which CS disrupts AECs in the respiratory tract and their impact on mucin secretion and airway function. Therefore, we analyzed lung homogenates of WT CS-LI mice left untreated or treated with CSP7 or CP for MUC5AC. Western blotting showed that CS-LI caused increased MUC5AC expression in WT mice (Fig. 4B). These changes were associated with reduced FOXA2 and acetylated alpha-tubulin, with augmented expression of HDAC6, SPDEF, and LC3BII, which were reversed following CSP7 treatment, either locally by nebulization or systemically by IP injection. We next analyzed lung homogenates for CAV1, p53, PAI-1, and LC3BII expression. Consistent with changes in AECs from COPD lungs, CS-LI in mice also increased CAV1, p53, and PAI-1. However, CS-LI mice treated with CSP7 via airways or systemically caused a marked inhibition of CAV1, p53, and PAI-1 (Fig. 4B). These changes were consistent with a significant reduction in MUC5AC, HDAC6, FOXA3, and CAV1 mRNA in CS-LI mice treated with CSP7, while increasing FOXA2 mRNA (Fig. 4C). Furthermore, IHC analysis of lung sections showed intense MUC5AC staining and thickening of the major airway in CS-LI mice. This was remarkably improved in CS-LI mice treated with CSP7 for 4 wk (Fig. 4D). This was further confirmed by visualization of goblet cells by PAS staining of the lung sections (Fig. 4F). We found increased staining for HDAC6, which was markedly reduced in WT mice with CS-LI exposed to CSP7 by either nebulization or IP injection (Fig. 4E). These changes were not evident in control CS-LI mice treated with CP. Immunofluorescence staining also revealed colocalization of MUC5AC and HDAC6 to thickened major airways in CS-LI mice, which was markedly reduced in those treated with CSP7, either via airways or by IP injection (Fig. 4G). Increased staining and colocalization of MUC5AC and HDAC6 in major airways were evident in mice exposed to placebo via airways or CP by intraperitoneal injection. Analysis of TECs isolated from CS-LI WT mice showed increased expression of MUC5AC and HDAC6 mRNA and was significantly reduced in CS-LI mice receiving CSP7 treatment (Fig. 4, H and I).
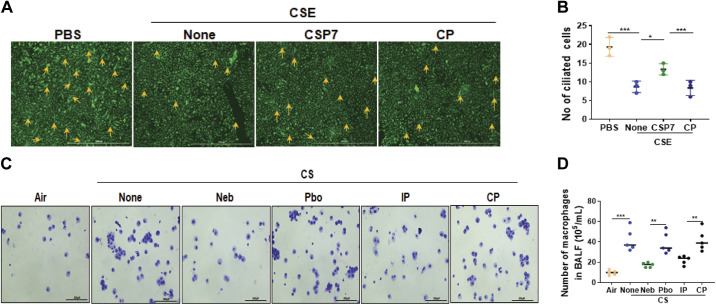
IHC analysis depicted reduced acetylated alpha-tubulin (Fig. 5A), and increased LC3B (Fig. 5B) and CAV1 (Fig. 5C) antigen staining, in lung sections of CS-LI WT mice. This was reversed after the treatment of CS-LI mice with CSP7. IHC analyses of tracheal sections showed a decrease in acetylated alpha-tubulin staining in WT mice with CS-LI, which was improved following treatment of these mice with CSP7 (Fig. 5D). Similarly, we found a marked decrease in the number of acetylated alpha-tubulin-positive AECs in WT CS-LI mice compared with their levels in ambient AIR kept control mice, suggesting the loss of ciliated cells due to lung injury. This trend was significantly reversed after the treatment of CS-LI WT mice with CSP7. CSP7 treatment also increased the number of ciliated TECs in WT mice with CS-LI (Fig. 5, E and F). Immunofluorescence staining further revealed a decrease in acetylated alpha-tubulin in AECs treated with CSE that was reversed with CSP7 treatment (Fig. 6, A and B). Staining of bronchoalveolar lavage cells from these mice revealed increased accumulation of macrophages in the lungs of mice with CS-LI (Fig. 6, C and D), which was significantly reduced in those treated with CSP7.
Figure 5.
CSP7 treatment attenuates airway epithelial injury in WT mice with CS-LI. IHC images (scale bar 100 µM) showing the expression of acetylated α-tubulin (cilia) (A) and LC3B (B) in lung sections of WT mice kept in ambient Air or exposed CS (CSE) for 20 wk with or without CSP7 (Neb and IP) treatment. C: IHC staining (scale bar 100 µM) for CAV1 in lung sections of CSE WT mice left untreated or treated with CSP7 (Neb) or placebo (Pbo) via airways by nebulization or treated with CSP7 or CP by intraperitoneal injection. Ambient air kept mice were used as controls. D: IHC analyses of lung trachea sections showed decrease in acetylated α-tubulin staining in CSE WT mice, which is reversed by CSP7 treatment (scale bar 200 µM). E: immunofluorescence imaging for acetylated α-tubulin (Ac-Tub) for cilia in TECs isolated from CSE WT mice treated as in A (scale bar 1,000 µm). F: graph showing number of Ac-Tub-positive cells. Experiments were repeated at least two times, and data are presented as means + SD and *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. CAV1, caveolin‐1; CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; WT, wild type.
Figure 6.
CSP7 inhibits cilia shortening in human AECs exposed to CSE in vitro, and macrophages accumulation in CS-LI mice. A: immunofluorescence staining (scale bar 1,000 µm) revealed decrease in acetylated α-tubulin (cilia) expression in AECs exposed to CSE that is reversed with CSP7 treatment. B: bar graph represents the changes in the number of ciliated cells under various treatment conditions. C: bronchoalveolar lavage (BAL) fluids were collected from WT mice (n = 10/group) kept in ambient Air or exposed to CS and treated with formulated CSP7 or placebo (Pbo) alone 2 h daily 5 days a week for 4 weeks using a Neb tower, or IP injected with 1.5 mg/kg of CSP7 or CP daily 5 days a week for 4 weeks. The BAL cells were stained with Diff-quick staining (scale bar 200 µM). D: bar graph represents the changes in the number of macrophages under various treatment conditions. Each experiment was repeated at least two to three times, and data are presented as means + SD and *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. AECs, airway epithelial cells; CSE, cigarette smoke extract; CS-LI, cigarette smoke-induced lung injury; CSP7, caveolin-1 scaffolding domain peptide; WT, wild type.
