Keywords: chronic hypoxia, hypoxia-inducible factor-1α, kidney injury, microRNA, vascular endothelial growth factor
Abstract
Previously, we found that the incidence of kidney injury in patients with chronic hypoxia was related to the partial pressure of arterial oxygen. However, at oxygen concentrations that contribute to kidney injury, the changes in the relationship between microRNAs (miRNAs) and the hypoxia-inducible factor-1α (HIF-1α)-vascular endothelial growth factor (VEGF) axis and the key miRNAs involved in this process have not been elucidated. Therefore, we elucidated the relationship between VEGF and kidney injury at different oxygen concentrations and the mechanisms mediated by miRNAs. Sprague–Dawley rats were exposed to normobaric hypoxia and categorized into six groups based on the concentration of the oxygen inhaled and injection of the angiogenesis inhibitor bevacizumab, a humanized anti-VEGF monoclonal antibody. Renal tissue samples were processed to determine pathological and morphological changes and HIF-1α, VEGF, and miRNA expression. We performed a clustering analysis of high-risk pathways and key hub genes. The results were validated using two Gene Expression Omnibus datasets (GSE94717 and GSE30718). As inhaled oxygen concentration decreased, destructive changes in the kidney tissues became more severe. Although the kidney possesses a self-protective mechanism under an intermediate degree of hypoxia (10% O2), bevacizumab injections disrupted this mechanism, and VEGF expression was associated with the ability of the kidney to repair itself. rno-miR-124-3p was identified as a crucial miRNA; a key gene target, Mapk14, was identified during this process. VEGF plays an important role in kidney protection from injury under different hypoxia levels. Specific miRNAs and their target genes may serve as biomarkers that provide new insights into kidney injury treatment.
NEW & NOTEWORTHY Renal tolerance to hypoxic environments is limited, and the degree of hypoxia does not show a linear relationship with angiogenesis. VEGF plays an important role in the kidney’s self-protective mechanism under different levels of hypoxia. miR-124-3p may be particularly important in kidney repair, and it may modulate VEGF expression through the miR-124-3p/Mapk14 signaling pathway. These microRNAs may serve as biomarkers that provide new insights into kidney injury treatment.
INTRODUCTION
Vascular endothelial growth factor (VEGF) expression is induced by hypoxia-inducible factor-1α (HIF-1α) under hypoxic conditions, and this HIF-1α/VEGF axis is associated with renal repair (1–4). Our previous study found that the incidence of kidney injury in patients with chronic hypoxia was related to the partial pressure of arterial oxygen () (5). Another study found that kidneys have a particular ability to self-repair in a hypoxic environment, and this repair ability is closely related to the HIF-1α/VEGF axis (6). However, we previously demonstrated limited renal tolerance to a hypoxic environment. Although HIF-1α expression increased in a severe hypoxic environment, it was not linearly correlated with VEGF expression, and the corresponding kidney injury was aggravated (6). Previous studies have shown that angiogenesis plays a crucial role in kidney repair (7, 8). We speculate that severe hypoxia may affect VEGF expression and disrupt the balance of renal self-repair, whereas mild hypoxia can elicit adaptive repair responses in rat kidneys. Bevacizumab, a humanized anti-VEGF monoclonal antibody, has been approved for inhibiting VEGF from binding to its receptors [Fms-related tyrosine kinase 1 (Flt-1) and kinase insert domain containing receptor (KDR)] on the endothelial cell surface to reduce angiogenesis (9). A previous study has shown that bevacizumab could neutralize VEGF-A and thus induce VEGF-A-depletion in rat kidneys (10). This contributes to the method for exploring the relationship between VEGF expression and kidney injury and repair.
The regulation of VEGF expression is complex and is affected by multiple factors, including hypoxia, signal transducer and activator of transcription (STAT) signaling, and microRNAs (miRNAs) (11). Recently, miRNAs have attracted increasing attention for their potential role in kidney injury. Several studies have demonstrated that miRNAs are involved in almost all physiological and pathological processes of kidney injury, including apoptosis, proliferation, and metabolism (12–14). For example, Lin et al. (15) reported that miR-423-5p induced cell cycle arrest and apoptosis by inhibiting the AKT/CDK2-FOXO1 pathway after severe renal damage, whereas Peng et al. (16) reported that the expression levels of VEGF appeared to be related to those of AKT. Xue et al. (17) showed that miR-376c improved renal function after ischemia-reperfusion injury by promoting angiogenesis through the HIF-1α/VEGF pathway, whereas Li et al. (18) found that overexpression of miR-126-5p could decrease the expression of VEGF. Different miRNAs have various effects on VEGF. However, currently, it is unclear how miRNA expression changes in specific networks impact VEGF expression in kidneys at different oxygen concentrations.
Therefore, in this study, we aimed to understand the significance of VEGF and analyze differences in miRNA expression levels in the kidneys under different degrees of hypoxia, thereby exploring the relationship between miRNAs and VEGF and establishing a foundation for treating kidney injury.
MATERIALS AND METHODS
Animals and Experimental Procedures
Sixty 5-wk-old male Sprague–Dawley rats (weighing 142.3 ± 7.2 g, Xinhua Hospital Experimental Animal Center) were used in this study. The chronic hypoxia model was developed according to methods described in our previous study (6). Rats were randomly distributed into the following six groups according to the different concentrations of oxygen inhaled and bevacizumab injections (each group comprised five animals): 21% O2 and saline-containing placebo injection [normoxic condition + normal saline (N + NS)], 21% O2 and bevacizumab injection [normoxic condition + bevacizumab (N + Bev)], 10% O2 and saline-containing placebo injection [mild hypoxia condition + normal saline (M + NS)], 10% O2 and bevacizumab injection [mild hypoxia condition + bevacizumab (M + Bev)], 7% O2 and saline-containing placebo injection [severe hypoxia condition + normal saline (S + NS)], and 7% O2 and bevacizumab injections [severe hypoxia condition + bevacizumab (S + Bev)]. Each group was further categorized into two subgroups according to the duration of hypoxia (1 or 2 wk).
Drug Injection and Sample Collection
Bevacizumab was administered in accordance with a previously published method (10, 19). Rats received 5 mg/kg bevacizumab (HY-P9906, MCE, Monmouth Junction, NJ) two times a week via the tail vein. Bevacizumab was diluted in 0.9% physiological saline (5 mg/mL) that also served as a placebo, and the process was performed as described in the MCE procedure manual. After modeling, all rats were euthanized, blood samples were collected from the abdominal aorta using EDTA vacuum collecting tubes, and the kidneys were promptly excised. The left kidney was flash frozen and immediately stored in a liquid nitrogen container for miRNA sequencing analysis, whereas the right kidney was soaked in 4% paraformaldehyde solution for fixation before staining and immunohistochemical examination.
