Abstract
The N-terminal alpha-helix domain of the human immunodeficiency virus type 1 (HIV-1) Nef protein plays important roles in enhancement of viral infectivity, virion incorporation of Nef, and the down-regulation of major histocompatibility complex class I (MHC-I) expression on cell surfaces. In this study, we demonstrated that Met 20 in the alpha-helix domain was indispensable for the ability of Nef to modulate MHC-I expression but not for other events. We also showed that Met 20 was unnecessary for the down-regulation of CD4. These findings indicate that the region governing MHC-I down-regulation is proximate in the alpha-helix domain but is dissociated functionally from that determining enhancement of viral infectivity, virion incorporation of Nef, and CD4 down-regulation.
The nef gene is unique to primate lentiviruses and is shown to be associated with their pathogenesis. In macaque monkeys infected with simian immunodeficiency virus, its nef gene is required for maintaining high viral loads and inducing CD4+-lymphocyte depletion (16, 27). Similar findings have been obtained from a severe combined immunodeficiency-hu mouse model infected with human immunodeficiency virus type 1 (HIV-1) (12, 15) and HIV-1-transgenic mice (13). Recent findings that the nef gene of HIV-1 derived from long-term nonprogressive carriers is truncated suggest the requirement of the intact nef gene for disease progression (18, 21, 25).
Functional characterization of Nef protein in vitro has shown that Nef (i) down-regulates the expression of CD4 and major histocompatibility complex class I (MHC-I) molecules, (ii) affects cellular signal transduction pathways, and (iii) enhances viral infectivity (for review, see references 14 and 28). It has also been reported that Nef is incorporated into the virions, where it is cleaved by the viral protease between amino acids 57 and 58 (24, 30), although the biological implication of virion incorporation of Nef remains unknown (9, 22, 23). These effects of Nef are associated with its membrane anchoring, and N-terminal myristoylation of Nef is a determinant for the anchoring (14, 28). However, studies on other myristoylated proteins have indicated that myristoylation alone is not sufficient to stably anchor a protein into the membrane (26).
The N-terminal residues 6 to 22 adopt an alpha-helix structure (5). An N-terminal Arg-rich motif (I16RERMRR22), which is well conserved among the Nef proteins of major HIV-1 strains, is required for association with a protein complex containing Lck and serine kinases and for optimal viral infectivity in resting peripheral blood mononuclear cells (PBMCs) but is dispensable for down-regulation of CD4 (6). It has been shown that bipartite motifs in the N terminus of Nef (K4xxK7 and R17ERMRR22) play important roles in virion incorporation of Nef and in viral infectivity (29). Recently, Mangasarian et al. have shown that a mutant Nef protein in which residues 17 to 26 have been deleted fails to down-regulate MHC-I while retaining the ability to modulate CD4 levels (20), in agreement with previous reports (3, 6, 8, 10).
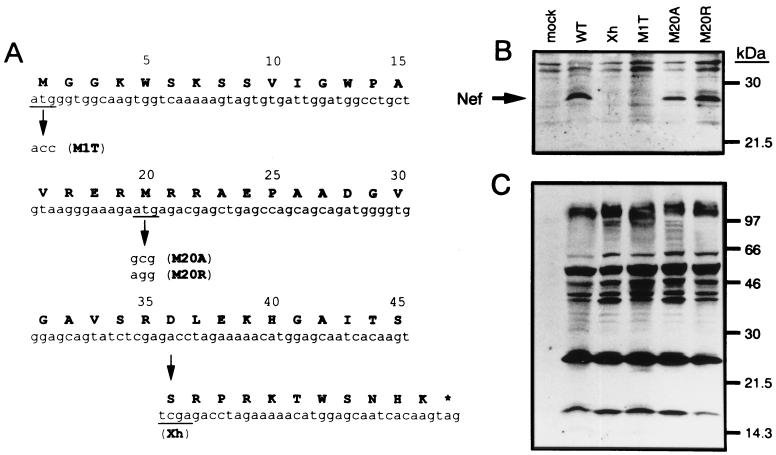
In this study, we investigated the functional role of the conserved methionine at position 20, which is present in the amphipathic alpha-helix domain of Nef protein. For this purpose, the second ATG codon, coding for Met 20, in the nef gene of HIV-1NL-432 (wild type [WT]) provirus clone pNL-432 (1) was mutated into GCG coding for Ala by using an LA PCR in vitro mutagenesis kit (Takara, Kusatsu, Japan). The mutant clone was designated pNL-M20A. This single amino acid exchange maintains hydrophobicity at this position. Considering the possibility that hydrophobicity could be important for proper function of the motif, the ATG codon was also changed into AGG coding for Arg to generate a pNL-M20R mutant. As controls, a Nef-defective mutant, pNL-Xh (referred to herein as Xh), having a frame shift at a XhoI site (2) and another Nef mutant, pNL-M1T (referred to herein as M1T), which lacks expression of Nef because of an alteration of the first ATG codon to ACC, were used. The mutations are summarized in Fig. 1A.