Effect of CSP7 on MH and Cilia Dysfunction in COPD Lung Tissues
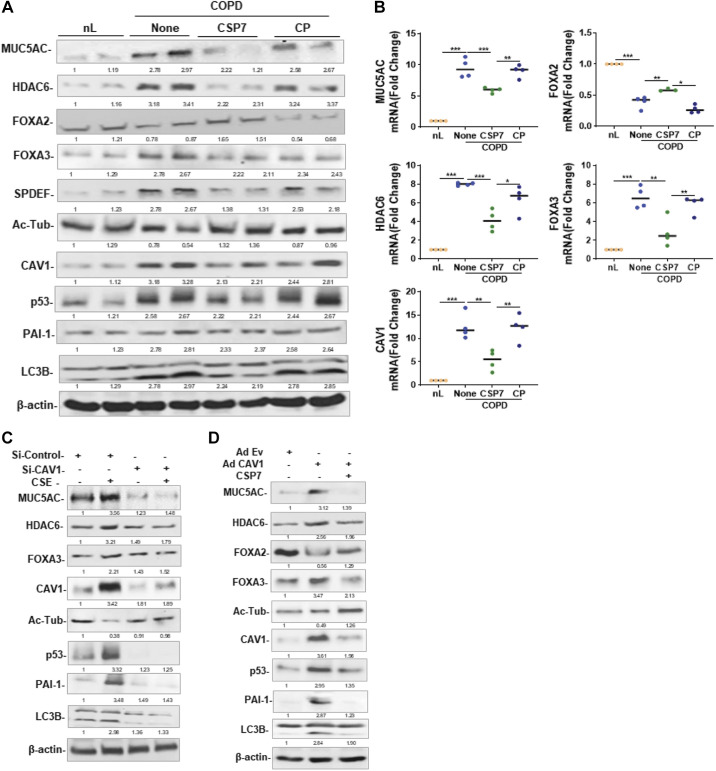
Human COPD lung tissues were treated with PBS or 10 µM CSP7 or CP ex vivo in culture dishes for 72 h. As shown in Fig. 7A, basal expressions of MUC5AC, HDAC6, SPDEF, FOXA3, and LC3BII were elevated, whereas expression of FOXA2 and acetylated alpha-tubulin was reduced in COPD tissues compared with their levels in nL tissues. Treatment of COPD tissues with CSP7 reduced MUC5AC and other mucous cell metaplasia (MCM) markers. These changes were associated with the suppression of baseline CAV1, p53, and PAI-1, suggesting that CSP7-mediated inhibition of p53 and downstream PAI-1 may provide a beneficial response. This was further confirmed by parallel inhibition of MUC5AC, HDAC6, FOXA3, and CAV1 mRNA with an increase in FOXA2 mRNA in CSP7-treated COPD tissues (Fig. 7B). However, COPD tissues failed to respond to CP treatment. AECs from COPD tissues showed increased CAV1, p53, and PAI-1 (Fig. 1A), suggesting CAV1-mediated induction of p53 and PAI-1 augments MUC5AC expression and MCM. To confirm this possibility, AECs from nL were transduced with CAV1 siRNA to block CAV1 expression. These cells were later treated with CSE. As shown in Fig. 7C, CSE failed to induce MUC5AC, HDAC6, and FOXA3 in AECs exposed to CAV1 siRNA. CSE also failed to inhibit acetylated alpha-tubulin in CAV1 siRNA-treated AECs. Consistent with the lack of acetylated alpha-tubulin, CAV1 siRNA-treated AECs exposed to CSE failed to induce p53 and PAI-1 expression. To confirm whether increased CAV1 can induce MUC5AC and MCM, we overexpressed CAV1 in AECs by adenovirus expressing CAV1 (Ad-CAV1). As shown in Fig. 7D, transduction of AECs with Ad-CAV1 increased MUC5AC, HDAC6, FOXA3, and SPDEF while reducing FOXA2 and acetylated alpha-tubulin, suggesting MCM. These changes were associated with the induction of p53 and PAI-1. Since CAV1 induces both p53 and PAI-1, and CSP or CSP7 inhibits CS-induced p53, PAI-1, and MUC5AC, we treated Ad-CAV1-treated AECs with CSP7 and found that CSP7 reduced CAV1-induced MUC5AC, HDAC6, FOXA3, and LC3BII while restoring FOXA2 and acetylated alpha-tubulin. In addition, CSP7 treatment of Ad-CAV1 transduced also reduced the expression of p53 and PAI-1.
Figure 7.
Effects of CSP7 on MUC5AC and MCC markers in human COPD tissues and AECs ex vivo. Human nL (n = 4) tissues from control donors and COPD lung (n = 4) tissues were left untreated or treated with 10 µM CSP7 or CP in dishes for 72 h. A: Western blotting of COPD lung tissues homogenates for MUC5AC, HDAC6, FOXA2, FOXA3, SPDEF, acetylated tubulin (Ac-Tub), CAV1, p53, PAI-1, LC3BII, and β-actin proteins. B: total RNA from nL and COPD tissues treated as in A was analyzed for MUC5AC, HDAC6, FOXA2, FOXA3, and CAV1 mRNAs by qPCR. C: AECs isolated from nL tissues were treated with control siRNA (siControl) or CAV1 siRNA (siCAV1), and cells were left untreated or treated with CSE. The lysates were immunoblotted for MUC5AC, HDAC6, FOXA3, Ac-Tub, CAV1, p53, PAI-1 LC3BII, and β-actin. D: AECs isolated from nL were transduced with Ad-Ev or Ad-Cav1. These cells were later treated with or without CSP7, and the lysates were tested for MUC5AC and other listed proteins by Western blotting. Experiment was repeated at least two times, and data are presented as means + SD and *P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. AECs, airway epithelial cells; CAV1, caveolin‐1; COPD, chronic obstructive pulmonary disease; CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; FOXA2, forkhead box protein A2; HDAC6, histone deacetylase 6; MUCAC, mucin 5AC; nL, “normal” lung; PAI-1, plasminogen activator inhibitor-1; SPDEF, domain-containing E26 transformation-specific like factor.