Histopathological Examinations
Hematoxylin and eosin (H&E) and Masson trichrome (MT) stainings were used to assess renal histological damage. Two pathologists (K.C. and J.Z.) blinded to the assigned groups’ identity evaluated the histological sections for signs of kidney injury to objectively examine histological changes. Renal damage was assessed using a semiquantitative scale according to the percentage of damaged tubules among total tubules as follows: 0, normal; 1, <25% damage; 2, 25–50% damage; 3, 50–75% damage; 4, 75–90% damage; and 5, >90% damage (20). For the quantitative analysis of fibrotic areas, the blue-stained areas in MT-stained sections were measured using an image analyzer (ImageJ, National Institutes of Health, Bethesda, MD). Expression levels of HIF-1α and VEGF were measured using Western blot analysis and immunohistochemistry. H&E staining, MT staining, immunohistochemistry staining, and Western blot analysis were all performed as previously described (6).
miRNA Sequencing and Analysis
Kidneys from four groups (M + NS, M + Bev, S + NS, and S + Bev) of rats euthanized on day 14 were obtained for miRNA analysis. Specifically, we first analyzed all rats that received bevacizumab compared with those that received normal saline. Subsequently, we compared miRNAs between different oxygen concentrations in rats that received bevacizumab and those that received normal saline. The mature miRNAs were identified through alignment against the miRBase v22 database (http://www.mirbase.org/) (21), and their expression patterns in different samples were analyzed. Differentially expressed miRNAs were determined using the Audic Claverie test [q value < 0.05, fold change (FC) > 2, or FC < 0.5]. Moreover, small RNA sequencing and analysis were conducted by OE Biotech (Shanghai, China). The raw data are available on National Center for Biotechnology Information (NCBI) BioProject (PRJNA915839). We performed three repeated trials for the same stimulation and three biological replicates for expression analysis to obtain reliable experimental data and reduce experimental error.
Quantitative RT-PCR
The quantification of individual miRNAs was performed using a two-step reaction process as follows: reverse transcription and PCR. The primer sequences were designed in the laboratory and synthesized by TsingKe Biotech based on the following mRNA sequences obtained from the NCBI database: 5′- GCACGCGGTGAATGCCAAA-3′ (rno-miR-124-3p forward primer) and 5′- GATCGCCCTTCTACGTCGTAT-3′ (rno-miR-124-3p reverse primer). miRNA expression was normalized to expression levels of 5S rRNA and calculated using the 2−ΔΔCt method (where Ct is threshold cycle), where ΔΔCt = (ΔCt experimental sample − ΔCt control sample) and ΔCt = (Ct sample − Ct endogenous control) (22).
Prediction of miRNA Target Genes
Target gene prediction was performed using information from the following databases: DIANA TOOLS (http://diana.imis.athena-innovation.gr/), TargetScan (http://www.targetscan.org/vert_71/), and miRDB (http://mirdb.org/). In addition, genes identified as targets in the three databases were analyzed. Gene ontology (GO) biological process enrichment analysis was performed using DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/). Significantly enriched GO terms (false discovery rate < 0.05) were represented as a bubble plot generated from http://www.bioinformatics.com.cn, a free online platform for data analysis and visualization. Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to annotate protein pathways; these pathways were classified according to the KEGG website (http://www.kegg.jp/) pathway hierarchy classification method.
The Search Tool for the Retrieval of Interacting Genes/Proteins was used to construct and visualize the protein-protein interaction network using Cytoscape software (version 3.9.0, http://www.cytoscape.org/, Boston, MA). The Maximal Clique Centrality algorithm in the cytoHubba plugin was used to explore important nodes in the network. In cytoHubba, we selected the top five nodes and excluded genes with degree < 10. KEGG pathway maps were customized by uploading KEGG orthology numbers through the user data mapping function on the KEGG website.
Plasmids and Luciferase Assay
Target relationships between miRNAs and target genes were validated using a dual-luciferase reporter assay. The inserts for the constructs were obtained using PCR amplification (PCR primer sequences are provided in Supplemental Table S1), directly cloned into the pmirGLO vector (Wuhan BioHealth Technology, Wuhan, China), and subsequently transformed into Escherichia coli strain DH5a (E. coli DH5a, Wuhan BioHealth Technology). Rat kidney-52E (NRK-52E) cells were acquired from Procell Life Science and Technology (Wuhan, China). Cells were passaged once they reached 80–90% confluency; subsequently, stable transfection was performed. NRK-52E cells were cotransfected with wt-3′-UTR with either rno-miR-124-3p or NC and mut-3′-UTR with either rno-miR-124-3p or NC. After cotransfection for 48 h, a dual-luciferase reporter assay kit (Cat. No. DD1205-01, Vazyme Biotech) was used to determine luciferase activity.
Validation Using Gene Expression Omnibus Datasets
Two independent datasets from Gene Expression Omnibus (GEO) databases (GSE94717 and GSE30718) were used to validate the prognostic value of the miRNA and its target gene to predict kidney injury. The validation data set was downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Expression values for the miRNA were extracted from GSE94717, whereas its target gene was extracted from GSE30718. Subsequently, binary logistic regression models were constructed to evaluate the diagnostic value of the miRNA and its target gene. Receiver operating characteristic (ROC) curves were created to evaluate the prognostic value of the miRNA and its target gene expression in the context of kidney injury. Furthermore, the area under the curve (AUC) was calculated for the model.
Statistical Analysis
Data are presented as means ± SD. Normal distribution was tested using the Shapiro–Wilk test. Comparisons between groups were performed using the nonparametric Kruskal–Wallis test. Moreover, Pearson’s correlation analysis was used to examine the relationship between changes in serum biochemical markers relevant to kidney injury and those in miRNA expression. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 23.0, IBM, Armonk, NY). Statistical significance was set at P < 0.05.
Ethics Approval and Consent to Participate
This study was conducted in strict accordance with the recommendations of the guidelines of the Institutional Animal Care and Use Committee of Xinhua Hospital Affiliated with Shanghai Jiao Tong University School of Medicine (Shanghai, China), and the experimental protocol was approved by the Ethics Committee of the same institution (XHEC-F-2021-001). GEO belongs to public databases. The patients involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles.
