Abstract
The induction of strong cytotoxic T-lymphocyte (CTL) and humoral responses appear to be essential for the elimination of persistently infecting viruses, such as hepatitis C virus (HCV). Here, we tested several vaccine regimens and demonstrate that a combined vaccine regimen, consisting of HCV E2 DNA priming and boosting with recombinant E2 protein, induces the strongest immune responses to HCV E2 protein. This combined vaccine regimen augments E2-specific immunoglobulin G2a (IgG2a) and CD8+ CTL responses to a greater extent than immunizations with recombinant E2 protein and E2 DNA alone, respectively. In addition, the data showed that a protein boost following one DNA priming was also effective, but much less so than those following two DNA primings. These data indicate that sufficient DNA priming is essential for the enhancement of DNA encoded antigen-specific immunity by a booster immunization with recombinant E2 protein. Furthermore, the enhanced CD8+ CTL and IgG2a responses induced by our combined vaccine regimens are closely associated with the protection of BALB/c mice from challenge with modified CT26 tumor cells expressing HCV E2 protein. Together, our results provide important implications for vaccine development for many pathogens, including HCV, which require strong antibody and CTL responses.
Hepatitis C virus (HCV) is a major causative agent of non-A, non-B hepatitis (7, 18). Previous studies indicate that the development of chronic liver disease and hepatocellular carcinoma is closely associated with persistent infection of HCV (30). Currently, the lack of efficient antiviral treatment against HCV makes the development of a vaccine highly desirable. It is unclear which type of immunity is essential for HCV resolution. Recombinant protein vaccination facilitates strong antibody responses and stimulates primarily Th2 cells, which are defined by their secretion of the cytokines interleukin-4 (IL-4), IL-5, and IL-10. Protein vaccination with HCV envelope glycoproteins E1 and E2 induced protective immunity against homologous virus challenge in chimpanzees (8). In this model, the protection appeared to be correlated to the titers of anti-E2 antibodies, suggesting that antibody responses are important for protection against HCV infection. Furthermore, there are growing evidences that Th1 and cytotoxic T-lymphocyte (CTL) responses to HCV proteins may play a key role in virus resolution during natural infection (9, 11, 24, 28). It has been reported that the prevalent cytokine pattern of circulating HCV-specific CD4+ T cells is Th1-like in patients who recovered from acute hepatitis, as exhibited by the secretion of IL-2 and gamma interferon (11). In addition, chimpanzees which generated high levels of CTL responses to HCV proteins eliminated HCV infection (9). Thus, an effective HCV vaccine must elicit both strong humoral and cell-mediated immune responses, especially Th1 and CTL responses.
Since it has been documented that DNA immunization preferentially induces Th1 immunity and CTL responses to many viral antigens (5, 26, 36), DNA vaccine approaches have been applied to generate protective immunity to a variety of pathogens (10, 12, 33). However, DNA immunization was also demonstrated to generate weaker antibody and CTL responses than did protein and live attenuated vaccinations, respectively (22, 32). In general, immunity generated by DNA vaccination alone appeared to be sufficient to protect against pathogens in only a few animal models (2, 23). To circumvent these limits of DNA vaccination, many groups have employed combinatorial vaccination regimens (3, 22, 29, 32). Antibody avidity and neutralizing antibody (nAb) titers to human immunodeficiency virus (HIV) gp160 were greatly enhanced in rabbits by DNA priming followed by protein boosting as compared to either DNA or protein immunization only (29). Furthermore, recombinant protein booster immunization of DNA-primed macaques had an enhancement of antibody responses of approximately 100-fold and protection from nonpathogenic simian/human immunodeficiency virus infection (22). Although antibody responses were greatly modulated quantitatively as well as qualitatively by protein boosting in DNA-primed animals, the effects of protein boosting on the induction of T-cell-based immunity, especially CD8+ CTL responses, have not been investigated. Here, we performed DNA priming-protein boosting vaccination regimens against HCV E2 to assess the effects of protein booster immunization on both antibody and CTL responses in mice. Both CTL and bulk antibody responses, especially immunoglobulin G2a (IgG2a) responses, were strongly increased by an E2 protein boosting in mice primed twice with E2 DNA. Moreover, the group of mice given the DNA priming-protein boosting vaccine regimen had the highest protection rate against challenge by tumor cells expressing HCV E2 protein. Even though other factors, such as antigen dosage, route of immunization, and kind of adjuvant, could affect the immune responses, our DNA priming-protein boosting may provide a general vaccination regimen for the induction of strong antibody and CTL responses.