Role of p53 and PAI-1 in CS-LI-Induced MH and Cilia Dysfunction
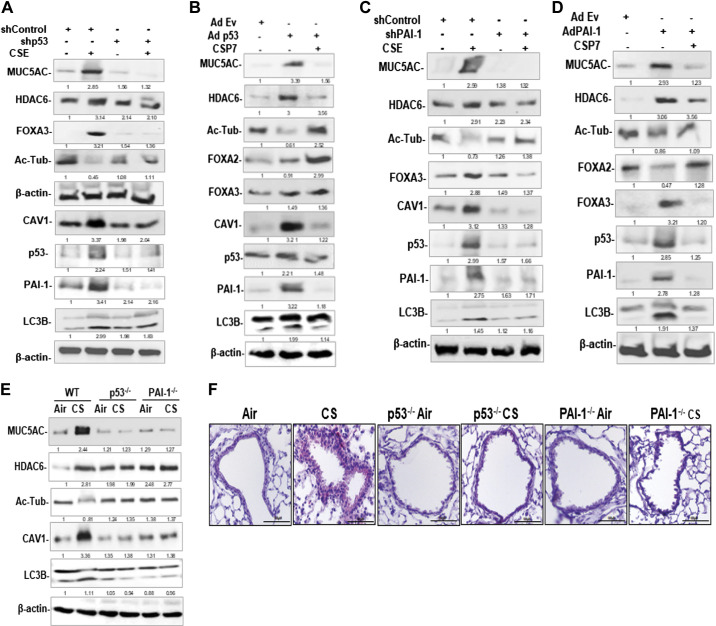
Since CS and CAV1 induce p53 expression in AECs, we investigated whether blockade of p53 mitigates MUC5AC and MCM. As shown in Fig. 8A, exposure of AECs from nL to CSE increased MUC5AC, HDAC6, FOXA3, acetylated alpha-tubulin, CAV1, p53, PAI-1, and LC3BII. However, transduction of AECs with lentivirus (Lv), expressing p53 shRNA (Lv-p53 shRNA), reduced CSE-induced MUC5AC, HDAC6, FOXA3, and LC3BII along with suppression of CAV1, p53, and PAI-1. These changes were associated with the restoration of acetylated alpha-tubulin, suggesting a reversal of MCM. Because CS-LI augments p53, and inhibition of p53 mitigates MUC5AC in AECs exposed to CSE, we sought whether overexpression of p53 mimics responses of CS exposure injury in AECs. As shown in Fig. 8B, overexpression of p53 in AECs from nL induced MUC5AC and inhibited acetylated alpha-tubulin and FOXA2. These changes were associated with a parallel increase in baseline CAV1 and PAI-1 in Ad-p53-treated cells. However, treatment of Ad-p53 transduced AECs with CSP7, reduced MUC5AC, and restored acetylated alpha-tubulin and FOXA2, while inhibiting CAV1, p53, and PAI-1. p53 augments PAI-1 expression by upregulating both mRNA transcription and stabilization. We, therefore, treated AECs with Lv-PAI-1 shRNA to prevent CSE-induced PAI-1. We found that inhibition of PAI-1 reduced MUC5AC, HDAC6, FOXA3, and LC3BII in AECs exposed to CSE while restoring acetylated alpha-tubulin (Fig. 8C). These changes were associated with inhibition of CAV1 and p53 in PAI-1-shRNA-treated AECs. To directly confirm increased PAI-1 contributes to MCM and MH, we transduced AECs isolated from nL with Ad-PAI-1. Control cells were exposed to Ad-Ev. As shown in Fig. 8D, overexpression of PAI-1 alone induced MUC5AC, HDAC6, FOXA3, p53, and LC3BII and reduced acetylated alpha-tubulin. These changes were reversed by treatment of Ad-PAI-1 transduced AECs with CSP7. To determine whether increased p53 or p53-mediated downstream induction of PAI-1 augments MUC5AC expression, we tested AECs isolated from WT and p53- and PAI-1-deficient mice exposed to CS for MUC5AC and MCM markers (HDAC6, FOXA3, and SPDEF) and compared the responses with AECs extracted from ambient AIR kept control mice. We found that CS-induced MUC5AC and MCM markers in WT mice. This induction was not observed in mice lacking p53 or PAI-1 expression (Fig. 8E). These changes were associated with resistance of p53- and PAI-1-deficient mice to CS-induced inhibition of LC3BII. IHC analysis of lung sections further confirmed resistance of p53- and PAI-1-deficient mice to CS-induced MH (Fig. 8F).
Figure 8.
Role of p53 and PAI-1 in CS-induced MH and cilia dysfunction in mice. A: AECs isolated from nL were transduced with Lv-p53 shRNA or control nonspecific shRNA. These cells were treated with or without CSE. AEC lysates were immunoblotted for MUC5AC, HDAC6, FOXA3, acetylated tubulin (Ac-Tub), CAV1, p53, PAI-1, and LC3BII. Same samples were analyzed for β-actin to assess equal loading. B: AECs from nL transduced with Ad-Ev or Ad-p53 were left untreated or treated with CSP7. Lysates of Ad-Ev, Ad-p53, and Ad-p53+CSP7 treated AECs were immunoblotted for listed proteins. C: AECs from nL transduced with Lv-PAI-1 shRNA or control nonspecific shRNA were later treated with or without CSE. The lysates were tested for above proteins by Western blotting. D: AECs transduced with Ad-Ev or Ad-PAI, and Ad-PAI-1-exposed AECs were later treated with or without CSP7. Lysates were immunoblotted for listed proteins. E: AECs isolated from ambient Air kept or CS-exposed (CS) WT, and p53- and PAI-1-deficient mice were immunoblotted for MUC5AC, HDAC6, Ac-Tub, CAV1, LC3BII, and β-actin. F: lung sections of WT, and p53- and PAI-1-deficient mice treated as in E were subjected to IHC staining for MUC5AC (scale bar 100 µM). AECs, airway epithelial cells; CAV1, caveolin‐1; CS, cigarette smoke; CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; HDAC6, histone deacetylase 6; MH, mucus hypersecretion; MUCAC, mucin 5AC; nL, “normal” lung; PAI-1, plasminogen activator inhibitor-1; WT, wild type.