FIG. 1.
Analysis of cellular expression of Nef proteins. (A) Sequences of HIV-1 nef mutants used here are shown. The N-terminal 45 residues of Nef and the corresponding nucleic acid sequence are depicted. The asterisk indicates the stop codon. (B and C) Lysates containing an equal amount of p24 capsid antigen were prepared from HeLa cells transfected with the respective proviral DNA clones and were analyzed by Western blotting with a rabbit anti-Nef antiserum (B) and a human anti-HIV-1 antiserum (C).
We initially examined the expression of the Nef protein in cells. HeLa cells were transfected with each proviral DNA clone by the calcium phosphate coprecipitation method (31). The lysates of transfected HeLa cells were quantified for the amount of p24 capsid antigen by a p24 antigen enzyme-linked immunosorbent assay kit (Cellular Products, Buffalo, N.Y.). The lysates containing an equal amount of the viral antigen were subjected to electrophoresis in a sodium dodecyl sulfate-gradient polyacrylamide gel. The separated proteins were blotted onto nitrocellulose membranes, treated with antibodies to HIV-1 proteins, and visualized using an ECL system (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). Anti-Nef antisera were provided by R. Swanstrom (through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) and Y. Takebe (National Institute of Infectious Diseases, Tokyo, Japan). Total virus antigens were detected by an anti-HIV-1 antiserum. The results of this experiment showed that mutations of Met 20 to Ala and Arg did not much affect the expression of the 27-kDa Nef protein, which was detected in pNL-432-transfected cells (Fig. 1B). The Xh- or M1T-transfected HeLa cells did not express the Nef protein (Fig. 1B). In addition, similar levels of the major viral antigens were observed among the cells transfected with the WT or the Nef mutants (Fig. 1C).
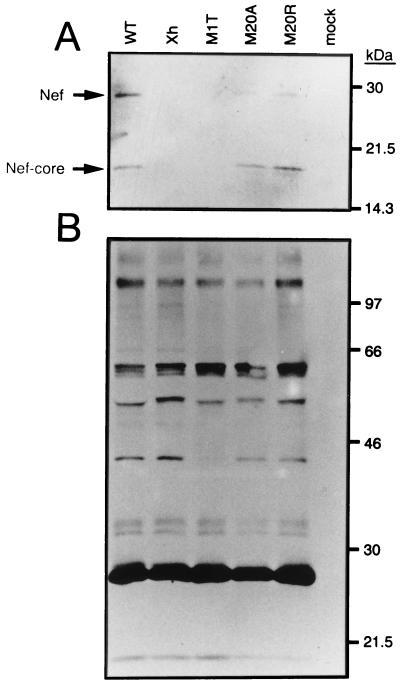
To determine whether Met 20 of the conserved amphipathic motif plays a role in virion incorporation of Nef, we examined the ability of the WT and mutant Nef proteins to be incorporated into virions. For this purpose, the lysates of the WT and Nef mutant viruses produced from HeLa cells transfected with the respective proviral DNA plasmids were subjected to Western blotting analysis as described above. Both a full-length Nef and its C-terminal core, which is a 20-kDa product cleaved in the virion by the viral protease, were demonstrated in the pNL-M20A and pNL-M20R viruses as well as the WT virus (Fig. 2A). These Nef proteins were not detected in Xh and M1T viruses (Fig. 2A). No major difference in the viral proteins was observed among the WT and its Nef variants (Fig. 2B). These results indicated the dispensability of Met 20 for virion incorporation of Nef.
