Abstract
Background
An important unmet need for new treatment options remains for patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M-HNSCC) previously treated with both platinum-based chemotherapy and anti-programmed cell death protein 1 (PD-1) antibody. Retrospective studies suggest that previous treatment with immune checkpoint inhibitor might augment the efficacy of subsequent chemotherapy. Here, we conducted a phase II trial aimed to evaluate the efficacy and safety of paclitaxel plus biweekly cetuximab for patients in this setting.
Patients and methods
This was a single-arm, multicenter, phase II trial. Key eligibility criteria were R/M-HNSCC, and previous treatment with both platinum-based chemotherapy and PD-1 antibody. Paclitaxel plus biweekly cetuximab consisted of weekly paclitaxel 100 mg/m2 (days 1, 8, 15) and biweekly cetuximab 500 mg/m2 (days 1, 15) with a cycle of 28 days until progression or unacceptable toxicity. Primary endpoint was objective response rate (ORR). Secondary endpoints included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and adverse events (AEs) (Common Terminology Criteria for Adverse Events version 5.0).
Results
Between August 2020 and August 2022, 35 patients were enrolled, of whom 33 were assessable for response. ORR was 69.6% (95% confidence interval 51.2% to 84.4%). With a median follow-up period for survivors of 16.6 months, median PFS and OS were 5.5 and 13.3 months, respectively. DCR was 93.7%. Twenty-three patients (65%) experienced grade 3 or 4 AEs, including neutropenia (34%), infection (14%), leukopenia (11%), mucositis (8%), and pneumonitis (8%). Eight patients discontinued study treatment due to treatment-related AEs, and no treatment-related death was observed.
Conclusions
Paclitaxel plus biweekly cetuximab showed highly encouraging efficacy and manageable toxicities in R/M-HNSCC patients previously treated with both platinum-based chemotherapy and PD-1 antibody. This combination therapy warrants further investigation in this setting.
Key words: head and neck cancer, PD-1 inhibitors, cisplatin, paclitaxel, cetuximab
Highlights
-
•
Paclitaxel plus biweekly cetuximab demonstrated promising efficacy for patients with R/M-HNSCC.
-
•
ORR was 69.6%, which met the primary endpoint.
-
•
PFS and OS were 5.5 and 13.3 months, respectively.
-
•
This combination is a potential treatment option after progression on platinum and PD-1 antibody.
Introduction
Patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M-HNSCC) have a poor prognosis of <1 year.1 In recent years, immune checkpoint inhibitors have improved treatment outcomes for patients with R/M-HNSCC. After the failure of platinum-based chemotherapy, nivolumab and pembrolizumab showed a survival benefit.2,3 In the KEYNOTE-048 trial, pembrolizumab alone improved overall survival (OS) in the programmed death-ligand 1 (PD-L1)-positive population and pembrolizumab combination chemotherapy improved OS in the total population as compared with the EXTREME regimen, namely combination chemotherapy with 5-fluorouracil, platinum, and cetuximab.4
However, for patients with R/M-HNSCC with failure of platinum-based chemotherapy and immune checkpoint inhibitors, prognosis is dismal. A retrospective study showed that median OS for these patients who could not receive subsequent chemotherapy was only 3.1 months.5 Furthermore, no standard treatment has been established. Nevertheless, recent retrospective studies have suggested that previous treatment with immune checkpoint inhibitor might augment the efficacy of subsequent chemotherapy.6, 7, 8
Combination chemotherapy with paclitaxel and cetuximab has potential as an attractive subsequent chemotherapy option for R/M-HNSCC.5,9 In the majority of studies, cetuximab was administrated under a weekly schedule with a loading dose of 400 mg/m2 followed by a weekly dose of 250 mg/m2. In Japan, a biweekly schedule of cetuximab was approved for colorectal cancer and head and neck cancer. In patients with colorectal cancer, the pharmacokinetics and pharmacodynamics of biweekly administration of cetuximab 500 mg/m2 are equivalent to those of the weekly administration of 400 mg/m2 followed by 250 mg/m2,10. Biweekly dosing suggested no significant difference in efficacy and adverse events (AEs) from weekly dosing.11,12 Further, a retrospective study showed that biweekly dosing retained efficacy and improved treatment compliance in patients with head and neck cancer.13
Here, we conducted a phase II trial to evaluate the efficacy and safety of paclitaxel plus biweekly cetuximab for patients with R/M-HNSCC who were previously treated with both platinum-based chemotherapy and programmed cell death protein 1 (PD-1) antibody.
