Abstract
We have utilized combination antiretroviral therapy following human immunodeficiency virus type 1-induced human CD4+ thymocyte depletion in the SCID-hu mouse to examine the immune competence of reconstituting thymocytes which appear following administration of combination therapy. These cells express a normal distribution of T-cell receptor variable gene families and are responsive to costimulatory signals. These results suggest that normal thymic function may be restored following antiretroviral treatment.
Administration of effective antiretroviral therapy to individuals infected with human immunodeficiency virus (HIV) results in a decrease in plasma viremia and an increase of circulating CD4+ T lymphocytes (10, 28). In adult patients, much of this increase, seen shortly following treatment, may be due to expansion or relocalization of preexisting memory rather than naive cells in the periphery (3, 22), which would have a limited potential for antigen reactivity. It would be ideal if, following antiretroviral therapy, naive T cells with broad antigen specificity were produced in the thymus and subsequently exported to the periphery. Evidence is mounting that de novo synthesis of naive cells occurs in HIV-infected patients following highly active antiretroviral therapy (HAART) and that thymic mass increases in response to CD4-cell decline, suggesting continuing thymic output (3, 9, 19, 23). It is thus of great interest to establish if HIV infection perturbs the function of the thymic microenvironment and influences the ability of this organ to direct development of a broad T-cell repertoire.
The process of thymopoiesis involves multiple differentiation steps culminating in the expression of rearranged T-cell receptor (TCR) alpha and beta genes (or gamma and delta chains for γδ T cells), which determine antigen reactivity. Thymocytes in many different stages of development express CD4, the receptor for HIV, as well as high levels of the HIV coreceptor CXCR4 (15, 30). Some thymocytes also express the other major HIV coreceptor, CCR5 (4, 15, 16, 24, 30); thus, the majority of these cells are susceptible to HIV infection. Due to the anatomic location and the complexity of the thymus, it is difficult to determine the pathogenic effects of HIV on thymic function by analyzing patient samples.
We and others have previously used the SCID-hu mouse system (20, 21) to model the effects of HIV infection in the thymus and on the function of the thymic microenvironment (1, 7, 27, 29). In this model, HIV infection causes severe depletion of CD4-bearing thymocytes (1, 7) and degeneration of the thymic epithelial cell supporting network (27), similar to that seen in infected humans (13). In previous studies, we found that administration of a powerful combination of antiretroviral drugs following total HIV-induced depletion of CD4-bearing thymocytes allowed for a transient resurgence of new thymopoiesis (29). This reconstitution could be derived from both endogenous and exogenously added hematopoietic progenitor cells. However, it has not yet been established if these reconstituting cells develop normally and are responsive to antigenic stimulation.
To determine which thymocytes were depleted by HIV type 1 (HIV-1) infection and subsequently reconstituted following combination antiretroviral therapy in the SCID-hu system, a series of Thy/Liv implants were infected with the highly aggressive CXCR4-tropic NL4-3 strain of HIV-1. Sequential biopsies of the Thy/Liv tissue were taken at various time points. Thymocytes obtained from single-cell suspensions of biopsied tissue were initially stained with monoclonal antibodies specific for human CCR5 (clone 2D7), CXCR4 (clone 12G5) (Pharmingen, San Diego, Calif.), CD4, and CD8 (Becton Dickinson, San Jose, Calif.).
