Abstract
Introduction
The aim of this study was to investigate if a negative test result for MYD88 L265P mutation, associated with vitreoretinal lymphoma (VRL) and primary CNS lymphoma, in liquid biopsies from intraocular fluids can be a useful adjuvant test to diagnose chronic lymphocytic leukemia in clinically challenging cases.
Case Presentations
We selected patients with a past medical history or examinations findings suspicious for intraocular lymphoma. We evaluated both vitreous and aqueous humor-derived (AHD) MYD88 L265P mutation from patients that had suspected intraocular lymphoma that warranted a liquid biopsy procedure. Gold-standard cytopathology, flow cytometry, and gene rearrangement studies were also performed. All 4 patients had negative AHD MYD88 L265P mutation testing. Gold-standard testing (cytology) either showed paucicellular specimens (1/4) or specimens with high background inflammation (3/4). One case showed a rare B-cell clonal population (CD5+, Kappa-restricted by flow cytometry), but this was not sufficient to make any definitive diagnosis. All patients were subsequently initiated on systemic therapy and had improvement in their disease burden.
Conclusions
Negative AHD MYD88 L265P mutation testing can serve as an adjuvant molecular test to diagnose difficult cases of intraocular CLL.
Keywords: Adult ocular oncology, Eye and systemic disease, Intraocular tumors, Lymphoma
Introduction
Chronic lymphocytic leukemia (CLL) is a hematologic malignancy due to abnormal proliferation of mature B-cells within the bone marrow, lymphoid tissue, and blood [1]. Based on epidemiological studies in 2020, there were over 20,000 new cases and 4,000 deaths from CLL in the USA (average age at diagnosis – 70). Similarly, CLL has the highest incidence in developed nations around the world (between 4 and 5 cases per 100,000 individuals) [2]. CLL usually presents in patients as incidental findings of lymphocytosis (and/or anemia, neutropenia, or thrombocytopenia) on routine bloodwork without contextual symptoms. However, many patients present to their physicians with classic “B-symptoms” including fevers, night sweats, fatigue, and weight loss [3]. In more advanced stages of CLL, patients can become more susceptible to infections due to a weakened immune system or transformation of CLL into diffuse large B-cell lymphoma (DLBCL) or Hodgkin’s lymphoma [4, 5]. The latter is referred to as Richter’s transformation and has a poor prognosis.
Compared to traditional types of lymphomas that manifest in ocular tissues such as mucosal-associated lymphoid tissue-derived lymphoma or DLBCL, it is rare to have patients present with ocular involvement of CLL [6]. Even more, there is notable lack of any pattern of preferential CLL targeting to a particular ocular tissue in that the invasion of ocular tissue does not appear to correlate with the stage of CLL [7]. A recent review published in 2020 identified 123 cases of ocular-involving CLL and concluded that this rare event could be underreported [8]. There was no report of posterior uveitis in cases with CLL. However, one case who showed Richter’s transformation to DLBCL developed posterior uveitis.
As such, the ability to diagnose intraocular-involving CLL quickly and efficiently can make a critical difference in the care for such patients, particularly if their CLL was considered in remission and ocular symptoms are the first, and perhaps only, features indicating the return of disease, Richter’s transformation, or the development of intraocular infections. To the best of our knowledge, we report for the first time 4 CLL patients, otherwise in remission, who presented with posterior uveitis/vitritis and used liquid biopsies from aqueous and vitreous humor testing for MYD88 L265P to differentiate from Richter’s transformation of DLBCL [9–16], thus indicating intraocular CLL.
Case Series Presentations
Case 1
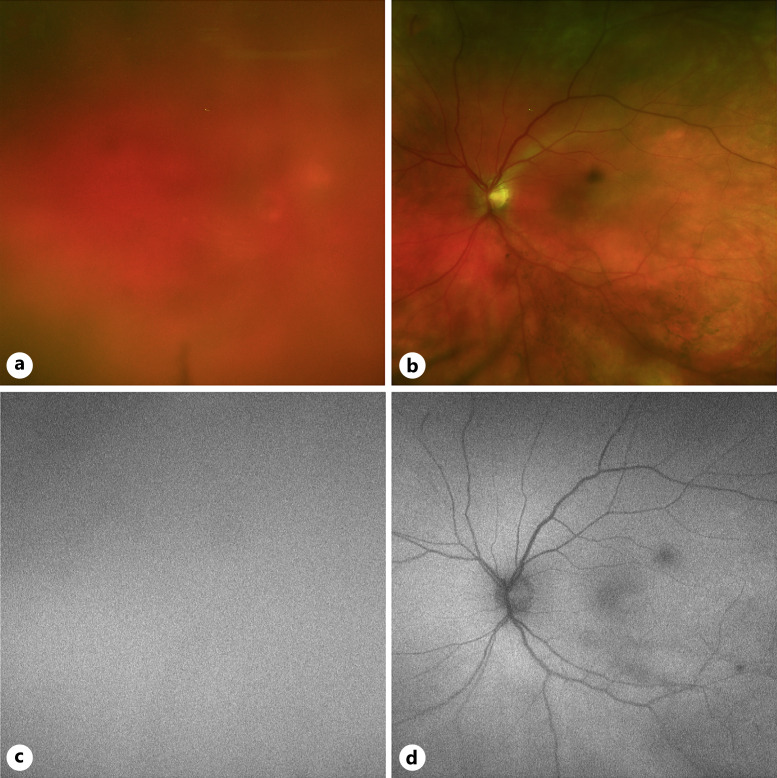
A 66-year-old woman with a history of both rheumatoid arthritis and osteoarthritis was diagnosed 5 years ago with stage 0 CLL after incidental finding of an elevated white count with a predominance of mature-appearing lymphocytes. Two years later, she was asymptomatic when her laboratory work demonstrated a steady rise in leukocyte counts with concurrent decreasing hemoglobin and platelet counts, followed by mild adenopathy and splenomegaly. Her CLL was reclassified as stage 2–3 and she was started on ibrutinib. Two and half years later (4 and a half years prior to her first ophthalmological examination), her ibrutinib dose decreased and she was reclassified as stage 0. Six months after she was restaged as stage 0, the patient noted a large cotton ball-shaped floater in her right eye without any sign of systemic problems. Her best corrected visual acuity (BCVA) was 20/60 OD and 20/20 OS. On fundus exam, there were 3+ vitreous cells in both eyes. In the right eye, there was a large subretinal hemorrhage around the superior margin of the optic nerve head, scattered peripapillary intraretinal hemorrhages, and significant macular edema including subretinal fluid in the nasal macula. There were peripheral scattered RPE deposits and subretinal white lesions in both eyes (Fig. 1).