Role of PP2A in CSP7-Mediated Inhibition of CS-LI-Induced MH and Ciliary Disassembly
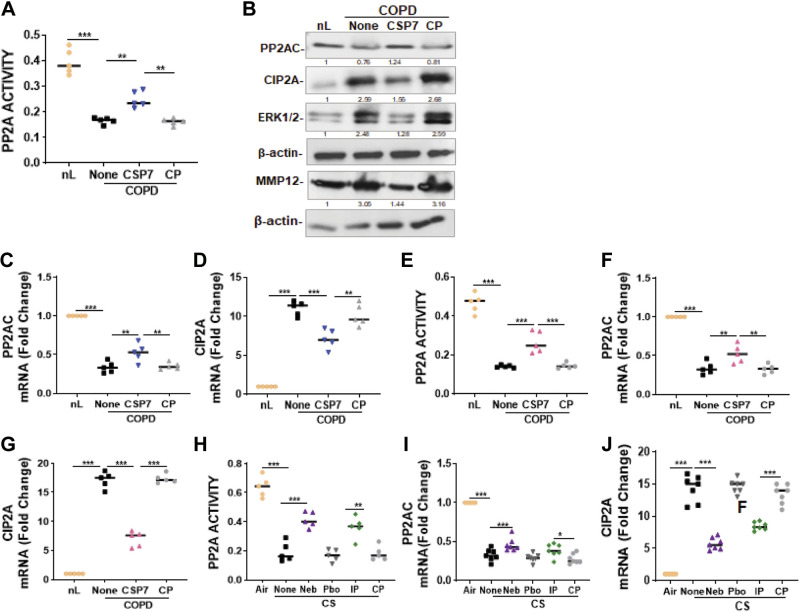
Since PP2A regulates ATM kinase activity and p53 expression during lung injury, we assessed PP2A activity and found a significant decrease in PP2A activity in AECs from COPD lungs compared with AECs from nL (Fig. 9A). This was significantly improved following treatment with CSP7, whereas treatment with CP failed to increase the PP2A activity in these cells. Western blotting of AEC lysates revealed a reduction in PP2A protein levels in AECs of COPD lungs, compared with AECs from nL (Fig. 9B). In addition to the reduction in PP2A protein, there was a marked increase in the expression of CIP2A; an endogenous inhibitor of PP2A. This was reversed by the treatment of AECs with CSP7. Consistent with a loss of PP2A function, ERK phosphorylation was increased in AECs from COPD lungs. This was reversed in AECs exposed to CSP7, while those treated with CP still showed elevated phospho-ERK1/2. These observations suggest that both ERK-mediated phosphorylation of PP2A, and CIP2A-mediated inhibition of PP2A activity, contribute to the loss of PP2A function during CS-LI. Since COPD pathogenesis is intricately linked to pulmonary MMP12 levels, we also investigated whether MMP12 was affected by the treatment of AECs from COPD lungs with CSP7. We found increased expression of MMP12 in AECs from COPD lungs and treatment with CSP7 markedly reduced the MMP12 level. This increase was markedly reduced after the treatment of AECs with CSP7. To further confirm the loss of PP2A expression in AECs from COPD lungs, we analyzed AECs for PP2A mRNA. Consistent with the suppression of PP2A protein, we found significant inhibition of PP2A mRNA in AECs from COPD lungs. This was improved in AECs treated with CSP7 (Fig. 9C). We also found a significant suppression of CIP2A mRNA following the treatment of AECs from COPD lungs with CSP7, which was otherwise increased in AECs of COPD lungs (Fig. 9D). This suggests that increased CIP2A likely reduced PP2A activity in AECs of COPD lungs.
Figure 9.
CSP7 restores PP2A activity by altering PP2A and CIP2A expression in COPD tissues, and in mice exposed to CS. A: AECs from nL and COPD lungs were treated with or without CSP7 or CP for 48 h and tested for PP2A activity. B: AEC lysates treated as in A were immunoblotted for PP2A, CIP2A, ERK1/2, and MMP12. Same samples were analyzed for β-actin to assess equal loading. Total RNA from AECs treated as in A was analyzed for PP2A (C) and CIP2A (D) mRNA. E: human nL (n = 5) and COPD lung (n = 5) tissues treated with PBS or 10 µM CSP7 or CP ex vivo in dishes for 72 h were tested for PP2A activity. RNA from nL and COPD tissues treated as in E were analyzed for PP2A (F) and CIP2A (G) mRNA. H: the lung homogenates from WT mice (n = 10/group) kept in ambient AIR or exposed to CS (CS) left untreated (None) or treated with CSP7 or placebo by nebulization, or by IP injection as in Fig. 4 were tested for PP2A activity. RNA for ambient Air and CS mice treated with or without CSP7 or CP was analyzed for PP2A (I) or CIP2A (J) mRNA by qPCR. Experiments were performed at least two to three times, and data are presented as means + SD and *P < 0.05, **P < 0.01 and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. AECs, airway epithelial cells ; COPD, chronic obstructive pulmonary disease; CSP7, caveolin-1 scaffolding domain peptide; nL, “normal” lung; PP2A, protein phosphatase 2 A; WT, wild type.
We then analyzed human lung tissues and found that PP2A activity was also reduced in lung tissues of patients with COPD compared with nL tissues from control donors. However, treatment of COPD lung tissues with CSP7 ex vivo caused a significant improvement in PP2A activity while CP had minimal effect in these tissues (Fig. 9E). Since reduced PP2A and increased CIP2A expression lowers PP2A activity in AECs isolated from COPD lungs (Fig. 9, A and B), we analyzed total RNA from nL and COPD tissues treated with or without CSP7 or CP for PP2A and CIP2A mRNA. Consistent with loss of PP2A activity, we found that PP2A mRNA levels were significantly reduced, whereas CIP2A mRNA was increased in COPD tissues compared with their level in nL (Fig. 9, F and G). Furthermore, treatment of COPD tissues with CSP7 significantly reversed both PP2A and CIP2A mRNA levels.
Consistent with the loss of PP2A activity in COPD tissues, we found that WT mice with CS-LI demonstrated a significant reduction in PP2A activity compared with ambient air kept control mice (Fig. 9H). Furthermore, WT mice with CS-LI, treated with CSP7 either locally using a nebulization tower or by IP injection for 4 wk, improved PP2A activity. Lungs of WT mice with CS-LI, exposed to placebo or CP, still showed loss of PP2A activity. Analysis of lung RNA for PP2A or CIP2A mRNA revealed that CS-LI inhibited PP2A mRNA while increasing CIP2A mRNA (Fig. 9, I and J). This was reversed in CS-LI mice that received CSP7.
CSP7 Inhibits p53 and Increases A2C Viability in COPD Lung Tissues
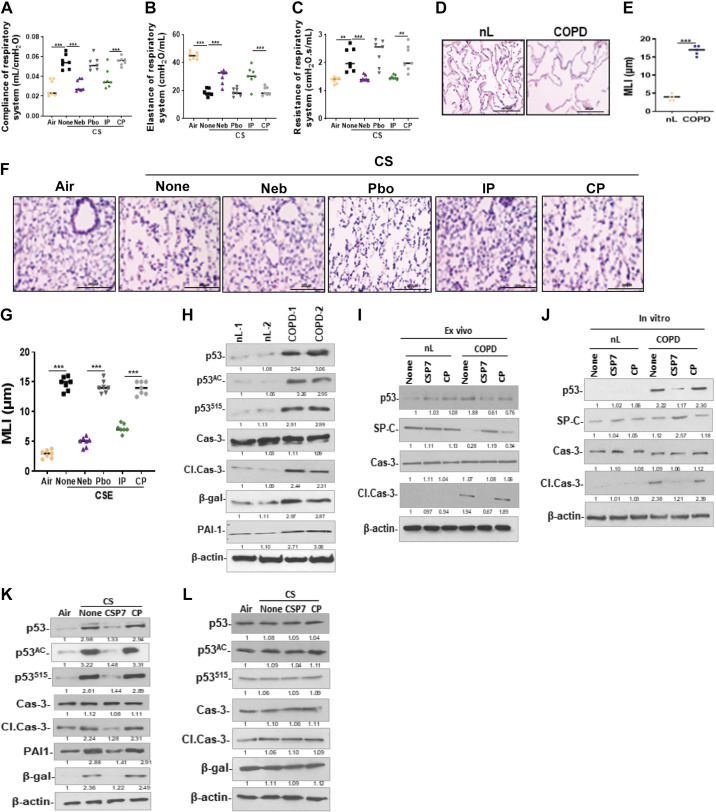
Since mice subjected to quantitative chest µCT 20 wk postexposure to CS showed increased lung volume, suggesting distal lung damage (Fig. 4A), we measured compliance, elastance, and resistance (Fig. 10, A–C). Pulmonary function testing by SCIREQ suggested alveolar damage reflected by increased lung volume and compliance, and reduced elastance. These pulmonary functions were significantly improved in mice with CS-LI following treatment with CSP7. Increased alveolar damage was reflected by the significant increase in the mean linear intercept (MLI) of COPD lung sections compared with the corresponding MLI of nL (Fig. 10, D and E). Consistent with alveolar damage in human COPD, analysis of H&E-stained lung sections (not shown), and measurement of MLI (Fig. 10, F and G) revealed a significant (P < 0.0001) alveolar damage in WT mice with CS-LI. Alveolar damage reflected by increased MLI in CS-LI mice was significantly (P < 0.0001) reduced after treatment with CSP7. However, CS-LI mice treated with CP continued to show alveolar damage.
