Abstract
Background
Metabolic syndrome (MetS) could increase the risk of cardiovascular disease (CVD) by 2-fold. Ideal control of modifiable risk factors in Life's Simple 7 (LS7) could reduce the CVD risk among the general population. This study aimed to investigate the effects of controlling modifiable risk factors using LS7 in MetS to prevent CVD.
Methods
44463 participants in NHANES 1999–2018 were included. The primary endpoint was a composite of CVD, including angina pectoris, coronary artery disease, myocardial infarction, congestive heart failure, and stroke. Multivariable weighted logistic regression analyses estimated the associations. The diagnosis of MetS complied with Harmonized International Diabetes Federation Criteria. Measurement of modifiable risk factors used the 2010 American Heart Association LS7 guideline and was indicated by cardiovascular health (CVH).
Results
14034 individuals were diagnosed with MetS. 4835 participants had CVD. The weighted mean CVH was 8.06 ± 0.03. Intermediate and poor CVH were associated with increased risk for CVD in participants with similar metabolic states compared to ideal CVH. By taking participants with metabolic health and ideal CVH as health control, participants with MetS and poor CVH were demonstrated to have a 3-fold (adjusted odds ratio, 4.00; 95 % confidence interval, 3.21–4.98) greater risk for CVD. Notably, under the condition of ideal CVH, the risk of having CVD was comparable between metabolic health and MetS after fully adjusted.
Conclusion
Ideal control of Life's Simple 7 in metabolic syndrome contributes to a comparable risk of cardiovascular disease with healthy subjects. LS7 could be recognized as a guideline for secondary prevention in MetS.
Keywords: Metabolic syndrome, Cardiovascular disease, Cardiovascular health, Life's simple 7
1. Introduction
Cardiovascular disease (CVD) remains a major threat to global health. According to Heart Disease and Stroke Statistics Update, CVD has affected 607.64 million individuals globally and accounted for 19.05 million deaths in 2020 [1]. The growing epidemic of CVD also presents substantial challenges to public healthcare, with which the estimated annual cost of CVD reached $407.3 billion in the United States (U.S.) [1].
Metabolic syndrome (MetS) is a clustering of cardiometabolic risk factors manifested as insulin resistance, dyslipidemia, hypertension, and abdominal obesity [2]. Growing evidence supports that MetS could increase the risk of CVD by 2-fold and further contribute to mortality from CVD causes [3]. Currently, MetS is becoming hyperendemic, with nearly a quarter of the world population affected [4]. Thus, early prevention in MetS might be beneficial in reducing the overall CVD burden.
Cardiovascular health (CVH), defined by the American Heart Association Life's Simple 7 (LS7), has been shown to reduce the risk of incident CVD among populations with diverse clinical manifestations [5,6]. There are seven modifiable risk factors in LS7 which include health behaviors (smoking, diet, physical activity) and biomarkers (body mass index (BMI), blood pressure, total cholesterol, fasting glucose). Previous studies demonstrated that ideal control of physical activity could lead to a remarkable risk reduction of CVD in MetS [7,8]. It remains a need, however, to investigate the combined effects of modifiable risk factors in LS7 among people with MetS.
The present study aimed to examine the risk of CVD among U.S. adults classified by metabolic states and control of modifiable risk factors, thus providing evidence-based advice in utilizing LS7 as a management tool in preventing CVD in MetS.
2. Methods
2.1. Data source and Study population
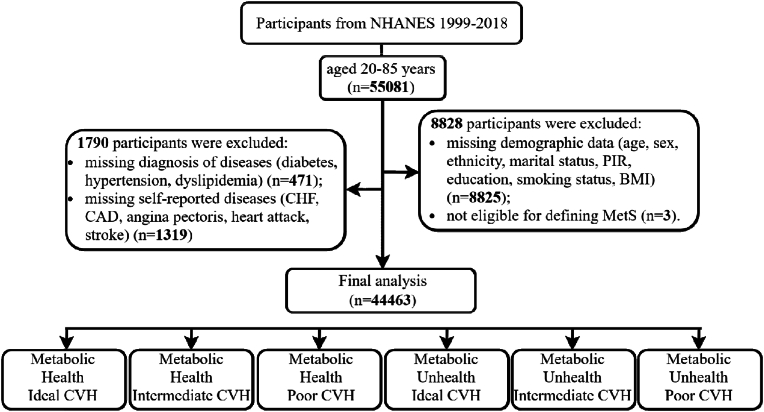
The National Health And Nutrition Examination Survey (NHANES) is an ongoing, multiple cycles, cross-sectional study in the U.S [9]. Participants in NHANES were sampled through a stratified, multistage probability design to be representative of the noninstitutionalized U.S. civilian population [10]. The current study primarily enrolled participants aged 20 to 85 from NHANES 1999–2018 with data on demographics, biometric measures, and health questionnaires. A total of 10618 participants with missing baseline and follow-up information were excluded. Overall, 44463 participants were included in the final analysis. The screening process is shown in Fig. 1.