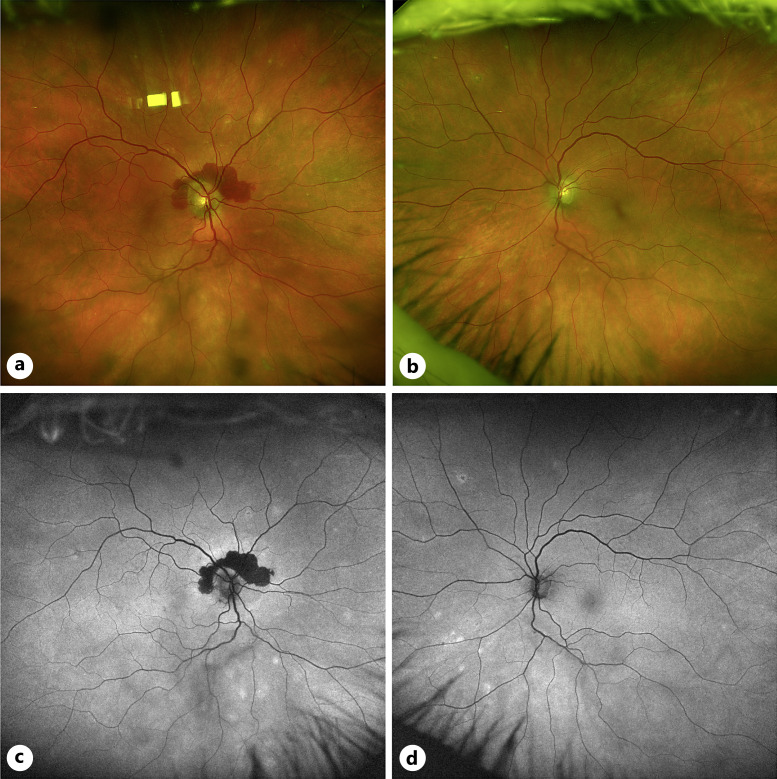
Fig. 1.
Fundus images of case 1. a Color fundus photo of the right eye with notable peripapillary hemorrhage and mid-peripheral RPE changes. b Color fundus photo of the left eye with mid-peripheral RPE changes. c Fundus autofluorescence imaging showing hypoautofluorescent peripapillary signal corresponding to the hemorrhage in (a) with hyperautofluorescent changes in the mid-periphery correspond with RPE changes in (a). d Fundus autofluorescence imaging showing hyperautofluorescent changes in the mid-periphery correspond with RPE changes in (b).
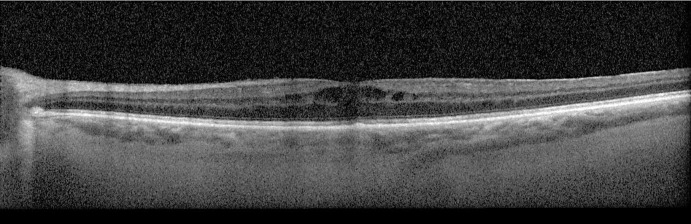
OCT of the macula and the optic nerve head confirmed the presence of subretinal fluid as well as a peripapillary subretinal hyperreflective membrane (Fig. 2). Differential diagnosis at this visit included the ocular involvement of CLL, Richter’s transformation to DLBCL, sarcoid-like reaction, choroidal neovascular membrane, and a drug reaction to ibrutinib. The patient underwent an anterior chamber tap for MYD88 L265P testing, which was found to be negative (refer to online suppl. materials. 1; for all online suppl. material, see https://doi.org/10.1159/000535951 for detailed methods applied to this case and all subsequent cases). She received one injection of Avastin in the right eye and used ketorolac four times a day in the right eye. Five months after the start of her ocular symptoms, her ocular complaints progressed and she was found to have 3+ vitreous cells in both eyes and persistent macular edema in the right eye, although the peripapillary subretinal hemorrhage resolved. The patient underwent pars plana vitrectomy for vitreous biopsy in her right eye. There was an intraoperative finding of vitreous snowballs. Cytopathology of the right vitreous was paucicellular with 8 leukocytes and no atypical features, B-cell clonality testing was negative for IGH and IGK rearrangements, flow cytometry profiling was negative (although the sample was paucicellular, but contained no B-cells and had few CD3+ T-cells), and MYD88 L265P mutation was negative. The patient completed a uveitic workup including QuantiFERON gold, angiotensin converting enzyme, lysozyme, and FTA-Abs, all of which were negative, thus ruling out infection and sarcoid-like reaction. Chest imaging obtained was also found to be normal. The patient’s oncologist viewed the findings as isolated and did not warrant more systemic workup and their staging of CLL was not commented on. At post-op month one, the patient had a BCVA of 20/20 OD. The peripapillary hemorrhage had resolved and there was only trace macular edema in the right eye. No vitreous cell was noted. After 6 months of follow-up, she continues to do well with 140 mg of ibrutinib.
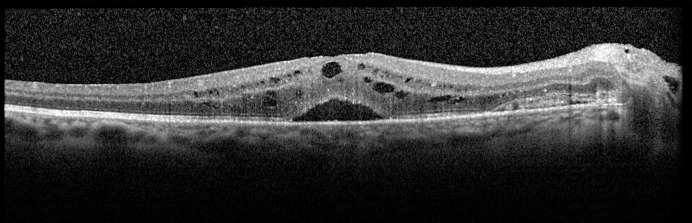
Fig. 2.
OCT macula of the right eye for case 1. OCT of the right eye macula shows central subretinal fluid with foveal and parafoveal inner retinal fluid pockets. Nasally, a subretinal hyperreflective membrane corresponding to the peripapillary hemorrhage.
Case 2
A 58-year-old woman was diagnosed with stage 0 CLL following non-specific abdominal symptoms 22 years ago. She was observed for 10 years. Then, she experienced new-onset abdominal pain, followed by a lymph node biopsy confirming proliferating CLL, and subsequent administration of six cycles of fludarabine, cyclophosphamide, and rituximab, plus Neulasta support. She remained in remission for 1 year.