Preparation of DNA vaccine constructs and recombinant proteins.
The DNA vaccine vector pTV2sE2t was constructed to encode HCV E2 sequences (amino acid [aa] residues 384 to 719) of type 1b (Korean isolate) (19) fused to the herpes simplex virus type 1 glycoprotein D (gD) signal sequence (aa residues 1 to 34) (20). To obtain recombinant herpes simplex virus type 1 gD and HCV E2 proteins, stable Chinese hamster ovary (CHO) cell lines expressing gD and gDE2t were constructed. Briefly, the cDNA fragment encoding the C-terminal truncated gD protein (aa 1 to 316) was amplified by PCR from the KOS-1 strain by using primers 5′-ATC CTG CAG GTC TCT TTT GT-3′ and 5′-CGC GAA TTC CTG GAT CGA CGG GAT-3′ and was inserted into pMT3 to produce pMT3-gD (19). To construct pMT3-gDE2t, the E2 region spanning aa residues 386 to 693 was obtained by PCR using E2N (5′-CCA TAT GCG CGT GAC AGG AGG AAC G-3′) and 2420A (5′-TGT TCT AGA GGA GGT GGA TTA ACC CA-3′) primers and fused in-frame to aa residue 326 of gD in pMT3-gD. The resulting constructs were used to establish recombinant CHO cell lines expressing either gD or gDE2t protein as previously described (19). The recombinant CHO cell lines were initially screened by immunoblotting and enzyme-linked immunosorbent assay (ELISA) using anti-gD (Fitzgerald Inc., Concord, Mass.) and anti-E2 monoclonal antibodies (mAbs) (19) and were subjected to five subsequent rounds of methotrexate (Sigma, St. Louis, Mo.) selection. To purify gD and gDE2t, the gD mAb affinity column was prepared by coupling the gD mAb to the CNBr-activated Sepharose-4B (Pharmacia Biotech Inc.). The secreted gD and gDE2t proteins were purified to 90% purity by repeating immunoaffinity chromatography twice (19).
Antibody responses induced by different combinatorial vaccinations.
To investigate the effect of protein boosting in DNA-primed mice, we compared antibody responses induced by several vaccination regimens, as shown in Table 1. Briefly, 6-week-old female BALB/c mice were injected either intramuscularly (i.m.) in the anterior tibialis muscles with 100 μg of DNA following pretreatment with bupivacaine-HCl (ASTRA) and/or subcutaneously (s.c.) with 5 μg of protein, with alum hydroxide serving as adjuvant. The booster immunizations were performed either once or twice with the same amount of DNA or protein at 1-month intervals. Sera were collected by eye bleeding at selected time points and assayed for the presence of E2-specific antibodies by ELISA using E2 and human growth hormone (hgh) fusion protein, which was purified from a recombinant CHO cell line (19). The end-point titrations were performed by ELISA with serial dilutions of pooled sera to determine the E2-specific antibody responses semiquantitatively. Mice given an injection with pTV2sE2t DNA (designated E2 DNA) induced a weak antibody response to E2 protein, which was enhanced approximately 4- to 12-fold by consecutive booster immunization with the same DNA (Table 1, group V). The gDE2t recombinant protein immunizations (group VII) elicited antibody responses that were five times higher than those induced by E2 DNA injections. Interestingly, when a booster immunization with gDE2t protein was performed after two rounds of E2 DNA injections, the antibody titer was dramatically increased (approximately 29-fold), which was even higher than that of gDE2t protein immunizations alone (Table 1, groups IV and VII). The protein priming-DNA boosting regimen induces a lower level (approximately four- to fivefold) of antibodies than those induced by DNA priming-protein boosting (Table 1, groups IV and VI), which supports a previous report that the specific order of immunization is important for the induction of optimal immune responses in prime-boost immunization strategies with different vaccine preparations (32). In addition, when a gDE2t booster immunization was performed in mice given a single E2 DNA injection, the antibody titer was increased to three times higher than that induced by E2 DNA boosting, but it was still lower than that induced by two rounds of recombinant gDE2t protein immunizations alone (Table 1, group VIII). These experiments demonstrate that booster-immunization-amplified antibody responses correlated with the type of priming regimen (DNA or protein) or upon the number of rounds of DNA priming that were administered. As expected, the effect of a gDE2t protein boosting was not observed in mice primed two times with control pTV2 DNA (Table 1, group II). Taken together, our data suggest that the enhancement of total IgG by a protein booster immunization is specific to antigen which is primed by DNA and not due to the nonspecific bystander activation of B cells by immunostimulatory effects of bacterial DNA (15).