Fig. 1.
Flowchart of study participants. BMI, body mass index, CHF, congestive heart failure, CAD, coronary artery disease, CVH. Cardiovascular health, MetS, metabolic syndrome, NHANES, national health and nutritional examination surveys, PIR, poverty income ratio.
2.2. Endpoints and measurements
The primary endpoint was a composite of CVD, including angina pectoris, coronary artery disease (CAD), myocardial infarction (MI), congestive heart failure (CHF), and stroke. The individual components of the primary endpoint were analyzed separately as secondary endpoints. Identification of these conditions was based on self-reported medical history via home interviews. Participants who answered “yes” to question MCQ160B–F, “Have you ever been told by a doctor or health professional that you had CAD/angina (angina pectoris)/heart attack (MI)/stroke/CHF?” were perceived as having a history of CVD.
Diagnosis of MetS conformed to the Harmonized International Diabetes Federation Criteria [2], in which participants have≥3 the following components were considered as having MetS: (1) triglyceride level≥150 mg/dl; (2) high-density lipoprotein-cholesterol<40 mg/dl in male and<50 mg/dl in female; (3) systolic blood pressure (SBP)≥130 mmHg or diastolic blood pressure (DBP)≥85 mmHg or treated; (4) fasting blood glucose (FBG)≥100 mg/dl or treated; (5) waist circumference≥102 cm in male and≥88 cm in female. Conditions of MetS in participants were categorized as metabolic unhealth or otherwise metabolic health.
Control of modifiable risk factors was indicated by CVH. We used the 2010 American Heart Association LS7 guideline to evaluate CVH [11]. LS7 components were assigned a score of 2 if total cholesterol (TC) < 200 mg/dL and untreated, FBG<100 mg/dL and untreated, SBP<120 mmHg and DBP<80 mmHg, 18.5<BMI<25 kg/m2, never smoked or quit≥1 year, physical activity>150 min/week of moderate and vigorous intensity, or healthy diet components≥4. LS7 components were assigned a score of 1 if 200≤TC < 239 mg/dL or treated, 100≤FBG<125 mg/dL or treated, 120≤SBP<139 mmHg or 80≤SBP<89 mmHg or treated, 25≤BMI<30 kg/m2, quit smoking<1 year, 1≤physical activity≤149 min/week of moderate and vigorous intensity, or 3≤healthy diet components<4. LS7 components were assigned a score of 0 if TC ≥ 240 mg/dL, FBG≥126 mg/dL, SBP≥140 mmHg or DBP≥90 mmHg, BMI≥30 kg/m2, current smoking, none of the physical activity, or healthy diet components≤1. The healthy diet components were derived from Dietary Approaches to Stop Hypertension eating pattern [12]. Overall scores≥10 were regarded as ideal CVH, <5 were poor CVH and otherwise were intermediate CVH.
2.3. Statistical analysis
Considering the stratified and multistage probability sampling in NHANES, we used the full sample mobile examination center exam weight and the Taylor series linearization method to estimate national estimates for all analyses and standard errors (SEs), respectively [[13], [14], [15], [16]].
The Kolmogorov-Smirnov test assessed the distribution of continuous variables. The baseline characteristics were summarized through descriptive statistics, reporting the weighted mean and SEs for normally distributed continuous variables, while non-normally distributed variables were presented as weighted medians and interquartile ranges. The categorical variables were reported as numbers and weighted proportions. The Rao-Scott χ2 tests compared the differences between groups in categorical variables. The two-sample Student's t-test investigated the difference between normally distributed variables and the Mann-Whitney U test was used for non-normally distributed variables. The comparisons across multiple groups for continuous variables were performed by the one-way analysis of variance (ANOVA).
Multivariable weighted logistic regression analyses were used to estimate odds ratios (ORs) and 95 % confidence intervals (CIs) for the associations between metabolic CVH groups and endpoints. Covariates included in the multivariable model were based on clinical importance and previously published studies: age (continuous), sex (male or female), ethnicity (White or Non-White), marital status (living with a spouse/partner or living without a spouse/partner), poverty income ratios (PIR) (low1.3, 1.3<middle<3.5, hig≥3.5), and educational level (<9th grade, 9–11th grade, high school graduate, some college or AA degree, ≥college graduate). Model 1 was unadjusted. Model 2 was adjusted with robust adjustment for the above covariates to control potential confounding effects.
We used R (Version 4.2.2) for all statistical analyses. The complexity of the sampling design was considered in each analysis by specifying primary sampling units, strata, and sample weights using the R package ‘survey’ (Version 4.1–1). Two‐sided P values < 0.05 were considered statistically significant.