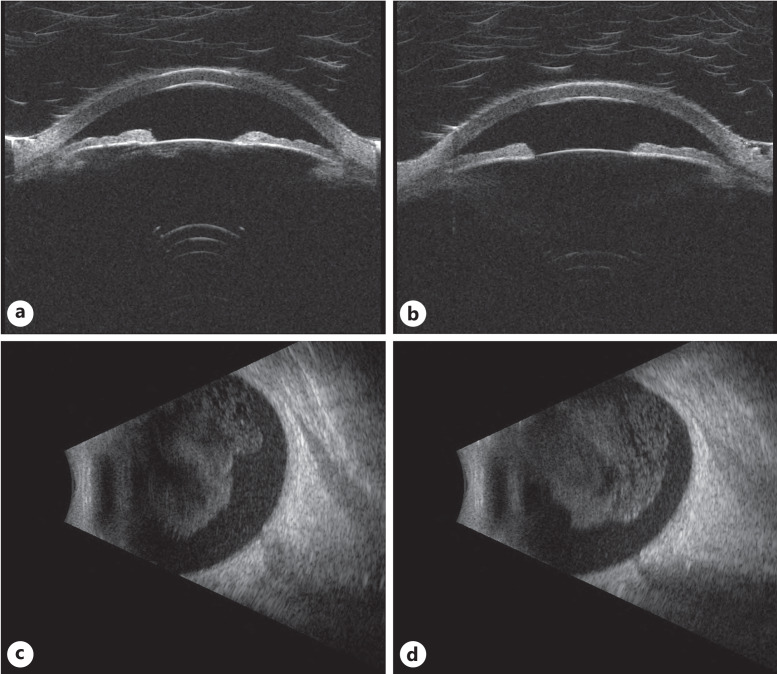
After her one-and-half-year follow-up, the patient noted vision changes (“looking through a dirty window”). On examination, her BCVA was 20/30-1 OU. Her anterior segment was notable for bilateral 2+ nuclear sclerosing cataract. On fundus exam of the right eye, there was 2–3+ vitreous cell, depigmentation inferior to the fovea with sclerotic vessels superonasal and inferonasal to the optic nerve (Fig. 3a, c). On the fundus exam of the left eye, there was 1–2+ vitreous cell, mild pigmentary changes and microaneurysms within the macula, a few sclerotic vessels plus a cotton wool spot in the superior arcade, and a subretinal lesion emanating off the superior arcade near a sclerotic vessel (Fig. 3b, d). OCT of the macula had minimal vitreous cells in both eyes and thickening of the retina along the superotemporal arcade in the left eye (Fig. 4). Ultrasound biomicroscopy was also unremarkable, but B-scan ultrasound showed moderate dense vitreous opacities in both eyes (Fig. 5). The differential diagnosis at the time included the ocular involvement of CLL, Richter’s transformation to DLBCL, sarcoid-like reaction as well as hyperviscosity, autoimmune, or chronic viral (e.g., CMV) etiologies. The patient underwent an anterior chamber tap for MYD88 L265P testing, which was negative, and underwent a pars plana vitrectomy of the right eye a few days later. Vitreous biopsy samples were negative for MYD88 L265P. However, cytopathology noted numerous mature T cells (CD4/8 = 3) and a rare B-cell clonal population. The patient also demonstrated CD5+ B-cells that were noted to be rare and kappa-restricted, which was consistent with the patient’s prior CLL diagnosis. Chest imaging was reported as normal. Laboratory workup included: complete blood count (white blood cell of 110.9), FTA-Abs (negative), angiotensin converting enzyme (within normal limits), anti-neutrophilic cytoplasmic antibody (negative), tuberculosis (negative), and HLA-B51 (negative), thus ruling out infection and sarcoid-like reaction. The patient’s vision steadily improved over a 2-month period with a final BCVA of 20/25-2 OD, 20/30-1 OS, and she was subsequently started on ibrutinib (420 mg daily) by her oncologist due to the systemic white blood cell count. Over the following 6 months, all the patient’s retinal lesions and vitreous cell resolved and she continues to do well.
Fig. 3.
Fundus images of case 2. a Color fundus photo of the right eye illustrating vitreous debris and notable attenuated vessels. b Color fundus photo of the left eye with similar vitreous debris, cotton wool spot along the superior arcade, and vessel attenuation. c Fundus autofluorescence imaging of the right eye. d Fundus autofluorescence imaging of the left eye.
Fig. 4.
OCT macula scans for case 2. OCT of the right eye (a) and left eye (b). There was minimal vitreous hyperreflective signal in both eyes.
Fig. 5.
Ultrasound imaging for case 2. Ultrasound biomicroscopy of the right (a) and left (b) eyes, as well as B-scan imaging of the right (c) and left (d) eyes, showed vitreous opacities consistent with slit lamp examination and fundus imaging.
