Abstract
Background:
Repurposing dantrolene to treat Alzheimer’s disease has been shown to be effective in amyloid transgenic mouse models but has not been examined in a model of tauopathy.
Objective:
The effects of a nanoparticle intranasal formulation, the Eagle Research Formulation of Ryanodex (ERFR), in young adult and aged wild type and PS19 tau transgenic mice was investigated.
Methods:
The bioavailability of intranasal ERFR was measured in 2 and 9–11-month-old C57BL/6J mice. Blood and brain samples were collected 20 minutes after a single ERFR dose, and the plasma and brain concentrations were analyzed. Baseline behavior was assessed in untreated PS19 tau transgenic mice at 6 and 9 months of age. PS19 mice were treated with intranasal ERFR, with or without acrolein (to potentiate cognitive dysfunction), for 3 months, beginning at 2 months of age. Animal behavior was examined, including cognition (cued and contextual fear conditioning, y-maze), motor function (rotarod), and olfaction (buried food test).
Results:
The dantrolene concentration in the blood and brain decreased with age, with the decrease greater in the blood resulting in a higher brain to blood concentration ratio. The behavioral assays showed no significant changes in cognition, olfaction, or motor function in the PS19 mice compared to controls after chronic treatment with intranasal ERFR, even with acrolein.
Conclusions:
Our studies suggest the intranasal administration of ERFR has higher concentrations in the brain than the blood in aged mice and has no serious systemic side effects with chronic use in PS19 mice.
Keywords: Alzheimer’s disease, blood-brain barrier, calcium, cognition, dantrolene, pharmacokinetics, PS19 mice, ryanodine receptor, tau protein, therapeutics
INTRODUCTION
Over 95% of patients with Alzheimer’s disease (AD) suffer from sporadic AD, which is typically in people over 65 years of age, while only about 5% of AD patients present with familial AD (FAD), which usually occurs decades earlier [1], though the hallmarks of amyloid plaque and tau tangles are similar for both forms. The combined accumulation of amyloid-beta and tau leads to increased intracellular Ca2+, inducing neuronal death and cognitive dysfunction. On the other hand, Ca2+ dysregulation in AD further exacerbates the amyloid and tau pathology, forming a vicious cycle [2]. Despite the tremendous international effort to develop drugs for the treatment of AD, no disease-modifying drugs are available currently. One of the proposed strategies for the development of new AD drug treatments is targeting the primary upstream AD pathology in the brain, such as cellular Ca2+ dysregulation.
Dantrolene is the only FDA-approved treatment for malignant hyperthermia, a severe reaction to anesthesia caused by overactivation of the type 1 ryanodine receptor (RyR-1) Ca2+ channels located on the sarcoplasmic reticulum (SR) membrane in skeletal muscle cells [3]. Overactivation of other RyR subtypes lead to common diseases, such as diabetes, asthma, and cardiac arrhythmia [4, 5]. While all RyR subtypes exist in the human brain, the type 2 RyR are more prevalent [6, 7]. The ryanodine receptor number and function are also increased in AD animal models [6, 8, 9]. This abnormal and excessive overactivation of RyR in AD, leading to the pathological elevation of cytosolic and mitochondrial Ca2+ concentrations, the depletion of ER Ca2+, and the associated downstream mitochondrial dysfunction [10], oxidative stress and ferroptosis [11, 12], impaired autophagy [7], ER stress [13, 14], synaptic dysfunction [7, 15], and eventually cognitive dysfunction [16-18].
While Ca2+ homeostasis has not been examined in the PS19 mouse model of tauopathy, cognitive dysfunction has been reported [19, 20]. Our hypothesis, based on other animals of AD with cognitive dysfunction, is that this cognitive dysfunction is due to Ca2+ dysregulation. Abnormal elevation of the cytosolic Ca2+ concentration activates Ca2+- dependent calpain and then GSK-3β, leading to tau protein phosphorylation and tau pathology [21-23]. Mitochondrial Ca2+ overloading in AD also wors-ens the tau pathologies [24]. Calcium dysregulation in the cytosol and mitochondria and associated tau pathology further damage mitochondrial function to produce ATP for cell energy and pathologically elevates cytosolic reactive oxygen species [25, 26]. Calcium dysregulation and tau pathology form a vicious cycle to be important and critical AD pathologies [2], which could be effective drug therapy targets [27, 28].
Dantrolene has been demonstrated to be neuroprotective in AD models, including cell culture [7, 29] and FAD animal models [16-18, 30, 31]. Although dantrolene is a small lipid-soluble molecule, it is limited ability to pass through the blood-brain barrier and enter the central nervous system (CNS) makes it a less effective treatment. However, our previous studies in 2-month-old wild type mice have demonstrated that the intranasal administration of dantrolene in a nanoparticles solution significantly increased its brain concentration and brain/blood concentration ratio, compared to oral or subcutaneous injections of dantrolene [16, 32]. Furthermore, with aging and with AD, the blood-brain barrier becomes increasingly more permeable, favoring drug penetration into the CNS in AD patients [33], especially with intranasal drug delivery.
The effects of intranasal dantrolene have been investigated in an aggressive amyloid model of AD, the 5XFAD model. We found that the chronic intranasal administration of intranasal dantrolene abolished the learning and memory impairment in 5XFAD mice, even when treatment was initiated after the onset of AD pathologies and cognitive dysfunction [16]. The effects of dantrolene on amyloid plaque levels in AD mouse models has been inconsistent, with no change in 5XFAD [16] mice to increased [34] and decreased amyloid levels in different amyloid transgenic AD models [17, 18]. The effects of dantrolene to restore cognition in a tau mouse model, another hallmark of AD, has not been studied before.
In this study, we employed the intranasal administration of an Eagle Research Formulation of Ryanodex (ERFR), a dantrolene nanoparticle formulation, to investigate the effects of age brain dantrolene concentrations and the effects on cognitive function in the commonly used tau PS19 tau transgenic mouse model [19, 35].
MATERIALS AND METHODS
Animals and treatment groups
All procedures were conducted in accordance with the ARRIVE guidelines and protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania. Male and female mice, including wild-type C57BL/6J (B6J) mice (Strain # 000664) at 2 and 9–11 months of age, C57BL/6J mice at 2 months of age, tau point mutation transgenic mice (P301S, Line PS19) at 2, 6, and 9 months of age, and non-transgenic (NT) littermates at 6 and 9 months of age, weighing 29–44 g, were used in these experiments. Mice (1–5 mice per cage) were maintained in the University of Pennsylvania laboratory animal research housing at 21–22°C with a 12–h light-dark cycle and provided with food and water ad libitum and nestlets for enrichment. The animal facility personnel changed the cages weekly and mice were separated into different cages if fighting was observed. Body weights were monitored every week. All efforts were made to minimize the suffering and number of mice according to IACUC guidelines. After mice were weaned, they were managed by an investigator twice a week to accustom the mice to being managed.
