Abstract
IgA-associated vasculitis (IgAV) known as Henoch - Schönlein purpura (HSP) disease is an inflammatory disorder of small blood vessels. It’s the most common type of systemic vasculitis in children which can be associated with the inflammatory process following infections. IgA vasculitis is a rare and poorly understood systemic vasculitis in adults. Coronavirus disease 2019 (COVID-19) has been associated with HSP in both adults and children. A 58-year-old woman was diagnosed with HSP, fulfilling the clinical criteria: palpable purpura, arthritis, hematuria. The disclosure of the HSP disease was preceded by a infection of the respiratory tract. COVID-19 infection was confirmed via the presence of IgM and IgG antibodies. This case indicates the possible role of SARS-CoV-2 in the development of HSP. The clinical course of IgAV in adults appears to be different from pediatric IgAV, especially due to higher risk of renal complications. Symptoms of the disease quickly resolved with low-dose of steroids.
Keywords: COVID-19, Henoch-Schönlein purpura, IgA vasculitis, Palpable purpura, Prognosis
Introduction
IgA vasculitis (IgAV), also known as Henoch-Schönlein purpura (HSP), is an inflammation of small vessels with deposition of immunoglobulin class A (IgA) in the blood vessels of the skin and internal organs. IgAV/HSP is the most common form of small vessel vasculitis in children, 10 times less common in adults. The annual incidence is about 15/100,000 in children and 1.3/100,000 in adults [1–3]. The etiology of IgAV/HSP is not fully understood. There is an association between IgAV and a history of bacterial infection, viral infection, vaccination, food allergens, and drug use. More than a dozen cases of IgAV after COVID-19 have recently been described [4]. A defect in the glycosylation of immunoglobulin IgA1 and its abnormal structure are basic phenomena in IgAV. Immunoglobulin IgA1 does not contain galactose molecules attached during glycosylation in its hinge region (galactose-deficient IgA1: Gd-IgA1). Abnormal IgA1 is formed after contact with antigens on the surface of mucous membranes of the upper respiratory tract and gastrointestinal tract [5]. The presence of IgA1 in blood induces the formation of autoantibodies, immune complexes. The pathogenesis of IgAV in the course of COVID-19 is associated with abnormal development of cellular response (Th2), increased activation of B cells and production of antibodies. This leads to type 3 hypersensitivity reactions, deposition of antigen-antibody complexes, usually in blood vessels, activation of the complement cascade and release of anaphylatoxins (C3a and C5a) [6]. Cytokines released during COVID-19 infection (IL-1, IL-6 and TNF) can also lead to B-cell proliferation and IgA1 production [7]. COVID-19 infection can lead to the development of IgAV after direct viral infection of endothelial cells via angiotensin-converting enzyme 2 receptors or indirectly via the inflammation induced by an immune response [8, 9]. The main symptoms of the disease are palpable purpura, arthritis, abdominal pain and glomerulonephritis. The diagnosis of IgAV is established on the basis of clinical symptoms and histopathological presentation of a skin or kidney specimen. According to the current SHARE (Single Hub and Access point for pediatric Rheumatology in Europe) guidelines, the 2010 EULAR/PRINTO/PRES (European Alliance of Associations for Rheumatology - EULAR, Pediatric Rheumatology International Trials Organization - PRINTO, Pediatric Rheumatology European Society - PRES) criteria are used to diagnose IgAV. These criteria are usually used for the diagnosis of IgAV in children and are of limited value in adult patients. However, they are more sensitive and specific than the 1990 ACR IgAV criteria. To diagnose IgAV, the presence of purpura and at least 1 of four symptoms: abdominal pain, arthritis/pain, renal involvement or histopathologically confirmed IgA-mediated vasculitis/glomerulonephritis is required [10, 11]. IgA vasculitis is a mild, self-limiting disease, especially in children. The course of the disease in adults is more severe, usually complicated by gastrointestinal and renal involvement. Gastrointestinal vasculitis can lead to intestinal perforation, hemorrhage and is the main risk of IgAV death in adults. Renal involvement is associated with an increased risk of progression to chronic renal failure [12, 13]. More than a dozen cases of IgAV after COVID-19 have been described so far. This virus can attack almost any organ and lead to skin lesions, renal, cardiovascular and neurological complications [14, 15].