Patients and methods
Study design
The study was conducted under a single-arm, multicenter, phase II design in five institutions in Japan (Japan Registry of Clinical Trials: jRCTs051200040). Eligible patients were ≥18 years old and had an Eastern Cooperative Oncology Group performance status of 0 or 1, histologically confirmed squamous cell carcinoma of the head and neck with distant metastasis or recurrence which was not amenable to curative locoregional treatment, refractory or intolerant to both platinum-based chemotherapy and PD-1 antibody, and no previous taxane-based chemotherapy for R/M-HNSCC. Additional inclusion and exclusion criteria are listed in Supplementary Appendix S1, available at https://doi.org/10.1016/j.esmoop.2024.103476.
Procedures
Paclitaxel plus biweekly cetuximab consisted of weekly paclitaxel 100 mg/m2 (days 1, 8, 15) and biweekly cetuximab 500 mg/m2 (days 1, 15) with a cycle of 28 days and continued until progression or unacceptable toxicity. Weekly paclitaxel at a dose of 100 mg/m2 was selected as this is the approved dose in Japan based on the results of a phase II trial.14 Cetuximab was administrated in 2 h followed by paclitaxel in 1 h. Dose modifications of study drugs were allowed in accordance with the severity of AEs (Supplementary Appendix S2, available at https://doi.org/10.1016/j.esmoop.2024.103476).
Tumor response was assessed every 8 weeks after registration by computed tomography or magnetic resonance imaging. AEs were assessed throughout study treatment, 7 days after last administration, and until improvement of treatment-related AEs. Severe AEs were also reported during study treatment.
Outcomes
Primary endpoint was objective response rate (ORR), defined as the best complete (CR) or partial response (PR) according to RECIST version 1.1.
Secondary endpoints were progression-free survival (PFS), OS, disease control rate (DCR), and AEs graded as per the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analyses
Previous studies have shown that around 10% of platinum-refractory R/M-HNSCC patients respond to chemotherapeutic agents2,4,15 and that 30% of platinum-refractory R/M-HNSCC patients respond to chemotherapy after progression with immune checkpoint inhibitors.6 The binomial analysis set for the null hypothesis had an ORR of 10% and alternative of 30% with a one-sided α of 0.05 and a power of 90%. Accordingly, the target accrual size was 33 patients. Allowing for a potential drop-out rate of 10%, we planned to enroll 35 patients in total. Binominal confidence intervals (CIs) for ORR and DCR were estimated by the exact method.
Time-to-event (PFS, OS) analyses were calculated by the Kaplan–Meier method with 95% CIs. All analyses were carried out using SPSS version 28.0.1.0 (IBM Corp., Armonk, NY).
The study protocol was approved by the Kobe University Clinical Research Ethical Committee and each participating hospital. This study was carried out in accordance with the international ethical recommendations stated in the Declaration of Helsinki and the Japanese Ethical Guidelines for Clinical Research. All patients who were enrolled in this study provided written informed consent to participate in the trial.
Results
Patients
Between August 2020 and August 2022, 35 patients met the eligibility criteria and were enrolled, of whom 33 were assessable for response (date of data cut-off was 15 August 2023). Two patients were not assessable for response because one patient was lost to follow-up and the second experienced severe paclitaxel allergy before the first disease evaluation. Table 1 shows patient characteristics at baseline. All patients had received treatment with both platinum-based chemotherapy and PD-1 antibody. Six patients had received previous treatment with cetuximab-containing chemotherapy for R/M-HNSCC. Among these six patients, five patients responded to the treatment and all six discontinued the treatment due to disease progression. At study entry, 12 patients had locoregional recurrence, 16 patients had distant metastases, and 7 patients had both locoregional recurrence and distant metastases. Major sites of metastasis were lung (31%) and lymph nodes (28%). At the time of analysis, 4 patients (11%) were still receiving study treatment, and 13 patients (37%) were receiving subsequent anticancer treatment (Supplementary Figures S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.103476).
Table 1.