Figure 10.
CSP7 inhibits p53 and improves viability in AT2 cells from mice with CS-LI and in human COPD lung tissues. WT mice (n = 10/group) were exposed to CS for 4 h/day 5 days a week. After 16 wk, CS-exposed (CS) WT mice were left untreated (None) or treated via airways to formulated CSP7 (Neb) or placebo (Pbo) alone 2 h daily 5 days a week for 4 wk using a nebulization tower. WT mice with CS-LI and IP injected CSP7 or CP daily 5 days a week for 4 wk were used as controls for comparison. Compliance of respiratory system (Crs; A), elastance of respiratory system (Ers; B), and resistance of respiratory system (Rrs; C). microscopic images of H&E-stained sections (scale bar 200 µM; D) and bar graph showing increased MLI in COPD lung than nL (E). Microscopic images of H&E-stained lung sections (scale bar 200 µM; F) and bar graph showing MLI in mice with CS-LI vs those kept in ambient AIR (G). H: AT2 cells isolated from nL and COPD lung tissues were analyzed for p53, PAI-1, senescence (β-Gal), apoptosis (Cl. Cas-3), and β-actin by Western blotting. I: AT2 cells isolated from human control (nL) and COPD lung tissues left untreated (None) or treated with CSP7 or CP for 72 h ex vivo were immunoblotted for p53, SP-C, Cl. Cas-3, Cas-3, and β-actin. J: lysates of AT2 cells isolated from nL and COPD lung tissues treated in vitro were immunoblotted for p53, SP-C, apoptosis, and β-actin. AT2 isolated from ambient AIR kept, or CS WT (K) or PAI-1-deficient (L) mice left untreated or treated with CSP7 or CP by IP injection were analyzed for above proteins by Western blotting. Each experiment was repeated at least two times, and data are presented as means + SD, and **P < 0.01 and ***P < 0.001 were obtained by one-way ANOVA with Tukey’s multiple comparison test and log-rank tests, respectively. COPD, chronic obstructive pulmonary disease; CSE, cigarette smoke extract; CSP7, caveolin-1 scaffolding domain peptide; HDAC6, histone deacetylase 6; H&E, hematoxylin and eosin; PAI-1, plasminogen activator inhibitor-1; WT, wild type.
Next, we analyzed AT2 cells isolated from the patients with COPD, which showed a marked increase in the total, acetylated, and serine 15 phosphorylated p53 protein, as well as activation of caspase-3 and β-galactosidase. Thus, implicating induction of p53 due to stabilization through posttranslational modifications such as acetylation and serine phosphorylation in increased apoptosis and senescence in AT2 cells (Fig. 10H). We next treated human COPD lung tissues with CSP7 ex vivo and compared the effects with similarly treated nL tissues from control subjects. We found that AT2 cells isolated from COPD lungs treated with CSP7 ex vivo showed reduction in p53 while restoring baseline SP-C expression (Fig. 10I). Similarly, ex vivo treatment of COPD lung tissues with CSP7 reduced active caspase-3 in AT2 cells, which are otherwise increased in AT2 cells isolated from COPD lung tissues left untreated or treated with CP, suggesting protection of AT2 cells through inhibition of apoptosis. Interestingly, CSP7 had minimal effect in nL tissues treated with CSP7 ex vivo. To further confirm that CSP7 inhibits p53 and apoptosis in injured AT2 cells, we isolated AT2 from COPD lung tissues and exposed to CSP7 in vitro. Consistent with changes observed in Fig. 10J, p53 expression and activation of caspase-3 were markedly increased in AT2 cells isolated from COPD lung tissues compared with their levels in AT2 cells from nL tissues (Fig. 10J). Furthermore, treatment of AT2 cells isolated from COPD lung tissues with CSP7 in vitro caused marked suppression of p53 and apoptosis, thereby restoring baseline SP-C level. However, p53 level and apoptosis remained elevated in AT2 cells from COPD lung tissues that were left untreated or treated with CP. AT2 cells isolated from nL tissues from subjects without COPD failed to respond to CSP7 treatment, suggesting its target specificity in injured AT2 cells with altered expression of p53.
Consistent with the changes observed in AT2 cells from human COPD lung tissues, immunoblotting of lysates of AT2 cells isolated from WT mice exposed to 20 wk of CS showed a marked increase in total, acetylated, and serine phosphorylated p53 along with induction of cleaved caspase-3 and β-galactosidase (Fig. 10K), suggesting reduced viability due to increased p53 in AT2 cells. This was markedly suppressed in AT2 cells of CS-LI mice exposed to CSP7 but not in those subjected to CP treatment. Consistent with loss of CS-induced p53, we found that that the expression of PAI-1 was reduced in AT2 cells of WT mice with CS-LI treated with CSP7. The expression of p53 and PAI-1 is increased in AT2 cells of mice following CS-LI; and p53 induces PAI-1 expression. We therefore exposed PAI-1−/− mice to CS for 20 wk to test the importance of increased PAI-1 expression in AT2 cells injury. As shown in Fig. 10L, Western blotting of isolated AT2 cells lysates showed little or no difference in the expression of p53, Acp53, S15p53, cleaved caspase-3, β-galactosidase in PAI-1-deficient mice kept in ambient AIR versus those exposed to CS with or without CSP7 or CP treatment, suggesting resistance of PAI-1-deficient mice to CS-LI. The findings suggest an intricate link between p53-mediated induction of major inhibitor of urokinase-type plasminogen activator (uPA)-fibrinolytic system and alveolar epithelial damage.