Our study aims to conduct a review of case reports on IgAV caused by COVID-19. We will assess how the age of onset affects the clinical spectrum of the disease and compare the symptoms of IgAV in COVID-19 cases with those caused by other factors. Identification of this patient group may enable us to investigate prognostic factors and develop suitable therapeutic methods in the future. So far, the lack reviews on this topic.
Case study
The patient, 58 years old woman, was brought to the ED in December 2021 because of worrisome skin lesions (Fig. 1). A few days earlier, the patient had symptoms of respiratory tract infection: dry cough, subfebrile states. She was not taking antibiotics. Five days after the symptoms of infection, she developed a rash on the skin of the lower extremities and forearms with the character of raised purpura, pain and swelling of the left knee joint. The patient denied abdominal pain, hematuria, gastrointestinal bleeding. Test results showed: elevated inflammatory markers, thrombocytosis, leukocyturia and erythrocyturia (Table 1). Lung CT showed diffuse foci of lung parenchymal consolidation located subpleurally with bronchial lumen dilatation in segment 3 of both lungs, the right lower lobe and in segments 4P, 8 L, 9 L, as well as features of peripheral pulmonary embolism. An abdominal ultrasound showed no abnormalities. A positive antibody result for COVID-19 and a positive PCR test were obtained. The patient had not been vaccinated against COVID-19. A diagnosis of pneumonia due to COVID-19 infection was made, and the patient was transferred to the Isolation Unit. During hospitalization, virological and immunological diagnostics were performed (Table 2). On the basis of clinical symptoms and laboratory results: raised purpura, arthritis, hematuria, sterile leukocyturia, IgAV was diagnosed. The woman did not consent to a skin biopsy. The patient met the criteria for the diagnosis of the disease according to EULAR/PRINTO/PRES (2010). Treatment included glucocorticoids (GCs) methylprednisolone at the initial dose of 16 mg/once a day (after 3 days the dose was reduced to 8 mg/once a day), low molecular weight heparin at a dose of 1 mg/kg body weight/twice a day and then rivaroxaban at a dose of 15 mg/twice a day. The patient did not require oxygen therapy. After 10 days of treatment, a significant reduction in skin lesions was achieved. Further observation of the patient was carried out in the setting of a rheumatology outpatient clinic where the dose of GCs was reduced. After 2 months, complete remission of skin and joint symptoms, normalization of laboratory test results was achieved. GCs were discontinued.
Fig. 1.