RESULTS
General Condition and Blood Parameters of the Rats
Figure 1A shows the testing procedures and grouping. No deaths occurred during the study period. The weights of 10 rats were included in the analysis for the first 7 days. Five rats in both groups were euthanized on day 7 in both groups. Therefore, the weights of the remaining five rats were considered in the analyses for the remaining 7 days (Fig. 1B). When the experiment commenced, the weight of N + NS rats rapidly increased to 274.0 ± 5.7 g on day 14, whereas rats in the S + Bev group experienced a gradual weight gain, with their final weight ranking the lowest at 198.0 ± 20.4 g on day 14. During the first 7 days, the weight of rats in the S + NS group increased slowly, and then the rate of increase was significantly faster than in rats in the S + Bev group, reaching 220.8 ± 8.9 g on day 14. Rats in the M + NS group showed a significant increase in at 130.3 ± 4.2 mmHg on day 14 compared with 116.3 ± 5.9 mmHg on day 7. of rats in the S + NS and S + Bev groups was relatively lower than in those in the other groups on day 14 (82.0 ± 15.1 and 70.0 ± 49.4 mmHg, respectively; Fig. 1C). Kidney weight was lower in the S + Bev group than in the other groups, and the difference was statistically significant between the N + NS and S + Bev groups (left kidney: 1.1 ± 0.1 vs. 0.7 ± 0.1, respectively, P < 0.05; right kidney: 1.1 ± 0.1 vs. 0.8 ± 0.1, respectively, P < 0.05; Fig. 1, D–E).
Figure 1.
A: schematic representation of the experimental schedule. Briefly, in the experiment, rats were subjected to different concentrations of oxygen (21% O2, 10% O2, and 7% O2) for 1 or 2 wk. Saline-containing placebo or bevacizumab was injected via the tail vein two times a week. B: the growth rate of rats. C: arterial partial pressure of oxygen () in rats. D: left kidney weight of rats. E: right kidney weight of rats. The following groups of rats are shown: 21% O2 and saline-containing placebo injection [normoxic condition + normal saline (N + NS)], 21% O2 and bevacizumab injection [normoxic condition + bevacizumab (N + Bev)], 10% O2 and saline-containing placebo injection [mild hypoxia condition + normal saline (M + NS)], 10% O2 and bevacizumab injection [mild hypoxia condition + bevacizumab (M + Bev)], 7% O2 and saline-containing placebo injection [severe hypoxia condition + normal saline (S + NS)], and 7% O2 and bevacizumab injections [severe hypoxia condition + bevacizumab (S + Bev)]. A two-tailed P < 0.05 was considered statistically significant; compared with the N + NS group, aP < 0.05; compared with the N + Bev group, bP < 0.05; compared with the M + NS group, cP < 0.05; compared with the M + Bev group, dP < 0.05; compared with the S + NS group, eP < 0.05; and compared with the S + Bev group, fP < 0.05. D, day.
Morphological Changes in the Kidneys
We first analyzed serum biochemical markers relevant to kidney injury. A substantial increase was found in serum creatinine levels in the S + NS and S + Bev groups, particularly in the S + Bev group, which was significantly higher on day 14 than in the N + NS and M + NS groups (Fig. 2A). Similarly, expression levels of serum uric acid, urea, and neutrophil gelatinase-associated lipocalin (NGAL) in the S + NS and S + Bev groups were significantly higher than in the other groups (Fig. 2, B–D). Tissue sections of kidneys stained with H&E are shown in Fig. 2E. N + NS kidneys showed a normal structure, and the cortical glomeruli were round or nearly round on both days 7 and 14 (Fig. 2E, i and vii). N + Bev kidneys exhibited mild desmoplasia and minimal immune cell infiltration; lesser tubular atrophy was observed, and occasional vacuolar degeneration was noted in renal tubular epithelial cells (Fig. 2E, ii). By day 14, the progression and development of renal injuries continued, and tubular epithelial cells showed swelling and watery degeneration (Fig. 2E, viii). Kidney lesions were similar between the N + Bev and M + NS groups on day 7 (Fig. 2E, ii and iii), whereas evident injury to the cortical glomeruli and renal tubular epithelial cells were rare in M + NS kidneys on day 14 (Fig. 2E, ix). We observed clear damage in kidney tissue from the M + Bev, S + NS, and S + Bev groups compared with the N + Bev group, particularly in the S + Bev group, where heavy infiltration of immune cells and loss of the brush border and vacuolar degeneration in tubular epithelial cells were observed, and injury severity increased with prolonged exposure to hypoxia (Fig. 2E, vi and xii). We further quantified the severity of histological kidney damage and found that bevacizumab treatment considerably increased the injury score among groups (Fig. 2H). MT staining revealed increased levels of collagenous components in the kidneys of rats treated with bevacizumab compared with those of the control groups (Fig. 2F). Notably, we observed no obvious interstitial collagen deposition (blue stain) in M + NS kidneys on day 14 compared with day 7 (Fig. 2F, iii and ix). Quantitative analysis of MT-stained areas confirmed these findings (Fig. 2G).
Figure 2.
A: serum creatinine (SCr) level in rats. B: blood urea nitrogen (BUN) level in rats. C: serum uric acid (UA) level in rats. D: concentration of serum neutrophil gelatinase-associated lipocalin (NGAL) in rats. E: kidney sections stained with hematoxylin and eosin. Magnification: ×40. ▴, vacuolar degeneration of renal tubular epithelial cells; Δ, shrunken tubules; →, representative lymphocytic infiltrate. F: Masson’s trichrome staining. Magnification: ×63. G: semiquantitative analysis of Masson’s trichrome staining in glomerulotubular areas of kidney sections from rats. H: pathological kidney injury scores of representative kidney samples of each group. The following groups of rats are shown: 21% O2 and saline-containing placebo injection [normoxic condition + normal saline (N + NS)], 21% O2 and bevacizumab injection [normoxic condition + bevacizumab (N + Bev)], 10% O2 and saline-containing placebo injection [mild hypoxia condition + normal saline (M + NS)], 10% O2 and bevacizumab injection [mild hypoxia condition + bevacizumab (M + Bev)], 7% O2 and saline-containing placebo injection [severe hypoxia condition + normal saline (S + NS)], and 7% O2 and bevacizumab injections [severe hypoxia condition + bevacizumab (S + Bev)]. A two-tailed P < 0.05 was considered statistically significant; compared with the N + NS group, aP < 0.05; compared with the N + Bev group, bP < 0.05; compared with the M + NS group, cP < 0.05; compared with the M + Bev group, dP < 0.05; and compared with the S + NS group. D7, day 7; D14, day 14.