Case 3
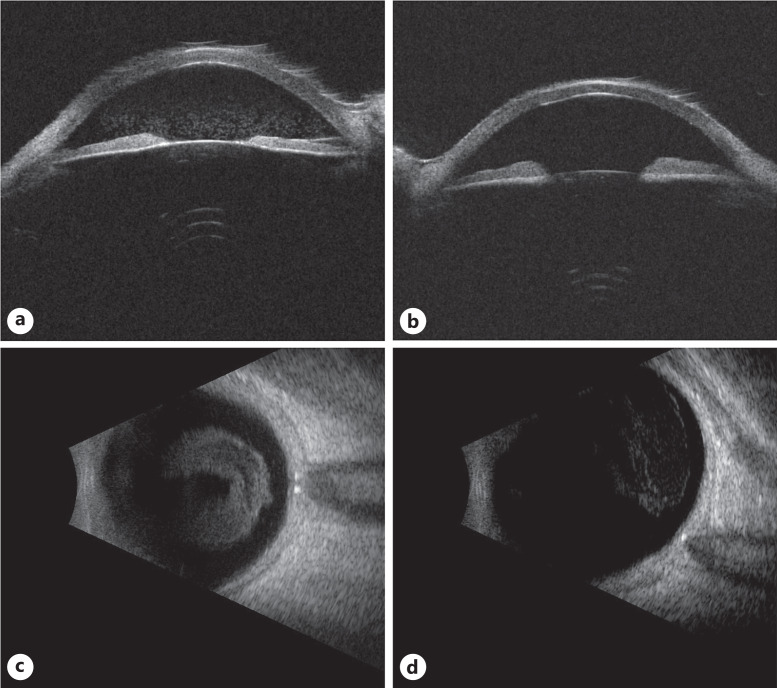
Five years prior, a 73-year-old woman was diagnosed with CLL and started on chemotherapy infusions and was maintained on daily dosing of ibrutinib (infusions included fludarabine, cyclophosphamide, rituximab, and dexamethasone). Four years later, the patient experienced an intermittent cloud in her vision over the course of a year, but she could not specify which eye was affected. On examination, her BCVA was 20/40 OD and 20/60 OS. There was dense vitreous debris in both eyes that precluded a good fundus exam, but the optic nerves were documented as normal (Fig. 6a–d). OCT of the macula showed no view in the right eye, but there was mild cystic foveal intraretinal fluid in the left eye (Fig. 7). Ultrasound biomicroscopy showed dense dispersed opacities in the anterior chamber in the right eye but was clear for the left eye (Fig. 8a, b). On B-scan ultrasound, there were mild vitreous opacities without uveal/scleral thickening or mass in both eyes (Fig. 8c, d). The differential diagnosis at the time included the ocular involvement of CLL, Richter’s transformation to DLBCL, and sarcoid-like reaction. The patient underwent an anterior chamber tap for MYD88 L265P, which was negative, and underwent a pars plana vitrectomy of the right eye a few days later. Vitreous biopsy was also negative for MYD88 L265P. Cytopathology showed polymorphous infiltration of discernable T-cells and mononuclear histiocytes, although no equivocal malignant cells were identified. Flow cytometry reported a paucicellular specimen with CD3+ T-cells (CD4/8 = 4) and no B-cells. B-cell clonality was also negative. The patient underwent a general laboratory workup to cover the differential diagnosis and was reported to be normal, including angiotensin converting enzyme and chest imaging for potential sarcoid-like reaction. However, after 6 months follow-up, she had no sign of recurrence and has been maintained on her daily dose of ibrutinib, thus decreasing suspicion for infectious or sarcoid-like reaction etiologies.
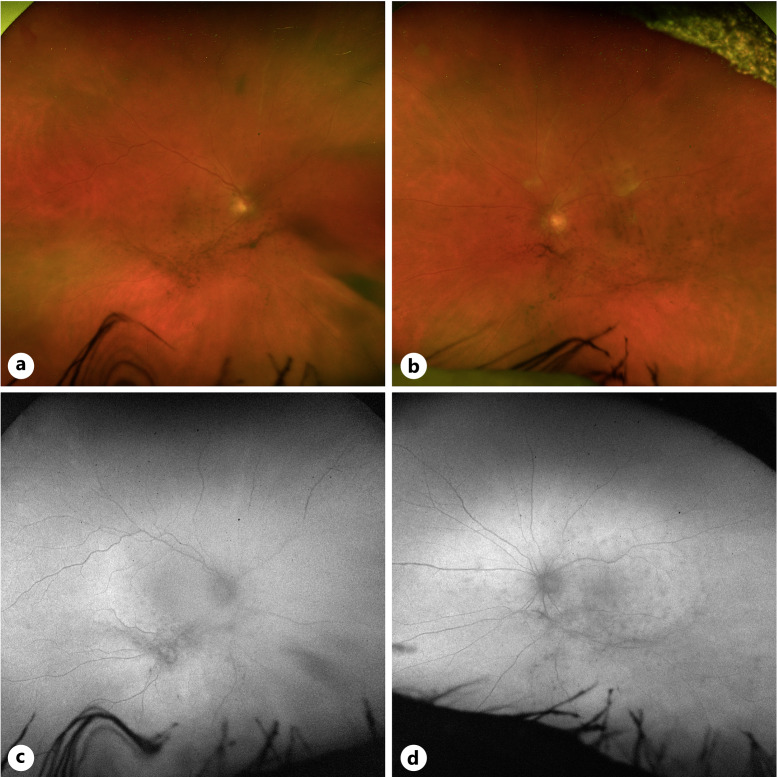
Fig. 6.
Fundus images of case 3. a Color fundus photo of the right eye was obstructed due to dense vitreous cell. b Color fundus photo of the left eye with vitreous opacities, blunted foveal reflex, and inferior periphery with reticular pigmentary changes. c Fundus autofluorescence imaging of the right eye yielded no diagnostic information due to vitreous cell. d Fundus autofluorescence imaging showing autofluorescent changes aligned with vitreous debris.
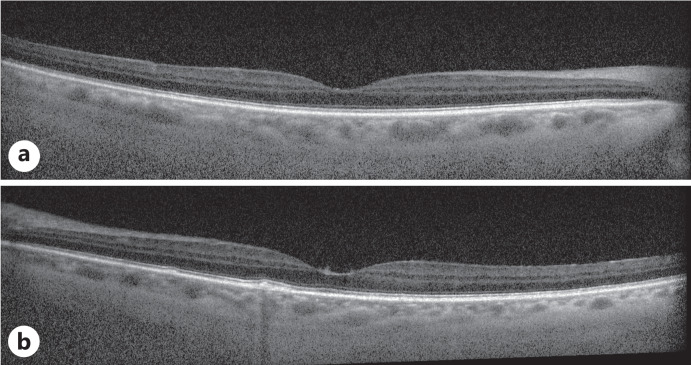
Fig. 7.
OCT macula of the left eye for case 3. OCT of the left eye macula shows foveal and parafoveal inner retinal fluid pockets. There is also notable vitreous hyperreflectivity corresponding to vitreous cell.
Fig. 8.
Ultrasound imaging for case 3. Ultrasound biomicroscopy demonstrated anterior and posterior chamber debris in the right eye (a), while only posterior chamber debris in the left eye (b). B-scan imaging of the right (c) and left eye (d) showed dense vitreous opacities, right greater than left.
Case 4
A 60-year-old gentleman was diagnosed with CLL following symptomatic weight loss and lymphadenopathy 22 years ago. He was treated with fludarabine and rituximab for six cycles and was in remission for 18 years. Three years prior, he experienced a CLL recurrence and initiated ibrutinib. The patient was under maintenance therapy for ibrutinib and monthly immunoglobulin therapy without any recurrence.