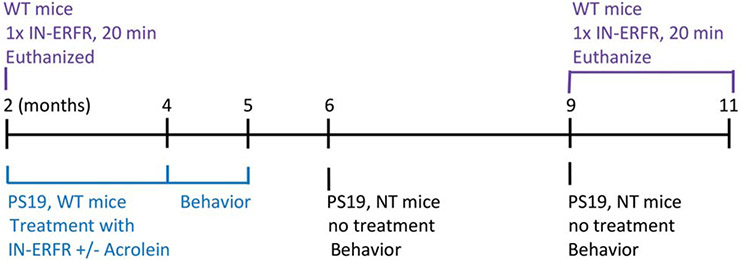
The animal treatment groups are described as follows. For the brain/blood concentration study (Fig. 1, purple group), the intranasal ERFR was administered one time to C57BL/6J mice (WT) at 2 months of age (N = 10) and 9–11 months of age (N = 9). Tau PS19 transgenic mice and non-transgenic littermates (NT) at 6 and 9 months of age (Fig. 1, Black group) underwent behavioral assays to determine baseline cognition, olfaction, and motor function (Fig. 1, black). For the behavioral assays, the number of NT mice used was n = 20 at 6 months and n = 14 at 9 months of age, and for the PS19 mice, the n = 19 at 6 months and n = 12 at 9 months of age. Another cohort of mice, beginning at 2 months of age, were given the intranasal ERFR daily for 3 months, with or without acrolein, to determine the effect of ERFR on cognitive function (Fig. 1, blue group). Acrolein is an endogenous aldehyde found to be increased in AD brains and to exacerbate cognitive function in AD animal models [36-38]. When we did not demonstrate significant baseline cognitive dysfunction in PS19 mice, we included acrolein gavages along with IN-ERFR to exacerbate cognitive dysfunction in the PS19 mouse model. PS19 and WT (C57BL/6J) mice were given either vehicle (water), acrolein alone, intranasal ERFR alone, or intranasal ERFR plus oral acrolein (Fig 1, blue group). For all 4 experimental groups, n = 6 for WT mice and n = 5 for PS19 mice.
Fig. 1. Experimental design timeline.
The dantrolene concentrations in the brain and blood were measured 20 min after a single dose of intranasal Eagle Research Formulation of Ryanodex (IN-ERFR) in wild type mice (WT) at 2 and 9–11 months of age (purple group). Baseline cognition was evaluated in untreated PS19 tau transgenic mice and non-transgenic littermates (NT) at 6 and 9 months of age (Black group). PS19 mice and WT mice were treated with IN-ERFR and acrolein for 3 months, beginning at 2 months of age and behavioral assays conducted from 4–5 months of age (blue group).
Intranasal administration of ERFR
Intranasal administration of dantrolene was performed using a method we have described previously [7, 16]. Briefly, the intranasal ERFR (IN-ERFR) formulation was provided by Eagle Pharmaceuticals, Inc. Aliquots were stored at −80°C and thawed at room temperature for a one-time use. ERFR was shielded from light using aluminum-wrapped vials and pipette tips. Animals were weighed before each dosing. A P200 analytical pipette with a standard 2–200 μL pipette tip was used to deliver one μL per gram of body weight. The mouse was held with one hand in a suspended supine position. Small droplets of ERFR were dripped very slowly into the nose until fully absorbed, with a 2–3 s break period between each droplet delivery. Each mouse was held still for 10–15 s after dosing completion. Total administration time per mouse was between 1–3 min. Mice for the brain/plasma concentration study were given one dose (Fig. 1, purple group) and euthanized after 20 min. The mice for the behavioral study were given IN-ERFR 1 dose/day (5 mg/kg), 5 days/week, for three consecutive months, starting at 2 months of age. Later on the same day or at the same time, acrolein (2 mg/kg) was administered by gavage daily for 5 days per week for three months [37] (Fig. 1, blue group).
Dantrolene brain/blood concentration ratio study
For the brain and blood concentration study, 20 min after the completion of each intranasal one-time administration of ERFR, each animal was individually anesthetized in a 2–4% isoflurane in 30% oxygen carrying gas chamber until breathing rate reached approximately 50% of normal rate. The animal was removed from the chamber and placed in a supine position on the operating platform in a fume hood. An isoflurane nose cone was used to maintain a surgical plane of anesthesia as determined by lack of response to a toe pinch. Isoflurane concentration was kept at 1–2% and was adjusted, as necessary. Two 1-inch, 22G needles were attached to 3mL syringes and washed with 1000 IU/mL heparin for blood collection. A 30G needle was attached to a peristaltic pump, and the intake tube was placed in a 0.9% saline/heparin (3 IU/mL) solution. The skin over the chest cavity was removed and the chest cavity opened to expose the chest cavity and heart. A syringe was inserted into the left ventricle to collect arterial blood into heparin-washed microfuge tubes. Additional blood in the chest cavity was collected with a syringe into heparin-washed tubes and placed on ice.
After the blood collection, while the animal was still under anesthesia, the animal was euthanized by inserting the pump needle into the left ventricle, the right atrium was cut, and cardiac perfusion and exsanguination was performed with a saline/heparin solution for 30 s at 10mL/min. The brain was removed, weighed, rapidly frozen in liquid nitrogen then stored at −80°C. The anticoagulated blood samples were then centrifuged at 2500G for 15 min at 4°C and the plasma supernatant was collected and transferred to Micronics vials for storage at −80°C. All brain and plasma samples were subsequently shielded from light until assayed.