Expression of HIF-1α and VEGF
Expression levels of HIF-1α increased with the duration of hypoxia as the oxygen concentration decreased, and a significant increase in HIF-1α levels was observed in the presence of the VEGF inhibitor (Fig. 3, A and C). HIF-1α was highly expressed in the S + Bev group on day 14 (Fig. 3Axii), and a significant difference was observed compared with the N + NS group. Among groups using a saline-containing placebo, VEGF expression first showed an increasing trend, followed by a decline as the oxygen concentration decreased, and expression in the M + NS group was highest on day 14 (Fig. 3, B and D). Conversely, bevacizumab has been shown to decrease VEGF expression levels. Each group showed increased VEGF expression levels over time except for the M + Bev group, which had the lowest VEGF expression levels on day 14. These results were verified using Western blot analysis (Fig. 3, E and H).
Figure 3.
Expression levels of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) in rat kidneys. A: localization of HIF-1α in kidneys (immunohistochemistry). Magnification: ×63. B: localization of VEGF in kidneys (immunohistochemistry). Magnification: ×63. C: relative levels of HIF-1α. D: relative levels of VEGF. E: expression of VEGF measured using Western blot analysis on day 7 (D7). F: quantitative analysis of VEGF expression using Western blot analysis on D7. G: expression of VEGF measured using Western blot analysis on day 14 (D14). H: quantitative analysis of VEGF expression using Western blot analysis on D14. The following groups of rats are shown: 21% O2 and saline-containing placebo injection [normoxic condition + normal saline (N + NS)], 21% O2 and bevacizumab injection [normoxic condition + bevacizumab (N + Bev)], 10% O2 and saline-containing placebo injection [mild hypoxia condition + normal saline (M + NS)], 10% O2 and bevacizumab injection [mild hypoxia condition + bevacizumab (M + Bev)], 7% O2 and saline-containing placebo injection [severe hypoxia condition + normal saline (S + NS)], and 7% O2 and bevacizumab injections [severe hypoxia condition + bevacizumab (S + Bev)]. A two-tailed P < 0.05 was considered statistically significant; compared with the N + NS group, aP < 0.05; compared with the N + Bev group, bP < 0.05; compared with the M + NS group, cP < 0.05; and compared with the M+Bev group.
Identification of Differentially Expressed miRNAs in the Renal Injury Rat Model
The transformed data set was subjected to partial least squares discriminant analysis to investigate the possibility of clustering samples, which revealed significant separation between groups (Fig. 4, A–C). A volcano plot revealed that in comparisons between bevacizumab and normal saline groups, four miRNAs were upregulated and six miRNAs were downregulated (Fig. 4D); when the M + NS and S + NS groups were compared, six miRNAs were upregulated and eight miRNAs were downregulated (Fig. 4E); in comparisons between the M + Bev and S + Bev groups, 10 miRNAs were upregulated and four miRNAs were downregulated (Fig. 4F). We found that rno-miR-124-3p was specifically expressed in all three heatmaps. Pearson’s correlation analysis revealed that rno-miR-124-3p expression was negatively correlated with NGAL, blood urea nitrogen, and serum creatinine levels (Fig. 4, G–I). Quantitative RT-PCR analysis was used to verify rno-miR-124-3p expression. Expression of rno-miR-124-3p was lower in the Bev groups than in the NS groups, and this difference was statistically significant (Fig. 4J). Expression of rno-miR-124-3p also tended to be downregulated in the S + NS and S + Bev groups, although not statistically significant (Fig. 4, K and L).
Figure 4.
The following groups of rats are shown: 21% O2 and saline-containing placebo injection [normoxic condition + normal saline (N + NS)], 21% O2 and bevacizumab injection [normoxic condition + bevacizumab (N + Bev)], 10% O2 and saline-containing placebo injection [mild hypoxia condition + normal saline (M + NS)], 10% O2 and bevacizumab injection [mild hypoxia condition + bevacizumab (M + Bev)], 7% O2 and saline-containing placebo injection [severe hypoxia condition + normal saline (S + NS)], and 7% O2 and bevacizumab injections [severe hypoxia condition + bevacizumab (S + Bev)]. A–C: partial least squares-discriminant analysis score plots of volatile compounds from Bev and NS group comparisons (A), S + NS and M + NS group comparisons (B), and S + Bev and M + Bev group comparisons (C). D–F: differential miRNA expression data are displayed using volcano plots: Bev vs. NS (D); S + NS vs. M + NS (E); and S + Bev vs. M + Bev (F). Red, green, and gray indicate upregulated miRNAs, downregulated miRNAs, and no significant changes in miRNA expression, respectively. G–I: the left heatmap depicts high-expression microRNA (red) and low-expression microRNA (blue) significantly altered between groups. The right heatmap shows correlation coefficient (ρ) values ranging from −1.0 (negative correlation, green panels) to 1.0 (positive correlation, purple panels) between serum biochemical markers relevant to kidney injury [serum creatinine, serum urea nitrogen in rats, serum uric acid in rats, and serum neutrophil gelatinase-associated lipocalin (NGAL)] and microRNA expression [Bev vs. NS groups (G), S + NS vs. M + NS groups (H), and S + Bev vs. M + Bev groups (I)]. J–L: levels of rno-miR-124-3p were determined using quantitative RT-PCR [Bev vs. NS groups (J), S + NS vs. M + NS groups (K), and S + Bev vs. M + Bev (L)]. *P < 0.01; **P < 0.001. ns, not significant (P > 0.05).
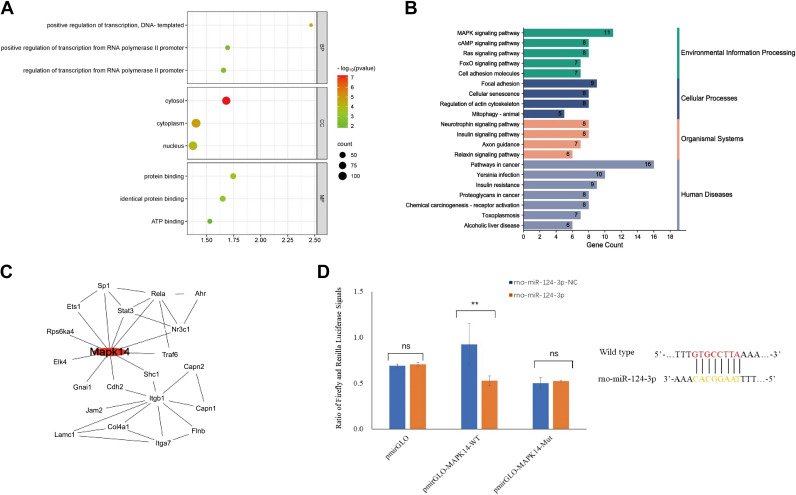
miRNA Target Gene Analysis and Prediction of Biological Functions
We extracted 306 intersected target genes of rno-miR-124-3p from databases, including TargetScan, miRDB, and DIANA microT (Supplemental Table S2). The results of the gene enrichment analyses are shown in Fig. 5A, including the following three GO categories: biological processes, cellular components, and molecular function. The differentially expressed genes were assigned to 31 KEGG pathways for KEGG enrichment analysis, mainly involving environmental information processing, cellular processes, organismal systems, and human diseases. Among these pathways, pathways in cancer (rno05200) and the MAPK signaling pathway (rno04010) were the top three kidney injury risk pathways (Fig. 5B). Based on the Search Tool for the Retrieval of Interacting Genes/Proteins database, a protein-protein interaction network of associated key genes was built, and Mapk14 was identified as a key hub gene in the network with the highest degree (Fig. 5C). Luciferase assays were performed to confirm relationships between rno-miR-124-3p and Mapk14 expression. The results showed that rno-miR-124-3p significantly reduced luciferase activity in the wild-type group but not in the mutant group (Fig. 5D). The relationship between VEGF and Mapk14 in the KEGG database (rno05200 and rno04010) is provided in Supplemental Fig. S1.
