Abstract
Transcripts of most intron-bearing cellular genes must be processed by the splicing machinery in order to efficiently accumulate and gain access to the cytoplasm. However, we found that herpes simplex virus induces cytoplasmic accumulation of both spliced and unspliced polyadenylated α-globin RNAs in infected HeLa cells. Accumulation of the unspliced RNA required the immediate-early protein ICP27, and ICP27 was sufficient (in combination with ICP4) to produce this effect in a transient-transfection assay. However, expression of ICP27 did not markedly alter the levels of fully spliced α-globin transcripts in infected cells. These data demonstrate that the previously documented effects of ICP27 on the cellular splicing apparatus do not greatly inhibit splicing of α-globin RNA and argue that ICP27 induces a splicing-independent pathway for α-globin RNA accumulation and nuclear export.
Herpes simplex virus (HSV) is a large enveloped DNA virus that infects a wide variety of mammalian cells in tissue culture (reviewed in reference 46). HSV executes a complex genetic regulatory program during lytic infection: three temporal classes of viral genes (immediate early [IE], early [E], and late [L]) are sequentially activated in a regulatory cascade driven by viral gene products, while expression of most host cell genes is strongly suppressed. The HSV lytic program is initiated by the virion transactivator VP16, which acts in combination with host factors to stimulate transcription of the five IE genes (reviewed in references 16 and 60). Four of the IE proteins (ICP0, ICP4, ICP22, and ICP27) then play key roles in orchestrating expression of the viral E and L genes (10, 30, 48, 49, 55, 59, 63).
Early studies demonstrated that the HSV lytic cascade involves both transcriptional and posttranscriptional controls of mRNA metabolism (64). Mounting evidence suggests that the immediate-early (IE) protein ICP27 contributes to the posttranscriptional component of the viral regulatory program (57; reviewed in reference 51). ICP27 is an essential regulator that stimulates accumulation of a subset of viral E and L mRNAs and is required for efficient viral DNA replication (12, 30, 34, 43, 44, 48, 56, 62). A clue to the mechanism of ICP27 action was provided by Sandri-Goldin and Mendoza (53), who showed that ICP27 enhances expression of constructs bearing a synthetic and incomplete polyadenylation signal and inhibits expression of reporter genes bearing certain introns in transient-cotransfection assays. A complementary series of studies by Clements and colleagues demonstrated that HSV infection alters the specificity of the host polyadenylation machinery in an ICP27-dependent fashion, thereby allowing more efficient use of a subset of viral polyadenylation signals (31–33). These findings indicate that ICP27 modulates intranuclear processing of pre-mRNAs and suggest that it inhibits splicing of at least some intron-bearing transcripts (53).
The hypothesis that ICP27 impairs RNA splicing has been supported by a considerable amount of data showing the following: (i) unspliced pre-RNA derived from the intron-bearing HSV genes encoding ICP0 and UL15 accumulate in the nuclei of some cell types infected with wild-type HSV but not with an ICP27 mutant (20, 21, 40), (ii) ICP27 colocalizes with and redistributes snRNPs in HSV-infected cell nuclei (38), (iii) ICP27 coimmunoprecipitates with splicing factors that react with anti-Sm antisera and appears to alter the phosphorylation status of some of these proteins (52), and (iv) nuclear extracts prepared from cells infected with wild-type HSV carry out in vitro splicing reactions less efficiently than those prepared from uninfected cells, and this reduction requires ICP27 (21). The majority of HSV genes lack introns, and therefore, as originally proposed by Sandri-Goldin and Mendoza (53), impairment of splicing by ICP27 could contribute to the selective expression of HSV genes by blocking the processing of most host pre-mRNAs. Consistent with this view, ICP27 mutants are defective in delayed shutoff of host cell protein synthesis (20, 48) and fail to induce the decline in the levels of cellular mRNAs observed during infection with wild-type HSV (20, 21).
ICP27 binds RNA through an RGG box (36) and shuttles between the nucleus and cytoplasm of infected cells (35, 39, 50, 58). Recent studies suggest that ICP27 stimulates the cytoplasmic accumulation of intron-less viral mRNAs by binding to these transcripts and mediating their nuclear export (50, 58). The shuttling and RNA export activities of ICP27 depend on a leucine-rich nuclear export sequence that is similar to that of human immunodeficiency virus (HIV) Rev (50). It is not yet clear how the nuclear export activity of ICP27 relates to its previously described effects on the intranuclear processing of viral and cellular pre-mRNAs.