Patient characteristics at baseline
| Characteristic (N = 35) | |
|---|---|
| Age (years), median (range) | 72 (45-81) |
| Sex, male, n (%) | 28 (80) |
| Primary site, n (%) | |
| Hypopharynx | 13 (37) |
| Oral cavity | 11 (31) |
| Nasal/paranasal sinus | 6 (17) |
| Oropharynxa | 2 (6) |
| Larynx | 2 (6) |
| External auditory canal | 1 (3) |
| ECOG PS, n (%) | |
| 0 | 9 (26) |
| 1 | 26 (74) |
| Platinum refractory, n (%) | 31 (89) |
| Platinum intolerable, n (%) | 4 (11) |
| Previous PD-1 Ab, n (%) | |
| Nivolumab | 19 (54) |
| Pembrolizumab | 16 (46) |
| Previous cetuximab, n (%) | |
| For R/M-HNSCC | 6 (17) |
| Site of disease, n (%) | |
| Locoregional only | 12 (34) |
| Metastasis only | 16 (46) |
| Locoregional and metastasis | 7 (20) |
Ab, antibody; ECOG PS, Eastern Cooperative Oncology Group score for performance status; PD-1, programmed cell death protein 1; R/M-HNSCC, recurrent and metastatic head and neck squamous cell carcinoma.
One patient was p16 positive.
Efficacy
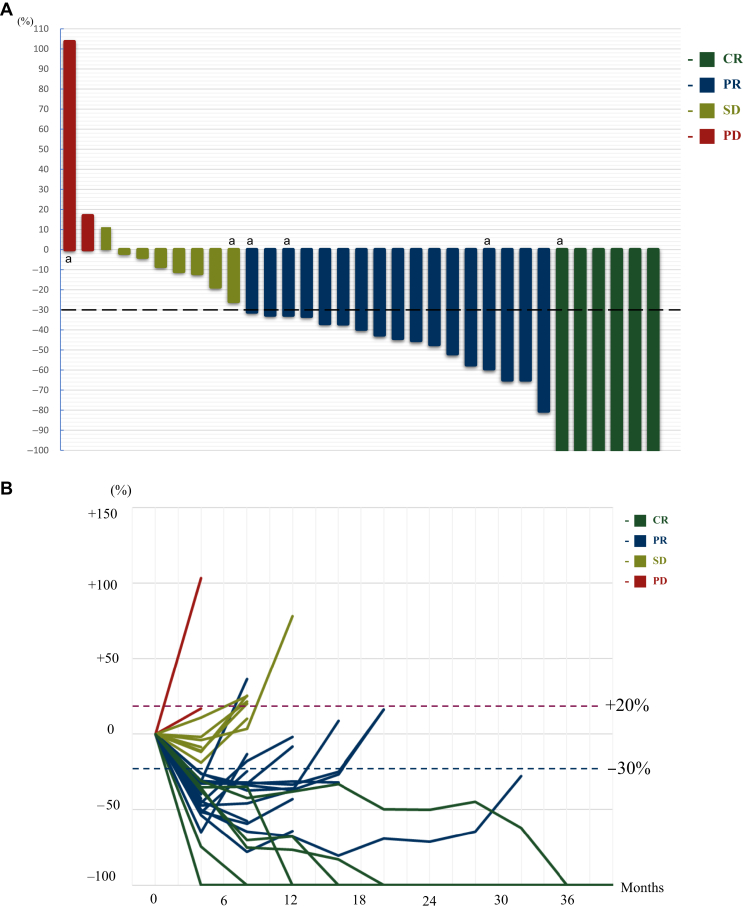
Of the 33 patients assessable for response, response was observed in 23 patients. ORR was 69.6% (95% CI 51.2% to 84.4%), which met the prespecified criteria for the primary endpoint. DCR was 93.7% (95% CI 79.7% to 99.2%) (Table 2 and Figure 1A and B).
Table 2.
Objective response in assessable patients
| Response | N = 33 n (%) |
|---|---|
| CR | 6 (18) |
| PR | 17 (51) |
| SD | 8 (24) |
| PD | 2 (6) |
| In the efficacy analysis population ORR: 69.6% (95% CI 51.2% to 84.4%) DCR: 93.7% (95% CI 79.7% to 99.2%) | |
Of 35 patients, 2 patients were not assessable.
CI, confidence interval; CR, complete response; DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Tumor response by waterfall plot and spider plot. (A) Best percentage change from baseline in the target lesion in the efficacy analysis population (N = 33). (B) Longitudinal changes in target lesion size from baseline in the efficacy analysis population (N = 33). Responses were calculated using RECIST version 1.1. The black dashed line means 30% reduction and the red dashed line means 20% increase.
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
aPatients who were resistant to a cetuximab-containing regimen.