Figure 5.
A: Gene Ontology analysis of differentially regulated target miRNAs. BP, biological process; CC, cellular component; FDR, false discovery rate; MF, molecular function. B: Kyoto Encyclopedia of Genes and Genomics pathway enrichment of differentially expressed genes. C: protein-protein interaction network construction and hub genes selection. The red node represents hub genes. D: effect of miR-NC and rno-miR-124-3p on luciferase activity in NRK-52E cells transfected with either the wt-3′-UTR or mut-3′-UTR. ns, not significant.
Prognostic Value of miRNAs and Their Target Genes With the GEO Data Set
To validate our findings, we downloaded a data set from the GEO database (GSE94717) to compare miRNA-expressing data from six blood samples from patients with sepsis-induced acute kidney injury with those of nine controls. For miR-124-3p, the value was 0.593 (0.275–0.911; Fig. 6A). We downloaded another data set from the GEO database (GSE30718) to compare Mapk14 expression data from kidney biopsies from 28 transplants with acute kidney injury with those of 11 pristine protocol biopsies of stable transplants. The AUC-ROCs of Mapk14 were 0.859 (0.745–0.973; Fig. 6B).
Figure 6.
Receiver-operator characteristic curves (ROC) for prediction of kidney injury. A: ROC of miR-211-5p and miR-124-3p for GSE94717. B: ROC of Gnai1, Sos2, Stat3, Mapk14, and Itgb1 for GSE30718.
DISCUSSION
This study expands on our previous research in important ways (5, 6). First, VEGF inhibition using bevacizumab disrupted the repair potential of the kidney, which further confirmed that the hypoxia-induced expression of VEGF plays an important role in the self-protective mechanism of the kidney. Additionally, we explored the miRNAs in the kidneys and identified miR-124-3p as a differentially expressed miRNA. Moreover, the correlation analysis demonstrated that miR-124-3p expression was negatively associated with kidney injury marker levels. Mapk14 was also identified using bioinformatics analysis as a hub gene, and the results of the luciferase reporter assay confirmed that Mapk14 was directly bound by miR-124-3p. GEO data indicated that miR-124-3p and Mapk14 expression levels were closely linked to renal injury.
Animal Model Construction
Commonly used animal models of kidney injury include cisplatin injury (23–24), glycerol injury (25–26), unilateral ureteral obstruction (27), ischemia-reperfusion kidney injury (28), 5/6 nephrectomy (29–30), and lipopolysaccharide-induced acute kidney injury (31–32). However, these models are inappropriate for studying patients with chronic hypoxia. In this study, we constructed a chronic/hypoxic renal injury model using a hypoxia chamber with a low oxygen concentration. To assess the effects of chronic hypoxia on the kidneys, we sequentially reduced the oxygen concentration from normoxia (21% O2) to severe hypoxia (7% O2). To date, most research on this topic has used this method (33–36); however, important limitations associated with this method should be acknowledged. Because the actual hypoxia levels in kidneys are unknown in this model, the extent of hypoxia and duration of exposure are controversial. For example, Takahashi et al. (33) housed db/db mice in a hypoxia chamber (12% O2) for up to 16 wk to induce advanced glomerulosclerosis with accelerated albuminuria, whereas Mazzali et al. (34) found that serum uric acid levels increased and subtle tubulointerstitial inflammation occurred as early as 24 h after the rats were placed into a 10% O2 chamber. Based on these findings and the results of previous literature reviews, we found that hypoxia typically requires 7 days before visible pathologically altered renal physiology is observed. Under actual operation conditions, kidney injury under an oxygen concentration ranging from 21% to 10% was not obvious. However, this impairment was aggravated by further decreasing oxygen concentrations, with deaths beginning to occur at concentrations below 7%.
Therefore, further research on the degree of hypoxia in kidneys is needed to develop more accurate disease models. Currently, the methods available for detecting hypoxia in the kidney include polarographic microelectrodes, fluorescence optodes, phosphorescence lifetime measurement, and pimonidazole immunohistochemistry (37).
Expression of HIF-1α and VEGF Under Different Degrees of Hypoxia
In this study, we found that the positive correlation between HIF-1α and VEGF expression was disrupted as the degree of hypoxia increased. Rats treated with 7% O2 tended to strongly express HIF-1α and relatively weakly express VEGF compared with those that inhaled 10% O2. Particularly, the high expression of VEGF helped reduce renal injury after hypoxia. We used bevacizumab, a VEGF-specific angiogenesis inhibitor, to investigate whether VEGF played a role in kidney repair. Reportedly, bevacizumab decreased VEGF expression and inhibited angiogenesis in the kidneys (9–10). In our model, we found that bevacizumab suppressed renal angiogenesis to a significantly greater extent than that of controls. Therefore, the results of our study were confirmed using bevacizumab. We believe that several factors may be responsible for the different expression patterns of HIF-1α and VEGF as hypoxia increases.
First, cells regulate the transcription of VEGF and erythropoietin genes by upregulating the expression of HIF-1α after hypoxic stimulation, thereby resulting in adaptive reactions, such as stimulating erythropoietin and angiogenesis (4). However, HIF-1α overexpression can also activate other pathways that aggravate kidney injury, including the transforming growth factor-β signaling pathway, which leads to kidney fibrosis (4, 38). These dual effects suggest that the adaptive response of the kidney depends on the physiological function and interaction of the downstream signaling pathways regulated by HIF-1α rather than on the expression of HIF-1α.