3. Results
3.1. Participants characteristics
A total of 44463 participants (weighted mean age 46 years, 50.46 % female) were included. There were 14304 individuals (weighted proportion 29.80 %) diagnosed with MetS. Among the metabolic CVH groups, individuals without MetS but ideal CVH were presented as younger, female, highly educated, and living with a spouse/partner. In addition, the PIR<1.3, current smoking, BMI≥25 kg/m2, and prevalence of co-morbidities of hypertension, dyslipidemia, diabetes, and CVD were less common in the metabolic health participants with ideal CVH (Table 1).
Table 1.
Characteristics of participants by metabolic state and cardiovascular health.
| Total (n = 44463) | Metabolic Health |
Metabolic Unhealth |
|||||
|---|---|---|---|---|---|---|---|
| Ideal CVH (n = 9898) | Intermediate CVH (n = 18123) | Poor CVH (n = 2138) | Ideal CVH (n = 1124) | Intermediate CVH (n = 11092) | Poor CVH (n = 2088) | ||
| Age, yrs | 46.90 ± 0.19 | 38.53 ± 0.26 | 45.26 ± 0.20 | 51.99 ± 0.43 | 51.83 ± 0.63 | 56.01 ± 0.22 | 57.93 ± 0.34 |
| Female, n (%) | 22437(51.26) | 5542(56.53) | 8689(49.10) | 1117(53.08) | 567(49.56) | 5449(48.70) | 1073(52.65) |
| White ethnicity, n (%) | 20080(69.27) | 4581(70.19) | 7885(66.79) | 837(62.29) | 577(75.32) | 5376(73.48) | 824(65.20) |
| Living alone, n (%) | 17716(36.11) | 3877(36.09) | 7259(37.12) | 1003(44.53) | 375(29.01) | 4262(33.33) | 940(39.74) |
| Education, n (%) | |||||||
| ≥college graduate | 9794(22.03) | 3547(42.49) | 3272(22.90) | 215(12.17) | 387(40.29) | 2179(25.18) | 194(11.13) |
| some college or AA degree | 12803(28.79) | 3045(30.70) | 5222(31.59) | 522(27.89) | 321(31.14) | 3155(31.44) | 538(30.37) |
| high school graduate | 10313(23.19) | 1787(17.28) | 4505(26.62) | 549(29.75) | 206(17.61) | 2729(26.13) | 537(29.39) |
| 9–11th grade | 6491(14.6) | 930(6.43) | 2946(12.87) | 483(20.21) | 117(7.62) | 1564(10.83) | 451(18.71) |
| <9th grade | 5062(5.58) | 589(3.10) | 2178(6.03) | 369(9.98) | 93(3.34) | 1465(6.41) | 368(10.40) |
| PIR, n (%) | |||||||
| <1.3 | 13631(21.28) | 2535(17.78) | 5997(23.95) | 905(33.09) | 246(13.22) | 3150(18.37) | 798(29.88) |
| 1.3–3.5 | 16962(38.15) | 3465(31.89) | 7018(37.24) | 848(40.93) | 407(32.58) | 4364(36.87) | 860(39.70) |
| ≥3.5 | 13870(31.19) | 3898(50.33) | 5108(38.80) | 385(25.98) | 471(54.21) | 3578(44.76) | 430(30.42) |
| Smoking habits, n (%) | |||||||
| Current | 9465(21.65) | 790(7.92) | 5318(31.73) | 947(49.35) | 28(2.31) | 1578(14.70) | 804(41.57) |
| Former | 11021(24.79) | 1351(15.47) | 4384(24.25) | 615(27.13) | 186(17.59) | 3670(34.23) | 815(37.76) |
| Never | 23977(53.93) | 7757(76.61) | 8421(44.01) | 576(23.53) | 910(80.10) | 5844(51.07) | 469(20.67) |
| BMI, kg/m2, n (%) | |||||||
| ≥25 | 31176(68.76) | 3732(36.96) | 12799(71.79) | 1939(91.95) | 761(72.26) | 9897(90.68) | 2048(98.55) |
| 18.5–25 | 12578(28.29) | 5841(59.78) | 5009(26.60) | 180(7.40) | 354(27.07) | 1156(8.96) | 38(1.40) |
| <18.5 | 709(1.59) | 325(3.26) | 315(1.61) | 19(0.65) | 9(0.66) | 39(0.36) | 2(0.05) |
| Hypertension, n (%) | 18785(36.85) | 938(8.46) | 5988(28.97) | 1392(61.73) | 555(45.79) | 8085(69.91) | 1827(84.14) |
| Diabetes, n (%) | 7626(12.60) | 149(1.16) | 2186(8.63) | 801(33.87) | 81(4.97) | 3183(23.41) | 1226(53.90) |
| Dyslipidemia, n (%) | 30748(68.50) | 4380(44.07) | 13366(74.83) | 1376(65.39) | 651(59.48) | 9025(82.90) | 1950(94.67) |