DISCUSSION
In the present study, we investigated the therapeutic potential of the novel peptide CSP7, a 7-mer deletion fragment of CAV1 using human AECs from patients with COPD and mouse model of CS-LI. We found elevated expressions of MUC5AC, SPDEF, and FOXA3, whereas FOXA2 was markedly downregulated in AECs from COPD lungs. FOXA3 affects mucus production and is involved in allergic airway hyperresponsiveness and is often detected in airway goblet cells of patients with COPD. In addition, FOXA3 binds and induces SPDEF transcription. The observed effects of FOXA3 on mucus-related gene expression are likely mediated, at least in part, by its ability to induce SPDEF despite FOXA3 being sufficient to induce goblet cell metaplasia (32, 33). Disruption of FOXA2 in epithelial cells causes airspace enlargement, neutrophilic infiltration, and MCM. We found that treatment of AECs from COPD lungs, or those exposed to CSE with CSP7 targeted genes most significantly associated with MCM.
Emerging evidence suggests that autophagy plays an important role in lung diseases, including COPD (34, 35). HDAC6 controls diverse cellular processes via deacetylating and destabilizing microtubules, thus facilitating the retrograde transport of ubiquitinated proteins into aggresomes (aggregation of misfolded proteins) and enhancing autophagosome-lysosome fusion (36, 37). Lam et al. (38) demonstrated that CS shortens cilia through an autophagy-dependent process termed “ciliophagy” mediated by HDAC6. In our study, we found that CSP7 inhibited the expression of HDAC6 and the autophagy pathway. In chronic oxidative stress, ciliary proteins are delivered to the lysosome for degradation or recycling, resulting in a shortening of airway cilia leading to impaired MCC (38, 39). We observed an increase in HDAC6 and upregulation of autophagy markers, with cilia shortening in AECs of COPD and CS-LI mouse lungs. Immunofluorescence imaging showed the colocalization of acetylated alpha-tubulin and LC3B, suggesting an interaction of cilia components and autophagosomes, which is reversed following the treatment with CSP7. Our group and others reported that CS induces AT2 cell damage. The process involves increased expression of CAV1 through activation of the ATM-p53-p21 pathway (40). In addition, CS-induced activation of EGFR and AKT through phosphorylation can contribute to AEC proliferation and metaplasia. The downregulation of CAV1 inhibits MUC5AC production, while conversely, CAV1 enhances CS-induced MH through the activation of EGFR and AKT (41). CAV1 reportedly regulates airway inflammation and aggravates lung injury. In our study, we sought to determine whether CAV1 modulates MH and ciliary dysfunction induced by CS. Our results revealed that elevated expression of CAV1 alone augments MH and ciliary disassembly in AECs often associated with COPD, suggesting CAV1 as a novel therapeutic target. We also found that forced expression of CAV1 enhanced MUC5AC and MCC-related genes. Conversely, the inhibition of CAV1 using siRNA reduced MH and ciliary dysfunction in AECs exposed to CSE.
A study by Siganaki et al. (42) reported an increase in p53 protein levels in COPD lungs. Our data demonstrate increased p53 and PAI-1 levels in COPD lungs. We also observed an increased p53 and PAI-1 expression in the in vitro and in vivo models of CS-LI. A gain-of-function experiment using a p53 and PAI-1 expressing adenoviral transfection demonstrated that overexpression of either p53 or PAI-1 in the AECs increased MUC5AC and MCM-related genes. A loss-of-function using transfection with p53 and PAI-1 shRNA decreased CS-induced secretion of MUC5AC. We showed that inhibition of p53 from binding to the endogenous PAI-1 mRNA in AECs, by either suppressing the expression of p53 or blockade of p53 interactions with the PAI-1 mRNA, mitigates CS-LI (25, 27). Furthermore, our earlier reports and others reveal that CAV1 expression is required for CS-induced activation of the p53 and PAI-1 pathways (43, 44). We demonstrated that CSP7 mitigates cilia shortening and improves MCC by interfering with CAV1-mediated induction of p53 and downstream PAI-1 expression by increased p53 in AECs.
We observed that AECs from subjects with COPD have reduced PP2A activity as well as increased CIP2A expression. An increase in CIP2A leads to phosphorylation of ERK and secretion of MMP12, with loss of PP2A activity (28, 45). Moreover, increased CAV1 induced the expression p53 and PAI-1 in AECs. This is often associated with COPD pathogenesis. CSE elicits binding of CAV1 to the catalytic subunit of PP2A, which in turn could downregulate PP2A activity (46). In addition, enhanced CIP2A suppresses PP2A activity in AECs. Furthermore, we observed phosphorylation of ERK and secretion of MMP12 ensue. Also, CS-induced stress can lead to cilia damage, ubiquitination, and the formation of intracellular protein aggregates. Moreover, HDAC6 recognizes damaged ciliary proteins and delivers them to the autophagosome, a process reliant on autophagy proteins. The damaged ciliary proteins are eventually delivered into the lysosome for degradation or recycling. As a result of the stress induced by CS, ciliary proteins are degraded, resulting in a shortening of airway cilia that contributes to impaired MCC. We, therefore, presume CAV1 to be a key player of a novel signaling pathway that links CS to MH and ciliary disassembly.
Our study clearly reveals that CSP7 mitigates cilia shortening and impaired MCC by interfering with CAV1-mediated AEC signaling pathways. This includes the downregulation of the ERK phosphorylation, expression of MMP12, and the inhibitor of PP2A, CIP2A. These findings provide not only new insights on how CSP7 regulates complex interrelationships between p53, PAI-1, autophagy, and primary cilia, but also the possibilities for the treatment of the ciliopathies often associated with MH. Our findings suggest that CSP7 could be a potential new therapeutic intervention to improve airway dysfunction associated with COPD pathogenesis.
Evidence from several studies indicates that AEC and AT2 cell apoptosis and defective alveolar fibrinolysis due to a disproportionate increase in PAI-1 expression are often associated with CS-LI, including COPD. Along these lines, PAI-deficient mice also resisted AT2 cell apoptosis, and the responses of p53- and PAI-1-deficient mice were very similar. The proportion of AT2 cells undergoing apoptosis and senescence is markedly increased in lungs of patients with COPD compared with healthy subjects. CS augments both PAI-1 and p53 expression in AT2 cells. We further found that treatment of isolated AT2 cells from COPD tissues in vitro as well as ex vivo treatment of COPD tissues with CSP7 improved viability by inhibiting senescence and apoptosis. The process involves suppression of CS-induced p53 and downstream PAI-1 expression. Our findings not only clarify the potential mechanisms of CS-induced alveolar and airway damage but also extend the understanding about how CSP7 mitigates the airway and alveolar epithelial injury. Considering the potential therapeutic properties of CSP7, our research provides several lines of evidence for the application of CSP7 in treatment of COPD and CS-LI. Our studies using AECs, AT2 cells, and human COPD tissue explants, as well as mouse model of CS-LI in vivo suggest that CSP7 has the potential to target MH and distal lung damage often associated with COPD.