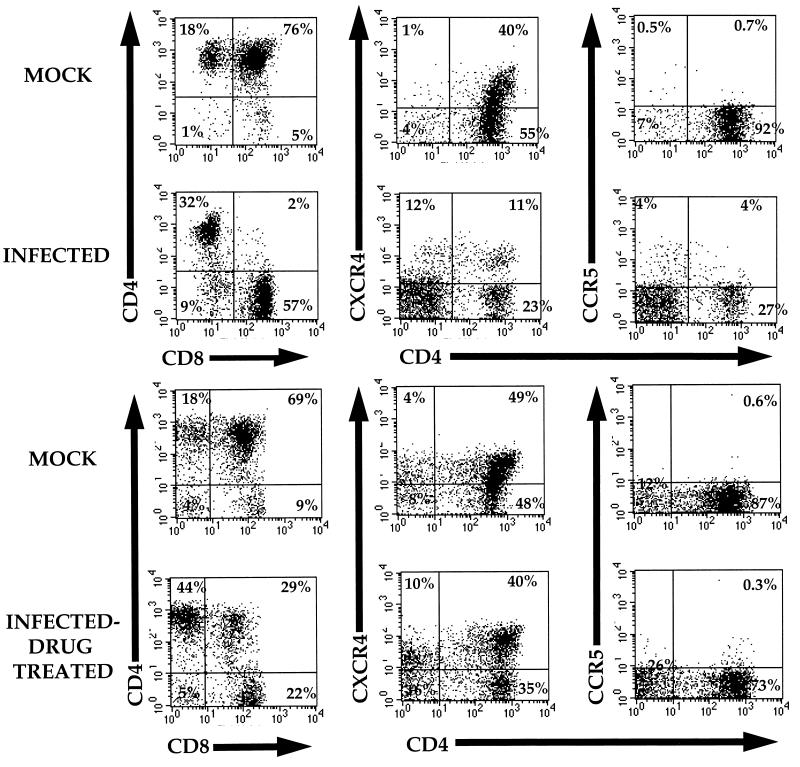
As previously shown, CD4-CD8 double-positive (DP) thymocytes were severely depleted 8 weeks following infection (Fig. 1A; Table 1). This resulted in a relative increase in CD4+-CD8− and CD4−-CD8+ mature thymocytes. The relative sparing of the CD4+-CD8− subset is likely due to the paucity of CXCR4 on these cells and their low level of overall transcription as a result of maturation (4, 15). The depletion of DP thymocytes resulted in a decrease of CD4+ cells bearing the coreceptor for this strain, CXCR4. Coincidentally, in some implants this resulted in a relative increase of thymocytes expressing the alternate HIV coreceptor CCR5, which is expressed on some CXCR4− cells (4, 15, 16, 24, 30).
FIG. 1.
Effects of HAART on thymocytes. SCID-hu mice were constructed, as described previously, at the University of California–Los Angeles (1, 12). Thy/Liv implants were infected with HIV-1NL4-3, and biopsies were taken at 8 weeks (A) and 11 weeks (B) postinfection and analyzed by flow cytometry. Immediately following the biopsies 8 weeks postinfection, mice were administered a daily combination of AZT, ddI and indinavir for the remainder of the experiment. Cells at each time point were stained with antibodies specific for CCR5 (FITC), CXCR4 (PE), CD4 (Red613), and CD8 (APC). The panels show flow cytometry profiles of a representative mock-infected animal (animal no. 175-28, top row) and the flow cytometry profiles of an HIV-1NL4-3-infected animal (animal no. 175-29, lower row). Percentages of cells within each relevant quadrant are provided.
TABLE 1.