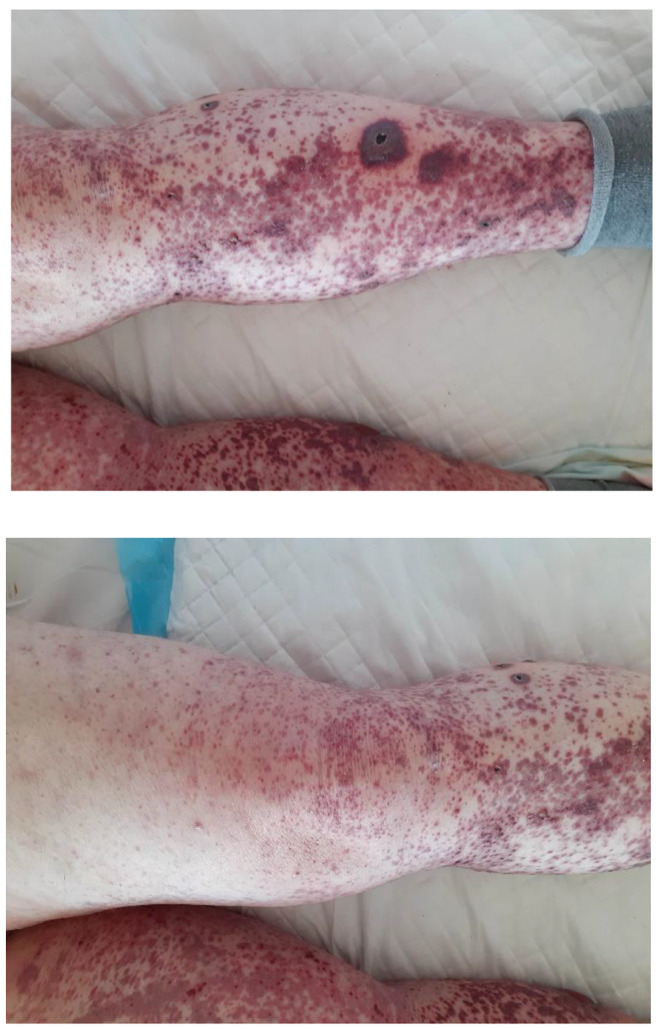
Palpable skin rash on the legs
Table 1.
Laboratory data
| Parameter | At the beginning of hospitalization | At the end of hospitalization |
|---|---|---|
| Hemoglobin (g/dl) | 12.2 | 11.8 |
| Total leukocyte count (cells/mcL) | 6300 | 4200 |
| Neutrophils (%/cells/mcL) | 72.1/4500 | 52.7/2200 |
| Lymphocytes (%/cells/mcL) | 17.7/1130 | 35.3/1500 |
| Platelets (cells/mcL) | 537 000 | 330 000 |
| Erythrocyte Sedimentation Rate (mm/1h) < 10 | 27 | |
| C-Reactive Protein (mg/L) 0–5 | 10.5 | 0.3 |
| Procalcitonin (ng/mL) < 0.5 | < 0.02 | 0.08 |
| IL- 6 (pg/ml) < 4.4 | 8.4 | 2.7 |
| Erytrocyturia | 20–25 | 1–3 |
| Leukocyturia | 15–20 | 1–3 |
| 24 h urinary protein (mg/24 h) | 950 | |
| Urine culture | negative | |
| Total bilirubin (mg/dl) 0.3–1.2 | 0.5 | 0.3 |
| Aspartate aminotransferase (IU/L) < 31 | 38 | 57 |
| Alanine aminotransferase (IU/L) > 34 | 20 | 159 |
| Lactate dehydrogenase (U/L) 120–246 | 252 | 213 |
| Creatine phosphokinase (U/L) 120–180 | 130 | |
| Ferritin (ng/mL) 10–291 | 119 | |
| Serum Creatinine (mg/dL) 0.5–1.1 | 0.6 | 0.34 |
| Protein (g/dL) 5.7–8.2 | 7 | |
| Albumin (g/dL) 3.8–5.4 | 4.3 | |
| Prothrombin time (sec.) 9.4–12.5 | 12.9 | 12.8 |
| Kaolin-kephalin time (sec.) 25.4–36.9 | 27.4 | 29.1 |
| Fibrynogen (g/l) 2-3.9 | 3.4 | |
| d-Dimer (ng/ml) 0-500 | 7247 | 987 |
Table 2.