| CVD, n (%) | 4835(8.44) | 279(2.34) | 1699(7.33) | 427(16.79) | 104(6.96) | 1783(13.63) | 543(24.10) |
| Angina pectoris, n (%) | 1263(2.40) | 71(0.61) | 420(1.96) | 109(4.60) | 29(2.00) | 489(4.12) | 145(7.16) |
| CAD, n (%) | 1811(3.34) | 94(0.81) | 630(2.82) | 152(6.00) | 45(3.25) | 706(5.81) | 184(8.89) |
| MI, n (%) | 1898(3.31) | 102(0.88) | 646(2.75) | 188(7.09) | 39(2.47) | 684(5.39) | 239(10.54) |
| CHF, n (%) | 1382(2.22) | 52(0.37) | 454(1.73) | 151(5.82) | 22(1.13) | 530(3.89) | 173(7.40) |
| Stroke, n (%) | 1680(2.76) | 93(0.75) | 590(2.42) | 167(6.51) | 35(2.27) | 595(4.12) | 200(8.68) |
| LS7 score | 8.06 ± 0.03 | 10.95 ± 0.02 | 7.48 ± 0.02 | 3.28 ± 0.03 | 10.39 ± 0.03 | 6.98 ± 0.02 | 3.47 ± 0.02 |
AA, academic assistant; ASCVD, atherosclerotic cardiovascular disease, BMI, body mass index; CVD, cardiovascular disease; CAD, coronary artery disease; CHF, congestive heart failure; CVH, cardiovascular health; LS7, life's simple 7; MI, myocardial Infarction; PIR, poverty income ratio. All p values were<0.001.
The CVD was found prevalent in 4835 participants (weighted proportion 8.44 %). In contrast to participants without CVD, those with CVD were more likely to be older, male, white ethnicity, less educated, living alone, current smokers, and BMI≥25 kg/m2. Moreover, the PIR<1.3, MetS, low CVH, and co-morbidities of hypertension, dyslipidemia, and diabetes were more frequent in participants with CVD (Table 2).
Table 2.
Characteristics of participants by the primary endpoint.
| CVD (n = 4835) | No-such Events (n = 39628) | |
|---|---|---|
| Age, yrs | 64.35 ± 0.29 | 45.29 ± 0.18 |
| Female, n (%) | 2093(46.30) | 20344(51.72) |
| White ethnicity, n (%) | 2731(76.23) | 17349(68.63) |
| Living alone, n (%) | 2108(39.19) | 15608(35.83) |
| Education, n (%) | ||
| ≥college graduate | 697(18.29) | 9097(29.19) |
| some college or AA degree | 1245(28.39) | 11558(31.37) |
| high school graduate | 1211(27.68) | 9102(23.66) |
| 9–11th grade | 875(15.56) | 5616(10.62) |
| <9th grade | 807(10.07) | 4255(5.16) |
| PIR, n (%) | ||
| <1.3 | 1778(28.14) | 11853(20.64) |
| 1.3–3.5 | 1981(41.14) | 14981(35.35) |
| ≥3.5 | 1076(30.72) | 12794(44.01) |
| Smoking habits, n (%) | ||
| Current | 972(21.76) | 8493(21.64) |
| Former | 1976(39.84) | 9045(23.28) |
| Never | 1887(38.40) | 22090(55.08) |
| BMI, kg/m2, n (%) | ||
| ≥25 | 3724(78.43) | 27452(67.87) |
| 18.5–25 | 1043(20.15) | 11535(30.48) |
| <18.5 | 68(1.42) | 641(1.65) |
| Hypertension, n (%) | 3741(73.94) | 15044(33.43) |
| Diabetes, n (%) | 1909(35.29) | 5717(10.51) |
| Dyslipidemia, n (%) | 4108(87.08) | 26640(66.79) |
| Angina pectoris, n (%) | 1263(28.39) | 0(0.00) |
| CAD, n (%) | 1811(39.59) | 0(0.00) |
| MI, n (%) | 1898(39.25) | 0(0.00) |
| CHF, n (%) | 1382(26.30) | 0(0.00) |
| Stroke, n (%) | 1680(32.64) | 0(0.00) |
| MetS | 2430(50.33) | 11874(27.91) |
| LS7 score | 6.53 ± 0.04 | 8.20 ± 0.03 |
| CVH | ||
| Ideal | 383(9.62) | 10639(31.04) |
| Intermediate | 3482(72.56) | 25733(62.50) |
| Poor | 970(17.82) | 3256(6.46) |
BMI, body mass index; CVD, cardiovascular disease; CAD, coronary artery disease; CHF, congestive heart failure; CVH, cardiovascular health; LS7, life's simple 7; MetS, metabolic syndrome; MI, myocardial Infarction; PIR, poverty income ratio.