Bioanalytical method
Brain and plasma samples were quantified by the LC-MS/MS method. The bioanalytical method involves the extraction of dantrolene and 5-OH-Dantrolene from mice plasma (K2EDTA and brain samples homogenized in 70% isopropyl alcohol in water) prior to extraction. The samples were extracted by protein precipitation using acetonitrile, containing the internal standard (Dantrolene-d4 and 5-OH-Dantrolene-d4). The analytes and internal standards are separated using a reversed phase liquid chromatography gradient and quantified with tandem mass spectrometric detection. The range of quantification for the method is 10 ng/mL-1000 ng/mL for plasma method and 1 ng/mL–1000ng/mL for the brain method. The analysis was performed on a triple-quadrupole LC-MS/MS (AB Sciex API 5500 QQQ) with Sciex ExionLC UPLC (ExionLC AD Pump) with Sciex ExionLC AD Multiplate Sampler and Sciex ExionLC AC Column Oven. The autosampler temperature was set at 4–10°C. Liquid chromatographic separation was performed on Restex Force Biphenyl (2.1 × 30 mm, 1.8 μm) and the temperature was set at 40°C. The mobile phase had a flow rate of 0.4mL/min and followed an elution gradient program using Mobile phase A (10 mM Ammonium Acetate + 0.1% Acetic Acid in Water) and Mobile Phase B (10 mM Ammonium Acetate + 0.1% Acetic Acid in 90: 10 ACN:Water). Peak area ratios of the analytes and the IS were used to calculate concentrations. The MS was operating in a negative electrospray ionization mode and multiple reaction monitoring (SRM) mode with a negative spray voltage of −4500 V.
Behavioral studies
Behavioral studies were conducted in untreated tau PS19 transgenic mice and non-transgenic littermates, at 6 and 9 months of age (Fig. 1, Black group). PS19 and C57BL/6J wild type control mice that began IN-ERFR, with or without acrolein, treatment at 2 months of age, were evaluated between 4–5 months of age, while treatment continued. The mice underwent several behavioral paradigms to evaluate learning and memory. The investigator was blinded to the experimental groups. All equipment was cleaned before and between subject trails with Clidox.
Fear conditioning test
The contextual and cued fear conditioning test is a behavioral paradigm used to assess associative fear, hippocampal and amygdala dependent and hippocampal independent, learning and memory in mice, as we have described previously [16]. On the first day, mice were acclimated to the testing environment for 1 h. The animals were placed in a conditioning chamber and given three pairings of a conditioned stimulus (auditory cue) and an aversive unconditioned stimulus (electric foot shock). On the second day, for the contextual fear conditioning test (hippocampal dependent memory), the mice were exposed to the same conditioning chamber without the auditory cue or foot shock and freezing time recorded. Two hours later, for the cued fear conditioning test (hippocampal independent memory), the mice were placed in a different chamber with no auditory cue or foot shock for the first 3 min, followed by 3 cycles of the previous auditory cue and freezing time recorded [16]. Freezing behavior during the tests was measured as an index of fear memory and were recorded using the ANY-maze Fear Conditioning System (Model: 46000-590, UGO Basile, Gemonio Italy) equipped with a video camera and ANY-maze software (V.4.99 Stoelting Co. Wood Dale, IL).
Y-maze spontaneous alternation behavior
Y-maze testing is used to measure short term spatial working memory. The test was carried out using a Y-shaped maze with three light-colored, opaque arms orientated at 120° angles from each other, with a method described previously with some modification [39]. Briefly, the mouse was introduced at a particular position on the maze and allowed to explore the arms freely for 5 min. If a mouse climbed on the maze walls, it was immediately returned to the abandoned arm. The start arm was varied between animals to avoid placement bias. An entry occurs when all four limbs of the mouse are within an arm. Re-entries into the same arm were rated as separate entries. An alternation is defined as consecutive entries into all three arms, and an overlapping technique was used. The number of arm entries and alternations were recorded to calculate the percentage of alternation behavior. The percent alternation was defined as consecutive entries in three different arms, divided by the number of alternations ((total arm entries minus 2) times 100). A high percentage was an indication of good working memory as this indicated that the mouse recalled which arms it had already visited. Mice with less than 8 arm entries during the 5-min trial were excluded from the analysis, considering the high risks of extreme cofounding factors rather than the cognitive function in these mice.
Motor function
Motor function was assessed for mice in all groups to see if there was any difference in the propensity of causing skeletal muscle weakness at 10 months of age with the rotarod (IITC Series 8, Life Sciences, Woodland Hills, CA), using a method we described previously [16, 17]. Mice were brought to the testing room at least 1 h before the test to get acclimated to the environment. Two 60-s training trials at a constant speed of 9 rpm were performed. All mice were trained on the first 60-s trial, followed by all mice being trained on the second 60-s trial, with a 30-min interval between the two trials for each mouse. Then, all mice were given three 120-s test trials with a gradually increasing speed (4–40 rpm). As before, all the mice underwent the first trial before starting the second and likewise for the third trial, and with approximately 60-min intervals between each of the three trials for a given mouse to reduce stress. The latency to fall from the rotarod was recorded and analyzed.
Olfactory function
To determine any impact of the intranasal ERFR on olfaction, the animals’ ability to smell volatile odors and their natural tendency to use olfactory cues for foraging was measured, as we have described previously [16]. The 3-day testing protocol consisted of an odor familiarization exercise on day one, food deprivation on day two, and testing on day three. On day one, mice were placed in a clean cage containing 3 cm of fresh bedding and normal chow and water. Three “cookies” per cage were placed in the bedding and left overnight. Cages were inspected on the morning of day two to record the number of cookies consumed. Later on day two, at approximately 4 pm, the normal mouse chow pellets were removed from the cages and the testing mice were fasted overnight, with free access to water during this time. The test was performed on day three at approximately 11 am, after 1 h acclimatization in the testing room. Mice were individually introduced into a clean cage containing clean bedding and allowed to acclimate to the cage for 5 min. In a second clean cage, a single cookie was buried beneath the bedding in a random corner of the cage and the mouse was placed in the second cage. Neophobia was not observed, and all mice explored the cage normally. The time for the animal to retrieve the cookie with its front paws was manually recorded (latency) up to a maximum of 15 min. All the mice located the food in under 15 min and immediately consumed the discovered food pellet.
Tau immunohistochemistry
Brain sections were examined for immunohistochemical localization of phosphorylated tau protein (ps396). The immunostaining method to detect phosphorylated tau proteins in brain sections are described previously in our publication [16], with some modification. Briefly, paraffin-embedded coronal brain sections (10 μm) were prepared, deparaffinized, and hydrated. Antigen retrieval was performed with the Antigen Unmasking Solution (H3300, Vector Lab, Newark, CA) in a pressure cooker. Then the sections were incubated in 10% normal goat serum for 30 min, in M.O.M Mouse Ig Blocking Reagent (PK-2200, Vector Lab) for 1 h, and in M.O.M diluent for 5 min, successively. Slides were incubated with the primary antibody, p-tau ps396 (1 : 500, Abcam, Cambridge, UK), at 4°C overnight, followed by incubation with M.O.M BiotinylatedAnti-Mouse IgG reagent (PK-2200, Vector Lab, Newark, CA) for 10 min and with VECTASTAIN ABC Reagent (PK-7200, Vector Lab, Newark, CA) for 5 min. Then the sections were dehydrated and cover-slipped with Per mount. All images were taken on an Olympus (BX51W1) microscope equipped with a Q Imaging Retiga 2000R digital camera and i Vision imaging software (BioVision Technologies, Exton, PA).