Immune reconstitution of SCID-hu micea
| Expt and mouse no. | HIV infected | Drug treated | Results at time point 1
|
Results at time point 2
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % CD4 SP | % CD4-CD8 DP | % CD4+, CXCR4+ | % CD4+, CCR5+ | % CD4 SP | % CD4-CD8 DP | % CD4+, CXCR4+ | % CD4+, CCR5+ | |||
| Expt 1 | ||||||||||
| 175-4 | + | − | 28 | 1.7 | 15 | 6.4 | 16.8 | 3.0 | 0.8 | 0.0 |
| 175-25 | + | − | 14 | 3 | 2.9 | 0.6 | 0.7 | 0.6 | 0.04 | 0.0 |
| 175-26 | + | + | 28 | 3 | 11 | 4.5 | 12.5 | 5.0 | 44 | 4.2 |
| 175-29 | + | + | 32 | 2.0 | 11 | 4.0 | 44 | 29 | 40 | 0.3 |
| 175-31 | + | + | 7.2 | 1.3 | 1.4 | ND | 17 | 31 | 31 | 5.9 |
| 175-32 | + | + | 33 | 3.9 | 13 | ND | 22 | 62 | 56 | 0.8 |
| 175-33 | − | + | 24 | 72 | 51 | ND | 15 | 83 | 58 | 0.2 |
| 175-36 | − | + | 20 | 77 | 58 | ND | 23 | 72 | 52 | 0.3 |
| 175-28 | − | + | 18 | 76 | 40 | 1.9 | 18 | 69 | 49 | 0.6 |
| Expt 2 | ||||||||||
| 179-8 | + | − | 7 | 10 | 2.1 | 3.7 | 8 | 2.4 | ||
| 179-10 | + | − | 9 | 10 | 2.3 | 5.1 | 1.2 | 13 | ||
| 179-1 | + | + | 11 | 0.7 | 6 | 12 | 70 | ND | ||
| 179-2 | + | + | 1.3 | 0.15 | 7 | 8 | 33 | ND | ||
| 179-4 | + | + | 1.3 | 0.3 | 15 | 7.7 | 56 | 3.1 | ||
| 179-6 | + | + | 8.1 | 0.6 | 7 | 18 | 29 | ND | ||
| 179-25 | + | + | 20 | 57 | 8.2 | 11 | 57 | 4.8 | ||
| 179-27 | + | + | 5.3 | 0.8 | 10 | 6.7 | 66 | ND | ||
| 179-28 | + | + | 2.1 | 2.9 | 13 | 11 | 48 | 7.9 | ||
| 179-30 | + | + | 12 | 1.3 | 6.4 | 17 | 29 | 7.5 | ||
| 179-9 | − | + | 2.9 | 84 | 0.04 | 32 | 46 | ND | ||
| 179-7 | − | + | 5 | 79 | 0.1 | 7.8 | 81 | 0.9 | ||
| 179-12 | − | − | 4.2 | 79 | 0.04 | 7.7 | 67 | 0.5 | ||
Results from two separate experiments, donor series numbers 175 and 179, are shown. Some SCID-hu mice were infected with HIV-1NL4-3 (+), and biopsies were taken at 8 weeks (time point 1) and 11 weeks (time point 2, experiment 1) or 12 weeks (time point 2, experiment 2) postinfection and analyzed by flow cytometry. Mock-infected mice (−) from the same donor series were analyzed in parallel. Immediately following the biopsies 8 weeks postinfection, indicated mice were administered a daily combination of AZT, ddI, and indinavir for the remainder of the experiment. Cells at each time point were stained with antibodies specific for CCR5 (FITC), CXCR4 (PE), CD4 (Red613), and CD8 (APC). Percentages of CD4 single positive (SP) and CD4-CD8 DP cells are indicated for each time point, as are the percentages of CXCR4- and CCR5-expressing cells.
To determine whether the population of thymocytes originally depleted following infection was replaced following therapy, we administered a combination of zidovudine (AZT), dideoxyinosine (ddI), and the protease inhibitor indinavir, as previously described (29), following the 8-week biopsy. Animals were again biopsied and thymocytes were assessed at 11 to 12 weeks following therapy. We observed a striking increase in CD4-CXCR4 DP thymocytes and a corresponding relative decrease in CCR5-bearing cells, such that reconstituted implants appeared similar to mock-infected tissues (Fig. 1B and Table 1). Thus, the reconstituting thymocyte population would presumably again be susceptible to infection with the NL4-3 strain. This is consistent with our other studies showing that renewed virus infection is associated with the secondary loss of reconstituting thymocytes at later times posttherapy (2).