FIG. 2.
Incorporation of Nef proteins into virions. HIV-1 WT and nef mutant viruses were prepared in HeLa cells transfected with the respective proviral DNA clones. Virus lysates containing an equal amount of p24 capsid antigen were analyzed by Western blotting with a rabbit anti-Nef antiserum (A) and a human anti-HIV-1 antiserum (B).
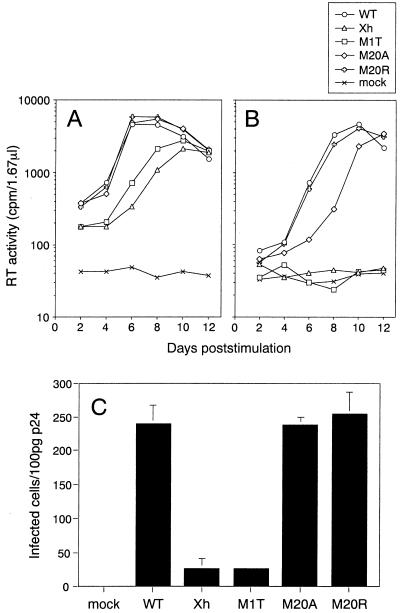
We then investigated the effect of mutations in the nef gene on viral infectivity in PBMCs. Resting PBMCs were infected with appropriate amounts of viruses which were adjusted by reverse transcriptase (RT) activity (32). Two days after infection, infected PBMCs were stimulated for 2 days by the addition of 0.5 μg of phytohemagglutinin P (Difco, Detroit, Mich.) per ml. The cells were maintained by exchanging every 2 days half the volume with culture medium consisting of RPMI 1640 with 10% fetal bovine serum, l-glutamine, antibiotics, and 50 U of recombinant human interleukin-2 (Serotec, Oxford, United Kingdom) per ml. The kinetics of viral replication were monitored by measuring RT activity in the culture supernatants. When a relatively large amount of virus (105 cpm of RT activity) was used, Nef-defective viruses Xh and M1T showed delayed kinetics and about a three-times-lower peak of replication than the WT, pNL-M20A, and pNL-M20R viruses (Fig. 3A). A decrease of the amount of input viruses to one-fifth (2 × 104 cpm of RT activity) reduced the growth of the Xh and M1T viruses to undetectable levels (Fig. 3B), although the WT, pNL-M20A, and pNL-M20R viruses replicated comparably (Fig. 3B). The slightly delayed growth kinetics of pNL-M20A (Fig. 3B) could be due to the reduced expression of Nef of this mutant (Fig. 1B). These results suggested that mutations in Met 20 of Nef did not much affect viral infectivity. To further address the potential contribution of Met 20 to viral infectivity, the WT and Nef mutant viruses were examined for single-round infectivity by MAGI assay (17). MAGI cells were plated in 96-well plates and infected in triplicate with serially diluted viruses in a total of 200 μl of culture medium containing 20 μg of DEAE-dextran (Sigma, St. Louis, Mo.) per ml. Infected cells were incubated for 2 days at 37°C, fixed, and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Blue cells were counted as infected cells. It was demonstrated that the infectivity of the pNL-M20A and pNL-M20R viruses was comparable to that of WT virus (Fig. 3C). The Xh and M1T viruses showed about 10-times-lower infectivity than WT virus (Fig. 3C). Taken together, the results show that Met 20 is dispensable for cellular expression of Nef and its incorporation into virions and for the enhancing the effects of Nef on both single and multiple cycles of HIV-1 infection.