Statistics
Dantrolene brain and blood concentrations were measured and reported as mean ± 95% CI (confidence intervals) and were analyzed by the Mann Whitney test. The data for the behavioral studies were presented as mean ± 95% CI and analyzed using one-way or 2-way ANOVA followed by the Tukey’s or Šídák’s multiple comparisons tests, as described in the figure legends. Degrees of freedom and f values are given for the ANOVA analyses. The significance level for all analyses was set at 95% (p < 0.05). GraphPad Prism 9 (GraphPad Software Inc.) was used to conduct all statistical analyses.
RESULTS
Dantrolene brain/blood concentration ratio study
The dantrolene concentration in the brain and blood was measured after a single intranasal exposure in young adult and aged C57BL/6J mice (Fig. 1, purple group). Blood samples were obtained from both the heart and chest cavity prior to euthanasia. We found that there was no significant interaction between age and plasma collection site (p = 0.7970, F (1, 33) = 0.06724) and no difference in the dantrolene concentration found in the plasma from the left ventricle compared to the chest cavity at 2 months of age (adjusted p = 0.793, 95% CI = −154.4 to 263.7) nor at 9–12 months of age (Adjusted p = 0.966, 95% CI = −193.0 to 236.0) (Fig. 2A) and thus the dantrolene concentration in the plasma obtained from the heart was used for all analyses. However, there was an overall significant decrease in the dantrolene plasma concentration from 2 to 9–12 months of age (p = 0.0012, 95% CI 97.14 to 357.3, F (1, 33) = 12.63) (Fig. 2A). In comparison to young adult mice, the mean dantrolene concentration in the plasma of aged mice was significantly lower (476 ng/ml versus 232 ng/ml, respectively), (p = 0.0083, 95% CI 37.0–437.0) (Fig. 2B) while the dantrolene concentration in the brain was significantly greater at 2 months compared with 9–12 months of age (p = 0.0400, 95% CI 1.700 to 39.10) (Fig. 2C). The ratio of the dantrolene concentration in the brain to the concentration in the blood was used to compare dantrolene’s penetration through the blood brain barrier at each age. The brain/blood dantrolene concentration ratio trended higher in aged mice (Fig. 2D, p = 0.114, 95% CI −15.53 to 1.560) primarily due to the significantly lower dantrolene concentrations in the plasma.
Fig. 2. Dantrolene concentrations in the blood and brain with age.
A) There was a significant decrease in the dantrolene plasma concentration at 9–11 months of age compared to 2 months of age in C57BL/6J mice. There was no statistical difference in the dantrolene concentration found in the plasma from the left ventricle compared to the chest cavity at 2 or 9–11 months of age. At 2 months, n = 9 (LV, left ventricle), n = 9 (CC, chest cavity), 9–11 months, n = 9 (left ventricle and chest cavity). Data were analyzed by two-way ANOVA, with Šídák’s multiple comparisons test. B) The concentration of dantrolene in the plasma was significantly greater at 2 months compared with 9–12 months of age. C) Likewise, the dantrolene concentration in the brain was significantly greater at 2 months compared with 9–11 months of age. Data for B) and C) were analyzed with the Mann Whitney test, n = 9 for all groups. D) The brain to plasma ratios at 2 months compared to 9–11 months were not significantly different. Data were analyzed with the Mann Whitney test, n = 9 (2 months) and n = 9 (9–11 months of age). All Data in the figure were expressed as the mean ± 95% CI.
Effects of intranasal ERFR on behavior
Cognitive function
Baseline cognitive function was first evaluated in tau transgenic PS19 mice and non-transgenic littermates at 6 and 9 months of age in the absence of intranasal ERFR (Fig. 1, Black group). Interestingly, with the hippocampal-dependent contextual fear condition test, there was a significant difference with age, with increased freezing times at 9 months compared to 6 months of age (p = 0.0020, 95% CI −117.3 to −27.60, F (1, 62) = 10.43) but no differences found with genotype (p = 0.8586, 95% CI −40.06 to 47.94, F (1,62) = 0.032 (Fig. 3A). There were significantly increased freezing times between NT mice from 6 to 9 months of age (adjusted p = 0.104, 95% CI −131.7 to −13.22) and between PS19 mice from 6 to 9 months of age (adjusted p = 0.0104, 95% CI −131.7 to −13.22). However, there were no significant differences in freezing times between the PS19 mice and their non-transgenic littermates at either 6 months (adjusted p = 0.9979, 95% CI −54.25 to 62.05) or 9 months of age (adjusted p = 0.9979, 95% CI −54.17 to 62.05). For the cued fear conditioning test, there was also a significant interaction with age, with increased freezing times at 9 months compared to 6 months (p = 0.0016, 95% CI −82.44 to −20.29, F (1, 62) = 10.92) but no significant differences were found with genotype (p = 0.2002, 95% CI −10.74 to 50.22, F (1, 62) = 1.677) (Fig. 3B). Likewise, the freezing times were significantly increased between NT mice from 6 to 9 months of age (adjusted p = 0.0084, 95% CI −92.4 to −10.32) and between PS19 mice from 6 to 9 months of age (adjusted p = 0.0084, 95% CI −92.4 to −10.32). There were also no significant differences in cued freezing times between the PS19 mice and their NT littermates at either 6 months (adjusted p = 0.5697, 95% CI −20.51 to 60.00) or 9 months of age (adjusted p = 0.5697, 95% CI −20.51 to 60.00). To further investigate what was driving the behavioral differences with age, sex was examined. We detected significantly increased freezing times with age for the female mice for both the contextual (p = 0.0045,95% CI −157.2 to −31.61, F (1, 31) = 9.403) (Fig. 3C) and cued fear conditioning (Fig. 3E) (p = 0.0001), 95% CI −126.6 to −46.02, F (1,31) = 19.10). In contrast, there were no significant differences with age for male mice for either the contextual (p = 0.1224, 95% CI −108.4 to 13.56, F (1,28) = 2.537) (Fig. 3D) or cued fear conditioning (p = 0.6247, 95% CI −54.77 to 33.46, F (1,28) = 2.446) (Fig. 3F), implying that the differences in cognition with the fear conditioning tests was primarily due to increased freezing times in female mice. With the Y-maze, we did not detect any significant interaction with age (p = 0.2578, 95% CI −3.759 to 13.69, F (1, 46) = 1.313) or genotype (p = 0.4385, 95% CI −4.304 to 9,768, F (1,46) = 0.6109) (Fig. 3G) or any significant differences in alternation percentage between the PS19 mice and their NT littermates at either 6 months (adjusted p = 0.8624, 95% CI −6.585 to 12.05) or 9 months of age (adjusted p = 0.8624, 95% CI −6.5859 to 12.05) (Fig. 3G).