Shortly before presentation, the patient noticed cloudy vision in his right eye. On examination, his BCVA was 20/20 OD and 20/25 OS. On fundus exam, there was dense syneresis, right greater than left eye, as well as 1+ cell in both eyes (Fig. 9). B-scan showed moderate vitreous opacities in the right eye, but there were no mass lesions or uveal thickening (Fig. 10). After 8 months of observation, the patient had developed increased abdominal pain and CT imaging demonstrated enlarged lymph nodes. He was restarted on venetoclax and rituximab. At the same time, he noted a slight decrease in his vision OD (20/30) and there were 2+ vitreous cells in both eyes, which raised the suspicion for ocular involvement of CLL versus Richter’s transformation to DLBCL. The patient thus underwent pars plana vitrectomy of the right eye to obtain a vitreous biopsy. Cytopathology showed atypical, enlarged lymphocytes containing hyperchromatic nuclei with finely dispersed chromatin containing delicate chromocenters, features that were suspicious for CLL. Anterior chamber tap was negative for MYD88 L265P. The patient then subsequently underwent pars plana vitrectomy of the left eye. Cytopathology showed inflammatory infiltrate containing atypical small lymphoid cells, degenerating lymphoid cells, numerous T-cells, and scatted histiocytes. Vitreous biopsy was also negative for MYD88 L265P. No systemic laboratories studies were explored by oncology other than complete blood count monitoring which showed a slightly elevated white blood cell count (11.3, upper limit of normal 11), but no neutropenia on differential, thereby decreasing suspicion for sarcoid-like reaction or odds of having an infection. The patient’s oncologist viewed the findings as isolated and did not warrant more systemic workup and their staging of CLL was not commented on.
Fig. 9.
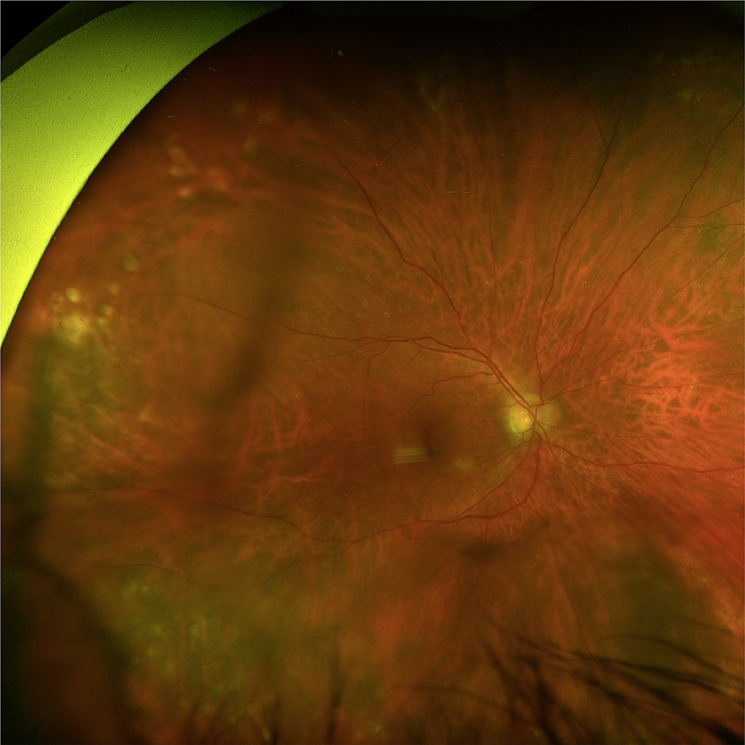
Fundus image of case 4. Color fundus photo of the right eye with dense syneresis. Posteriorly, there was a Weiss ring and lattice degeneration without tears or detachments. A large choroidal nevus was seen in the inferonasal periphery.
Fig. 10.
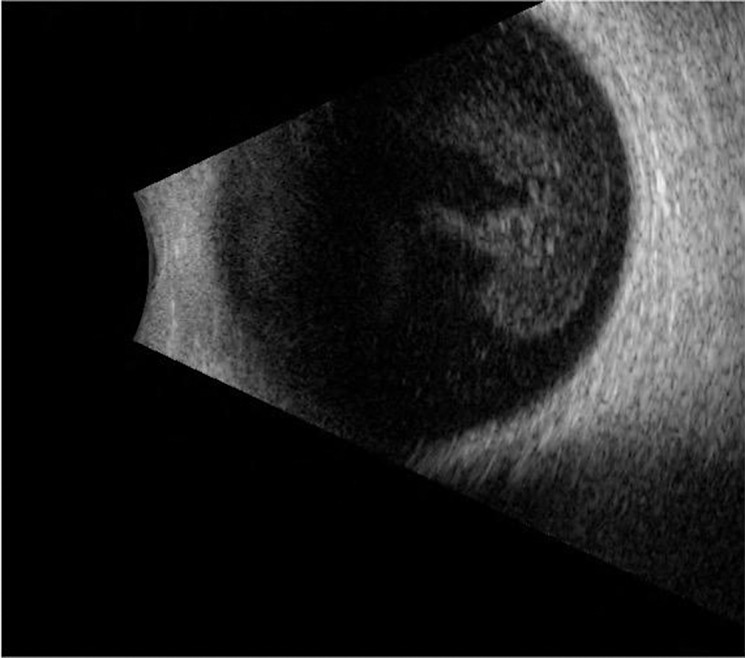
Ultrasound imaging for case 4. B-scan imaging of the right eye showed dense vitreous opacities, but no uveal/scleral thickening.
On follow-up, he developed new peripapillary subretinal hemorrhages in both eyes. The hemorrhages in the right eye continued to enlarge and developed macular edema. He was first treated with Avastin injection and later Eylea injections. His vision improved to BCVA of 20/20 OD.
Discussion
CLL involvement of the eye is rare. Patients might present with nonspecific ocular symptoms that overlap with many other leukemias and lymphomas. Seminal work conducted nearly 50 years ago compiled a comprehensive set of examination findings in the posterior segments of patients with CLL [17–19]. These included intraretinal and subretinal hemorrhages, microaneurysms, cotton wool spots, retinal vasculature tortuosity and engorgement, and vitreous hemorrhage. More recent studies with the advent of advanced neuroradiological techniques also report a strong preponderance for optic nerve involvement of CLL with or without the addition of retinal and choroidal findings. Such cases include a patient with bilateral optic nerve edema with MRI showing optic nerve enhancement and confirmed CLL on optic nerve sheath biopsy [20], and a patient with optic nerve pallor and MRI confirmation of optic nerve enhancement [21]. One case from 1982 even showed optic nerve changes on dilated slit-lamp examination correlating with CT imaging [22].