Second, the high expression of HIF-1α does not imply that the downstream VEGF is fully activated. Previous studies have demonstrated that the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is not only involved in the synthesis of HIF-1α but is also the main downstream signaling pathway of VEGF (39–40). Therefore, as both PI3K and AKT are activated following hypoxic stimulation, understanding whether the expression of PI3K or AKT changes as hypoxia increases is difficult. Yan et al. (41) also found that the binding of early growth response gene 1 (Egr-1) and HIF-1α to the VEGF promoter was a significantly greater stimulus for the induction of VEGF than was the binding of HIF-1α alone. In addition, Bai et al. (42) found that VEGF could activate the entire Akt/endothelial nitric oxide synthase/nitric oxide signaling pathway, providing a protective effect on the kidneys by improving renal microperfusion.
Third, not only can HIF-1α regulate the expression of VEGF in anoxic environments, but other HIF-1α-independent pathways may also be involved in angiogenesis, including the peroxisome proliferator-activated receptor-γ/VEGF (43) and peroxisome proliferator-activated receptor-γ coactivator-1β/VEGF signaling pathways (44). However, it is unclear whether oxygen concentration affects the expression of these pathways.
miR-124-3p and Mapk14
VEGF is highly regulated by multiple signaling pathways under different degrees of hypoxia, making kidney repair studies extremely difficult. miRNAs are endogenous, small, noncoding, single-stranded RNA molecules that are evolutionarily conserved. They can target a large number of genes and regulate several signal pathways simultaneously (12). Based on the characteristics of miRNAs, we hypothesize that specific miRNAs exhibit differential expression under various degrees of hypoxia and that they regulate the expression of VEGF.
We compared the expression of miRNAs between the bevacizumab and normal saline groups under different degrees of hypoxia (21%, 10%, and 7% O2). We found that miR-124-3p was specifically expressed in various kidney tissues, and significantly lower expression was observed in the bevacizumab group than in the normal saline group. In addition, we found that miR-124-3p levels correlated with kidney injury marker levels. As a widely explored miRNA, miR-124-3p has been shown to play critical roles in hypoxia-induced injury (45, 46). For example, Wu et al. (47) found that miR-124-3p attenuated inflammation, oxidative stress, and apoptosis in lipopolysaccharide-treated H9C2 cells by modulating the SP1/HDAC4/HIF-1α axis. Shu and Zhang (48) described that the miR-124/AKT/Nrf2 pathway played an important role in preventing oxygen deprivation-induced injury. A study has also highlighted that miR-124 reverses doxorubicin resistance by targeting STAT3 to control the HIF-1α signaling pathway (49). However, no evidence supports a direct link between miR-124-dependent transcription and VEGF expression.
Therefore, to further understand the molecular function of miR-124-3p in kidney repair, we explored miR-124-3p target genes using TargetScan, miRDB, and DIANA microT, which identified Mapk14 as a key target gene. Furthermore, the results of the luciferase reporter assay confirmed that miR-124-3p directly binds to Mapk14. Previous studies have demonstrated that MAPK14 and VEGF are in a regulatory circuit where MAPK14 inhibition enhances VEGF-induced angiogenesis and decreases vascular permeability (50, 51). Previously, Yang et al. (52) demonstrated that platelet-derived miR-200a-3p modulated the expression of VEGF-A in endothelial cells by targeting Mapk14. Moreover, Pan et al. (53) found that miR-124 could alleviate the symptoms of acute lung injury by inhibiting the activation of the MAPK signaling pathway via subsequent targeting of Mapk14. Based on these findings, we further refined the study hypothesis to state that miR-124-3p affects kidney repair by targeting Mapk14, which indirectly regulates VEGF expression. These findings provide a new perspective regarding this puzzling challenge. However, further investigation needs to be performed to validate this hypothesis.
Limitations
This study had some limitations. First, rats were exposed to normal air during feeding/cleaning (10 min every 48 h) and the surgical operation (30 min maximum). The possibility that this exposure might have affected our results cannot be ruled out. Second, no cell-specific identification of miR-124-3p was conducted. Therefore, ongoing and future efforts toward in vitro and in vivo research will be required to confirm or refine the inferences of our study. Third, we cannot predict whether our results in rats can be generalized to humans. However, some human experiments have highlighted correlations among miR-124-3p, Mapk14, and VEGF (54–56). Therefore, we are currently conducting follow-up studies in in vivo and in vitro modeling systems to assess this hypothesis.
Conclusions
In conclusion, we described changes in the expression levels of HIF-1α and VEGF, showing the important role of VEGF in the protective mechanism of the kidney under different degrees of hypoxia. Notably, miR-124-3p was a key miRNA that positively affected kidney repair. miR-124-3p may affect kidney repair by targeting Mapk14, indirectly regulating VEGF expression.
DATA AVAILABILITY
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Fig. S1 and Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.22347778.v3.
GRANTS
This work was supported by the Youth Project of the National Natural Science Foundation of China (Grant 82200754) and the Xinhua Hospital Development Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the institutions to which the authors are affiliated.
AUTHOR CONTRIBUTIONS
Y.X. and Y.Z. conceived and designed research; Y.Z. performed experiments; H.M., J.L., and J.Z. analyzed data; J.X., X.Z., and X.K. interpreted results of experiments; Y.X. and J.X. prepared figures; Y.X. and Y.Z. drafted manuscript; X.Z. and X.K. edited and revised manuscript; X.Z. and X.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the technical support provided by Kaiqin Chen. We thank Editage (www.editage.cn) for English language editing.