Fig. 3. Baseline cognitive function in PS19 tau transgenic mice with age.
Significant differences were noted in the contextual (A) and cued fear conditioning (FC) tests (B) with age, with increased freezing times at 9 months compared to 6 months of age. There were no significant differences in freezing times between the PS19 mice and their non-transgenic (NT) littermates at either 6 or 9 months of age for either the contextual or cued FC tests. Female mice had significantly increased freezing times with age on the contextual (C) and cued FC tests (E) compared with males which showed no significant differences (D, F). (A−D: NT mice, n = 20 (6 months), n = 14 (9 months); PS19 mice n = 19 (6 months), n = 12 (9 months) or the cued FC test (B NT, n = 15 (6 months), n = 9 (9 months); PS19 n = 18 (6 months), n = 12(9 months)). G) There was no significant difference in the Y-Maze with age and no significant differences in alternation percentage between the PS19 mice and their non-transgenic littermates at either age. NT, n = 6 (6 months), n = 5 (9 months); PS19 n = 6 (6 months), n = 5(9 months). All data are expressed as the mean ± 95% CI and analyzed with a 2-way ANOVA, with Tukey’s multiple comparisons test.
Acrolein treatment has been suggested as a novel animal model of sporadic AD [36]. Rats treated with acrolein daily for 8 weeks developed cognitive impairment [37]. Thus, to accelerate cognitive impairment in the PS19 tau transgenic mouse model, acrolein was given to PS19 mice and non-transgenic littermates starting at 2 months of age, in conjunction with intranasal dantrolene (ERFR), and cognitive function assessed from 4–5 months of age (Fig. 1, blue group). For the hippocampal and amygdala-dependent contextual fear conditioning test, there was no significant interaction between treatment and genotype (p = 0.5026, 95% CI −13.98 to 90.99, F (3, 36) = 0.7989). There were no significant differences in the freezing times between the WT and PS19 mice with any treatment (vehicle, adjusted p = 0.4736,95% CI −61.07 to 271.8; acrolein, adjusted p > 0.9999, 95% CI −167 to 165.1; ERFR, adjusted p = 0.9997, 95% CI −141.3 to 191.5; ERFR + Acrolein, adjusted p > 0.9997, 95% CI −141.5 to 191.3). Furthermore, there were no significant differences between the PS19 ERFR treated mice compared to PS19 vehicle controls (adjusted p = 0.9465, 95% CI −234.7 to 112.9), or compared to the PS19 ERFR + acrolein treated mice (adjusted p > 0.9999, 95% CI −164.0 to 183.7). There was no significant difference between the PS19 ERFR + Acrolein treated mice and PS19 Acrolein control treated mice (p = 0.9694, 95% CI −119.1 to 228.6). In addition, there was no significant difference between the WT ERFR treated mice and WT vehicle treated control mice (p > 0.9999, 95% CI −139.3 to 178.0) (Fig. 4A). Similarly, for the cued fear conditioning test, hippocampal-independent fear conditioning, there was no interaction between treatment and genotype (p = 0.2440, 95% CI −0.8919 to 44.73, F (3, 36) = 1.452). There were no significant differences in freezing times between the WT and PS19 mice with any treatment (vehicle, adjusted p = 0.4210, 95% CI −24.60 to 120.1; acrolein, adjusted p = 0.5898, 95% CI −30.64 to 114.0; ERFR, adjusted p > 0.9999, 95% CI −65.86 to 78.81; ERFR + Acrolein, adjusted p > 0.999). Furthermore, there were no significant differences between the PS19 ERFR treated mice compared to PS19 vehicle controls (adjusted p = 0.5954, 95% CI −118.9 to 32.21), or compared to the PS19 ERFR + acrolein treated mice (adjusted p > 0.9999, 95% CI −71.49 to 79.61). There was no significant difference between the PS19 ERFR + Acrolein treated mice and PS19 Acrolein control treated mice (p = 0.9274, 95% CI −103.7 to 47.41). In addition, there was no significant difference between the WT ERFR treated mice and WT vehicle treated control mice (p > 0.9999, 95% CI −71.05 to 66.88) (Fig. 4B). For the y-maze, there was an overall significant decrease in alternation percentage between the WT mice and the PS19 mice (p = 0.0030, 95% CI 3.760 to 16.96, F (1, 35) = 10.16) suggesting impaired short-term spatial working memory in the PS19 mice However, the post-hoc tests indicated that there were no significant differences in the alternation percentage between the WT and PS19 mice for any treatment ((vehicle, adjusted p = 0.7456, 95% CI −10.40 to 31.01; acrolein, adjusted p = 0.4503, 95% CI −7.372 to 34.04; ERFR, adjusted p = 0.9846, 95% CI −15.62 to 27.63; ERFR + Acrolein, adjusted p = 0.6022, 95% CI −8.914 to 32.50). Furthermore, there were no significant differences between the PS19 ERFR treated mice compared to PS19 vehicle controls (adjusted p > 0.9999, 95% CI −23.49 to 19.76), or compared to the PS19 ERFR + Acrolein treated mice (adjusted p = 0.9997, 95% CI −18.36 to 24.90). There was also no significant difference between the PS19 ERFR + Acrolein treated mice and PS19 Acrolein control treated mice (p = 0.9985, 95% CI −25.72 to 17.53). In addition, there was no significant difference between the WT ERFR treated mice and WT vehicle treated control mice (p > 0.9999, 95% CI −18.28 to 23.13) (Fig. 4C).
Fig. 4. Cognitive function after chronic intranasal ERFR and acrolein treatment in PS19 mice.