FIG. 3.
Analysis of the infectivity of the WT and nef mutants of HIV-1. (A and B) Replication kinetics of the viruses in PBMCs. Viruses were obtained from HeLa cells transfected with WT and various nef mutant proviral clones. Unstimulated PBMCs (105) were infected with 1 × 105 (A) and 2 × 104 (B) cpm of RT from viruses, and 2 days later, cells were stimulated by the addition of 0.5 μg of phytohemagglutinin P per ml. A half volume of the culture supernatants was harvested and refed with the same volume of culture medium with 50 U of recombinant human interleukin-2 per ml every 2 days. Kinetics of RT production in the culture supernatants are indicated. (C) Single-round infectivity of the viruses. Infectivity was determined by counting blue foci of X-Gal-treated MAGI cells 2 days after inoculation with the viruses. Averages and standard deviations of triplicate titrations of the same viral stock are shown.
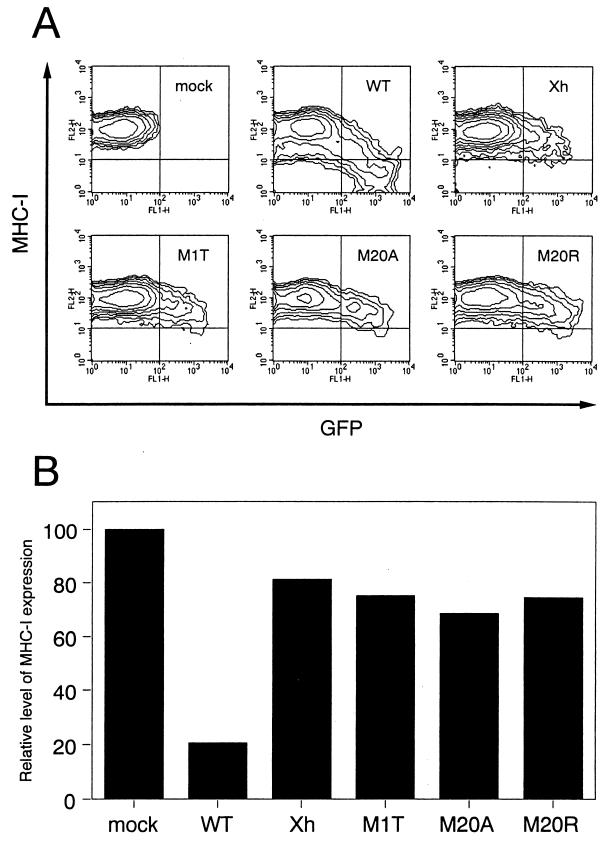
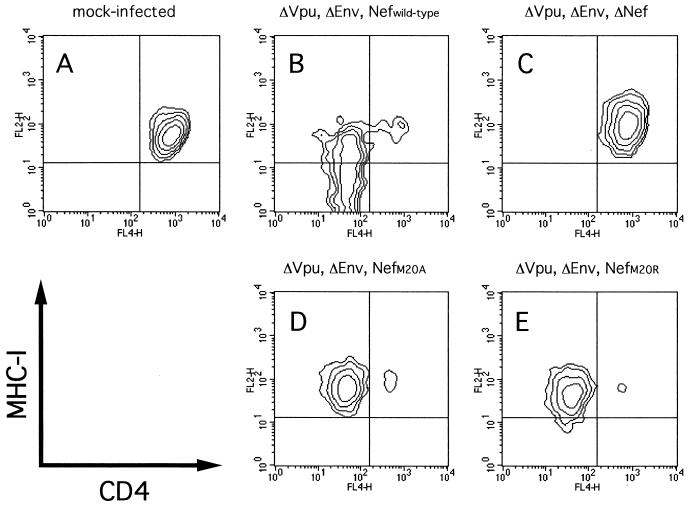
In the course of our study, Mangasarian et al. reported that a mutant Nef protein with a deletion of the N-terminal alpha-helix domain at residues 17 to 26 fails to down-regulate MHC-I while retaining the ability to modulate the CD4 level (20). We therefore examined whether Met 20 contributes to the ability of Nef to down-regulate MHC-I expression on the cell surface. Since CEM-GFP cells contain an HIV-1 long terminal repeat-driven green fluorescence protein (GFP) cDNA and GFP expression is inducible by Tat (11), we used it for measuring directly the level of MHC-I expression on HIV-1-infected cells. The CEM-GFP cell line was provided from J. Corbeil through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. CEM-GFP cells (105) were infected with 5 × 105 cpm of RT from the WT or the Nef mutant viruses prepared from transfected HeLa cells. When syncytium formation was observed a few days after infection, the cells were treated with an R-phycoerythrin (RPE)-conjugated mouse anti-MHC-I monoclonal antibody (W6/32; Dako, Glostrup, Denmark) at 4°C for 1 h. The cells were washed and fixed with 1% formaldehyde, and the fluorescence intensity for GFP and MHC-I was detected by a FACSCalibur (Becton Dickinson, Mountain View, Calif.). As shown in Fig. 4A, in the case of WT virus infection, down-regulation of MHC-I expression on GFP-positive cells was obvious, and especially the fluorescence intensity for GFP was reversely correlated with the level of MHC-I expression (Fig. 4A). In sharp contrast, infection with either pNL-M20A or pNL-M20R scarcely down-regulated MHC-I expression on GFP-positive