TABLE 1.
The end point titers of antibodies in the immunized mice
| Group no. | DNA and/or protein (no. of injections)a | No. of mice | Anti-E2 IgG titer (102) 3 weeks afterb:
|
||
|---|---|---|---|---|---|
| First injection | Second injection | Third injection | |||
| I | pTV2 (3) | 17 | 2 ± 1 | 4 ± 2 | 9 ± 3 |
| II | pTV2 (2), gDE2t (1) | 8 | 2 ± 1 | 4 ± 2 | 277 ± 67 |
| III | pTV2sE2t (2), gD (1) | 8 | 53 ± 9 | 199 ± 33 | 312 ± 45 |
| IV | pTV2sE2t (2), gDE2t (1) | 17 | 61 ± 12 | 245 ± 44 | 7,003 ± 2,234 |
| V | pTV2sE2t (3) | 17 | 57 ± 11 | 212 ± 33 | 673 ± 121 |
| VI | gDE2t (2), pTV2sE2t (1) | 8 | 232 ± 42 | 1,098 ± 212 | 1,520 ± 235 |
| VII | gDE2t (3) | 17 | 267 ± 55 | 1,123 ± 234 | 3,121 ± 458 |
| VIII | pTV2sE2t (1), gDE2t (1) | 8 | 55 ± 11 | 601 ± 173 | |
Female BALB/c mice were injected with 100 μg of DNA (i.m.) or 5 μg of protein (s.c.) and then boosted one time (group VIII) or two times (groups I to VII) with an identical dose of DNA or protein at 4-week intervals.
Data shown represent geometric mean titers ± standard errors for each group of animals.
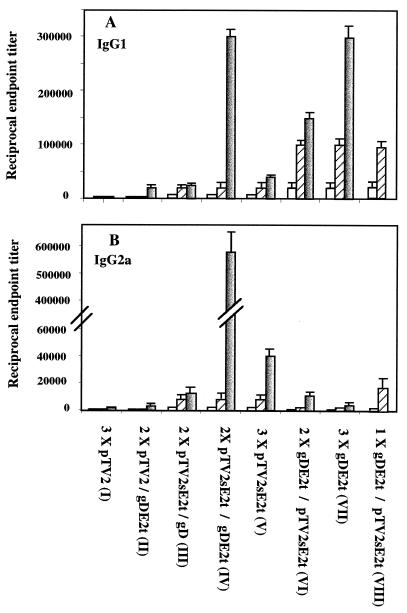
It is likely that DNA immunization predominantly induces IgG2a antibodies, which is generally accepted as an indicator of Th1 immunity (26). The presence of E2-specific IgG isotypes were assayed by ELISA using horseradish peroxidase-conjugated sheep anti-mouse IgG1 or IgG2a secondary antibodies (Southern Biotechnology Associates). As expected, the dominant IgG subclasses induced by gDE2t protein and E2 DNA immunizations were IgG1 and IgG2a, respectively (Fig. 1). Interestingly, a booster immunization with gDE2t protein in E2 DNA-primed mice dramatically increased E2-specific IgG1 and IgG2a isotype titers (Fig. 1). Although this enhanced IgG1 titer was comparable to that raised in mice that received three rounds of gDE2t protein immunizations alone, the level of IgG2a titer was approximately 15 and 150 times higher than that of mice given three rounds of E2 DNA and gDE2t protein immunizations, respectively. However, a reverse-ordered immunization regimen which performed booster immunizations with E2 DNA after priming twice with gDE2t protein slightly enhanced both IgG1 and IgG2a titers, which were approximately 2 and 40 times lower than those induced by the DNA priming-protein boosting regimen, respectively (Fig. 1). Our data strongly suggest that the predetermined dominance of the IgG2a isotype was not significantly changed by booster immunization with antigens which induce the IgG1 isotype or vice versa. Our data, together with a previous report that β-galactosidase (β-Gal) protein booster immunization in β-Gal DNA-primed mice significantly enhanced the IgG2a titer and caused secretions of smaller amounts of IL-4 and IL-5 than those from mice given protein immunization only (26), suggest that the Th1-dominated immune responses raised after DNA priming was preserved by protein booster immunization. This DNA priming-protein boosting regimen has great potential for inducing Th1-like immunity, which is likely to be essential for the clearance of intracellular pathogens. It has been previously reported that antibodies directed to hypervariable region 1 (HVR1) of the E2 protein have neutralizing activity (31) and that a booster immunization with HIV gp120 protein in gp120 DNA-primed mice strongly enhanced the nAb titers (29). To determine whether antibodies specific to HVR1 peptide are enhanced by a gDE2t protein booster immunization in E2 DNA-primed mice, we analyzed HVR1-specific antibodies following this regimen. Although we previously reported that immunization with E2 DNA induces both homologous and heterologous HVR1-specific antibodies in Buffalo rats (20, 21), mice did not generate HVR1-specific antibodies in our present experiments (data not shown). These conflicting results may stem from differences in the genetic backgrounds of mice and rats.