PS19 mice and wild type C57BL/6J mice (WT), beginning at 2 months of age, were given 3 months of intranasal dantrolene (ERFR), with or without acrolein (Acro) treatments, and controls were given vehicle (water) or acrolein alone. A) For contextual fear conditioning, there was no significant interaction between treatment and genotype and no significant differences in the freezing times between the WT and PS19 mice with any treatment. Furthermore, there were no significant differences between any of the groups. WT mice n = 6 and PS19 mice n = 5, for all groups. B) For cued fear conditioning, there was no interaction between treatment and genotype and no significant differences in freezing times between the WT and PS19 mice with any treatment. Furthermore, there were no significant differences between any of the groups. WT mice n = 6 and PS19 mice n = 5, for all groups. C) For the y-maze, there was an overall significant decrease in alternation percentage between the WT mice and the PS19 mice, indicating impaired spatial working memory. However, the post-hoc tests indicated that there were no significant differences between any of the groups. WT mice, n = 6 for all groups except n = 5 for Acrolein; PS19 mice, n = 5 for all groups. All data in the figure were expressed as the mean ± 95% CI and were analyzed using 2-way ANOVA with Tukey’s Multiple Comparisons test.
Side effects of intranasal administration of ERFR and acrolein
Overall, mice were observed to have good general health as evidenced by being well groomed and appropriate food intake and social behavior.
Olfaction
There was an overall significant effect of age on olfaction, with the older mice taking longer to find the buried food (p = 0.0115, 95% CI −114.2 to −15.16, F (1, 50) = 6.883), though all mice discovered the buried food in under the allotted time, 15 min. However, there were no effects on olfactory function after intranasal ERFR treatment in PS19 mice compared to untreated non-transgenic littermates (NT) at either 6 months (adjusted p = 0.8362, 95% CI −54.25 to 87.00) or 9 months of age (adjusted p = 0.5221, 95% CI −49.34 to 128.8) (Fig. 5A).
Fig. 5. Olfaction and motor function in PS19 tau transgenic with and without treatment.
A) There was an overall significant interaction of age with olfaction, with the older mice taking longer to find the buried food. However, there were no effects in PS19 mice compared to non-transgenic littermates (NT) at either 6 or 9 months of age in untreated PS19 mice. PS19 mice, n = 18 (6 months), n = 12 (9 months); NT controls, n = 15 (6 months), n = 9 (9 months). Data were analyzed using 2-way ANOVA with Šídák’s multiple comparisons test. B) No significant differences were found on rotarod performance between untreated NT controls and PS19 mice at either age. PS19 mice, n = 18 (6 months), n = 8 (9 months); NT controls, n = 15 (6 months), n = 8 (9 months). Data were analyzed using 2-way ANOVA with Šídák’s multiple comparisons test. C) No significant differences in motor function were found between genotype or ERFR and acrolein (Acro) treatment groups. PS19 (n = 5), NT mice n = 6, for all groups. Data were analyzed using 2-way ANOVA with Tukey’s post hoc test. All data in the figure are expressed as the mean ± 95% CI.
Motor function
Rotarod is a standard test to measure motor function in mice. After 3 months of treatment with ERFR, there was a significant overall difference in genotype, with the NT controls spending less time on the rotarod than the PS19 mice (p = 0.0267, 95% CI −12.95 to −0.8315, F (1, 45) = 5.246). However, there were no significant differences between the NT controls and PS19 mice at either 5 months (adjusted p = 0.3592, 95% CI −12.46 to 3.487) or 9 months of age (p = 0.1276, 95% CI −20.70 to 2.115) (Fig. 5B). There was no significant interaction between genotype and treatment on rotarod performance in the PS19 and wild type (c57BL/6J) mice treated for 3 months with intranasal dantrolene (ERFR), with or without acrolein (p = 0.2108,95% CI −4.632 to 4.187, F (3, 36) = 1.581) (Fig. 5C).
Confirmation oftau pathology in PS19 mice
Immunohistochemical analysis of brain sections from non-transgenic littermates (NT) and PS19 mice at 6 months of age demonstrated a significant number of phosphorylated tau ps396 positive cells in both the hippocampus and cortex in the PS19 mice, but negative immunostaining in the NT mice (Supplementary Figure 1).
DISCUSSION
This study finds that the dantrolene concentration in an older brain was greater than in the blood, which correlates with our previous pharmacokinetic study in younger mice [16, 32]. We also found that in PS19 tau transgenic mice, after chronic treatment with intranasal ERFR, even with acrolein, there were no significant changes in cognition, olfaction, or motor function. However, we also found that at baseline, untreated PS19 mice did not exhibit cognitive deficits, as reported previously [20, 40].
Considering the need for the chronic use of therapeutics for AD patients, the higher dantrolene concentration in the CNS compared to the blood in the`tau transgenic mice strengthens its therapeutic efficacy. Further understanding of the pharmacokinetics of intranasal dantrolene in the brain and peripheral blood in animals at different ages is important for the therapy design of dantrolene as a potential treatment for AD.
Our previous studies in mice clearly demonstrated a higher and prolonged dantrolene concentration in the brain with intranasal administration compared to oral use [16, 32]. Additionally, the intranasal dantrolene nanoparticle formulation provided better neuroprotection against cognitive dysfunction compared to the subcutaneous approach in 5XFAD mice. Furthermore, no significant side effects were detected in the nasal mucosa, or with olfactory, motor, or liver function, or morbidity and mortality, with up to 10 months intranasal administration [16, 41]. In this current collaborative research work, we used a dantrolene formulation, ERFR, created by Eagle Pharmaceuticals, Inc., to study a potential intranasal formulation of dantrolene for administration in future clinical studies. In comparison to our previous studies in 2-month-old mice [16, 32], the intranasal administration of ERFR achieved significantly less brain or blood dantrolene concentrations at 20 min after administration with a higher, though not significant, brain/blood dantrolene concentration ratio. One difference with our previous studies is the technique used to measure the brain and blood dantrolene concentrations [16, 32]. The current study demonstrated that the intranasal administration of ERFR achieved significantly lower blood dantrolene concentrations in aged mice compared to young adult mice, due to the decreased number of blood vessels to the nasal mucosa in aged mice. However, though the brain dantrolene concentration was significantly less in aged mice compared to that in young adult mice, the relative brain to blood ratio was higher, indicating a greater concentration of dantrolene in the brain than the blood. One possible mechanism is that the blood brain barrier in aged mice, and in AD patients, is disrupted and allows more uptake of dantrolene which typically has limited penetration into the CNS [42]. Nevertheless, this study suggests that intranasal administration of ERFR may provide adequate neuroprotective effects, with minimal systemic side effects.
The lack of sporadic AD animal models is challenging for the AD field and a limitation for the development of treatments. Drugs that have proven effective in animal studies have not translated to the effective treatment for AD patients, 95% of whom have sporadic AD [1]. Although dantrolene has been shown to be neuroprotective in multiple FAD animal models [16, 17, 30, 31, 43], its effects on a tauopathy animal model have not been tested previously. Neuronal tau accumulation is one of the hallmarks of Alzheimer’s disease and thought to contribute to brain atrophy and neurodegeneration. Among currently available tau pathology animal models, P301S mice (PS19) have been widely used for mechanistic studies and cognitive dysfunction has been reported at 5–6 months of age [19, 35, 40, 44]. Therefore, we hypothesized that intranasal dantrolene administration would ameliorate cognitive dysfunction in the PS19 tau transgenic mouse model.