However, there is paucity of any case or retrospective studies documenting vitritis in the setting of CLL, and the reasons are not clear as to why this is so. One of the earliest case reports diagnosing CLL (specifically Richter’s syndrome) by diagnostic vitreous biopsy was in 1996 [23]. An 84-year-old woman was found to persistent vitreous cell, yellow sub-macular lesions, and keratic precipitates in the setting of significant reduced vision in her right eye. Her clinical findings were minimally responsive to topical steroids. An oral steroid course was successful in reducing her vitritis, but she had subsequent rebound vitritis not only in her right eye but also new onset vitritis in her left eye. She subsequently underwent a diagnostic vitrectomy that found large B-cells in her vitreous, hence confirming CLL, and was determined to be Richter’s syndrome after blood tests showed a mildly elevated white blood cell count (13,000) and a bone marrow biopsy showing stage 0 CLL. Of note, radiological and CSF studies were negative prior to bone marrow biopsy. This case beautifully illustrates the challenge for diagnosing CLL in patients with minimal system symptoms in conjunction with limited vision symptoms that can masquerade as idiopathic inflammation (regardless of any past CLL diagnosis). Furthermore, the utility of testing vitreous fluid presents a new accessible biopsy site to make critical diagnoses as was the case for the 4 patients we presented.
Gold-standard testing to diagnose suspect intraocular lymphoma (regardless of type) in the context of clinical suspicion involves cytopathological analysis of ocular fluid as well as flow cytometry with clonality testing either by cell surface markers or immunoglobulin gene rearrangement testing. Similar to challenges in the diagnosis of CNS involvement of lymphoma, flow cytometry requires a large quantity of cells to provide an accurate and reliable characterization, which is not always possible in vitreous biopsy [24]. Second, background inflammatory cells can dilute the signal such that detecting rare lymphoma cells does not meet the minimum threshold to make a formal diagnosis [25]. These two problems also affect cytopathology when either paucicellular samples or samples with high background inflammatory cells make it difficult to identify neoplastic cells [26]. Third, the timing of vitreous biopsy can influence if lymphoma is detected in a sample, depending on when the patient is presenting relative to their symptom onset and the cell burden was altered due to corticosteroid or chemotherapeutic use.
Richter’s transformation to DLBCL is another differential diagnosis in CLL patients with posterior uveitis/vitritis. Analysis of vitreous by cytopathology and flow cytometry, plus immunoglobulin gene rearrangement, is the standard test for the diagnosis of DLBCL. New developments in detection of VRL from vitreous liquid biopsies have focused on adjuvant molecular testing for specific biomarkers such as MYD88 L265P [27–29]. More recently, work by our group using cell-free fraction PCR of MYD88 L265P from vitreous and aqueous biopsies have picked up VRL diagnoses in paucicellular samples [16]. In contrast, intraocular CLL does not have a unique biomarker for molecular testing. Because of the cytopathological and flow cytometry overlap with DLBCL (and by extension VRL) [30–32], there is a need for molecular diagnostic testing to distinguish the two entities. The four cases we present demonstrate that negative MYD88 L265P can be a crucial adjuvant test to narrow a final diagnosis of intraocular CLL. In cases 1 and 3, both liquid biopsies were paucicellular and contained T-cells with minimal background inflammatory infiltrate lacking atypical features. Clonality and flow cytometry were negative for detection of a hematological malignancy. Conversely, cases 2 and 4 were not paucicellular but had notable lymphocytic cell populations. In case 2, a rare CD5+ clonal B-cell population was detected but is not a distinguishing feature between CLL and DLBCL. In case 4, numerous atypical, enlarged lymphocytes with hyperchromatic nuclei were seen, but repeat testing showed general inflammatory infiltrate. From the records we had access to, none of the oncologists commented that the CLL staging was changed presumably due to the fact the CLL was isolated to the intraocular space, Furthermore, all of 4 cases were MYD88 L265P negative, which is significant because on average 74% of VRL tumors are positive for this mutation from tumor cell DNA (unpublished meta-analysis). Hence, with high clinical suspicion for CLL and liquid biopsies without definitive cytopathological and flow cytometry metrics indicative of CLL (including samples that yield a paucity of cells for analysis), negative MYD88 L265P can help rule out DLBCL. It should be noted that this test should be used as a simultaneous adjuvant test during aqueous and/or vitreous biopsies given that more long-term, larger cohort studies utilizing MYD88 L265P testing are needed before considering it a separate gold-standard diagnostic test.
One of the differential diagnoses for ocular CLL, sarcoidosis or sarcoid-like reaction, remains a key concern when attempting to make a confirmed ocular CLL diagnosis. This has been reported on in the literature with the earliest published report by Brincker [33] in 1972 who reviewed 1,500 cases of malignant lymphoma and evaluated for the number of cases that had systemic sarcoidosis or sarcoid-like reactions. He reported 19 cases of either diagnosis but noted several key caveats: the malignancy long preceded the presentation of sarcoid-like reaction or sarcoidosis by several years, the majority of patients had no clinical evidence at the time of examination for sarcoid-like reaction or sarcoidosis (albeit it should be noted that this study was performed several decades prior to modern laboratory and clinical imaging techniques), and this study had no ocular CLL reports. Brincker followed up his initial study with an additional literature review that reported 17 cases of sarcoidosis or sarcoid-like reaction in patients to malignant lymphoma, of which three had eye lesions [34]. Other groups have reported rare cases of ocular involvement of sarcoidosis or sarcoid-like reactions; however, they lack specific descriptions of the eye findings and do not report ocular tissue biopsy for evaluation (2 of 5 cases by Karakantza et al. [35]). The most recent specific study addressing sarcoid-like reactions and the eye in the context of cancer was reported in 2015 by Balasubramaniam and colleagues [36]. They reported a case series of 5 patients that had sarcoid-like reaction following the diagnosis of cancer. One patient was diagnosed with sarcoid-like reaction 34 months after initial diagnosis and treatment of B-cell CLL, which presented with similar vitreous findings as our patients. One patient had uveal melanoma and renal cell carcinoma, but the granulomatous findings were not ocular involving. Of note, the remaining 3 patients had endometrial cancer, mucosal-associated lymphoid tissue lymphoma, and renal cell carcinoma, all of which had ocular findings (choroidal granulomas, intermediate uveitis, and retinal vasculitis, respectively), and all of which presented after formal cancer diagnosis. Collectively, while rare to have both a neoplasm and sarcoidosis or sarcoid-like reaction, these few cases illustrate the utmost caution ophthalmologists should have in making an ocular CLL diagnosis.