Immunological tests
| Variables | Result |
|---|---|
| Anti-SARS CoV-2 in IgM class (> 1) | 6.2 |
| Anti-SARS CoV-2 in IgG class (> 7.1) | 120 |
| SARS-CoV-2 PCR | Positive |
| Rapid SARS-CoV-2 antigen test | Negative |
| Cryoglobulins | Negative |
| Human Immunodeficiency Virus | Negative |
| Hepatitis B surface antigen | Negative |
| Anti-hepatitis C virus | Negative |
| EBV IgM antibodies | Negative |
| CMV IgM antibodies | Negative |
| Influenza A – RNA | Negative |
| Influenza B – RNA | Negative |
| RSV – RNA | Negative |
| ANA | Negative |
| pANCA | Negative |
| cANCA | Negative |
| Anti-CCP | Negative |
| Rf-IgM | Negative |
| Complement levels C3 (mg/dl) 90–170 | 109.2 |
| Complement levels C4 (mg/dl) 12–36 | 28.7 |
| IgA (mg/dl) 40–350 | 280 |
| IgM (mg/dl) 50–300 | 137 |
| IgG (mg/dl) 650–1600 | 1250 |
Search strategy
PubMed, Scopus and Web of Science databases were searched for articles containing case reports published between December 1, 2019 and July 31, 2023 using the following keywords: ‘COVID-19’, ‘SARS-CoV-2’, ‘IgA vasculitis’, ‘Henoch-Schönlein purpura’, and ‘palpable purpura’. Only clinical case studies written in English and accessible in full text were included. The quality of clinical cases were evaluated by Equator network’s Clinical Case Reporting Guideline Development-CARE checklist.
Discussion
The association between COVID-19 infection and development of autoimmune diseases is widely debated. To date, an increased risk of inflammatory myopathy, systemic scleroderma, and systemic lupus erythematosus has been demonstrated [16–19]. Various forms of systemic vasculitis have also been described in the literature, including several cases of IgAV in patients infected with COVID-19 [20]. IgA vasculitis is a rare and poorly understood systematic vasculitis in adults. The data indicate that clinical presentation and outcome of IgAV in adults differ from children.
We present a case of a 58-year-old woman who developed IgAV with skin, joint and kidney involvement after COVID-19 infection, successfully treated with GCs.
The first case of an IgAV patient was published by Allez et al. It was a 24-year-old man with Crohn’s disease treated with TNFα inhibitors, who developed skin lesions, joint complaints, and abdominal pain during COVID-19 infection. Histopathological examination of the skin confirmed IgAV. In the case described, a link between IgA vasculitis and Crohn’s disease or treatment with TNFα inhibitors could not be ruled out [21]. One of the first cases of IgA nephropathy (IgAN) in the course of COVID-19 infection was described by Suso et al. in a 78-year-old man. Symptoms of vasculitis (skin lesions, arthritis, hematuria, proteinuria, renal failure) occurred e few weeks after COVID-19 [22]. The most data on the co-occurrence of IgAV and COVID- 19 are provided by the works of Faroog et al., Messova et al., Wonga et al. Faroog et al. described 13 patients with IgAV and IgAN associated with COVID-19 infection. The mean age was 23.8 years (range 1–78), the majority of patients were male (77%). Nearly half of the patients (46%) were over 18 years of age. In 10 patients (77%), symptoms of COVID-19 infection and/or positive PCR co-occurred with symptoms of IgAV. In the remaining three patients (23%), COVID-19 infection resolved before the onset of IgAN/IgAV symptoms. The most common clinical manifestations were skin lesions (85%) and gastrointestinal symptoms (62%). Less common were joint pain (54%) and arthritis with swelling (31%). Renal involvement was present in about half of the patients. Most patients received GCs and reported recovery or improvement; 2 patients in early childhood died [23]. Messova et al. analyzed a group of patients infected with COVID-19 between December 1, 2019 and December 1, 2021, who met criteria for IgA vasculitis. Among 13 patients (8 adults and 5 children), 12 patients were male. More than half of the patients had symptoms of kidney damage: proteinuria, hematuria, acute renal failure. Four patients had no respiratory symptoms. In their analysis, Messova et al. showed that all elderly patients had symptoms of kidney damage, and 80% of them developed acute renal failure. The results of this analysis are similar to other studies: COVID-19-associated IgAV mainly affects adults and is characterized by a more severe disease course with skin, joint and kidney damage. A positive effect of GCs has been reported in most individuals [24]. An interesting analysis was conducted by Jedlowski et al. They compared the course of IgAV induced by COVID − 19 in children and adults in a group of 10 patients (5 children, 5 adults). The time from the onset of COVID-19 symptoms to the development of IgAV was 2–37 days. There was no significant difference between the two groups in terms of overall organ involvement, except for joint pain and proteinuria, which were more common in adults. Like other researchers, they showed that COVID-19-associated IgAV compared to classical IgAV was significantly more common in adults and male subjects. As in the population of patients with classical IgAV, the course of the disease in adults is more severe, complicated by renal involvement. The prognosis of IgAV patients with COVID infection is fairly good; GCs are effective in most cases [25].