cells; the same was true for the Xh and M1T Nef-defective mutants (Fig. 4A). The level of MHC-I expressed on each GFP-positive cell population was calculated as geometric mean fluorescence using CELLQuest software (Becton Dickinson), and the relative level of MHC-I expression compared with that on mock-infected cells is shown (Fig. 4B). The level of MHC-I expression on WT-infected cells was 20% of that on uninfected cells, while that on any of the mutant-infected cells was about 70 to 80% of that on uninfected cells. From this result, it is clearly demonstrated that Met 20 in the N-terminal alpha-helix domain is essential for the ability of Nef to modulate MHC-I expression. We further determined whether Met 20 is important for the ability of Nef to down-regulate CD4 expression. For an assay of CD4 down-regulation, we made other DNA constructs lacking expression of Env and Vpu, which affect the CD4 level. The BamHI-NcoI DNA fragments of the mutants containing the mutated nef genes (M1T, M20A, and M20R) were inserted into the corresponding region of pNL43-Ude1.K1, which is defective for both vpu and env (7). These mutants were designated pNL43-Ude1.K1.nM1T, pNL43-Ude1.K1.nM20A, and pNL43-Ude1.K1.nM20R, respectively. The envelope glycoproteins of vesicular stomatitis virus (VSV-G)–HIV-1 pseudotyped viruses were then prepared by cotransfection of VSV-G expression vector pCMV-G (33) and the nef mutants into HeLa cells as previously described (4). CEM-GFP cells (105) were infected with 2.5 × 107 cpm of RT from various pseudotyped viruses, and after 2 days, the cells were stained with RPE-conjugated W6/32 and allophycocyanin-conjugated anti-CD4 (Leu-3A; Becton Dickinson) monoclonal antibodies. The fluorescence intensity for CD4 and MHC-I on the cells expressing GFP was then determined as described above. As can be clearly seen in Fig. 5, the pNL-M20A and pNL-M20R mutants retained the ability to down-regulate CD4 expression while failing to down-modulate the MHC-I level.
FIG. 4.
Effect of mutations in Nef protein on MHC-I down-regulation on the surfaces of HIV-1-infected cells. (A) CEM-GFP cells (105) were infected with 5 × 105 cpm of RT from each virus obtained from transfected HeLa cells, and 3 to 5 days later the cells were reacted with an RPE-labeled anti-MHC-I antibody at 4°C for 1 h. The cells were washed, fixed with 1% formaldehyde, and analyzed for fluorescence intensity by flow cytometry. (B) The level of MHC-I expression on the GFP-positive population in virus-inoculated CEM-GFP cells was evaluated as geometric mean fluorescence using CELLQuest software (Becton Dickinson).