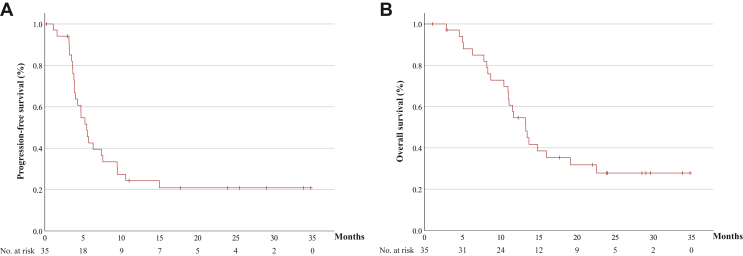
With a median follow-up period for survivors of 16.6 months, the median PFS and OS were 5.5 months (95% CI 4.3-6.6 months) and 13.3 months (95% CI 10.7-15.8 months), respectively (Figure 2A and B). One-year PFS and OS were 24.2% and 54.6%, respectively. Median time to response and duration of response were 1.7 months and 5.7 months (95% CI 0.9-10.5 months), respectively. Treatment response according to the time from last administration of PD-1 antibody was CR for 42 days (range 25-182 days), PR for 32 days (7-266 days), stable disease for 38.5 days (14-544 days), and progressive disease (PD) for 105 days (76-134 days), respectively (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103476). The ORR of patients who received study treatment within 60 days from the last administration of PD-1 antibody was 73% and that of the patients over 60 days was 57% (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103476).
Figure 2.
Survival outcomes for patients receving study teatment. Kaplan–Meier curves for progression-free survival (A) and overall survival (B) in the total population (N = 35). The x-axis represents months.
Among the six patients resistant to previous cetuximab-containing chemotherapy, four patients (66.6%) responded to the protocol treatment.
Safety
Safety data were available for all 35 patients. The most common AEs of any grade were skin rash (65%), peripheral nerve neuropathy (45%), neutropenia (40%), fatigue (40%), and paronychia (37%). Twenty-three of 35 patients (65%) experienced grade 3 or 4 AEs, including neutropenia (34%), leukopenia (11%), infection (8%), mucositis (8%), and pneumonitis (8%). Any grade of pneumonitis (interstitial lung disease) occurred in four patients (11%) (Table 3, Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103476). Twenty-four patients (68%) required a reduction in paclitaxel or cetuximab dose due to AEs; of these, 22 patients required a reduction in paclitaxel and 11 patients required a reduction in cetuximab.
Table 3.
All AEs ≥10% and all grade 3/4 AEs
| AEs (N = 35) | Grade 1/2 n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|
| Any AE | 35 (100) | 19 (54) | 4 (11) |
| Non-hematologic AEs | |||
| Skin rash | 22 (62) | 1 (2) | 0 |
| Dry skin | 21 (60) | 0 | 0 |
| Peripheral nerve neuropathy | 15 (42) | 1 (2) | 0 |
| Fatigue | 12 (34) | 2 (2) | 0 |
| Mucositis | 11 (31) | 3 (8) | 0 |
| Paronychia | 12 (24) | 1 (2) | 0 |
| Loss of appetite | 9 (25) | 1 (2) | 0 |
| Alopecia | 9 (25) | 0 | 0 |
| Dysgeusia | 9 (25) | 0 | 0 |
| Hypomagnesemia | 8 (22) | 0 | 0 |
| Infection | 3 (8) | 3 (8) | 1 (2) |
| Dysphagia | 5 (14) | 1 (2) | 0 |
| Diarrhea | 5 (14) | 1 (2) | 0 |
| Pneumonitis | 1 (2) | 3 (8) | 0 |
| Hand–foot skin reaction | 4 (11) | 0 | 0 |
| Infusion reaction/allergy | 3 (8) | 1 (2) | 0 |
| Myalgia | 4 (11) | 0 | 0 |
| Hypokalemia | 0 | 1 (2) | 0 |
| Pharyngeal fistula | 0 | 1 (2) | 0 |
| Cerebral infarction | 0 | 0 | 1 (2) |
| Hematologic AEs | |||
| Leukopenia | 4 (11) | 4 (11) | 0 |
| Neutropenia | 2 (5) | 8 (22) | 4 (11) |
| Anemia | 4 (11) | 3 (8) | 0 |
| Febrile neutropenia | — | 1 (2) | 0 |
AEs, adverse events.