Inconsistent with previous studies using this model [19] with reported cognitive dysfunction and motor deficits at around 6 months of age, the PS19 mice in our study did not demonstrate significant cognitive impairment or impaired motor function even at 9 months of age. It is possible that genetic drift is occurring, which has been reported in this model [35]. Although acrolein has been used to establish cognitive dysfunction like AD in WT rodents [37], the current study did not demonstrate acrolein’s neurotoxic effects to enhance cognitive dysfunction in either WT or PS19 transgenic mice. Thus, we could not determine whether intranasal ERFR was protective against cognitive dysfunction. Our study questions the use of PS19 tau transgenic mice as a dependable animal model to assess cognitive function and the therapeutic effectiveness of new or repurposed drugs for the treatment of AD.
Consistent with our previous study using a similar intranasal dantrolene nanoparticle formulation chronically [16], intranasal ERFR for up to 3 months did not affect body weight and general health, nor olfactory and motor function. So, intranasal ERFR may be safe for chronic use, an initial but crucial step for the development of intranasal ERFR as a potential drug for the treatment of AD in patients, though further studies are needed.
This study has the following limitations: 1)Dantrolene concentrations were measured at a single time point, so the full brain/plasma pharmacokinetics of intranasal ERFR were not obtained. However, we previously performed a full pharmacokinetic study in young mice [32] and, based on that study, we chose the 20-min time point to determine the intranasal ERFR concentrations in the brain and blood in older mice; 2) The aim of our study was to determine the effects of treatment on cognition in the PS19 mice and due to the lack of cognitive dysfunction, even with acrolein treatment, we did not further examine the tau pathology; 3) The literature did not report confounding effects of acrolein and we elected to use the same concentration to promote cognitive dysfunction. A higher dose may be necessary to produce cognitive dysfunction; 4) Allowing the mice to age longer than 11 months may be necessary to examine cognitive decline, but the reported high mortality by 12 months prevented us from designing the experiments with older mice [37]; 5) Some aggressive PS19 male mice had to be singly housed which may cause depressive-like behaviors. However, these mice had the benefit of nestlets and the ability to see, hear, and smell mice in adjoining cages to help alleviate those behaviors; 6) Finally, though we included both sexes in our study, sex differences were not the aim of this study, and we did not design the study to have the power to examine sex differences. Combining the sexes could also be a confounder. This will be included in future study designs.
Conclusions
Our study suggests that the intranasal administration of ERFR provides a favorable dantrolene brain concentration in aged mice compared to young adult mice and has no serious systemic side effects with chronic use, suggesting its potential as a treatment for older AD patients. Further studies are necessary to examine the efficacy of intranasal ERFR as a potential chronic therapy for AD patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ipsita Das, University of Pennsylvania for her help with technical support.
FUNDING
This work was supported by grants to H.W. from the National Institution Aging (NIA R01AG061447 and 3R01AG061447-03S1), and Eagle Pharmaceutical, Inc.-UPENN Collaborative Research Fund.
Footnotes
CONFLICT OF INTEREST
Drs. Huafeng Wei, Maryellen Eckenhoff, and Ge Liang are listed as inventors of patents application entitled “Intranasal Administration of Dantrolene for Treatment of Alzheimer’s Disease” in multiple countries by The Trustees of the University of Pennsylvania. Drs. Veron Browne, Vinolia Chellaraj, Douglas Gisewhite, Michael Greenberg, Sudhir Ranjan, and Gaozhong Zhu own stock options in Eagle Pharmaceuticals along with patents/royalties of the Ryanodex drug used in this study. They are all full-time employees of Eagle Pharmaceuticals. All other authors have no conflicts of interest.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-231337.
DATA AVAILABILITY
Data is available upon request from the corresponding author.
REFERENCES
- [1].2021 Alzheimer’s disease facts and figures (2021). Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [2].Webber EK, Fivaz M, Stutzmann GE, Griffioen G (2023) Cytosolic calcium: Judge, jury and executioner of neurode-generation in Alzheimer’s disease and beyond. Alzheimers Dement 19,3701–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (2004) Dantrolene – A review of its pharmacology, therapeutic use and new developments. Anaesthesia 59, 364–373. [DOI] [PubMed] [Google Scholar]
- [4].Marks AR (2023) Targeting ryanodine receptors to treat human diseases. J Clin Invest 133, e162891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kushnir A, Wajsberg B, Marks AR (2018) Ryanodine receptor dysfunction in human disorders. Biochim Biophys Acta Mol Cell Res 1865, 1687–1697. [DOI] [PubMed] [Google Scholar]
- [6].Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 33, 1001.e1–1001.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H (2020) Dantrolene ameliorates impaired neurogenesis and synap-togenesis in induced pluripotent stem cell lines derived from patients with Alzheimer’s disease. Anesthesiology 132, 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yao J, Chen SRW (2023) RyR2-dependent modulation of neuronal hyperactivity, a potential therapeutic target for treating Alzheimer’s disease. J Physiol, doi: 10.1113/JP283824 [DOI] [PubMed] [Google Scholar]
- [9].Yao J, Sun B, Institoris A, Zhan X, Guo W, Song Z, Liu Y, Hiess F, Boyce AKJ, Ni M, Wang R, Ter Keurs H, Back TG, Fill M, Thompson RJ, Turner RW, Gordon GR, Chen SRW (2020) Limiting RyR2 open time prevents Alzheimer’s disease-related neuronal hyperactivity and memory loss but not beta-amyloid accumulation. Cell Rep 32, 108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gibson GE, Thakkar A (2017) Interactions of mitochondria/metabolism and calcium regulation in Alzheimer’s disease: A calcinist point of view. Neurochem Res 42,1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].SanMartin CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, Garcia A, Hartel S, Hidalgo C, Paula-Lima AC (2017) RyR2-mediated Ca2+release and mitochondrial ROS generation partake in the synaptic dysfunction caused by amyloid beta peptide oligomers. Front Mol Neurosci 10, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sun Y, Yan C, He L, Xiang S, Wang P, Li Z, Chen Y, Zhao J, Yuan Y, Wang W, Zhang X, Su P, Su Y, Ma J, Xu J, Peng Q, Ma H, Xie Z, Zhang Z (2023) Inhibition of ferroptosis through regulating neuronal calcium homeostasis: An emerging therapeutic target for Alzheimer’s disease. Ageing Res Rev 87, 101899. [DOI] [PubMed] [Google Scholar]