FIG. 1.
Determination of anti-E2 isotype antibodies in the immunized mice. IgG1 (A) and IgG2a (B) isotypes of anti-E2 antibodies in sera from immunized mice were determined by ELISA using purified hghE2t protein and isotype-specific secondary antibodies. The mean titers of anti-E2 antibodies of eight mice per group (plus standard error of the mean) obtained at week 3 after the first (□), second (▨), and final (■) injections are shown. The different vaccination regimens are at the bottom; the number of injections are indicated.
Enhancement of CTL responses by a protein booster immunization.
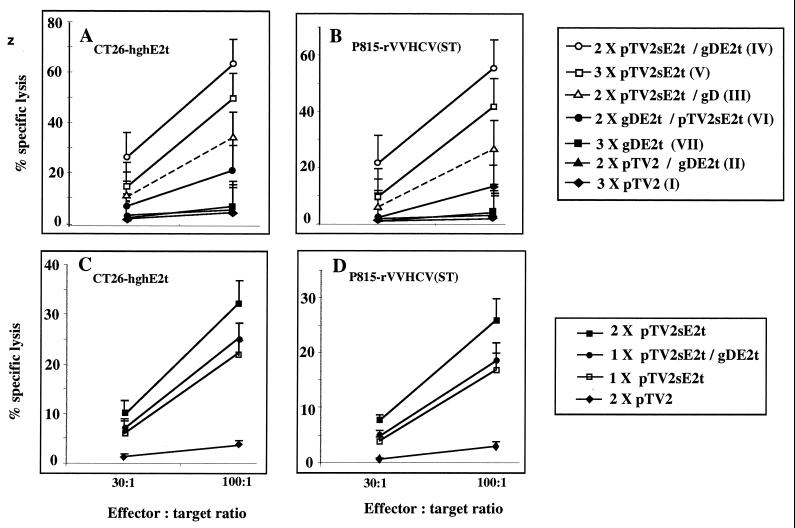
To investigate the effects of protein boosting on the level of CD8+ T-cell responses generated by DNA priming, we compared the level of CTL responses induced by various vaccination regimens. Briefly, eight mice from each group were tested by a conventional 51Cr-release assay (34) for the ability to generate CTLs at 3 weeks after the final immunization. CT26-hghE2t cell lines expressing HCV E2t which were fused to hgh (19) were generated under continuous selective pressure with 350 μg of G418 (GIBCO BRL) per ml and were used to raise CTLs by in vitro stimulation of spleen cells from the immunized mice for 7 days in the presence of 10 U of murine IL-2 (Pharmingen) per ml. As target cells, we used CT26, CT26-hghE2t, and P815, which was infected with recombinant vaccinia virus expressing β-Gal or HCV structural proteins (aa residues 1 to 740). As expected, mice immunized with E2 DNA, but not with recombinant gDE2t protein, elicited E2-specific CTL responses (Fig. 2). In order to determine whether different booster immunization regimens affect the generation of CTL responses in mice primed by DNA or protein injection, we analyzed CTL activities in mice primed twice with E2 DNA or gDE2t protein followed by a booster immunization with the same DNA or protein. A booster immunization with gDE2t protein, but not with control gD protein, in mice primed twice with E2 DNA significantly enhanced CTL activity (Fig. 2A and B, P value of <0.05 by Fisher's exact test between group III and IV). This activity was somewhat higher than that induced by three rounds of E2 DNA immunizations alone. In contrast, no group of mice showed specific CTL activities against two control targets, CT26 and P815, which were infected with recombinant vaccinia virus expressing β-Gal (data not shown). Interestingly, a booster immunization with gDE2t protein after priming once with E2 DNA did not significantly enhance CTL responses, which were much lower than those induced by two rounds of E2 DNA immunization alone (Fig. 2C and D). Together, these observations demonstrated that E2-specific CTL responses were significantly enhanced by gDE2t protein booster immunization in two rounds of E2 DNA-primed mice, suggesting that sufficient priming with E2 DNA immunization is necessary to enhance CTL activity by protein boosting, as was seen in antibody responses. An E2 DNA booster injection appeared to generate CTL activity in mice