In this study, 12 patients (34%) discontinued study treatment with paclitaxel plus biweekly cetuximab due to AEs (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103476). Additionally, no patients discontinued paclitaxel only and five patients (14%) discontinued cetuximab only. Of these, discontinuation was due to treatment-related AEs in eight patients. Four patients (11%) had treatment-related pneumonitis, from which all recovered following discontinuation of the study treatment and treatment with steroids. Other treatment-related discontinuations were infection in two, pharyngeal fistula in one, and grade 3 allergy against paclitaxel in one. Four patients (11%) discontinued study treatment due to non-treatment-related AEs. These AEs were manageable and no treatment-related death was observed.
Discussion
The results of this study showed that combination of paclitaxel plus biweekly cetuximab was highly effective for the treatment of R/M-HNSCC in patients who were previously treated with both platinum-based chemotherapy and PD-1 antibody. Although previous retrospective studies suggested that previous immunotherapy might augment the efficacy of salvage chemotherapy, this is the first prospective study to show promising efficacy for salvage chemotherapy after immunotherapy.
In first-line treatment of R/M-HNSCC with paclitaxel and anti-epidermal growth factor receptor (EGFR) antibody, median ORR, PFS, and OS have been reported as 37%-54%, 4.2-7.5 months, and 8.1-9.9 months, respectively.16, 17, 18 By contrast, in a retrospective study, the efficacy of paclitaxel with or without cetuximab for platinum-refractory R/M-HNSCC was modest as compared with the first-line setting, with an ORR of 6.2%-30%, a median PFS of 2.5-4.2 months, and a median OS of 4.2-6.9 months.2,19,20 Recently, retrospective studies have suggested that chemotherapy after immune checkpoint inhibitors showed better efficacy than a historical control of chemotherapy in the platinum-refractory setting. The ORR, median PFS, and median OS in such conditions were 30%-54%, 2.1-4.2 months, and 6.9-9.8 months, respectively.6,21, 22, 23 In addition to these reports, recent findings from a randomized phase II trial of ficlatuzumab, a humanized anti-hepatocellular growth factor monoclonal antibody, with or without cetuximab indicated that ficlatuzumab plus cetuximab demonstrated a better ORR of 19%, compared to the 4% ORR observed with ficlatuzumab alone, in patients with HNSCC treated with both platinum-based chemotherapy and immunotherapy.24 In the INTERLINK-1 trial, the addition of the anti-natural killer group 2A antibody monalizumab to cetuximab failed to show a survival improvement. However, cetuximab monotherapy in that trial showed an ORR of 19.1% for the patients with R/M-HNSCC who progressed after platinum and PD-1 antibody, which was numerically higher than the ORR with cetuximab alone in previous reports.25 Accordingly, the combination of chemotherapy with cetuximab appears to offer better efficacy than other chemotherapeutic options. In our current phase II study, paclitaxel plus biweekly cetuximab showed an ORR of 69.6%, a median PFS of 5.5 months, and a median OS of 13.3 months. To our knowledge, this is the first prospective demonstration of this highly encouraging efficacy for these populations.
The mechanism underlying how this combination chemotherapy worked well for patients treated with both platinum-based chemotherapy and PD-1 antibody remains unclear. The mechanisms of resistance to immune checkpoint inhibitors are associated with various factors, including tumor microenvironment and immune antigen presentation.26 Moreover, cetuximab increases not only PD-1-positive tumor-infiltrating lymphocytes but also causes the infiltration of suppressive regulatory T cells and myeloid-derived suppressor cells with increased expression of PD-1 and PD-L1, which could be inhibited by PD-1 antibody.27,28 Therefore, we focused on the PD-1 occupancy of peripheral T cells after administration of PD-1 antibody. The elimination half-life of PD-1 antibody is 12-20 days, whereas PD-1 occupancy of peripheral T cells with PD-1 antibody is sustained at 57%-70% for ∼60 days and PD-1 occupancy declines gradually over time.29,30 In the KEYNOTE-048 trial, PFS until progression under subsequent chemotherapy was longer for patients receiving pembrolizumab-containing treatment.31 Furthermore, combination of PD-1 antibody and cetuximab showed promising activity for platinum-refractory R/M-HNSCC.32 Indeed, in the present study, median duration from the last administration of PD-1 antibody was 35 days and no patient who received study treatment within 60 days after the last administration of PD-1 antibody experienced PD. Further, ORR was 73%, which was numerically higher than the ORR of 57% for patients over 60 days. On this basis, residual activity of PD-1 antibody might produce a synergistic effect with chemotherapy, resulting in enhancement of the immune microenvironment.33, 34, 35
In our present trial, previous treatment with cetuximab for R/M-HNSCC was allowed. Six patients had received previous cetuximab-containing chemotherapy for R/M-HNSCC. Although all six of these patients had discontinued cetuximab-containing chemotherapy due to disease progression, four patients (66.6%) responded to study treatment. Rechallenge of anti-EGFR antibody treatment has been investigated for patients with metastatic colorectal cancer, in whom it showed promising efficacy in late-line settings.36,37 In addition, rechallenge of cetuximab with chemotherapy for patients with R/M-HNSCC showed better ORR than those who received chemotherapy without cetuximab (16.4%-33.3% versus 6.2%, respectively).19,38 Consistent with these previous reports, rechallenge with cetuximab after PD-1 inhibitor appeared to yield a meaningful response in our study.