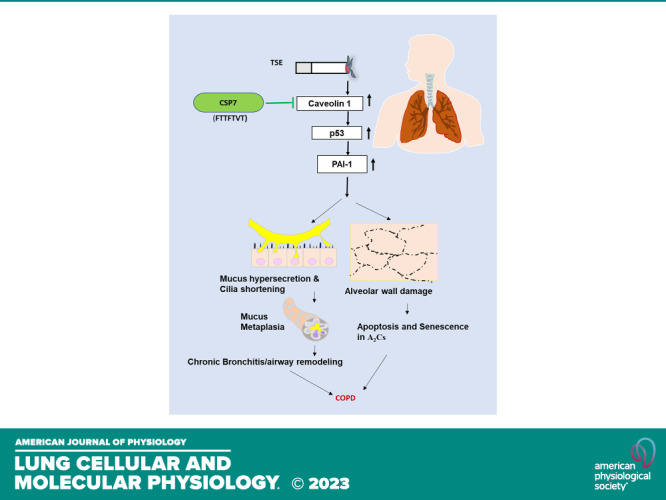
In summary, we show that elevated expression of CAV1 caused MCM and MH through induction of p53 and PAI-1 expression in AECs from the lungs of patients with COPD and mice with CS-LI. Furthermore, CS-LI downregulated PP2A activity and increased the expression of CIP2A leading to increased phosphorylation of ERK and secretion of MMP12. In addition, we found increased expression of p53 and PAI-1 and reduced viability due to induction of senescence and apoptosis in AT2 cells from lungs of patients with COPD or mice with CS-LI. These changes are all reversed by a seven amino acid (FTTFTVT) CAV1 scaffolding domain peptide, CSP7. We further show that CSP7 mitigates cilia shortening and improves MCC. These findings provide not only new insights on how CSP7 regulates complex interrelationships between p53 and PAI-1 expression, autophagy, and primary cilia in AECs and loss of AT2 cells viability but also the possibilities for the treatment of MH and associated ciliopathies and alveolar damage. Our findings suggest a dual role of CSP7 on AECs and AT2 cells as a potential new treatment to improve lung function during chronic lung diseases such as COPD through the maintenance of AEC proteostasis and modulation of the autophagic pathway and alveolar epithelial regeneration. Furthermore, our findings demonstrate that CS-LI can be reduced by systemic or airway delivery of CSP7. Phase 1 clinical trial testing of this peptide has been completed and phase 2 testing in patients with COPD is underway.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This work was supported in part by NIH Grants HL133067-01, HL15139701A1, ES025815, and ES032506, Department of Defense Award W81X WH-20-1-0142, and Flight Attendant Medical Research Institute (FAMRI) Clinical Innovator Award Grant 150063 (to S.S.).
DISCLOSURES
S. Shetty has patents issued for the use of the CSP, CSP7 for the treatment of chronic lung diseases, including idiopathic pulmonary fibrosis (IPF) and COPD. S. Shetty is a consultant and investor of Lung Therapeutics, Inc, a University of Texas start-up biotechnology firm that is commercializing CSP7 for the treatment of IPF. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
S.S. conceived and designed research; D.N.D., B.P., V.G., V.K., A.K.B., S.B., N.M., H.T., and L.F. performed experiments; D.N.D., A.K.B., S.B., G.J.C., N.M., H.T., L.F., and S.S. analyzed data; Y.S. and S.S. interpreted results of experiments; D.N.D. and L.F. prepared figures; D.N.D. and S.S. drafted manuscript; D.N.D., Y.S., N.V.K., and S.S. edited and revised manuscript; A.K.B., G.J.C., N.M., N.V.K., L.F., and S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank B. Starcher for editing the manuscript.
REFERENCES
- 1. Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 180: 3–10, 2009. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 2. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3. Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol 42: 268–275, 2010. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Decramer M, Janssens W. Mucoactive therapy in COPD. Eur Respir Rev 19: 134–140, 2010. doi: 10.1183/09059180.00003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mortaz E, Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, Folkerts G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim Biophys Acta 1812: 1104–1110, 2011. doi: 10.1016/j.bbadis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 6. Brüggemann TR, Fernandes P, Oliveira LM, Sato MN, Martins MA, Arantes-Costa FM. Cigarette smoke increases CD8α+ dendritic cells in an ovalbumin-induced airway inflammation. Front Immunol 8: 718, 2017. doi: 10.3389/fimmu.2017.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie B, Laxman B, Hashemifar S, Stern R, Gilliam TC, Maltsev N, White SR. Chemokine expression in the early response to injury in human airway epithelial cells. PLoS One 13: e0193334, 2018. doi: 10.1371/journal.pone.0193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Feng X, Zheng D, Li A, Li C, Li S, Zhao Z. Ergosterol attenuates cigarette smoke extract-induced COPD by modulating inflammation, oxidative stress and apoptosis in vitro and in vivo. Clin Sci (Lond) 133: 1523–1536, 2019. [Erratum in Clin Sci (Lond) 133: 2237, 2019]. doi: 10.1042/CS20190331. [DOI] [PubMed] [Google Scholar]
- 9. Yu X, Seow HJ, Wang H, Anthony D, Bozinovski S, Lin L, Ye JM, Vlahos R. Matrine reduces cigarette smoke-induced airway neutrophilic inflammation by enhancing neutrophil apoptosis. Clin Sci (Lond) 133: 551–564, 2019. doi: 10.1042/CS20180912. [DOI] [PubMed] [Google Scholar]
- 10. Ning Y, Shang Y, Huang H, Zhang J, Dong Y, Xu W, Li Q. Attenuation of cigarette smoke-induced airway mucus production by hydrogen-rich saline in rats. PLoS One 8: e83429, 2013. doi: 10.1371/journal.pone.0083429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen R, Liang Y, Ip MSM, Zhang KY, Mak JCW. Amelioration of cigarette smoke-induced mucus hypersecretion and viscosity by dendrobium officinale polysaccharides in vitro and in vivo. Oxid Med Cell Longev 2020: 8217642, 2020. doi: 10.1155/2020/8217642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70: 459–486, 2008. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 13. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 180: 388–395, 2009. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 117: 978–988, 2007. doi: 10.1172/JCI29176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest 125: 2021–2031, 2015. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131: 953–964, 2004. doi: 10.1242/dev.00966. [DOI] [PubMed] [Google Scholar]
- 17. Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol 184: 6133–6141, 2010. doi: 10.4049/jimmunol.1000223. [DOI] [PubMed] [Google Scholar]
- 18. Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189: 301–313, 2014. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6: 463–477, 2004. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 20. Roscioli E, Hamon R, Lester SE, Jersmann HPA, Reynolds PN, Hodge S. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD. Respir Res 19: 234, 2018. doi: 10.1186/s12931-018-0945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective rPole of heme oxygenase-1. Autophagy 4: 887–895, 2008. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 22. Ryter SW, Chen ZH, Kim HP, Choi AM. Autophagy in chronic obstructive pulmonary disease: homeostatic or pathogenic mechanism? Autophagy 5: 235–237, 2009. doi: 10.4161/auto.5.2.7495. [DOI] [PubMed] [Google Scholar]