To fully reconstitute immune function, de novo thymopoiesis must allow production of a broad distribution of TCR variable β (TCRVβ) gene families. We and others have previously shown that HIV infection did not result in the depletion of thymocytes expressing specific TCRVβ gene families by a superantigen-like effect (6, 17). However, renewed thymopoiesis could be affected differently by indirect effects on stromal elements rather than direct effects on thymocytes themselves. We assessed the newly reconstituting CD4-CD8 DP thymocyte population for the relative distribution of 21 different human TCRVβ gene families by using a panel of monoclonal antibodies specific for these molecules that has been demonstrated to cover approximately 60 to 70% of TCRVβ chains on T cells (S. Killian, L. Hultin, and J. V. Giorgi, unpublished data). Antibodies specific for the following, conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) comprised the panel as follows: Vβ1, -2, -5.1, -7.1, -8, -9, -11, -12, -13.1, -13.6, -14, -16, -17, -18, -20, -21.3, -22, -23 (obtained from Coulter/Immunotech, Westbrook, Maine), Vβ3, -5.2/5.3 (Pharmingen), Vβ5.1, and Vβ6.7 (Endogen, Woburn, Mass.). Vβ13.2 was generously donated by P. Marrack and was FITC conjugated by Sierra Lab Logics (Gilroy, Calif.). Antibodies specific for CD3 (FITC), CD4 (allophycocyanin [APC]), CD8 (peridinin chlorophyll protein [PerCP]), CD16 (FITC), CD56 (FITC), and CD45 (PE) (Becton Dickinson) were also used to identify the overall percentages and phenotype of human T cells in the single-cell suspensions, of which greater than 95% were human T cells. Four-color flow cytometry was performed with a FACSCalibur flow cytometer and analyzed with Cellquest software (Becton Dickinson). Forward versus side scatter was used to gate the live thymocyte population. Percentages of cells staining positive for each individual Vβ subtype in each population were determined by gating relative to appropriate isotype controls.
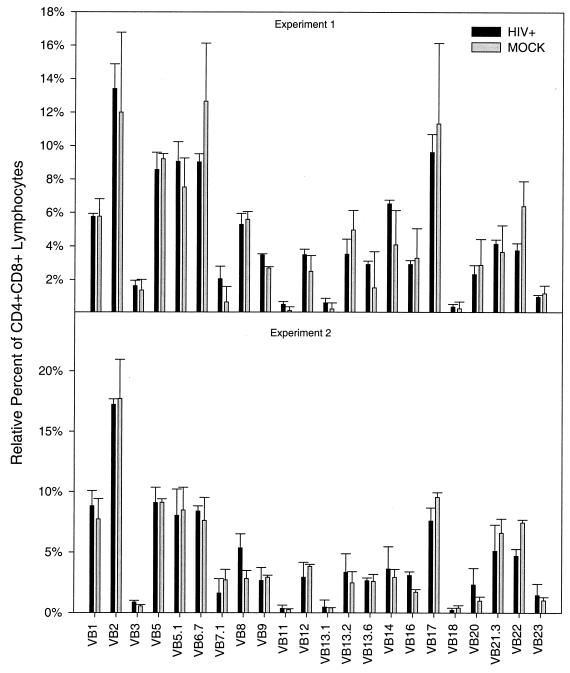
In two separate experiments, we compared the relative distribution of the TCRVβ families on mock-infected and newly reconstituted CD4-CD8 DP thymocytes. The animals in each experiment contained Thy/Liv implants derived from the same fetal tissue donor; thus, we could compare the effects of HIV on genetically identical stroma. As shown in Fig. 2, there was no statistically significant difference in the distribution of any of these TCRVβ gene families on DP thymocytes obtained from infected and reconstituted versus mock-infected implants in either experiment. The same analyses were performed on the mature CD4+-CD8− and CD4−-CD8+ populations, and similar results were obtained (data not shown). Thus, the reconstituting thymocytes appear to have no specific defect in TCR expression.
FIG. 2.