given by two rounds of gDE2t protein administration, which was comparable to what was induced by a single E2 DNA immunization. Therefore, it is likely that a DNA priming-protein boosting regimen would optimize the elicitation of high levels of HCV E2-specific CTL responses. In order to determine which population of effector CTLs was increased by a booster immunization with gDE2t protein after E2 DNA priming, we performed a CTL assay with either enriched CD4+ or CD8+ T cells sorted by the MACS system (Milteny Biotec), which yielded enriched T-cell populations of approximately 95%, as confirmed by fluorescence-activated cell sorter analysis (CellQuest software; Becton Dickinson). Enriched T-cell populations were stimulated by the addition of CT26-hghE2t cells and IL-2 (50 U/ml) in the presence of mitomycin C (Sigma)-treated naive splenocytes. These experiments indicated that only CD8+ effector cells, but not CD4+ cells, were demonstrated to generate E2-specific cytotoxicity (data not shown), suggesting that gDE2t protein booster immunization further increased the CD8+ CTL population primed by E2 DNA injection.
FIG. 2.
E2-specific CTL responses in the immunized BALB/c mice. Spleen cells obtained at week 3 after third (A and B) or second (C and D) immunizations were maintained in RPMI 1640 medium supplemented with 10 mM HEPES buffer, 5 × 10−5 M 2-mercaptoethanol, and 10% fetal bovine serum (GIBCO BRL). Responder cells (2.0 × 107) were restimulated in vitro with mitomycin C-treated (25 μg/ml) CT26-hghE2t cells (1.0 × 106) at 37°C. After a 1-week in vitro culture, effector cells were tested in a conventional cytotoxicity assay against two different target cells, such as CT26-hghE2t (A and C) or P815 infected with recombinant vaccinia virus expressing HCV core and E1 and E2 proteins (B and D). Data are represented as percentage of specific lysis (plus standard error of the mean) versus effector-to-target ratios, where n = 8.
Although the mechanism by which a protein booster immunization further enhanced CD8+ CTL responses in DNA-primed mice is unclear, there are several possible explanations. First, since DNA vaccination appeared to predominantly induce both Th1 and CD8+ CTL immunity, protein boosting in DNA-primed mice may further stimulate preformed memory Th1 cells which produce IL-2 and gamma interferon in secondary lymphoid organs. Next, these cytokines would make memory CD8+ CTL to further proliferate. Second, since the cross-priming ability of dendritic cells could be increased by Th1 cytokines and/or CD40L on activated Th cells (4, 6, 13, 17, 18), it is possible that these dendritic cells may cross-present the exogenous gDE2t protein to memory CD8+ T cells. Alternatively, the antigen-IgG immune complexes were demonstrated to enhance the ability of dendritic cells' cross-presentation via FcγR-mediated endocytosis (27). Since mouse IgG2a binds more strongly to FcγR than IgG1 (37), it is likely that the complexes of gDE2t with IgG2a induced by E2 DNA priming may be cross-presented.
Tumor protection studies.