Two limitations affect out study. First, a case series of 4 patients is a small number of patients to base a new recommendation for molecular testing to diagnose CLL. However, we acknowledge the intraocular CLL is a rare disease, with under 200 reported cases over a multi-decade period compared to roughly 400 cases per year for VRL [37]. We are not suggesting that negative MYD88 L265P molecular testing should replace gold-standard cytopathology or flow cytometry, but the aforementioned described cases provide a useful context of how negative MYD88 L265P testing can help clinicians provide excellent management of patients with challenging presentations of CLL, including initiation of potentially life-saving treatment. Second, 3 of the 4 patients we describe (cases 1, 3, and 4) were on ibrutinib. We highlighted earlier that the use of steroids can affect the tumor burden and potential mask its presence when performing routine lumbar puncture (or vitreous and/or aqueous liquid biopsy). Ibrutinib is a direct acting small molecular inhibitor used in the treatment of CLL, among other hematogenous cancers. One case report from 2021 showed how initiation of ibrutinib in a patient with choroidal infiltration of CLL resulted in resolution of disease, and following a period of cessation its recurrence in the choroid [38]. We are not of the opinion that the diagnostic yield for MYD88 L265P testing was adversely affected by the use of ibrutinib because these patients were on the medication for several years before their ocular symptoms presented. For the same reason, we are not of the opinion that any degree of CME seen in these patients was a result of ibrutinib. We believe the patient presentations represented a progression of disease that was not adequately controlled by ibrutinib, thus validating the utility of not only our molecular testing but also gold-standard cytopathology and flow cytometry, which is considered standard of care.
In conclusion, we report posterior uveitis/vitritis in 4 CLL patients who were otherwise under remission. In the context of nebulous or unequivocal gold-standard cytopathology and/or flow cytometry testing, molecular PCR testing for MYD88 L265P can be a useful adjuvant test for diagnosing ocular-involving CLL. We anticipate that with any future specific biomarker associated with CLL will utilize MYD88 L265P testing as a pertinent negative molecular test on liquid vitreous and aqueous biopsies.
Statement of Ethics
This study did not require ethics approval in accordance with local or national guidelines. Written informed consent was obtained from all patients for publication of their details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
H.D. was supported by the Richard N. and Marilyn K. Witham Professorship of Ophthalmology and Visual Sciences. R.C.R. was supported by the National Eye Institute (NEI) (R01EY030989), Research to Prevent Blindness (RPB), A. Alfred Taubman Medical Research Institute, the Beatrice and Reymont Paul Foundation, March Hoops to Beat Blindness, and Leonard G. Miller Endowed Professorship and Ophthalmic Research Fund at the Kellogg Eye Center. Additional support for this research was provided by Grossman, Elaine Sandman, Marek and Maria Spatz (endowed fund), Greenspon, Dunn, Avers, Boustikakis, Sweiden, and Terauchi research funds.
Author Contributions
D.A.B., R.C.R., and N.A.B.: data acquisition, data analysis, and manuscript preparation. V.M.E. and T.J.W.: data analysis and manuscript preparation. H.D.: research design, data acquisition, data analysis, and manuscript preparation
Funding Statement
H.D. was supported by the Richard N. and Marilyn K. Witham Professorship of Ophthalmology and Visual Sciences. R.C.R. was supported by the National Eye Institute (NEI) (R01EY030989), Research to Prevent Blindness (RPB), A. Alfred Taubman Medical Research Institute, the Beatrice and Reymont Paul Foundation, March Hoops to Beat Blindness, and Leonard G. Miller Endowed Professorship and Ophthalmic Research Fund at the Kellogg Eye Center. Additional support for this research was provided by Grossman, Elaine Sandman, Marek and Maria Spatz (endowed fund), Greenspon, Dunn, Avers, Boustikakis, Sweiden, and Terauchi research funds.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be direct to the corresponding author.
Supplementary Material
References
- 1. Kikushige Y, Ishikawa F, Miyamoto T, Shima T, Urata S, Yoshimoto G, et al. Self-renewing hematopoietic stem cell is the primary target in pathogenesis of human chronic lymphocytic leukemia. Cancer Cell. 2011;20(2):246–59. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. [DOI] [PubMed] [Google Scholar]
- 3. Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–37. [DOI] [PubMed] [Google Scholar]
- 4. Rossi D, Cerri M, Capello D, Deambrogi C, Rossi FM, Zucchetto A, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008;142(2):202–15. [DOI] [PubMed] [Google Scholar]
- 5. Parikh SA, Habermann TM, Chaffee KG, Call TG, Ding W, Leis JF, et al. Hodgkin transformation of chronic lymphocytic leukemia: incidence, outcomes, and comparison to de novo Hodgkin lymphoma. Am J Hematol. 2015;90(4):334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buchan J, McKibbin M, Burton T. The prevalence of ocular disease in chronic lymphocytic leukaemia. Eye. 2003;17(1):27–30. [DOI] [PubMed] [Google Scholar]
- 7. Singh AD. The prevalence of ocular disease in chronic lymphocytic leukaemia. Eye. 2003;17(1):3–4. [DOI] [PubMed] [Google Scholar]
- 8. Delestre F, Blanche P, Bouayed E, Bouscary D, Mouthon L, Brezin A, et al. Ophthalmic involvement of chronic lymphocytic leukemia: a systematic review of 123 cases. Surv Ophthalmol. 2021;66(1):124–31. [DOI] [PubMed] [Google Scholar]