3.2. CVH and primary and secondary endpoints in participants with similar metabolic states
In participants with MetS, intermediate and poor levels of CVH were found to be associated with an increased risk of CVD compared with the ideal CVH. After the adjustment for covariates, the intermediate and poor CVH increased the CVD risk by 65 % (OR, 1.65; 95 % CI, 1.28–2.13) and 162 % (OR, 3.10; 95 % CI, 2.32–4.14), respectively. Similar trends were observed for metabolic health participants (Table 3).
Table 3.
Risk of cardiovascular disease according to cardiovascular health in participants with similar metabolic states.
| CVH | Metabolic Health |
Metabolic Unhealth |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95 % CI | Adjusted OR | 95 % CI | OR | 95 % CI | Adjusted OR | 95 % CI | |
| Ideal | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref |
| Intermediate | 3.31 | 2.71–4.03 | 1.81 | 1.48–2.22 | 2.11 | 1.67–2.67 | 1.65 | 1.28–2.13 |
| Poor | 8.43 | 6.76–10.53 | 3.13 | 2.46–3.99 | 4.25 | 3.23–5.58 | 3.10 | 2.32–4.14 |
CI, confidence interval; CVH, cardiovascular health; OR, odds ratio. Adjusted OR for age (continuous), sex, ethnicity, marital status, poverty income ratio, and educational level. All p values < 0.001.
Significant and inverse associations between CVH and the components of CVD were shown regardless of the metabolic states. By taking the ideal CVH as a reference, it was identified that intermediate and poor CVH metrics were associated with elevated risk for angina pectoris, CAD, MI, CHF, and stroke (Table S1-S5).
3.3. Metabolic CVH and primary and secondary endpoints
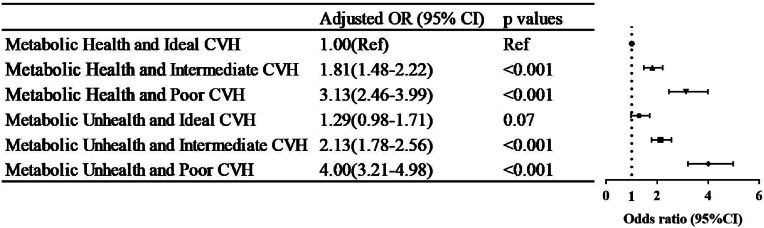
Correlations between the metabolic CVH and endpoints were summarized in Fig. 2. By taking the metabolic health participants with ideal CVH as the health control, participants with decreasing levels of CVH and/or MetS had elevated risk for CVD. Particularly, the cumulative effects of metabolic unhealth and poor CVH were demonstrated to have a 3-fold (adjusted OR, 4.00; 95 % CI, 3.21–4.98) greater risk for CVD than the health control. Of note, under the condition of ideal CVH, the risk of having CVD was comparable between metabolic health and MetS after fully adjusting for covariates.
Fig. 2.
Risk of cardiovascular disease by metabolic state and cardiovascular health. CVH. Cardiovascular health, CI, confidence interval; OR, odds ratio.
Similarly, elevated risk for angina pectoris, CAD, MI, CHF, and stroke in participants with unfavorable CVH and/or MetS was observed compared to the health control (all p < 0.05). After adjustment for the covariates, the risk of having angina pectoris, CAD, MI, CHF, and stroke was non-significant between metabolic health and MetS (all p > 0.05) (Tables S6–S10).
4. Discussion
This is the first study investigating the effects of LS7 in MetS. It was indicated that ideal control of LS7 was associated with decreased risk of CVD in participants with and without MetS. Moreover, the associations between MetS and CVD were attenuated under ideal CVH. Taken together, these observations suggested that the combined control of modifiable risk factors in LS7 contributed to protection against CVD, and LS7 could be recognized as a guideline for secondary prevention in MetS.
Modifiable risk factors, such as disturbed lipid and glucose metabolism and sedentary behaviors, are prominent cardiovascular risk factors. It has been shown that modifiable cardiovascular risk factors accounted for over 90 % risk for developing CVD [17]. Thus, modifications in metabolic disorders and lifestyle are the cornerstone of CVD management. Reports from clinical trials and epidemiological studies prove the positive impacts of controlling modifiable risk factors in preventing CVD among the general population and MetS [[18], [19], [20]]. Over the past decade, advances were further made in investigating the combined effects of modifiable risk factors. For instance, behavior counseling interventions of physical activity and a healthy diet were found to significantly benefit CVD risk reduction [21]. In this respect, the LS7 could act as a guideline for a comprehensive reflection of the modifiable risk factors and give an overall evaluation of control through CVH metrics. In support of this, evidence is accumulating on the role of ideal CVH in reducing incident CVD [22]. Additionally, results from case-control studies have discovered that the prevalence of MetS and unfavorable levels of CVH were significantly higher in patients with CVD compared to those free of CVD [23,24].