TCRVβ distribution in CD4-CD8 DP thymocytes following HAART. The top panel and bottom panel are two separate experiments involving tissue donors 175 and 179, respectively. SCID-hu mice were either infected with HIVNL4-3 or mock infected and biopsied 8 weeks postinfection to establish that depletion of the CD4-CD8 DP population had occurred (not shown). Mice with total CD4-CD8 DP-cell depletion were administered HAART for 3 weeks. Following therapy, mice were biopsied and analyzed by flow cytometry for CD4 (APC), CD8 (PerCP), and TCRVβ distribution. CD4-CD8 DP cells were gated and analyzed for positive staining of each Vβ receptor. The percentages of each Vβ subtype recognized in the CD4-CD8 DP population were summed to determine the total T-cell Vβ population. The relative abundance of each Vβ subtype was then calculated as a percentage of that total. The top panel represents data from three HIV-infected mice and two mock-infected mice. The bottom panel represents data from four HIV-infected mice and two mock-infected mice. Differences between percentages of each Vβ subtype of HIV- and mock-infected mice were not statistically significant by the Wilcoxon rank sum test (P > 0.4) (performed with SAS software; SAS Institute, Inc., Cary, N.C.).
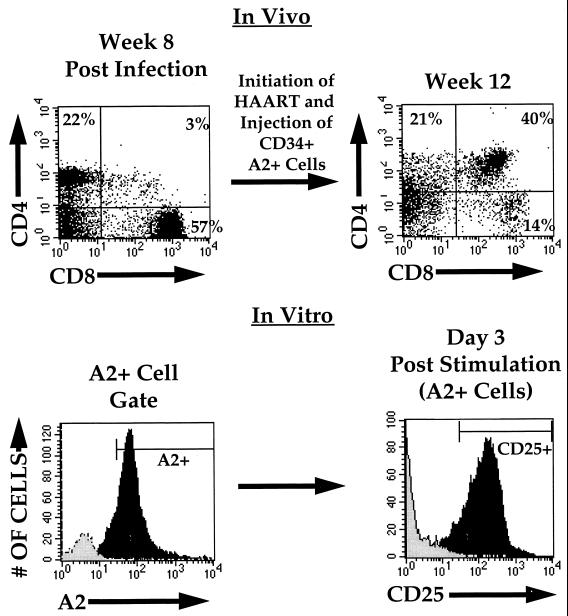
To assess the functionality of newly reconstituting thymocytes, we utilized simultaneous stimulatory signals directed towards the TCR (CD3) and the costimulatory receptor, CD28, an activating combination thought to be physiologically relevant (8, 26). To eliminate any possibility of potential reactivity by mature thymocytes remaining in the implant following HIV-induced depletion, we introduced exogenous CD34+, HLA-A2+ human hematopoietic progenitor cells into HIV-depleted, drug-treated HLA-A2− implants as previously described (2, 29) and allowed T-lymphoid differentiation to occur. The progeny of these progenitor cells could be differentiated from thymocytes derived from the original infected implant by using monoclonal antibody MA2.1, which is specific for HLA-A2 (2, 29). Three to five weeks following the administration of these progenitor cells to infected, drug-treated implants, resulting thymocytes were harvested and either costimulated or cultured unstimulated ex vivo, as previously described (14, 18). Three days following costimulation, donor-derived thymocytes (as determined by expression of HLA-A2) were assessed for expression of the activation marker CD25 (the α chain of the interleukin-2 receptor), which increases significantly following thymocyte activation. As seen in Fig. 3, the majority of these reconstituting cells responded to the costimulatory signal by expressing CD25. Thymocytes from control implants not receiving donor cells responded to stimulation with similar levels of CD25, but did not express HLA-A2 (not shown). Increased cellular proliferation and/or maintainence of viability was also observed in ex vivo-costimulated thymocyte cultures compared to unstimulated thymocyte cultures cultured in parallel (data not shown), further indicating functional response to T-cell costimulatory signals.
FIG. 3.