- 9. Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cani AK, Hovelson DH, Demirci H, Johnson MW, Tomlins SA, Rao RC. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget. 2017;8(5):7989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nayyar N, White MD, Gill CM, Lastrapes M, Bertalan M, Kaplan A, et al. MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas. Blood Adv. 2019;3(3):375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narasimhan S, Joshi M, Parameswaran S, Rishi P, Khetan V, Ganesan S, et al. MYD88 L265P mutation in intraocular lymphoma: a potential diagnostic marker. Indian J Ophthalmol. 2020;68(10):2160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vermaat JS, Somers SF, de Wreede LC, Kraan W, de Groen RAL, Schrader AMR, et al. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica. 2020;105(2):424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Visco C, Tanasi I, Quaglia FM, Ferrarini I, Fraenza C, Krampera M. Oncogenic mutations of MYD88 and CD79B in diffuse large B-cell lymphoma and implications for clinical practice. Cancers. 2020;12(10):2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balikov DA, Hu K, Liu CJ, Betz BL, Chinnaiyan AM, Devisetty LV, et al. Comparative molecular analysis of primary central nervous system lymphomas and matched vitreoretinal lymphomas by vitreous liquid biopsy. Int J Mol Sci. 2021;22(18):9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demirci H, Rao RC, Elner VM, Demirci FY, Axenov L, Betz B, et al. Aqueous humor-derived MYD88 L265P mutation analysis in vitreoretinal lymphoma: a potential less invasive method for diagnosis and treatment response assessment. Ophthalmol Retina. 2023;7(2):189–95. [DOI] [PubMed] [Google Scholar]
- 17. Duke JR, Wilkinson CP, Sigelman S. Retinal microaneurysms in leukaemia. Br J Ophthalmol. 1968;52(5):368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kincaid MC, Green WR. Ocular and orbital involvement in leukemia. Surv Ophthalmol. 1983;27(4):211–32. [DOI] [PubMed] [Google Scholar]
- 19. Schachat AP, Markowitz JA, Guyer DR, Burke PJ, Karp JE, Graham ML. Ophthalmic manifestations of leukemia. Arch Ophthalmol. 1989;107(5):697–700. [DOI] [PubMed] [Google Scholar]
- 20. Khan K, Malik AI, Almarzouqi SJ, Morgan ML, Yalamanchili S, Chevez-Barrios P, et al. Optic neuropathy due to chronic lymphocytic leukemia proven with optic nerve sheath biopsy. J Neuro Ophthalmol. 2016;36(1):61–6. [DOI] [PubMed] [Google Scholar]
- 21. Moazzam AA, Drappatz J, Kim RY, Kesari S. Chronic lymphocytic leukemia with central nervous system involvement: report of two cases with a comprehensive literature review. J Neuro Oncol. 2012;106(1):185–200. [DOI] [PubMed] [Google Scholar]
- 22. Currie JN, Lessell S, Lessell IM, Weiss JS, Albert DM, Benson EM. Optic neuropathy in chronic lymphocytic leukemia. Arch Ophthalmol. 1988;106(5):654–60. [DOI] [PubMed] [Google Scholar]
- 23. Hattenhauer MG, Pach JM. Ocular lymphoma in a patient with chronic lymphocytic leukemia. Am J Ophthalmol. 1996;122(2):266–8. [DOI] [PubMed] [Google Scholar]
- 24. Akintola-Ogunremi O, Whitney C, Mathur SC, Finch CN. Chronic lymphocytic leukemia presenting with symptomatic central nervous system involvement. Ann Hematol. 2002;81(7):402–4. [DOI] [PubMed] [Google Scholar]
- 25. Borowitz M, Bigner SH, Johnston WW. Diagnostic problems in the cytologic evaluation of cerebrospinal fluid for lymphoma and leukemia. Acta Cytol. 1981;25(6):665–74. [PubMed] [Google Scholar]
- 26. Deckert M, Brunn A, Montesinos-Rongen M, Terreni MR, Ponzoni M. Primary lymphoma of the central nervous system: a diagnostic challenge. Hematol Oncol. 2014;32(2):57–67. [DOI] [PubMed] [Google Scholar]
- 27. Bonzheim I, Giese S, Deuter C, Susskind D, Zierhut M, Waizel M, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015;126(1):76–9. [DOI] [PubMed] [Google Scholar]
- 28. Carreno E, Clench T, Steeples LR, Salvatore S, Lee RWJ, Dick AD, et al. Clinical spectrum of vitreoretinal lymphoma and its association with MyD88 L265P mutation. Acta Ophthalmol. 2019;97(1):e138–9. [DOI] [PubMed] [Google Scholar]
- 29. Shi H, Zhou X, Chen B, Xiao J, Li Y, Zhou X, et al. Clinical relevance of the high prevalence of MYD88 L265P mutated vitreoretinal lymphoma identified by droplet digital polymerase chain reaction. Ocul Immunol Inflamm. 2021;29(3):448–55. [DOI] [PubMed] [Google Scholar]
- 30. Kroft SH, Dawson DB, McKenna RW. Large cell lymphoma transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma. A flow cytometric analysis of seven cases. Am J Clin Pathol. 2001;115(3):385–95. [DOI] [PubMed] [Google Scholar]
- 31. Abdel-Ghafar AA, El Din El Telbany MA, Mahmoud HM, El-Sakhawy YN. Immunophenotyping of chronic B-cell neoplasms: flow cytometry versus immunohistochemistry. Hematol Rep. 2012;4(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seegmiller AC, Hsi ED, Craig FE. The current role of clinical flow cytometry in the evaluation of mature B-cell neoplasms. Cytometry B Clin Cytom. 2019;96(1):20–9. [DOI] [PubMed] [Google Scholar]
- 33. Brincker H. Sarcoid reactions and sarcoidosis in Hodgkin’s disease and other malignant lymphomata. Br J Cancer. 1972;26(2):120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brincker H. The sarcoidosis-lymphoma syndrome. Br J Cancer. 1986;54(3):467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karakantza M, Matutes E, MacLennan K, O'Connor NT, Srivastava PC, Catovsky D. Association between sarcoidosis and lymphoma revisited. J Clin Pathol. 1996;49(3):208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balasubramaniam SC, Salomao DR, Davies JB, Ramsay RC, Habermann TM, Chow GK, et al. Paraneoplastic sarcoid-like reactions and the eye. Retina. 2015;35(4):789–97. [DOI] [PubMed] [Google Scholar]
- 37. Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, et al. Primary vitreoretinal lymphoma: a report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist. 2011;16(11):1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sieburth RM, Weaver CD, Kirzhner M, Shildkrot Y. Ibrutinib for control of choroidal and orbital metastasis from chronic lymphocytic leukemia. Retin Cases Brief Rep. 2023;17(2):120–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be direct to the corresponding author.