We previously reported that the endogenous chromosomal human α-globin gene is activated during HSV infection of HeLa cells, leading to accumulation of correctly initiated α-globin RNAs (6). Analysis of viral mutants demonstrated that the IE proteins ICP0, ICP4, and ICP22 are each required for efficient induction. The α-globin gene contains two introns, and thus its transcript is a candidate for ICP27-mediated alterations in splicing. Indeed, since α-globin gene expression stringently requires the IE viral transactivators and is temporally regulated as a viral E gene (6), the effects of ICP27 on α-globin transcript processing could be highly relevant to both cellular and viral gene expression during HSV infection. A substantial amount of evidence has shown that intron-bearing genes require splicing for the efficient accumulation, polyadenylation, and nuclear export of their transcripts (1, 7–9, 19, 22, 23, 25, 27, 37, 47). In view of ICP27's putative role in inhibiting mRNA splicing, one might expect ICP27-deficient mutants to induce significantly higher levels of α-globin RNA than does wild-type HSV. However, we found that deletion of ICP27 had little effect on the accumulation of α-globin RNA (6). These considerations prompted us to study the splicing, polyadenylation, and subcellular location of the α-globin transcripts induced by HSV infection.
HSV-1 infection induces multiple α-globin transcripts in HeLa cells.
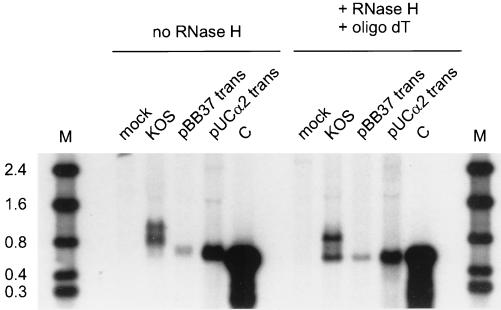
We used Northern blot analysis to examine the number and sizes of the α-globin transcripts present in HSV-1-infected HeLa cells. HeLa cells were infected with HSV-1 strain KOS at an input multiplicity of 10 in the presence of 300 μg of phosphonoacetic acid (PAA) per ml, and total RNA was extracted 6 h postinfection. PAA was included to block the reduction of globin RNA levels that otherwise occurs after the onset of viral DNA replication (6). Total RNA was also harvested from HeLa cells that were transfected with an ICP4 expression clone (pBB37) to induce expression of the endogenous α-globin genes or a plasmid bearing the α2-globin gene (pUCα2). pBB37 and pUCα2 have been previously described (6). Infection, transfection, and RNA extractions were done as previously described (6). RNA samples were analyzed by electrophoresis on a 1.5% agarose-formaldehyde gel, followed by Northern blot hybridization using a 1.5-kb PstI fragment bearing the human α2-globin gene as a probe. As shown in Fig. 1 (no RNase H), at least two diffuse bands of α-globin RNA were detected in HSV-infected HeLa cells. In contrast, α-globin RNA induced from the endogenous chromosomal α1-and α2-globin genes following transfection of the ICP4 expression vector (pBB37) migrated as a single band, as did the α-globin RNA expressed from the transfected human α2-globin gene (pUCα2). These data show that HSV-1 infection induces the accumulation of multiple α-globin RNA species and that one or more viral gene products in addition to ICP4 are required to produce this multiplicity of transcripts.
FIG. 1.
HSV-1 infection induces multiple α-globin RNA species in HeLa cells. Total RNA was harvested from HeLa cells that were either mock infected, infected with HSV-1 strain KOS, or transfected with an ICP4 expression clone (pBB37) or a plasmid bearing the α2-globin gene (pUCα2). The RNA samples were either treated with RNase H in the presence of oligo(dT) (+RNase H + oligo dT) or left untreated (no RNase H). The α-globin RNA species were examined by Northern blot analysis using a 1.5-kb PstI fragment bearing the human α2-globin gene as a probe. C, control blood RNA; M, RNA size markers (in kilobases).
We previously demonstrated that most of the α-globin transcripts present in HSV-infected HeLa cells initiate at the α-globin promoter and are processed at the globin 3′ polyadenylation signal (6). Therefore, the multiple RNA species observed in infected cells likely arise through differential splicing and/or variation in the length of the 3′ poly(A) tail. To examine these possibilities in more detail, we removed the poly(A) tails from the transcripts by treating the RNA samples with RNase H in the presence of oligo(dT). Ten micrograms of total RNA was hybridized to 300 ng of oligo(dT) (Pharmacia) in a buffer containing 20 mM Tris (pH 7.5), 10 mM MgCl2, 0.5 mM EDTA, 50 mM NaCl, 1 mM dithiothreitol, and 30 μg of bovine serum albumin per ml at 60°C. RNase H (Promega) was added to a final concentration of 0.025 U/μl, and the samples were incubated at 30°C for 1 h. The cleavage products were recovered by ethanol precipitation and examined by Northern blot analysis (Fig. 1, + RNase H). Removal of the poly(A) tail resolved the diffuse α-globin bands in KOS-infected cells into three discrete bands of approximately 580, 700, and 840 nucleotides (nt), with the 700-nt species being considerably fainter than the other two. These observations excluded the possibility that the multiple globin RNA species in infected cells are caused by heterogeneity in the length of the poly(A) tail and instead suggested that they arise through differential splicing. Consistent with this hypothesis, the 580-nt band comigrated with deadenylated globin mRNA from transfected cells and human blood (Fig. 1, + RNase H; also seen in Fig. 3 and 5 for blood RNA) and therefore likely corresponds to fully spliced globin mRNA (predicted size of 576 nt). Moreover, the estimated sizes of the ∼700- and ∼840-nt species closely correspond to those predicted for transcripts retaining one (688 or 718 nt) and both introns (835 nt) (see Fig. 3 for evidence confirming these assignments). The untreated and RNase H-oligo(dT)-treated transcripts found in infected cells differed in length by approximately 200 to 250 nt, thus documenting the length of the poly(A) tails of these RNAs. We noted that the transcripts present in the transfected cells had shorter poly(A) tails than those present in infected cells (Fig. 1, compare the most rapidly migrating abundant band in infected cells with the corresponding species in transfected cells, no RNase H). The basis for this difference remains to be precisely defined.