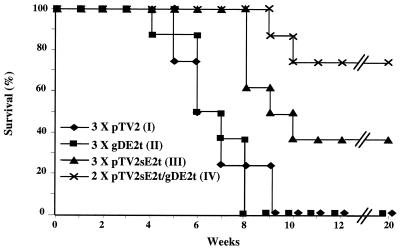
To confirm the antibody and CTL immunity induced by various vaccination regimens in vivo as previously reported (35), 2 × 106 CT26-hghE2t tumor cells were s.c. injected into the groups of the immunized mice at 4 weeks after the last immunization. As shown in Fig. 3, the groups of mice immunized three times with either control DNA or with gDE2t protein displayed tumor formation and began succumbing at approximately 4 to 5 weeks, and all displayed tumor formation and had succumbed by 9 weeks postinjection. However, the group of mice that received three rounds of E2 DNA injection were delayed in tumor formation and lethargy, resulting in a final survival rate of 44%, which is not statistically significant when compared to those of groups I and II (P = 0.082). The group of mice that were booster immunized with gDE2t protein, following E2 DNA priming two times, showed the highest survival rate: approximately 78% at week 20, which was significantly different from groups I and II (P < 0.03). Thus, these data suggest that HCV E2-specific antibody and CTL responses induced by a DNA priming and protein boosting regimen confers in vivo protection against modified tumor challenge. Although it is not easy to evaluate relative effects of antibody and CTL responses on tumor protection in vivo, it is likely that both contribute to the elimination of tumor cells. E2-specific CD8+ CTLs could directly kill the CT26-hghE2t cells, as shown in the in vitro CTL assay, and antibodies, especially IgG2a, which strongly binds to FcγR on macrophages and natural killer cells, could mediate antibody-dependent cell-mediated cytotoxicity (1, 14, 25).
FIG. 3.
Protection of immunized mice from modified CT26 tumor cells expressing hghE2t. BALB/c (nine per group) mice immunized with control vector, pTV2sE2t, and/or gDE2t protein were injected s.c. with 2.0 × 106 CT26-hghE2t tumor cells. The vitality of individual mice was monitored for 20 weeks after tumor cell injection.
In conclusion, we have shown that consecutive immunizations involving priming twice with HCV E2 DNA and boosting with a recombinant gDE2t protein elicits enhanced antibody and CTL responses which protected mice from a lethal tumor challenge. Our DNA priming and protein boosting vaccine strategy thus offers promise for vaccine development against many pathogens.
Acknowledgments
This work was supported by grants from the Ministry of Health and Welfare of Korea (grant 97-B-1-0004) and the Korean Green Cross Corp.
We express great thanks to Sang Chun Lee for elaborate animal care and support of animal experiments.
REFERENCES
- 1.Adams D O, Hall T, Steplewski Z, Koprowski H. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive form of antibody-dependent cytolysis. Proc Natl Acad Sci USA. 1984;81:3506–3510. doi: 10.1073/pnas.81.11.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsopp C E, Plebanski M, Gilbert S, Sinden R E, Harris S, Frankel G, Dougan G, Hioe C, Nixon D, Paoletti E, Layton G, Hill A V. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur J Immunol. 1996;26:1951–1959. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- 3.Barnett S W, Rajasekar S, Legg H, Doe B, Fuller D H, Haynes J R, Walker C M, Steimer K S. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15:869–873. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 4.Bevan M J. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987;325:192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- 5.Boyer J D, Wang B, Ugen K E, Agadjanyan M, Javadian A, Frost P, Dang K, Carrano R A, Ciccarelli R, Coney L, Williams W V, Weiner D B. In vivo protective anti-HIV immune responses in non-human primates through DNA immunization. J Med Primatol. 1996;25:25242–25250. doi: 10.1111/j.1600-0684.1996.tb00022.x. [DOI] [PubMed] [Google Scholar]
- 6.Brossart P, Bevan M J. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–1599. [PMC free article] [PubMed] [Google Scholar]
- 7.Choo Q-L, Kuo G, Weiner A, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 8.Choo Q-L, Kuo G, Ralston R, Weiner A, Chien D, Van Nest G, Han J, Berger K, Thudium K, Kao C, Kansopon J, McFarland J, Tabuzi A, Ching K, Moss B, Cummins L B, Houghton M, Muchmore E. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci USA. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper S, Erickson A L, Adams E J, Kansopon J, Weiner A J, Chien D Y, Houghton M, Parham P, Walker C M. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly J J, Friedman A, Martinez D, Montgomery D L, Shiver J W, Motzel S L, Ulmer J B, Liu M A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1993;6:583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari C, Penna A, Bertoletti A, Cavalli A, Missale G, Lamonaca V, Boni C, Valli A, Bertoni R, Urbani S, Scognamiglio P, Fiaccadori F. Antiviral cell-mediated immune responses during hepatitis B and hepatitis C virus infections. Recent Results Cancer Res. 1998;154:330–336. doi: 10.1007/978-3-642-46870-4_24. [DOI] [PubMed] [Google Scholar]