- 23. Shetty S, Idell S. Caveolin-1-related intervention for fibrotic lung diseases. Cells 12: 554, 2023. doi: 10.3390/cells12040554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galbiati F, Volonté D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell 12: 2229–2244, 2001. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shetty SK, Bhandary YP, Marudamuthu AS, Abernathy D, Velusamy T, Starcher B, Shetty S. Regulation of airway and alveolar epithelial cell apoptosis by p53-Induced plasminogen activator inhibitor-1 during cigarette smoke exposure injury. Am J Respir Cell Mol Biol 47: 474–483, 2012. doi: 10.1165/rcmb.2011-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marudamuthu AS, Bhandary YP, Fan L, Radhakrishnan V, MacKenzie B, Maier E, Shetty SK, Nagaraja MR, Gopu V, Tiwari N, Zhang Y, Watts AB, Williams RO 3rd, Criner GJ, Bolla S, Marchetti N, Idell S, Shetty S. Caveolin-1-derived peptide limits development of pulmonary fibrosis. Sci Transl Med 11: eaat2848, 2019. doi: 10.1126/scitranslmed.aat2848. [DOI] [PubMed] [Google Scholar]
- 27. Bhandary YP, Shetty SK, Marudamuthu AS, Midde KK, Ji HL, Shams H, Subramaniam R, Fu J, Idell S, Shetty S. Plasminogen activator inhibitor-1 in cigarette smoke exposure and influenza A virus infection-induced lung injury. PLoS One 10: e0123187, 2015. doi: 10.1371/journal.pone.0123187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nath S, Ohlmeyer M, Salathe MA, Poon J, Baumlin N, Foronjy RF, Geraghty P. Chronic cigarette smoke exposure subdues PP2A activity by enhancing expression of the oncogene CIP2A. Am J Respir Cell Mol Biol 59: 695–705, 2018. doi: 10.1165/rcmb.2018-0173OC. [DOI] [PubMed] [Google Scholar]
- 29. Lam HC, Choi AM, Ryter SW. Isolation of mouse respiratory epithelial cells and exposure to experimental cigarette smoke at air liquid interface. J Vis Exp 21: 2513, 2011. doi: 10.3791/2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prodromou NV, Thompson CL, Osborn DP, Cogger KF, Ashworth R, Knight MM, Beales PL, Chapple JP. Heat shock induces rapid resorption of primary cilia. J Cell Sci 125: 4297–4305, 2012. doi: 10.1242/jcs.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JY, Koga H, Kawaguchi Y, Tang W, Wong E, Gao YS, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao TP. HDAC6 controls autophagosome maturation essential for ubiquitin- selective quality control autophagy. EMBO J 29: 969–980, 2010. doi: 10.1038/emboj.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol 34: 527–536, 2006. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 is induced in asthma and promotes allergen induced mucin overproduction. Am J Respir Cell Mol Biol 47: 178–185, 2012. doi: 10.1165/rcmb.2011-0421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryter SW, Nakahira K, Haspel JA, Choi AM. Autophagy in pulmonary diseases. Annu Rev Physiol 74: 377–401, 2012. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 35. Wu YF, Li ZY, Dong LL, Li WJ, Wu YP, Wang J, Chen HP, Liu HW, Li M, Jin CL, Huang HQ, Ying SM, Li W, Shen HH, Chen ZH. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy 16: 435–450, 2020. doi: 10.1080/15548627.2019.1628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129: 1351–1363, 2007. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandey UB, Nie Z, Batlevi Y, McCray BA, Ritson GP, Nedelsky NB, Schwartz SL, DiProspero NA, Knight MA, Schuldiner O, Padmanabhan R, Hild M, Berry DL, Garza D, Hubbert CC, Yao TP, Baehrecke EH, Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447: 859–863, 2007. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 38. Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, An CH, Shan B, Franks JM, Haley KJ, Owen CA, Tesfaigzi Y, Washko GR, Quackenbush J, Silverman EK, Rahman I, Kim HP, Mahmood A, Biswal SS, Ryter SW, Choi AM. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest 123: 5212–5230, 2013. [Erratum in J Clin Invest 130:6189, 2020]. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cloonan SM, Lam HC, Ryter SW, Choi AM. Ciliophagy”: The consumption of cilia components by autophagy. Autophagy 10: 532–534, 2014. doi: 10.4161/auto.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volonte D, Galbiati F. Caveolin-1, cellular senescence and pulmonary emphysema. Aging (Albany NY) 1: 831–835, 2009. doi: 10.18632/aging.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu Q, Chen X, Fang X, Chen Q, Hu C. Caveolin-1 aggravates cigarette smoke extract-induced MUC5AC secretion in human airway epithelial cells. Int J Mol Med 35: 1435–1442, 2015. doi: 10.3892/ijmm.2015.2133. [DOI] [PubMed] [Google Scholar]
- 42. Siganaki M, Koutsopoulos AV, Neofytou E, Vlachaki E, Psarrou M, Soulitzis N, Pentilas N, Schiza S, Siafakas NM, Tzortzaki EG. Deregulation of apoptosis mediators' p53 and bcl2 in lung tissue of COPD patients. Respir Res 11: 46, 2010. doi: 10.1186/1465-9921-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Volonte D, Kahkonen B, Shapiro S, Di Y, Galbiati F. Caveolin-1 expression is required for the development of pulmonary emphysema through activation of the ATM-p53-p21 pathway. J Biol Chem 284: 5462–5466, 2009. doi: 10.1074/jbc.C800225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shetty S, Shetty P, Idell S, Velusamy T, Bhandary YP, Shetty RS. Regulation of plasminogen activator inhibitor-1 expression by tumor suppressor protein p53. J Biol Chem 283: 19570–19580, 2008. doi: 10.1074/jbc.M710268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 23: 9389–9404, 2003. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhandary YP, Shetty SK, Marudamuthu AS, Fu J, Pinson BM, Levin J, Shetty S. Role of p53-fibrinolytic system cross-talk in the regulation of quartz-induced lung injury. Toxicol Appl Pharmacol 283: 92–98, 2015. doi: 10.1016/j.taap.2015.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.