The course of IgAV in the patient reported here was relatively mild. To date, few descriptions of IgAV in women have been reported, with men predominating in each report. Symptoms of vasculitis appeared several days after the symptoms of COVID-19 infection (similar to the work of Jedlowski et al.). The clinical presentation was dominated by skin lesions, arthritis, and renal involvement (according to Faroog et al., the percentages were 85%, 54%, and about 50%, respectively). COVID-19 infection was complicated by pulmonary embolism. Symptoms of the disease quickly resolved with low-dose GCs, as in previously described cases.
Conclusions
COVID-19 infection can lead to IgAV, especially in men. Palpable purpura may be the first sign of the disease. Renal complications with proteinuria and renal failure development are more common in adults [24, 25]. Early diagnosis and use of GCs leads to rapid remission of the disease. It is recommended that future studies with larger sample sizes be conducted to further explore the relationship between COVID-19 and IgAV.
Limitations
The study has limitations due to the small number of clinical cases, making it challenging to draw definitive conclusions or establish a causal relationship between COVID-19 and IgAV. The quality of the work is significantly affected by the absence of a skin biopsy, which was not possible due to the lack of patient consent. Publication bias may be suspected, as clinicians are more likely to report clinically significant and challenging cases. As case reports make up the majority of the literature, it is difficult to generalise findings to the wider population.
Author contributions
All authors take full responsibility for the integrity and accuracy of all aspects of the work. The authors confirm contribution to the paper as follows: study conception and design: DS, AGC, JEM; data collection: DS, AK, KR, JS; analysis and interpretation of results: DS, AGC, JEM; draft manuscript preparation: DS. All authors reviewed the results and approved the final version of the manuscript.
Declarations
Conflict of interest
Authors - Dorota Suszek, Anna Grzywa-Celińska, Justyna Emeryk-Maksymiuk, Adam Krusiński, Katarzyna Redestowicz, Jan Siwiec declare that they have no conflict of interest. We are interested in publishing our work in an open access format.
Informed consent
The authors obtained written consent from the patient for their photographs and medical information to be published in print and online. Patient consent form is retained by the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kang Y, Park JS, Ha YJ, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schonlein purpura. J Korean Med Sci. 2014;29:198–203. doi: 10.3346/jkms.2014.29.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goretti M, Penido MG, Monteiro Pereira Palma L. IgA vasculitis in children. Braz J Nephrol. 2022;44(1):3–5. doi: 10.1590/2175-8239-jbn-2022-e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liyun Xu Y, Li X, Wu IgA vasculitis update: epidemiology, pathogenesis, and biomarkers. Front Immunol. 2022;13:921864. doi: 10.3389/fimmu.2022.921864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machura E, Krakowczyk H, Bąk-Drabik K, Szczepańska M. SARS-CoV-2 infection as a possible trigger for IgA-Associated Vasculitis: a Case Report. Child (Basel) 2023;10(2):344. doi: 10.3390/children10020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trimarchi H, Barratt J, Monteiro RC, Feehally J (2019) IgA nephropathy: State of the art: a report from the 15th International Symposium on IgA Nephropathy celebrating the 50th anniversary of its first description. Kidney Int 95(4): 750–756. 10.1016/j.kint.2019.01.007 [DOI] [PubMed]