FIG. 5.
Effect of mutations in the Nef protein on CD4 down-regulation on the surfaces of cells infected with VSV-G–HIV-1 pseudotyped virus. The pseudotyped viruses were prepared by cotransfection of pCMV-G and pNL43-Ude1.K1 (B), pNL43-Ude1.K1.nM1T (C), pNL43-Ude1.K1.nM20A (D), or pNL43-Ude1.K1.nM20R (E) into HeLa cells. CEM-GFP cells (105) were infected with 2.5 × 107 cpm of RT from each pseudotyped virus, and 2 days later, cells were treated at 4°C for 1 h with RPE-labeled anti-MHC-I antibody and allophycocyanin-labeled anti-CD4 antibody. The cells were then washed, fixed with 1% formaldehyde, and analyzed for fluorescence intensity for CD4 and MHC-I in the cell population expressing GFP by flow cytometry.
The present findings demonstrate that the down-regulation of MHC-I by Nef is a property genetically and functionally separate from virion incorporation of Nef and enhancement of viral infectivity by Nef. It has been shown that the N-terminal alpha-helix region is the determinant for these three effects (6, 20, 29). However, we showed that mutations in Met 20 cause the loss of the ability of Nef to modulate MHC-I expression but not the other effects (Fig. 2 to 4). We also showed that the mutations in Met 20 did not affect the ability of Nef to down-regulate the CD4 level (Fig. 5). Our results support and extend the report of Le Gall et al. on Nef (19) that shows the functional dissociation of MHC-I down-regulation from enhancement of viral infectivity. It is surprising that the region governing MHC-I down-regulation is proximate to but dissociated functionally from that determining virion incorporation of Nef and enhancement of viral infectivity by Nef. Baur et al. have shown that the N-terminal alpha-helix domain of Nef interacts with Lck and a serine kinase (6). Based on this finding, it is postulated that one of the two kinases is required for association of Nef with MHC-I and the other plays a critical role in virion incorporation of Nef and enhancement of viral infectivity by Nef. If so, it is possible that Lck phosphorylates a tyrosine-based motif of the MHC-I cytoplasmic domain to facilitate association between Nef and MHC-I. Alternatively, it can also be speculated that these kinases do not take part in the modulation of MHC-I expression. Mangasarian et al. have shown that treatment of HIV-1-infected cells with herbimycin A, an inhibitor of Src family protein kinases, does not block MHC-I down-regulation (20), which argues against the former hypothesis. In this case, it is probable that the IRERMRR motif including Met 20 associates with MHC-I and that the far N-terminal region in the alpha-helix domain, at residues 6 to 22, interacts with the kinases. Baur et al. have also shown that deletion of residues 16 to 22 reduces but does not completely abolish Lck binding (6), implying that the deletion maintains the Lck-binding domain but deteriorates affinity between Nef and Lck. This result supports the latter hypothesis. Further study on the functional roles of the kinases associated with the N terminus of Nef is required to prove these hypotheses.
Acknowledgments
We are grateful to R. Swanstrom (through the AIDS Research and Reference Reagents Program, Division of AIDS, NIAID, NIH) and Y. Takebe for providing anti-Nef antisera and to J. Corbeil (also through the AIDS Research and Reference Reagents Program, Division of AIDS, NIAID, NIH) and A. Miyanohara for the gift of CEM-GFP cells. We also thank S. Bour for helpful discussion and Kazuko Yoshida for editorial assistance.