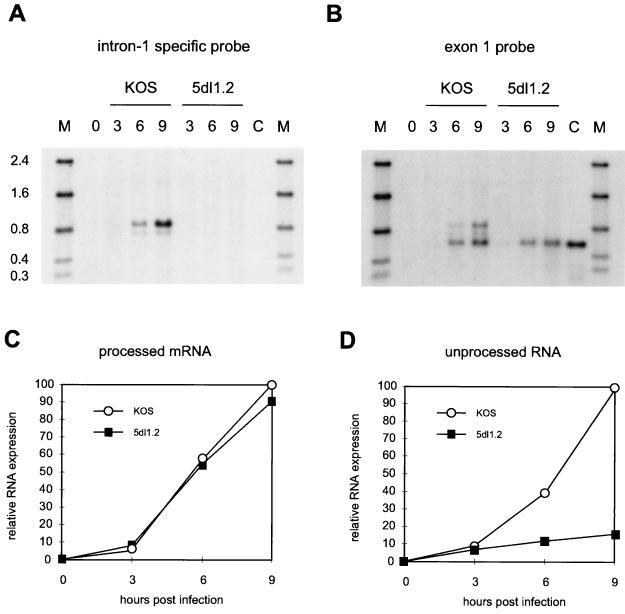
FIG. 3.
Time course of accumulation of spliced and unspliced α-globin RNA. HeLa cells were infected with KOS or 5dl1.2 in the presence of PAA. Total RNA harvested at the indicated times postinfection was treated with RNase H in the presence of oligo(dT) to remove the poly(A) tails and then analyzed by Northern blot hybridization using 32P-labeled oligonucleotide probes specific for intron 1 (A) or exon 1 (B). Lane C, control blood RNA; lane M, RNA size markers (in kilobases). Signals representing the fully processed (C) and unprocessed (D) α-globin transcripts detected in panel B were quantified by phosphorimager analysis, and normalized data were plotted.
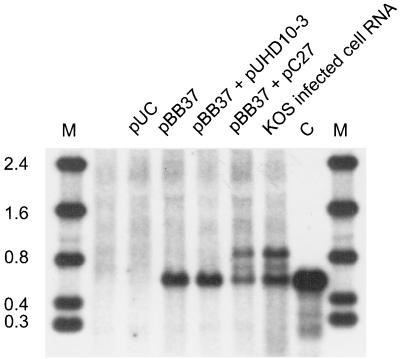
FIG. 5.
ICP27 induces accumulation of unspliced α-globin pre-mRNA in transfected cells. HeLa cells were transfected with the ICP4 expression clone (pBB37) in combination with pUC, the control vector pUHD10-3, or the ICP27 expression clone pC27, and total RNA was extracted at 36 h posttransfection. These RNA samples and RNA from KOS-infected cells were treated with RNase H and oligo(dT) and then analyzed for α-globin transcripts by Northern blot hybridization. C, control blood RNA; M, RNA size markers (in kilobases).
ICP27 induces cytoplasmic accumulation of unspliced α-globin RNA.