REFERENCES
- 1. Tanaka S, Tanaka T, Nangaku M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis (Basel) 1: 80–89, 2015. doi: 10.1159/000381515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shu S, Wang Y, Zheng M, Liu Z, Cai J, Tang C, Dong Z. Hypoxia and hypoxia-inducible factors in kidney injury and repair. Cells 8: 207, 2019. doi: 10.3390/cells8030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang B, Li ZL, Zhang YL, Wen Y, Gao YM, Liu BC. Hypoxia and chronic kidney disease. EBioMedicine 77: 103942, 2022. doi: 10.1016/j.ebiom.2022.103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol 21: 268–283, 2020. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaya X, Yueniu Z, Lili X, Wei XI, Xiaodong ZH. Study on kidney injury in cyanosis and acyanosis congenital heart disease after cardiopulmonary bypass operation: a single-center retrospective analysis. Chinese Pediatr Emerg Med 28: 6, 2021. doi: 10.3760/cma.j.issn.1673-4912.2021.01.007. [DOI] [Google Scholar]
- 6. Xu Y, Xiangmei K, Jiru L, Cui T, Wei Y, Xu J, Zhu Y, Zhu X. Mild hypoxia enhances the expression of HIF and VEGF and triggers the response to injury in rat kidneys. Front Physiol 12: 690496, 2021. doi: 10.3389/fphys.2021.690496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13: 2109–2114, 2009. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stefanik DF, Fellows WK, Rizkalla LR, Rizkalla WM, Stefanik PP, Deleo AB, Welch WC. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55: 91–100, 2001. doi: 10.1023/A:1013329832067. [DOI] [PubMed] [Google Scholar]
- 10. Zhao N, Xu Q, Wang M, Fei X, Pan Y, Chen X, Ma S. Mechanism of kidney injury caused by bevacizumab in rats. Int J Clin Exp Pathol 7: 8675–8683, 2014. [PMC free article] [PubMed] [Google Scholar]
- 11. Wu JB, Tang YL, Liang XH. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. Onco Targets Ther 11: 6901–6909, 2018. doi: 10.2147/OTT.S172042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo C, Dong G, Liang X, Dong Z. Epigenetic regulation in AKI and kidney repair: mechanisms and therapeutic implications. Nat Rev Nephrol 15: 220–239, 2019. doi: 10.1038/s41581-018-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan PC, Chen CC, Chen YC, Chang YS, Chu PH. MicroRNAs in acute kidney injury. Hum Genomics 10: 29, 2016. doi: 10.1186/s40246-016-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P, Chen Z, Zou Y, Wan X. Roles of non-coding RNAs in acute kidney injury. Kidney Blood Press Res 41: 757–769, 2016. doi: 10.1159/000450566. [DOI] [PubMed] [Google Scholar]
- 15. Lin Z, Liu Z, Wang X, Qiu C, Zheng S. MiR-21-3p plays a crucial role in metabolism alteration of renal tubular epithelial cells during sepsis associated acute kidney injury via AKT/CDK2-FOXO1 pathway. Biomed Res Int 2019: 2821731, 2019. doi: 10.1155/2019/2821731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng J, Yang K, Tian H, Lin Y, Hou M, Gao Y, Zhou X, Gao Z, Ren J. The mechanisms of Qizhu Tangshen formula in the treatment of diabetic kidney disease: network pharmacology, machine learning, molecular docking and experimental assessment. Phytomedicine 108: 154525, 2023. doi: 10.1016/j.phymed.2022.154525. [DOI] [PubMed] [Google Scholar]
- 17. Xue J, Zhu K, Cao P, Long C, Deng Y, Liu T, Yin G, Li X, Wang Z. Ischemic preconditioning-induced protective effect for promoting angiogenesis in renal ischemia-reperfusion injury by regulating miR-376c-3p/HIF-1α/VEGF axis in male rats. Life Sci 299: 120357, 2022. doi: 10.1016/j.lfs.2022.120357. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Zhang Z, Li A, Hu Y. Propofol attenuates renal ischemia/reperfusion injury by regulating the MALAT1/miR-126-5p axis. J Gene Med 23: e3349, 2021. doi: 10.1002/jgm.3349. [DOI] [PubMed] [Google Scholar]
- 19. Kocaturk H, Bedir F, Turangezli Ö, Arslan R, Çoban TA, Altuner D, Suleyman H. Effect of adenosine triphosphate, benidipine and their combinations on bevacizumab-induced kidney damage in rats. Adv Clin Exp Med 30: 1175–1183, 2021. doi: 10.17219/acem/140440. [DOI] [PubMed] [Google Scholar]
- 20. Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, Kim S, Kim NH, Cho ES, In Yook J, Yoo TH, Song E, Kim P, Shin EC, Chung K, Choi K, Choi C. Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality. Sci Adv 6: eaaz6980, 2020. doi: 10.1126/sciadv.aaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–D158, 2008. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23. Qu Y, An F, Luo Y, Lu Y, Liu T, Zhao W, Lin B. A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials 155: 41–53, 2018. doi: 10.1016/j.biomaterials.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 24. Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL. Recent advances in models, mechanisms, biomarkers, and interventions in cisplatin-induced acute kidney injury. Int J Mol Sci 20: 3011, 2019. doi: 10.3390/ijms20123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izuwa Y, Kusaba J, Horiuchi M, Aiba T, Kawasaki H, Kurosaki Y. Comparative study of increased plasma quinidine concentration in rats with glycerol- and cisplatin-induced acute renal failure. Drug Metab Pharmacokinet 24: 451–457, 2009. doi: 10.2133/dmpk.24.451. [DOI] [PubMed] [Google Scholar]
- 26. Sauriyal DS, Jaggi AS, Singh N, Muthuraman A. Investigating the role of endogenous opioids and KATP channels in glycerol-induced acute renal failure. Fundam Clin Pharmacol 26: 347–355, 2012. doi: 10.1111/j.1472-8206.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 27. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 28. Shiva N, Sharma N, Kulkarni YA, Mulay SR, Gaikwad AB. Renal ischemia/reperfusion injury: an insight on in vitro and in vivo models. Life Sci 256: 117860, 2020. doi: 10.1016/j.lfs.2020.117860. [DOI] [PubMed] [Google Scholar]
- 29. Ozcan A, Ware K, Calomeni E, Nadasdy T, Forbes R, Satoskar AA, Nadasdy G, Rovin BH, Hebert LA, Brodsky SV. 5/6 Nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. Am J Nephrol 35: 356–364, 2012. doi: 10.1159/000337918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gretz N, Meisinger E, Waldherr R, Strauch M. Acute renal failure after 5/6 nephrectomy: histological and functional changes. Contrib Nephrol 60: 56–63, 1988. doi: 10.1159/000414790. [DOI] [PubMed] [Google Scholar]
- 31. Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol 289: F1324–F1332, 2005. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- 32. Du J, Jiang S, Hu Z, Tang S, Sun Y, He J, Li Z, Yi B, Wang J, Zhang H, Li YC. Vitamin D receptor activation protects against lipopolysaccharide-induced acute kidney injury through suppression of tubular cell apoptosis. Am J Physiol Renal Physiol 316: F1068–F1077, 2019. doi: 10.1152/ajprenal.00332.2018. [DOI] [PubMed] [Google Scholar]