This work was supported by grants-in-aid for AIDS research from the Ministry of Education, Science, Sports and Culture of Japan and the Ministry of Health and Welfare of Japan.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi A, Ono N, Sakai H, Ogawa K, Shibata R, Kiyomasu T, Masuike H, Ueda S. Generation and characterization of the human immunodeficiency virus type 1 mutants. Arch Virol. 1991;117:45–58. doi: 10.1007/BF01310491. [DOI] [PubMed] [Google Scholar]
- 3.Aiken C, Krause L, Chen Y, Trono D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 4.Akari H, Uchiyama T, Fukumori T, Iida S, Koyama A H, Adachi A. Pseudotyping human immunodeficiency virus type 1 by vesicular stomatitis virus G protein does not reduce the cell-dependent requirement of Vif for optimal infectivity: functional difference between Vif and Nef. J Gen Virol. 1999;80:2945–2950. doi: 10.1099/0022-1317-80-11-2945. [DOI] [PubMed] [Google Scholar]
- 5.Barnham K J, Monks S A, Hinds M G, Azad A A, Norton R S. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36:5970–5980. doi: 10.1021/bi9629945. [DOI] [PubMed] [Google Scholar]
- 6.Baur A S, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin B M. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 7.Bour S, Strebel K. The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J Virol. 1996;70:8285–8300. doi: 10.1128/jvi.70.12.8285-8300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y-L, Trono D, Camaur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gervaix A, West D, Leoni L M, Richman D D, Wong-Staal F, Corbeil J A. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc Natl Acad Sci USA. 1997;94:4653–4658. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulizia R J, Collman R G, Levy J A, Trono D, Mosier D E. Deletion of nef slows but does not prevent CD4-positive T-cell depletion in human immunodeficiency virus type 1-infected human-PBL-SCID mice. J Virol. 1997;71:4161–4164. doi: 10.1128/jvi.71.5.4161-4164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 14.Harris M. From negative factor to a critical role in virus pathogenesis: the changing fortunes of Nef. J Gen Virol. 1996;77:2379–2392. doi: 10.1099/0022-1317-77-10-2379. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B M, Gao L, Bloch L M, Chen I S Y, Zack J A. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651–662. [DOI] [PubMed]
- 17.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 19.Le Gall S, Prevost M-C, Heard J-M, Schwartz O. Human immunodeficiency virus type 1 Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology. 1997;229:295–301. doi: 10.1006/viro.1996.8417. [DOI] [PubMed] [Google Scholar]
- 20.Mangasarian A, Piguet V, Wang J-K, Chen Y-L, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73:1964–1973. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller M D, Warmerdam M T, Ferrell S S, Benitez R, Greene W C. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology. 1997;234:215–225. doi: 10.1006/viro.1997.8641. [DOI] [PubMed] [Google Scholar]
- 23.Pandori M, Craig H, Moutouh L, Corbeil J, Guatelli J. Virological importance of the protease-cleavage site in human immunodeficiency virus type 1 Nef is independent of both intravirion processing and CD4 down-regulation. Virology. 1998;251:302–316. doi: 10.1006/viro.1998.9407. [DOI] [PubMed] [Google Scholar]
- 24.Pandori M W, Fitch N J S, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premkumar D R, Ma X Z, Maitra R K, Chakrabarti B K, Salkowitz J, Yen-Lieberman B, Hirsch M S, Kestler H W. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res Hum Retrovir. 1996;12:337–345. doi: 10.1089/aid.1996.12.337. [DOI] [PubMed] [Google Scholar]
- 26.Resh M D. Myristoylation and palmitoylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 27.Rud E W, Cranage M, Yon J, Quirk J, Ogilvie L, Cook N, Webster S, Dennis M, Clarke B E. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J Gen Virol. 1994;75:529–543. doi: 10.1099/0022-1317-75-3-529. [DOI] [PubMed] [Google Scholar]
- 28.Saksela K. HIV-1 Nef and host cell protein kinases. Front Biosci. 1997;2:606–618. doi: 10.2741/a217. [DOI] [PubMed] [Google Scholar]
- 29.Welker R, Harris M, Cardel B, Krausslich H G. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J Virol. 1998;72:8833–8840. doi: 10.1128/jvi.72.11.8833-8840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welker R, Kottler H, Kalbitzer H R, Krausslich H-G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral protease. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 31.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyl transferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]