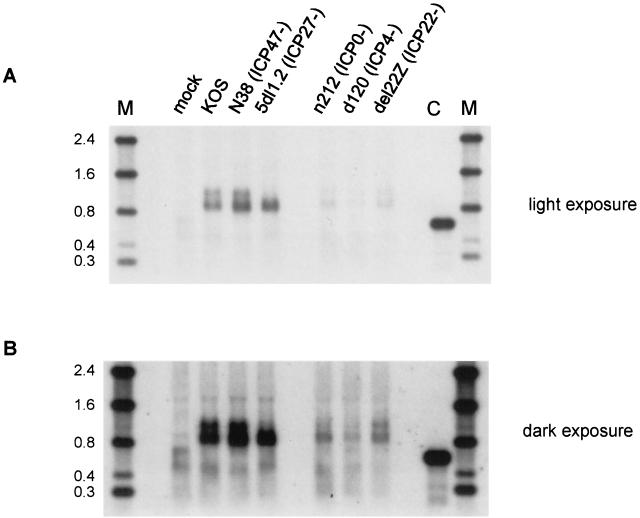
In order to determine which HSV-1 IE gene products are required for the accumulation of multiple α-globin transcripts during infection, we examined the α-globin transcripts in cells infected with a panel of HSV-1 strains that are each defective in one of the IE genes. HeLa cells were infected in the presence of PAA with strains KOS, N38 (61), 5dl1.2 (30), n212 (4), d120 (10), or del22Z (41) (wild type and defective in ICP47, ICP27, ICP0, ICP4, and ICP22, respectively), and total RNA was harvested 6 h later. Northern blot analysis showed identical patterns of α-globin RNAs in cells infected with wild-type strain KOS and strain N38 (ICP47-) (Fig. 2A). As demonstrated previously, the ICP0, ICP4, and ICP22-defective strains are severely compromised in their ability to accumulate α-globin RNA; nevertheless, a long exposure of the Northern blot (Fig. 2B) revealed a pattern similar to that observed in the KOS-infected cells (although the relative abundance of the larger transcripts was somewhat reduced with d120 and n212). In marked contrast, the ICP27-deficient mutant 5dl1.2 accumulated only the more rapidly migrating transcript.
FIG. 2.
Northern blot analysis of α-globin RNA from HeLa cells infected with various HSV mutants. HeLa cells were infected with the indicated HSV strains in the presence of PAA, and total RNA extracted 6 h postinfection was analyzed by Northern blot as described for Fig. 1. Panels are two different exposures of the same blot. C, control blood RNA; M, RNA size markers (in kilobases).
We tested the hypothesis that the larger α-globin transcripts correspond to unspliced and partially spliced derivatives by probing total RNase H-oligo(dT)-treated RNA from KOS- or 5dl1.2-infected cells with oligonucleotides specific for α-globin intron 1 (5′-AGGCGGCCTTGACGTTGGTCTTGTC-3′) or exon 1 (5′-GAGGGTGGCCTGTGGGTCCGGGCGGGCGAG-3′) (Fig. 3A and B). The two more slowly migrating bands present in KOS-infected cells hybridized to the intron 1 probe (Fig. 3A), while all three transcripts hybridized to the exon 1 probe (Fig. 3B). Taken in combination with the data presented and discussed above, these data confirm that the most rapidly migrating band corresponds to fully spliced mRNA while the two more slowly migrating bands correspond to transcripts retaining one and both introns. Only fully spliced RNA was observed in cells infected with the ICP27-deficient 5dl1.2 mutant (Fig. 3B and D). Thus, ICP27 is required for accumulation of the unspliced polyadenylated α-globin RNAs. This observation is, at first glance, generally consistent with the hypothesis that ICP27 inhibits splicing of α-globin pre-mRNA. However, we noted that cells infected with the ICP27 mutant and wild-type HSV-1 (KOS) displayed essentially identical amounts of fully spliced α-globin mRNA which accumulated at comparable rates for at least 12 h (Fig. 3C). Moreover, the unspliced RNA was substantially less abundant (≤50%) than the spliced transcript at all of the time points examined during infection with KOS (note that the exon-specific probe used in this experiment provides an accurate estimate of the relative molar abundance of the spliced and unspliced RNAs, while the genomic probe used in Fig. 1, 2, 4, and 5 overestimates the abundance of the unspliced RNA, because it contains both intron and exon sequences). Thus, while these data do not exclude a small inhibitory effect on splicing, they demonstrate that ICP27 does not greatly interfere with the production of spliced α-globin transcripts under our conditions of infection (discussed further below).
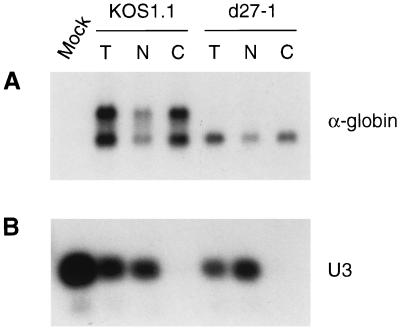
FIG. 4.
Unspliced α-globin RNA accumulates in the cytoplasm. Duplicate dishes of HeLa cells were infected with HSV-1 strain KOS1.1 or d27-1 in the presence of PAA. At 6 h postinfection, total RNA (T) was harvested from one dish and cytoplasmic (C) and nuclear (N) RNA fractions were isolated from the second dish. Cell equivalents of each RNA sample (10 μg of total RNA) were treated with RNase H and oligo(dT) and then analyzed by Northern blot hybridization for the presence of α-globin transcripts (A). As a cell fractionation control, the same samples were analyzed for their content of U3 snoRNA by hybridization to an oligonucleotide probe specific for U3 (B).