Functional response of the reconstituting thymocyte population. HIV-infected or mock-infected (not shown) thymic implants were biopsied 8 weeks postinfection and analyzed by flow cytometry for CD4 (biotin-Red613) and CD8 (APC) (upper left panel). HAART was initiated at this time, and CD34+/HLA-A2+ cells were injected into the implants at 8.5 weeks postinfection. A second biopsy was performed at 12 weeks postinfection, at which time cells were examined by flow cytometry (upper right panel) and cultured in the presence or absence of costimulation. Three days following costimulation, cells derived from implants not receiving HLA-A2+ cells (grey histogram) and those receiving HLA-A2+ CD34+ cells (black histogram) were analyzed for HLA-A2 (FITC) expression (lower left panel). HLA-A2+ cells in the implant, which in this mouse (animal no. 179-28) constituted the majority of cells, were gated and analyzed for CD25 (interleukin-2 receptor) (PE) expression (lower right panel) (black histogram). Unstimulated cells were cultured and analyzed in parallel (grey histogram). At this time, 92% of costimulated cells and 1% of unstimulated cells expressed CD25. CD25 expression was less than 1% in freshly isolated thymocytes prior to stimulation (not shown). Similar expression of CD25 was seen following costimulation of thymocytes from two mock-infected and six HIV-infected mice.
Recent studies have demonstrated an increase in naive CD4 cells in both peripheral blood and lymph nodes of infected adults following combination antiretroviral therapy (3, 9, 22, 23, 31). Several lines of evidence, including increased thymic mass correlating with decreased peripheral CD4 counts (19), phenotypic identification of maturing thymocytes in adults (5, 11), and evidence of peripheral T cells bearing recent TCR rearrangement (9, 11, 25, 31) suggest that these naive cells are derived from de novo thymopoiesis. These studies add hope that the HIV-infected immune system could be fully reconstituted with appropriate therapy. The studies performed herein were designed to determine potential inhibitory effects of previous HIV replication within the thymic microenvironment as well as the phenotypic and functional properties of the reconstituting thymocytes originating after administration of combination antiretroviral therapy. Due to effects of HIV on the thymus, altered differentiation pathways in the infected thymus could result in the expression of receptors unable to transduce appropriate signals into the cell. This could occur at the level of either the stromal cell or thymocyte. Changes in expression patterns of a number of surface molecules (i.e., major histocompatibility complex, adhesion molecules, cytokine receptors, etc.) could have dire consequences on thymopoiesis. Loss or altered representation of TCR gene families due to HIV-induced perturbation of the thymus might result in an inability to appropriately respond to a variety of antigenic epitopes.
The studies reported herein extend our previous observations and indicate that following administration of effective antiretroviral therapy to the HIV-infected thymus, reconstituting thymocytes are phenotypically and functionally normal. We definitively established that de novo-produced thymocytes were functional by assessing the response to costimulatory signals of thymocytes derived from exogenous hematopoietic precursor cells introduced into the HIV-infected, drug-treated implant. We determined that a full complement of TCRVβ gene families was expressed on reconstituting cells, suggesting that these cells might be able to respond to a wide variety of antigens. Our studies here focused on the reconstitution of fetal thymic implants. Recently, several groups, including ours, have shown that the adult thymus continues to export T cells to the periphery even late in life (9, 11, 25, 31). Together with these studies, the results shown herein suggest that effective antiretroviral therapy, coupled with a strategy to improve thymic function in adults, could result in immune reconstitution in HIV-infected patients.
Acknowledgments
We thank Lance Hultin for technical assistance and Christel Uittenbogaart for critical review of the manuscript.
This work was supported by NIH grants AI 36554 and AI 36059 and the UCLA CFAR. J.A.Z. is an Elizabeth Glaser scientist supported by the Pediatric AIDS Foundation. S.G.K. is a recipient of the UCLA Center for Clinical AIDS Research and Education HIV Pathogenesis Institutional Training Grant.