- 33. Takahashi N, Yoshida H, Kimura H, Kamiyama K, Kurose T, Sugimoto H, Imura T, Yokoi S, Mikami D, Kasuno K, Kurosawa H, Hirayama Y, Naiki H, Hara M, Iwano M. Chronic hypoxia exacerbates diabetic glomerulosclerosis through mesangiolysis and podocyte injury in db/db mice. Nephrol Dial Transplant 35: 1678–1688, 2020. doi: 10.1093/ndt/gfaa074. [DOI] [PubMed] [Google Scholar]
- 34. Mazzali M, Jefferson JA, Ni Z, Vaziri ND, Johnson RJ. Microvascular and tubulointerstitial injury associated with chronic hypoxia-induced hypertension. Kidney Int 63: 2088–2093, 2003. doi: 10.1046/j.1523-1755.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 35. Chu YT, Chen BH, Chen HH, Lee JC, Kuo TJ, Chiu HC, Lu WH. Hypoxia-induced kidney injury in newborn rats. Toxics 11: 260, 2023. doi: 10.3390/toxics11030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rathi V, Tiwari I, Kulshreshtha R, Sagi S. Hypobaric hypoxia induced renal injury in rats: Prophylactic amelioration by quercetin supplementation. PLoS One 18: e0279304, 2023. doi: 10.1371/journal.pone.0279304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sugahara M, Tanaka T, Nangaku M. Hypoxia-inducible factor and oxygen biology in the kidney. Kidney360 1: 1021–1031, 2020. doi: 10.34067/KID.0001302020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abe H, Takeda N, Isagawa T, Semba H, Nishimura S, Morioka MS, Nakagama Y, Sato T, Soma K, Koyama K, Wake M, Katoh M, Asagiri M, Neugent ML, Kim JW, Stockmann C, Yonezawa T, Inuzuka R, Hirota Y, Maemura K, Yamashita T, Otsu K, Manabe I, Nagai R, Komuro I. Macrophage hypoxia signaling regulates cardiac fibrosis via oncostatin M. Nat Commun 10: 2824, 2019. doi: 10.1038/s41467-019-10859-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li J, Zhang C, Jiang H, Cheng J. Andrographolide inhibits hypoxia-inducible factor-1 through phosphatidylinositol 3-kinase/AKT pathway and suppresses breast cancer growth. Onco Targets Ther 8: 427–435, 2015. doi: 10.2147/OTT.S76116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang L, Tian Y, Zhao P, Jin F, Miao Y, Liu Y, Li J. Electroacupuncture attenuates pulmonary vascular remodeling in a rat model of chronic obstructive pulmonary disease via the VEGF/PI3K/Akt pathway. Acupunct Med 40: 389–400, 2022. doi: 10.1177/09645284221078873. [DOI] [PubMed] [Google Scholar]
- 41. Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med 6: 1355–1361, 2000. [Erratum in Nat Med 7: 509, 2001]. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 42. Bai Y, Zhang Y, Yang S, Wu M, Fang Y, Feng J, Liu B. Protective effect of vascular endothelial growth factor against cardiopulmonary bypass-associated acute kidney injury in beagles. Exp Ther Med 15: 963–969, 2018. doi: 10.3892/etm.2017.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Zhang L, Zang X, Shen X, Li J, Chen L. Baohuoside I inhibits tumor angiogenesis in multiple myeloma via the peroxisome proliferator-activated receptor γ/vascular endothelial growth factor signaling pathway. Front Pharmacol 13: 822082, 2022. doi: 10.3389/fphar.2022.822082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rowe GC, Jang C, Patten IS, Arany Z. PGC-1β regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Physiol 301: E155–E163, 2011. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang YP, Liu Q, Xu GH, Zhang J, Chen Y, Hua FZ, Deng CQ, Hu YH. The lncRNA ROR/miR-124-3p/TRAF6 axis regulated the ischaemia reperfusion injury-induced inflammatory response in human cardiac myocytes. J Bioenerg Biomembr 51: 381–392, 2019. [Erratum in J Bioenerg Biomembr 53: 367, 2021]. doi: 10.1007/s10863-019-09812-9. [DOI] [PubMed] [Google Scholar]
- 46. Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther 22: 75, 2020. doi: 10.1186/s13075-020-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu M, Huang Z, Huang W, Lin M, Liu W, Liu K, Li C. microRNA-124-3p attenuates myocardial injury in sepsis via modulating SP1/HDAC4/HIF-1α axis. Cell Death Discov 8: 40, 2022. doi: 10.1038/s41420-021-00763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shu K, Zhang Y. Protodioscin protects PC12 cells against oxygen and glucose deprivation-induced injury through miR-124/AKT/Nrf2 pathway. Cell Stress Chaperones 24: 1091–1099, 2019. doi: 10.1007/s12192-019-01031-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu C, Xing H, Guo C, Yang Z, Wang Y, Wang Y. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle 18: 2215–2227, 2019. doi: 10.1080/15384101.2019.1638182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Issbrücker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M. p38 MAP kinase–a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J 17: 262–264, 2003. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 51. Wang L, Kwak JH, Kim SI, He Y, Choi ME. Transforming growth factor-beta1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase kinase 3-p38α and p38delta mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem 279: 33213–33219, 2004. doi: 10.1074/jbc.M403758200. [DOI] [PubMed] [Google Scholar]
- 52. Yang J, Xu H, Chen K, Zheng D, Liu S, Zhou X, Lin Y, Cheng H, Luo Q, Yang M, Yan X, Hao J. Platelets-derived miR-200a-3p modulate the expression of ET-1 and VEGFA in endothelial cells by targeting MAPK14. Front Physiol 13: 893102, 2022. doi: 10.3389/fphys.2022.893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pan W, Wei N, Xu W, Wang G, Gong F, Li N. MicroRNA-124 alleviates the lung injury in mice with septic shock through inhibiting the activation of the MAPK signaling pathway by downregulating MAPK14. Int Immunopharmacol 76: 105835, 2019. doi: 10.1016/j.intimp.2019.105835. [DOI] [PubMed] [Google Scholar]
- 54. Mucaj V, Lee SS, Skuli N, Giannoukos DN, Qiu B, Eisinger-Mathason TS, Nakazawa MS, Shay JE, Gopal PP, Venneti S, Lal P, Minn AJ, Simon MC, Mathew LK. MicroRNA-124 expression counteracts pro-survival stress responses in glioblastoma. Oncogene 34: 2204–2214, 2015. doi: 10.1038/onc.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang JS, Li L. MiR-124 and miR-506 are involved in the decline of protein C in children with extra-hepatic portal vein obstruction. Sci Rep 11: 12320, 2021. doi: 10.1038/s41598-021-91862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heredea RE, Melnic E, Cirligeriu LE, Berzava PL, Stănciulescu MC, Popoiu CM, Cimpean AM. VEGF pathway gene expression profile of proliferating versus involuting infantile hemangiomas: preliminary evidence and review of the literature. Children (Basel) 9: 908, 2022. doi: 10.3390/children9060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Tables S1 and S2: https://doi.org/10.6084/m9.figshare.22347778.v3.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.