Nuclear export of mRNAs appears to be intimately linked to splicing and polyadenylation, and unspliced pre-mRNAs are generally confined to the nucleus of eukaryotic cells (5). To determine the cellular location of the ICP27-induced α-globin pre-mRNAs, we prepared nuclear and cytoplasmic RNA fractions from cells infected with wild-type HSV-1 or an ICP27-null mutant strain. Duplicate dishes of HeLa cells were infected with HSV-1 strain KOS1.1 or d27-1 (45) in the presence of PAA. At 6 h postinfection, total RNA was harvested from one dish by using the RNeasy purification kit (Qiagen). Cytoplasmic and nuclear RNA fractions were isolated from the second dish of cells according to the RNeasy handbook (Qiagen). The combined yields of cytoplasmic and nuclear RNAs equalled that of the total RNA and represented approximately 60 to 70% and 30 to 40%, respectively, of the total RNA yield. Cell equivalents of total, nuclear, and cytoplasmic RNA were treated with RNase H and oligo(dT) and then analyzed by Northern blot hybridization for the presence of α-globin transcripts (Fig. 4A). Surprisingly, the majority of the unspliced α-globin RNA in cells infected with wild-type HSV-1 was found in the cytoplasmic fraction. Indeed, the subcellular distribution of the unspliced RNA could not be distinguished from that of the spliced transcript. The same RNA samples were analyzed for their content of the U3 snoRNA, which is exclusively nuclear, by probing with an oligonucleotide specific for U3 (5′-ACCACTCAGACCGCGTTCTCTCCCTCTCAC-3′) (Fig. 4B). As expected, the U3 snoRNA was located entirely in the nuclear RNA fraction, indicating that the cytoplasmic fraction was free of nuclear RNA contamination. These data indicate that HSV infection induces efficient cytoplasmic accumulation of polyadenylated unspliced α-globin RNA, and ICP27 is required for this effect. These findings are in contrast with the reports by others that ICP27 causes nuclear retention of unspliced pre-mRNAs of two intron-containing HSV-1 transcripts, ICP0 and UL15 (40, 50), and demonstrate that nuclear retention of intron-containing transcripts by ICP27 is not universal.
We used a transient-transfection assay to determine if ICP27 is able to induce accumulation of unspliced α-globin RNA in the absence of HSV infection. Expression of ICP4 suffices to activate the endogenous α-globin gene in transfected HeLa cells (6). We therefore cotransfected the ICP4 expression plasmid pBB37 with an ICP27 expression vector, pC27, into HeLa cells. pC27 bears the ICP27 open reading frame under the control of the TET-OFF promoter of pUHD10-3 (35). Although the TET-OFF promoter is inactive in HeLa cells (17), ICP27 expression was efficiently induced by coexpression of ICP4 (data not shown). RNA was extracted 36 h posttransfection and treated with RNase H and oligo(dT), and the α-globin transcripts were examined by Northern blot analysis (Fig. 5). Only fully spliced globin RNA was detected in cells expressing ICP4, while addition of ICP27 induced accumulation of the same unspliced α-globin species seen in HSV-infected cells. Furthermore, as in HSV-infected cells, the majority of the unspliced α-globin pre-mRNA was present in the cytoplasmic RNA fraction (data not shown). Thus, ICP27 is sufficient (in combination with ICP4) to induce cytoplasmic accumulation of unspliced polyadenylated α-globin RNA in the absence of other HSV gene products. We observed a 40% reduction in the total α-globin signal (spliced plus unspliced RNA) in the presence of ICP27, an effect that was not observed during infection with wild-type versus ICP27-deficient HSV. It is unlikely that this reflects a reduced splicing efficiency, since if such were the case, one would predict the total α-globin signal to be the same but with a decrease of the spliced message and a corresponding increase in the unspliced transcript. The possibility that the reduction in α-globin message stems from overexpression of ICP27 in the transfected cells is presently under investigation.
We report here two observations that have considerable bearing on the function of ICP27. First, our finding that expression of ICP27 has little effect on the rate of accumulation of fully spliced α-globin RNA demonstrates that the splicing apparatus retains the ability to process this transcript for extended periods in HSV-infected cells maintained in the presence of PAA. In contrast, previous work has shown that the expression of ICP27 greatly reduces accumulation of spliced transcripts derived from several other host intron-bearing genes during infection (20, 21), presumably due to defective splicing, and this effect is thought to be responsible (at least in part) for the ICP27-dependent delayed shutoff of host cell protein synthesis. It is unclear why ICP27 has varied effects on cellular transcripts. One possibility is that cellular genes vary in their sensitivity to the ICP27-induced alterations of the splicing apparatus. Perhaps splicing of the α-globin transcript is relatively resistant because α-globin pre-mRNA contains only two small introns, whereas most cellular genes contain many more introns, and these are on average much larger than those in the globin gene (see for example reference 18). Alternatively, the effects of ICP27 on the α-globin transcript may differ because, unlike with most other cellular genes, expression and accumulation of α-globin RNA is actively induced by the actions of viral gene products. Inasmuch as the α-globin gene is in this sense acting as a surrogate viral gene, its behavior may reflect a global distinction by ICP27 between viral and cellular transcripts.