Consistent with the prior findings, our study confirmed that CVH was inversely correlated with the risk of CVD in participants with and without MetS. Moreover, regarding metabolic health and ideal CVH as the health control, participants with different metabolic states and aggravating levels of CVH were associated with increased CVD risk, ranging from 81 % to 300 % after adjustment. Intriguingly, the ideal CVH offsets the CVD risk in participants with MetS. Taken together, our study extended the preexisting evidence that ideal control of the modifiable risk factors in LS7 could attenuate the risk of CVD in MetS. The LS7 might be a promising preventive strategy in MetS for CVD. Future studies are needed to validate our findings.
In a meta-analysis involving 87 studies and 951083 participants, MetS was associated with a 1.99-fold and 2.27-fold increased risk for MI and stroke, respectively [3]. Besides, physical activity, a leading modifiable risk factor, was discovered to correlate with the risk of CAD, CHF, and stroke in a dose-response way [25]. In concert with these, the present study observed similar trends between metabolic CVH groups and the risk of angina pectoris, CAD, MI, CHF, and stroke. However, the mechanisms behind the associations remain elusive. It was proposed that the increased CVD risk in MetS was attributable to co-morbidities of cardiovascular risk factors and was further compounded by poor CVH that comprises smoking, physical inactivity, and an unhealthy diet [26]. Modifying the modifiable risk factors indicated by ideal CVH could lead to metabolic profile improvements, vascular conditioning, reverse cardiac remodeling, and cardiomyocyte molecular adaptations, consequently improving cardiovascular outcomes [26]. Therefore, we concluded that the interactions between the metabolic CVH and the risk of CVD were biologically plausible and might be mediated through multiple pathophysiological mechanisms.
Although our study highlighted the combined effects of modifiable risk factors in LS7 for the risk of CVD among people with and without MetS after controlling for potential covariates, public health factors, such as socioeconomic status and educational level, play indispensable roles in the development of CVD in MetS [27,28]. Thus, it calls for more attention and effort in addressing the barriers to social determinants of health. Furthermore, LS7 could imply the accessibility to medications and healthy foods by the CVH score. In this case, the utilization of LS7 in the management of MetS might have implications in healthcare as well.
There are some limitations in our study. First, the associations in our study were not causal, given the cross-sectional design. Prospective research and randomized clinical trials are needed to validate our findings and the practicality of the LS7 in MetS. Second, restricted by the incomplete data on sleep and anxiety in NHANES, life's essential 8 [29] and circadian syndrome [30], the updated concepts of LS7 and MetS, could not be considered for investigation. Based on this, we hope our study established a base for future studies and provided preliminary evidence for the combined effects of modifiable risk factors in MetS. Third, there might be recall bias in the self-reported interviews. Fourth, although we have adjusted for known potential covariates, the possibility of unmeasured confounding remains. Finally, interpreting our results needs to be cautious as the present study focused on the US population.
5. Conclusion
In this nationwide population-based study, we discovered that ideal control of LS7, combining modifiable risk factors of lifestyle and biomarkers, could attenuate the risk of CVD in MetS. Our study recognized the significance and clinical implications of LS7 in the management of MetS. Further research is needed to validate our findings and provide complementary evidence.
Declarations
Ethic statements
The NHANES has been approved by the research ethics review board of the US Centers for Disease Control and Prevention National Center for Health Statistics. All participants in NHANES provided written informed consent to participate. The Ethics Committee of Fuwai Hospital determined that this study was exempt from the review, given the use of deidentified data.
Availability of data
All data are publicly available and can be accessed at the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx). Our code is available upon reasonable request to the corresponding author.
Funding
This work was supported by the National Key R&D Program of China (Grant number: 2021ZD0111003), Capital's Funds for Health Improvement and Research from the Beijing Municipal Health Commission (Grant number: SF 2022-2-4035), and the Capital Health Development Project of China grant (Grant number: SHF-2016-2-4032).
CRediT authorship contribution statement
Ruihuan Shen: Writing – review & editing, Software, Investigation, Formal analysis. Xuantong Guo: Writing – original draft, Visualization, Validation, Formal analysis. Tong Zou: Writing – review & editing, Supervision, Project administration, Conceptualization. Lihong Ma: Writing – review & editing, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors thank the NHANES and participants.
Handling editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2024.200283.