- 12.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 14.Kipps T J, Parham P, Punt J, Herzenberg L A. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985;161:1–17. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 16.Kuo G, Choo Q L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 17.Kurts C, Kosaka H, Carbone F R, Miller J F, Heath W R. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labeur M S, Roters B, Pers B, Mehling A, Luger T A, Schwarz T, Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 19.Lee K J, Suh Y A, Cho Y G, Cho Y S, Ha G W, Chung K H, Hwang J H, Yun Y D, Lee D S, Kim C M, Sung Y C. Hepatitis C virus E2 protein purified from mammalian cells is frequently recognized by E2-specific antibodies in patient sera. J Biol Chem. 1997;272:30040–30046. doi: 10.1074/jbc.272.48.30040. [DOI] [PubMed] [Google Scholar]
- 20.Lee S W, Cho J H, Sung Y C. Optimal induction of hepatitis C virus envelope-specific immunity by bicistronic plasmid DNA inoculation with the granulocyte-macrophage colony-stimulating factor gene. J Virol. 1998;72:8430–8436. doi: 10.1128/jvi.72.10.8430-8436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S W, Cho J H, Lee K J, Sung Y C. Hepatitis C virus envelope DNA-based immunization elicits humoral and cellular immune responses. Mol Cell. 1998;8:444–451. [PubMed] [Google Scholar]
- 22.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, Liu M A, Shiver J W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson D R, Marousis C G, Davis G L, Rice C M, Wong J, Houghton M, Lau J Y. The role of hepatitis C virus-specific cytotoxic T lymphocytes in chronic hepatitis C. J Immunol. 1997;1583:1473–1481. [PubMed] [Google Scholar]
- 25.Parham P, Kipps T J, Ward F E, Herzenberg L A. Isolation of heavy chain class switch variants of a monoclonal anti-DC1 hybridoma cell line: effective conversion of noncytotoxic IgG1 antibodies to cytotoxic IgG2 antibodies. Hum Immunol. 1983;8:141–151. doi: 10.1016/0198-8859(83)90009-5. [DOI] [PubMed] [Google Scholar]
- 26.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rehermann B, Chang K M, McHutchinson J, Kokka R, Houghton M, Rice C M, Chisari F V. Differential cytotoxic T-lymphocyte responsiveness to the hepatitis B and C viruses in chronically infected patients. J Virol. 1996;70:7092–7102. doi: 10.1128/jvi.70.10.7092-7102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richmond J F, Lu S, Santoro J C, Weng J, Hu S L, Montefiori D C, Robinson H L. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol. 1998;72:9092–9100. doi: 10.1128/jvi.72.11.9092-9100.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarselli E, Cerino A, Esposito G, Silini E, Mondelli M, Traboni C. Occurrence of antibodies reactive with more than one variant of the putative envelope glycoprotein (gp70) hypervariable region 1 in viremic hepatitis C virus-infected patients. J Virol. 1995;69:4407–4412. doi: 10.1128/jvi.69.7.4407-4412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:4397–4402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 33.Tedeschi V, Akatsuka T, Shih J W-K, Batlegay M, Feinstone S M. A specific antibody response to HCV E2 elicited in mice by intramuscular inoculation of plasmid DNA containing coding sequences for E2. Hepatology. 1997;25:459–462. doi: 10.1002/hep.510250234. [DOI] [PubMed] [Google Scholar]
- 34.Toes R E, Offringa R, Blom R J, Brandt R M, van der Eb A J, Melief C J, Kast W M. An adenovirus type 5 early region 1B-encoded CTL epitope-mediating tumor eradication by CTL clones is down-modulated by an activated ras oncogene. J Immunol. 1995;154:3396–3405. [PubMed] [Google Scholar]
- 35.Tokushige K, Wakita T, Pachuk C, Moradpour D, Weiner D B, Zurawski V R, Jr, Wands J R. Expression and immune response to hepatitis C virus core DNA-based vaccine constructs. Hepatology. 1996;24:14–20. doi: 10.1002/hep.510240104. [DOI] [PubMed] [Google Scholar]
- 36.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 37.Unkeless J C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]