- [13].Fonseca AC, Ferreiro E, Oliveira CR, Cardoso SM, Pereira CF (2013) Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim Biophys Acta 1832, 2191–2203. [DOI] [PubMed] [Google Scholar]
- [14].Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, Matute C (2013) Ca(2+)-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astro-cytes and in a model of Alzheimer’s disease. Aging Cell 12, 292–302. [DOI] [PubMed] [Google Scholar]
- [15].McDaid J, Mustaly-Kalimi S, Stutzmann GE (2020) Ca(2+) dyshomeostasis disrupts neuronal and synaptic function in Alzheimer’s disease. Cells 9, 2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi Y, Zhang L, Gao X, Zhang J, Ben Abou M, Liang G, Meng Q, Hepner A, Eckenhoff MF, Wei H (2020) Intranasal dantrolene as a disease-modifying drug in Alzheimer 5XFAD mice. J Alzheimers Dis 76, 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H (2012) Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F, Benfenati F, Chami M (2012) Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32, 11820–11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takeuchi H, Iba M, Inoue H, Higuchi M, Takao K, Tsukita K, Karatsu Y, Iwamoto Y, Miyakawa T, Suhara T, Trojanowski JQ, Lee VM, Takahashi R (2011) P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One 6, e21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lasagna-Reeves CA, de Haro M, Hao S, Park J, Rousseaux MW, Al-Ramahi I, Jafar-Nejad P, Vilanova-Velez L, See L, De Maio A, Nitschke L, Wu Z, Troncoso JC, Westbrook TF, Tang J, Botas J, Zoghbi HY (2016) Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron 92, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goni-Oliver P, Avila J, Hernandez F (2009) Memantine inhibits calpain-mediated truncation of GSK-3 induced by NMDA: Implications in Alzheimer’s disease. J Alzheimers Dis 18, 843–848. [DOI] [PubMed] [Google Scholar]
- [22].Kurbatskaya K, Phillips EC, Croft CL, Dentoni G, Hughes MM, Wade MA, Al-Sarraj S, Troakes C, O’Neill MJ, Perez-Nievas BG, Hanger DP, Noble W (2016) Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol Commun 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimers Dis 20 Suppl 2, S487–S498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Esteras N, Abramov AY (2020) Mitochondrial calcium deregulation in the mechanism of beta-amyloid and tau pathology. Cells 9, 2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: Implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28, 1563–1574. [DOI] [PubMed] [Google Scholar]
- [27].Calvo-Rodriguez M, Kharitonova EK, Bacskai BJ (2020) Therapeutic strategies to target calcium dysregulation in Alzheimer’s disease. Cells 9, 2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu M, Wang L, Gao J, Dong Q, Perry G, Ma X, Wang X (2019) Inhibition of calpain protects against tauopathy in transgenic P301S tau mice. J Alzheimers Dis 69,1077–1087. [DOI] [PubMed] [Google Scholar]
- [29].Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robin-son N, Martin GM, Mattson MP (1996) Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport 8, 379–383. [DOI] [PubMed] [Google Scholar]
- [30].Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG, Stutzmann GE (2012) Stabilizing ER Ca(2+) channel function as an early preventative strategy for Alzheimer’s disease. PLoS One 7, e52056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi Y, Wang Y, Wei H (2019) Dantrolene: From malignant hyperthermia to Alzheimer’s disease. CNS Neurol Disord Drug Targets 18, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang J, Shi Y, Yu S, Wang Y, Meng Q, Liang G, Eckenhoff MF, Wei H (2020) Intranasal administration of dantrolene increased brain concentration and duration. PLoS One 15, e0229156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bowman GL, Dayon L, Kirkland R, Wojcik J, Peyratout G, Severin IC, Henry H, Oikonomidi A, Migliavacca E, Bacher M, Popp J (2018) Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement 14, 1640–1650. [DOI] [PubMed] [Google Scholar]
- [34].Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I (2010) Role of presenilins in neuronal calcium homeostasis. J Neurosci 30, 8566–8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, Lee VM (2015) Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol 130, 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen C, Lu J, Peng W, Mak MS, Yang Y, Zhu Z, Wang S, Hou J, Zhou X, Xin W, Hu Y, Tsim KWK, Han Y, Liu Q, Pi R (2022) Acrolein, an endogenous aldehyde induces Alzheimer’s disease-like pathologies in mice: A new sporadic AD animal model. Pharmacol Res 175, 106003. [DOI] [PubMed] [Google Scholar]
- [37].Huang YJ, Jin MH, Pi RB, Zhang JJ, Ouyang Y, Chao XJ, Chen MH, Liu PQ, Yu JC, Ramassamy C, Dou J, Chen XH, Jiang YM, Qin J (2013) Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol Lett 217, 184–191. [DOI] [PubMed] [Google Scholar]
- [38].Nakamura M, Uemura T, Saiki R, Sakamoto A, Park H, Nishimura K, Terui Y, Toida T, Kashiwagi K, Igarashi K (2016) Toxic acrolein production due to Ca(2+) influx by the NMDA receptor during stroke. Atherosclerosis 244, 131–137. [DOI] [PubMed] [Google Scholar]
- [39].Kraeuter AK, Guest PC, Sarnyai Z (2019) The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol 1916, 105–111. [DOI] [PubMed] [Google Scholar]
- [40].Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ (2011) Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci 31, 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang B, Shi Y, Abou MB, Xu L, Liang G, Wei H (2022) Effects of chronic intranasal dantrolene on nasal mucosa morphology in mice. Eur Rev Med Pharmacol Sci 26, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wuis EW, Rijntjes NV, Van der Kleijn E (1989) Whole-body autoradiography of 14C-dantrolene in the marmoset monkey. Pharmacol Toxicol 64, 156–158. [DOI] [PubMed] [Google Scholar]
- [43].Wu Z, Yang B, Liu C, Liang G, Eckenhoff MF, Liu W, Pickup S, Meng Q, Tian Y, Li S, Wei H (2015) Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice. Alzheimer Dis Assoc Disord 29, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Makani V, Zhang B, Han H, Yao Y, Lassalas P, Lou K, Pater-son I, Lee VM, Trojanowski JQ, Ballatore C, Smith AB 3rd, Brunden KR (2016) Evaluation of the brain-penetrant microtubule-stabilizing agent, dictyostatin, in the PS19 tau transgenic mouse model of tauopathy. Acta Neuropathol Commun 4, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon request from the corresponding author.