Contributor Information
Tong Zou, Email: zoutong200l@163.com.
Lihong Ma, Email: malihongfuwai@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., Baker-Smith C.M., Beaton A.Z., Boehme A.K., Buxton A.E., Commodore-Mensah Y., Elkind M.S.V., Evenson K.R., Eze-Nliam C., Fugar S., Generoso G., Heard D.G., Hiremath S., Ho J.E., Kalani R., Kazi D.S., Ko D., Levine D.A., Liu J., Ma J., Magnani J.W., Michos E.D., Mussolino M.E., Navaneethan S.D., Parikh N.I., Poudel R., Rezk-Hanna M., Roth G.A., Shah N.S., St-Onge M.P., Thacker E.L., Virani S.S., Voeks J.H., Wang N.Y., Wong N.D., Wong S.S., Yaffe K., Martin S.S., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2023 update: a Report from the American heart association. Circulation. 2023 Feb 21;147(8):e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 2.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International diabetes Federation Task Force on Epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009 Oct 20;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 3.Mottillo S., Filion K.B., Genest J., Joseph L., Pilote L., Poirier P., Rinfret S., Schiffrin E.L., Eisenberg M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010 Sep 28;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 4.Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko H., Suzuki Y., Ueno K., Okada A., Fujiu K., Matsuoka S., Michihata N., Jo T., Takeda N., Morita H., Kamiya K., Node K., Yasunaga H., Komuro I. Association of Life's Simple 7 with incident cardiovascular disease in 53 974 patients with cancer. Eur J Prev Cardiol. 2022 Dec 21;29(18):2324–2332. doi: 10.1093/eurjpc/zwac195. [DOI] [PubMed] [Google Scholar]
- 6.Hasbani N.R., Ligthart S., Brown M.R., Heath A.S., Bebo A., Ashley K.E., Boerwinkle E., Morrison A.C., Folsom A.R., Aguilar D., de Vries P.S. American heart association's life's simple 7: lifestyle recommendations, polygenic risk, and lifetime risk of coronary heart disease. Circulation. 2022 Mar 15;145(11):808–818. doi: 10.1161/CIRCULATIONAHA.121.053730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekblom-Bak E., Halldin M., Vikström M., Stenling A., Gigante B., de Faire U., Leander K., Hellénius M.L. Physical activity attenuates cardiovascular risk and mortality in men and women with and without the metabolic syndrome - a 20-year follow-up of a population-based cohort of 60-year-olds. Eur J Prev Cardiol. 2021 Oct 13;28(12):1376–1385. doi: 10.1177/2047487320916596. [DOI] [PubMed] [Google Scholar]
- 8.Kim S.Y., Go T.H., Lee J.H., Moon J.S., Kang D.R., Bae S.J., Kim S.E., Lee S.J., Cho D.H., Park Y.J., Youn Y.J., Kim J.Y., Ahn S.G. Differential association of metabolic syndrome and low-density lipoprotein cholesterol with incident cardiovascular disease according to sex among Koreans: a national population-based study. Eur J Prev Cardiol. 2022 Feb 9;28(18):2021–2029. doi: 10.1093/eurjpc/zwaa114. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics . March 2019. Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index - 2019 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations.https://www.cdc.gov/nchs/data/datalinkage/public-use-linked-mortality-file-description.pdf Hyattsville, Maryland. [Google Scholar]
- 10.National Center for Health Statistics. National Health and Nutrition Examination Survey. US Centers for Disease Control and Prevention. Accessed April 18, 2023. https://www.cdc.gov/nchs/nhanes/index.htm. .