REFERENCES
- 1.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 2.Amado R G, Jamieson B D, Cortado R, Cole S W, Zack J A. Reconstitution of human thymic implants is limited by HIV breakthrough during antiretroviral therapy. J Virol. 1999;73:6361–6369. doi: 10.1128/jvi.73.8.6361-6369.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 4.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 5.Bertho J M, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol. 1997;179:30–40. doi: 10.1006/cimm.1997.1148. [DOI] [PubMed] [Google Scholar]
- 6.Boldt-Houle, D. M., B. D. Jamieson, G. M. Aldrovandi, C. R. Rinaldo, Jr., G. D. Ehrlich, and J. A. Zack. 1997. Loss of T cell receptor Vbeta repertoires in HIV type 1-infected SCID-hu mice. AIDS Res. Hum. Retrovir. 13:125–134. [DOI] [PubMed]
- 7.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 8.Chambers C A, Allison J P. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 9.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic output with age and during the treatment of HIV infection. Nature. 1999;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 10.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson B D, Douek D C, Killian S, Hultin L E, Scripture-Adams D D, Giorgi J V, Marelli D, Koup R A, Zack J A. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 12.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi V V, Oleske J M, Saad S, Gadol C, Connor E, Bobila R, Minnefor A B. Thymus biopsy in children with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110:837–842. [PubMed] [Google Scholar]
- 14.Kitchen S G, Korin Y, Roth M D, Landay A, Zack J A. Costimulation of CD8+ lymphocytes induces CD4 expression and allows HIV-1 infection. J Virol. 1998;72:9054–9060. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchen S G, Zack J A. Distribution of the human immunodeficiency virus coreceptors CXCR4 and CCR5 in fetal lymphoid organs: implications for pathogenesis in utero. AIDS Res Hum Retrovir. 1999;15:143–148. doi: 10.1089/088922299311565. [DOI] [PubMed] [Google Scholar]
- 17.Komanduri K V, Salha M D, Sekaly R P, McCune J M. Superantigen-mediated deletion of specific T cell receptor V beta subsets in the SCID-hu Thy/Liv mouse is induced by staphylococcal enterotoxin B, but not HIV-1. J Immunol. 1997;158:544–549. [PubMed] [Google Scholar]
- 18.Korin Y D, Zack J A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCune J M, Loftus R, Schmidt D K, Carroll P, Webster D, Swor-Yim L B, Francis I R, Gross B H, Grant R M. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Investig. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 21.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pakker N G, Notermans D W, de Boer R J, Roos M T, de Wolf F, Hill A, Leonard J M, Danner S A, Miedema F, Schellekens P T. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 23.Pantaleo G, Cohen O J, Schacker T, Vaccarezza M, Graziosi C, Rizzardi G P, Kahn J, Fox C H, Schnittman S M, Schwartz D H, Corey L, Fauci A S. Evolutionary pattern of human immunodeficiency virus (HIV) replication and distribution in lymph nodes following primary infection: implications for antiviral therapy. Nat Med. 1998;4:341–345. doi: 10.1038/nm0398-341. [DOI] [PubMed] [Google Scholar]
- 24.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulin J-P, Viswanathan M N, Harris J M, Komanduri K V, Wieder E, Ringuette N, Jenkins M, McCune J M, Sekaly R-P. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robey E, Allison J P. T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol Today. 1995;16:306–310. doi: 10.1016/0167-5699(95)80140-5. [DOI] [PubMed] [Google Scholar]
- 27.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 29.Withers-Ward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I S, Zack J A. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 30.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 31.Zhang L, Lewin S R, Markowitz M, Lin H-H, Skulsky E, Karanicolas R, He Y, Jin X, Tuttleton S, Vesanen M, Spiegel H, Kost R, van Lunzen J, Stellbrink H-J, Wolinsky S, Borkowsky W, Palumbo P, Kostrikis L G, Ho D D. Measuring recent thymic emigrants in the blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999;190:725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]