Second, we find that the unspliced polyadenylated α-globin pre-mRNA is exported from the nucleus as efficiently as the fully spliced message. This was a surprising observation because a large amount of evidence indicates that splicing is required for efficient accumulation, polyadenylation, and nuclear export of transcripts of intron-bearing genes in metazoan cells. Interestingly, the fully spliced α-globin message efficiently gained access to the cytoplasm in the absence of ICP27 (Fig. 4A). These data therefore raise the possibility that two modes exist for nuclear export of α-globin transcripts during HSV infection: the splicing-dependent pathway that intron-bearing genes normally utilize and an alternative splicing-independent pathway that requires ICP27 for accumulation and export of unspliced α-globin transcripts. In this regard, ICP27 may be acting analogously to the HIV Rev protein. Rev binds to a specific cis-acting element (the RRE) present in unspliced and partially spliced HIV RNAs and promotes nuclear export of these transcripts (reviewed in reference 42). Several naturally intronless mRNAs, both viral and cellular, similarly require specific cis-acting sequences that promote splicing-independent RNA export (24, 26, 27). For example, the cellular hnRNP L protein binds to multiple elements in the HSV-TK transcript that are each able to mediate splicing-independent cytoplasmic accumulation (27). Recent evidence indicates that such cis-acting elements suppress the use of cryptic splice sites and stimulate splicing-independent polyadenylation of these transcripts, as well as promoting their nuclear export (25). It is tempting to speculate that ICP27 acts in a fashion similar to Rev and hnRNP L. Consistent with this view, Sandri-Goldin (50) has shown that ICP27 binds to several intron-less HSV transcripts and mediates their splicing-independent RNA export. However, Sandri-Goldin found that transcripts of the intron-bearing viral ICP0 and UL15 genes did not detectably interact with ICP27 in these assays and were preferentially retained in the nucleus. These data were interpreted to indicate that ICP27 globally distinguishes between intron-bearing and intron-less RNAs and transports only the latter (50). Our findings suggest an alternative interpretation: perhaps ICP27 recognizes specific features that are present in only a subset of RNAs and transports these transcripts irrespective of the presence or absence of introns. Supporting this hypothesis are the recent data of Buisson et al. (2). These authors showed that the Epstein-Barr virus (EBV) homologue of ICP27, EB2, induces the cytoplasmic accumulation of unspliced RNA derived from some genes bearing constitutive splicing signals without reducing accumulation of spliced RNA. Interestingly, the EB2 protein also blocked the use of cryptic (noncanonical) splicing signals in transcripts lacking bona fide introns, thereby promoting cytoplasmic accumulation of unspliced transcripts of these intron-less genes.
Intron-containing transcripts are normally recognized by splicing factors and assembled into spliceosomes. The protein-bound pre-mRNA is then either productively spliced into mRNA or degraded by as yet poorly understood discard pathways (3, 28). In some cases, a substantial fraction of the intron-containing nuclear pre-mRNA is degraded rather than spliced (28). One interpretation of our data is that ICP27 rescues this subset of nuclear α-globin RNA from degradation, for example, by dissociating it from nonproductive interactions with the splicing machinery and promoting its polyadenylation and nuclear export. According to this view, the unspliced globin RNAs that accumulate in the presence of ICP27 derive from a subset of primary transcripts that would have been degraded in its absence. The proposed mechanism is again reminiscent of that used by the HIV Rev protein, which increases the intranuclear stability of unspliced HIV RNA as well as facilitates its export to the cytoplasm (11, 14, 28, 29, 54). Alternatively, ICP27 may intercept a fraction of globin pre-mRNAs before they engage the splicing apparatus and mediate their efficient polyadenylation and transport.
It seems possible that ICP27-induced cytoplasmic accumulation of unspliced transcripts plays a biological role during HSV infections. Phelan et al. (40) and Hardy and Sandri-Goldin (21) have shown that a fraction of the unspliced ICP0 and UL15 RNA is present in the cytoplasm, and Everett et al. obtained direct evidence that truncated forms of ICP0 are translated from partially unspliced ICP0 transcripts that retain intron 2 (13). More generally, it is interesting to speculate that the proposed “transcript rescue” function of ICP27 plays a fundamental role in HSV gene expression. Splicing signals are highly degenerate, and cryptic splice donor and acceptor signals are therefore likely to appear by chance in HSV transcripts that lack efficient or bona fide introns. Although insufficient for splicing, such signals are predicted to promote nonproductive interactions with the splicing machinery, leading to nuclear retention (5) or degradation through the discard pathway. The proposed rescue function would serve to counteract this effect. This proposal is consistent with the observation that EBV EB2 rescues transcripts bearing weak splicing signals (2). Intriguingly, a small fraction of HSV glycoprotein C mRNA is spliced, indicating the presence of weak splicing signals in this transcript (15). The gC protein is translated from the unspliced form of the RNA. It is therefore perhaps significant that accumulation of unspliced gC mRNA stringently requires ICP27 (44).