- 11.Fang N., Jiang M., Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int. J. Cardiol. 2016 Jul 1;214:279–283. doi: 10.1016/j.ijcard.2016.03.210. [DOI] [PubMed] [Google Scholar]
- 12.Sacks F.M., Svetkey L.P., Vollmer W.M., Appel L.J., Bray G.A., Harsha D., Obarzanek E., Conlin P.R., Miller ER 3rd, Simons-Morton D.G., Karanja N., Lin P.H., DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the dietary Approaches to Stop hypertension (DASH) diet. DASH-sodium collaborative research group. N. Engl. J. Med. 2001 Jan 4;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C.L., Paulose-Ram R., Ogden C.L., et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 14.Johnson C.L., Paulose-Ram R., Ogden C.L., et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 15.Vallance J.K., Winkler E.A., Gardiner P.A., Healy G.N., Lynch B.M., Owen N. Associations of objectively-assessed physical activity and sedentary time with depression: NHANES (2005-2006) Prev. Med. 2011;53(4–5):284–288. doi: 10.1016/j.ypmed.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Wolter K.M. Taylor Series Methods. second ed. Springer; New york: 2007. Introduction to variance estimation. [Google Scholar]
- 17.Yusuf S., Hawken S., Ounpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004 Sep 11-17;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S., Joseph P., Rangarajan S., Islam S., Mente A., Hystad P., Brauer M., Kutty V.R., Gupta R., Wielgosz A., AlHabib K.F., Dans A., Lopez-Jaramillo P., Avezum A., Lanas F., Oguz A., Kruger I.M., Diaz R., Yusoff K., Mony P., Chifamba J., Yeates K., Kelishadi R., Yusufali A., Khatib R., Rahman O., Zatonska K., Iqbal R., Wei L., Bo H., Rosengren A., Kaur M., Mohan V., Lear S.A., Teo K.K., Leong D., O'Donnell M., McKee M., Dagenais G. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020 Mar 7;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atar D., Jukema J.W., Molemans B., Taub P.R., Goto S., Mach F., CerezoOlmos C., Underberg J., Keech A., Tokgözoğlu L., Bonaca M.P. New cardiovascular prevention guidelines: how to optimally manage dyslipidaemia and cardiovascular risk in 2021 in patients needing secondary prevention? Atherosclerosis. 2021 Feb;319:51–61. doi: 10.1016/j.atherosclerosis.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Lavie C.J., Ozemek C., Carbone S., Katzmarzyk P.T., Blair S.N. Sedentary behavior, exercise, and cardiovascular health. Circ. Res. 2019 Mar;124(5):799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 21.US Preventive Services Task Force. Krist A.H., Davidson K.W., Mangione C.M., Barry M.J., Cabana M., Caughey A.B., Donahue K., Doubeni C.A., Epling J.W., Jr., Kubik M., Landefeld S., Ogedegbe G., Pbert L., Silverstein M., Simon M.A., Tseng C.W., Wong J.B. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US preventive services task force recommendation statement. JAMA. 2020 Nov 24;324(20):2069–2075. doi: 10.1001/jama.2020.21749. [DOI] [PubMed] [Google Scholar]
- 22.Wu S., An S., Li W., Lichtenstein A.H., Gao J., Kris-Etherton P.M., Wu Y., Jin C., Huang S., Hu F.B., Gao X. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw. Open. 2019 May 3;2(5) doi: 10.1001/jamanetworkopen.2019.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stojisavljević D., Janković J., Erić M., Marinković J., Janković S. Cardiovascular health status and metabolic syndrome in adults living in a transition European country: findings from a population-based study. J. Stroke Cerebrovasc. Dis. 2018 Mar;27(3):568–574. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.046. [DOI] [PubMed] [Google Scholar]
- 24.Del Brutto O.H., Mera R.M., Montalván M., Del Brutto V.J., Zambrano M., Santamaría M., Tettamanti D. Cardiovascular health status and metabolic syndrome in Ecuadorian natives/Mestizos aged 40 years or more with and without stroke and ischemic heart disease--an atahualpa project case-control nested study. J. Stroke Cerebrovasc. Dis. 2014 Apr;23(4):643–648. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Kraus W.E., Powell K.E., Haskell W.L., Janz K.F., Campbell W.W., Jakicic J.M., Troiano R.P., Sprow K., Torres A., Piercy K.L. PHYSICAL ACTIVITY GUIDELINES ADVISORY COMMITTEE*. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med. Sci. Sports Exerc. 2018;51(6):1270–1281. doi: 10.1249/MSS.0000000000001939. 2019 Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017 May;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaing W., Vallibhakara S.A., Attia J., McEvoy M., Thakkinstian A. Effects of education and income on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017 Jul;24(10):1032–1042. doi: 10.1177/2047487317705916. [DOI] [PubMed] [Google Scholar]
- 28.Hoveling L.A., Liefbroer A.C., Bültmann U., Smidt N. Understanding socioeconomic differences in incident metabolic syndrome among adults: what is the mediating role of health behaviours? Prev. Med. 2021 Jul;148 doi: 10.1016/j.ypmed.2021.106537. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., Black T., Brewer L.C., Foraker R.E., Grandner M.A., Lavretsky H., Perak A.M., Sharma G., Rosamond W., American Heart Association Life's essential 8: updating and enhancing the American heart association's construct of cardiovascular health: a presidential advisory from the American heart association. Circulation. 2022 Aug 2;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z., Tuomilehto J., Kronfeld-Schor N., Alberti G., Stern N., El-Osta A., Chai Z., Bilu C., Einat H., Zimmet P. The circadian syndrome is a significant and stronger predictor for cardiovascular disease than the metabolic syndrome-the NHANES survey during 2005-2016. Nutrients. 2022 Dec 14;14(24):5317. doi: 10.3390/nu14245317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are publicly available and can be accessed at the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx). Our code is available upon reasonable request to the corresponding author.