- 6.Roncati L, Ligabue G, Fabbiani L, et al. Type 3 hypersensitivity in COVID-19 vasculitis. Clin Immunol. 2020;217:108487. doi: 10.1016/j.clim.2020.108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugino H, Sawada Y, Nakamura M. IgA vasculitis: etiology, treatment, biomarkers and epigenetic changes. Int J Mol Sci. 2021;22(14):7538. doi: 10.3390/ijms22147538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farooq H, Aemaz Ur Rehman M, Asmar A, et al. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: a systematic review. J Taibah Univ Med Sci. 2022;17:1–13. doi: 10.1016/j.jtumed.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hočevar A, Rotar Z, Jurčić V, et al. IgA vasculitis in adults: the performance of the EULAR/PRINTO/PRES classification criteria in adults. Arthritis Res Ther. 2016;18(1):58. doi: 10.1186/s13075-016-0959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990;33(8):1114–1121. doi: 10.1002/art.1780330809. [DOI] [PubMed] [Google Scholar]
- 12.Yaseen K, Herlitz LC, Villa-Forte A. IgA vasculitis in adults: a Rare yet Challenging Disease. Curr Rheumatol Rep. 2021;23(7):50. doi: 10.1007/s11926-021-01013-x. [DOI] [PubMed] [Google Scholar]
- 13.Hočevar A, Tomšič M, Jurčić V, et al. Predicting gastrointestinal and renal involvement in adult IgA vasculitis. Arthritis Res Therapy. 2019;21:302. doi: 10.1186/s13075-019-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlers CG, Wang B, Howell DN, Choksi V. Extensive palpable Purpura Preceding Renal Dysfunction in Immunoglobulin A Vasculitis due to Coronavirus-19 infection. Am J Med. 2023;136(7):655–658. doi: 10.1016/j.amjmed.2023.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salem Y, Alam Z, Shalabi MM, et al. Vasculitis Associated with COVID-19. Cureus. 2023;15(5):e38725. doi: 10.7759/cureus.38725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saud A, Naveen R, Aggarwal R, Gupta L. COVID-19 and myositis: what we know so far. Curr Rheumatol Rep. 2021;23:63. doi: 10.1007/s11926-021-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamani B, Moeini Taba S-M, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. 2021;15:29. doi: 10.1186/s13256-020-02582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denton CP, Campochiaro C, Bruni C, et al. COVID-19 and systemic sclerosis: rising to the challenge of a pandemic. J Scleroderma Relat Disord. 2021;6(1):58–65. doi: 10.1177/2397198320963393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong K, Farooq Alam Shah MU, Khurshid M, et al. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg (Lond) 2022;74:103249. doi: 10.1016/j.amsu.2022.103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allez M, Denis B, Bouaziz JD, et al. COVID-19- related IgA vasculitis. Arthritis Rheumatol. 2020;72(11):1952–1953. doi: 10.1002/art.41428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suso AS, Mon C, Alonso IO, et al. IgA Vasculitis with Nephritis (Henoch – Schönlein Purpura) in a COVID-19 patient. Kidney Int Rep. 2020;5(11):2074–2078. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farooq H, Aemaz Ur Rehman M, Asmar A, et al. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: a systematic review. J Taibah Univ Med Sci. 2022;17(1):1–13. doi: 10.1016/j.jtumed.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messova A, Pivina L, Muzdubayeva Z, et al. COVID-19 and New Onset IgA Vasculitis: a systematic review of Case Reports. J Emerg Nurse. 2022;48(4):348–365. doi: 10.1016/j.jen.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jedlowski PM, Jedlowski MF. Coronavirus disease 2019-associated immunoglobulin a vasculitis/Henoch-Schönlein purpura: a case report and review. J Dermatol. 2022;49(1):190–196. doi: 10.1111/1346-8138.16211. [DOI] [PMC free article] [PubMed] [Google Scholar]