The hypothesis that ICP27 stimulates accumulation of unspliced α-globin mRNA by inducing a splicing-independent pathway for globin RNA accumulation and nuclear export predicts that accumulation of unspliced α-globin pre-mRNA could be uncoupled by mutation from the previously described intron-dependent “repression” function of ICP27. Experiments designed to test this prediction are under way.
Acknowledgments
P.C. and K.S.E. contributed equally to this work.
We thank Joanne Duncan, Carol Lavery, Holly Saffran, and Rob Maranchuk for superb technical assistance, Peter O'Hare for pBB37, and Steve Rice for pC27, stimulating discussions, and a critical review of an earlier version of the manuscript.
This research was supported by a grant from the Medical Research Council of Canada (MT-12172). P.C. held a studentship from the MRC, and J.R.S. was a Terry Fox Senior Scientist of the National Cancer Institute of Canada.
REFERENCES
- 1.Buchman A R, Berg P. Comparison of intron-dependent and intron-independent gene expression. Mol Cell Biol. 1988;8:4395–4405. doi: 10.1128/mcb.8.10.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buisson M, Hans F, Kusters I, Duran N, Sergeant A. The C-terminal region but not the Arg-X-Pro repeat of Epstein-Barr virus protein EB2 is required for its effect on RNA splicing and transport. J Virol. 1999;73:4090–4100. doi: 10.1128/jvi.73.5.4090-4100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess S M, Guthrie C. A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell. 1993;73:1377–1391. doi: 10.1016/0092-8674(93)90363-u. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Schaffer P A. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4570–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D D, Sharp P A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 6.Cheung P, Panning B, Smiley J R. Herpes simplex virus immediate-early proteins ICP0 and ICP4 activate the endogenous human α-globin gene in nonerythroid cells. J Virol. 1997;71:1748–1793. doi: 10.1128/jvi.71.3.1784-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiou H C, Dabrowski C, Alwine J C. Simian virus 40 late mRNA leader sequences involved in augmenting mRNA accumulation via multiple mechanisms, including increased polyadenylation efficiency. J Virol. 1991;65:6677–6685. doi: 10.1128/jvi.65.12.6677-6685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooke C, Alwine J C. The cap and the 3′ splice site similarly affect polyadenylation efficiency. Mol Cell Biol. 1996;16:2579–2584. doi: 10.1128/mcb.16.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke C, Hans H, Alwine J C. Utilization of splicing elements and polyadenylation signal elements in the coupling of polyadenylation and last-intron removal. Mol Cell Biol. 1999;19:4971–4979. doi: 10.1128/mcb.19.7.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerman M, Vazeux R, Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell. 1989;57:1155–1165. doi: 10.1016/0092-8674(89)90053-6. [DOI] [PubMed] [Google Scholar]
- 12.Everett R D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2, and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986;67:2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- 13.Everett R D, Cross A, Orr A. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology. 1993;197:751–756. doi: 10.1006/viro.1993.1651. [DOI] [PubMed] [Google Scholar]
- 14.Febler B K, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis G N. Rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci USA. 1989;86:1495–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frink R J, Eisenberg R, Cohen G, Wagner E K. Detailed analysis of the portion of the herpes simplex virus type 1 genome encoding glycoprotein C. J Virol. 1983;45:634–647. doi: 10.1128/jvi.45.2.634-647.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goding C R, O'Hare P. Herpes simplex virus Vmw65-octamer binding protein interaction: a paradigm for combinatorial control of transcription. Virology. 1989;173:363–367. doi: 10.1016/0042-6822(89)90548-5. [DOI] [PubMed] [Google Scholar]
- 17.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haig D. Do imprinted genes have few and small introns? Bioessays. 1996;18:351–353. doi: 10.1002/bies.950180504. [DOI] [PubMed] [Google Scholar]
- 19.Hamer D H, Leder P. Splicing and the formation of stable RNA. Cell. 1979;18:1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- 20.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hentschel C C, Birnstiel M L. The organization and expression of histone gene families. Cell. 1981;25:301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Carmichael G G. Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol. 1996;16:1534–1542. doi: 10.1128/mcb.16.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Carmichael G G. The mouse histone H2a gene contains a small element that facilitates cytoplasmic accumulation of intronless gene transcripts and of unspliced HIV-1-related mRNAs. Proc Natl Acad Sci USA. 1997;94:10104–10109. doi: 10.1073/pnas.94.19.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Wimler K M, Carmichael G G. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z M, Yen T S. Role of the hepatitis B virus posttranscriptional regulatory element in export of intronless transcripts. Mol Cell Biol. 1995;15:3864–3869. doi: 10.1128/mcb.15.7.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Mertz J E. HnRNP L binds a cis-acting RNA sequence element that enables intron-dependent gene expression. Genes Dev. 1995;9:1766–1780. doi: 10.1101/gad.9.14.1766. [DOI] [PubMed] [Google Scholar]
- 28.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malim M H, Hauber J, Le S-Y, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989;338:254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutant exhibits altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGregor F, Phelan A, Dunlop J, Clements J B. Regulation of herpes simplex virus poly(A) site usage and the action of immediate-early protein IE63 in the early-late switch. J Virol. 1996;70:1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLauchlan J, Loney P C, Sandri-Goldin R M, Clements J B. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J Virol. 1992;66:6939–6945. doi: 10.1128/jvi.66.12.6939-6945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLauchlan J, Simpson S, Clements J B. Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell. 1989;59:1093–1105. doi: 10.1016/0092-8674(89)90765-4. [DOI] [PubMed] [Google Scholar]
- 34.McMahan L, Schaffer P A. The repressing and enhancing functions of the herpes simplex virus regulatory protein ICP27 map to the C-terminal regions and are required to modulate viral gene expression very early in infection. J Virol. 1990;64:3471–3485. doi: 10.1128/jvi.64.7.3471-3485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mears W E, Rice S A. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242:128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 36.Mears W E, Rice S A. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J Virol. 1996;70:7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niwa M, Rose S D, Berget S M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 1990;4:1552–1559. doi: 10.1101/gad.4.9.1552. [DOI] [PubMed] [Google Scholar]
- 38.Phelan A, Carmo-Fonseca M, McLauchlan J, Lamond A I, Clements J B. A herpes simplex virus type-1 immediate early gene product IE63 regulates small ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90:9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phelan A, Clements J B. Herpes simplex virus type 1 immediate early protein IE63 shuttles between nuclear compartments and the cytoplasm. J Gen Virol. 1997;78:3327–3331. doi: 10.1099/0022-1317-78-12-3327. [DOI] [PubMed] [Google Scholar]
- 40.Phelan A, Dunlop J, Clements J B. Herpes simplex virus type 1 protein IE63 affects the nuclear export of virus intron-containing transcripts. J Virol. 1996;70:5255–5265. doi: 10.1128/jvi.70.8.5255-5265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poffenberger K L, Raichlen P E, Herman R C. In vitro characterization of a herpes simplex virus type 1 ICP22 deletion mutant. Virus Genes. 1993;7:171–186. doi: 10.1007/BF01702397. [DOI] [PubMed] [Google Scholar]
- 42.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 43.Rice S A, Knipe D M. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988;62:3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice S A, Knipe D M. Genetic evidence for two distinct trans-activation functions of the herpes simplex virus α protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice S A, Lam V. Amino acid substitution mutations in the herpes simplex virus ICP27 protein define an essential gene regulation function. J Virol. 1994;68:823–833. doi: 10.1128/jvi.68.2.823-833.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 47.Ryu W S, Mertz J E. Simian virus 40 late transcripts lacking excisable intervening sequences are defective in both stability in the nucleus and transport to the cytoplasm. J Virol. 1989;63:4386–4394. doi: 10.1128/jvi.63.10.4386-4394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sacks W R, Greene C C, Ashman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandri-Goldin R M. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 1998;12:868–879. doi: 10.1101/gad.12.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandri-Goldin R M. Interactions between a herpes simplex virus regulatory protein and cellular mRNA processing pathways. Methods. 1998;16:95–104. doi: 10.1006/meth.1998.0647. [DOI] [PubMed] [Google Scholar]
- 52.Sandri-Goldin R M, Hibbard M K. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70:108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandri-Goldin R M, Mendoza G E. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 1992;6:848–863. doi: 10.1101/gad.6.5.848. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz S, Felber B K, Pavlakis G N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–160. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the alpha-22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sekulovich R E, Leary K, Sandri-Goldin R M. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J Virol. 1988;62:4510–4522. doi: 10.1128/jvi.62.12.4510-4522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith I L, Hardwicke M A, Sandri-Goldin R M. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 58.Soliman T M, Sandri-Goldin R M, Silverstein S J. Shuttling of the herpes simplex virus type 1 regulatory protein ICP27 between the nucleus and cytoplasm mediates the expression of late proteins. J Virol. 1997;71:9188–9197. doi: 10.1128/jvi.71.12.9188-9197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stow N D, Stow E C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 60.Thompson C C, McKnight S L. Anatomy of an enhancer. Trends Genet. 1992;8:232–236. [Google Scholar]
- 61.Umene K. Conversion of a fraction of the unique sequences to part of the inverted repeats in the S component of the herpes simplex virus type 1 genome. J Gen Virol. 1986;67:1035–1048. doi: 10.1099/0022-1317-67-6-1035. [DOI] [PubMed] [Google Scholar]
- 62.Uprichard S L, Knipe D M. Herpes simplex ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes. J Virol. 1996;70:1969–1980. doi: 10.1128/jvi.70.3.1969-1980.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watson R J, Clements J B. A herpes simplex virus type 1 function continuously required for early and late virus RNA synthesis. Nature. 1980;285:329–330. doi: 10.1038/285329a0. [DOI] [PubMed] [Google Scholar]
- 64.Weinheimer S P, McKnight S L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987;195:819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]