With regard to the safety profile of paclitaxel plus biweekly cetuximab, this was generally manageable. However, ∼60% of patients required a reduction in study drug dosage. The main reason for dose reduction was peripheral neuropathy for paclitaxel and skin toxicity for cetuximab. These AEs could potentially affect the quality of life of patients. This regimen should be carefully managed accordingly, and dose adjustment in future clinical trials is considered. Moreover, attention should be given to the reasons for treatment discontinuation. Of 35 patients, 31 patients have discontinued study treatment and 4 patients are still on study treatment at the time of data cut-off. Eighteen patients (51%) discontinued because of PD. Eight patients (22%) discontinued due to treatment-related AEs, of whom four (11%) discontinued due to treatment-related pneumonitis, albeit that all four recovered with steroid treatment. After discontinuation, median time to progression was 177 days (range 50-665 days), while one patient showed a persistent response at the cut-off date. In a retrospective study of R/M-HNSCC, cetuximab-based chemotherapy showed a higher incidence of pneumonitis in the setting of chemotherapy after progression on immune checkpoint inhibitor than no prior immune checkpoint inhibitor, at 21.1%-25.0% versus 0%-7%, respectively.3,8,19,39, 40, 41 Similarly, in non-small-cell lung cancer, EGFR-tyrosine kinase inhibitor after progression of immune checkpoint inhibitor might have increased the incidence of pneumonitis as compared with EGFR-tyrosine kinase inhibitor without prior immune checkpoint inhibitor (25.7% versus 4.59%, odds ratio 4.31).42 Although the incidence of pneumonitis in this study appeared to be lower than in reports in R/M-HNSCC patients with prior immune checkpoint inhibitors and all patients recovered from pneumonitis, close monitoring of patients during treatment with paclitaxel plus biweekly cetuximab after immune checkpoint inhibitors is warranted.
This study has some limitations. Firstly, it was conducted under a non-randomized, single-arm design. Secondly, although paclitaxel plus biweekly cetuximab demonstrated a high ORR of 69.6%, evaluation of response was assessed by the investigators and almost all patients had human papillomavirus (HPV)-unrelated cancer. The EXTREME regimen showed no difference by HPV status,43 while the phase II trial of ficlatuzumab and trials of PD-1 antibody with cetuximab showed better efficacy for patients with HPV-unrelated cancer.24,44 Therefore, the difference in efficacy of paclitaxel plus biweekly cetuximab between HPV-related and HPV-unrelated cancer should be assessed in future trials. Thirdly, 11% of patients discontinued study treatment due to treatment-related pneumonitis. Hence, confirming the efficacy and safety of this combination would require a randomized comparison between paclitaxel plus biweekly cetuximab and paclitaxel alone in this setting.
In conclusion, paclitaxel plus biweekly cetuximab showed highly encouraging efficacy and a manageable toxicity profile in R/M-HNSCC patients who were previously treated with both platinum-based chemotherapy and PD-1 antibody. Paclitaxel plus biweekly cetuximab could be a potential treatment option and warrants further investigation in this setting.
Acknowledgements
The authors would like to thank the Clinical and Translational Research Center, Kobe University Hospital for consulting on statistical analysis. We also thank Guy Harris DO of Dmed (https//dmed.co.jp) for support with the editing of the manuscript.
Funding
None declared.
Disclosure
TK has received invited speaker fees from Shionogi, Merck Biopharma, and Nihon Medi-Physics. NK’s institution reports receiving research funding from Bristol Myers Squibb, AstraZeneca, Chugai Pharmaceutical, Bayer, Lilly, GSK, and Adlai Nortye; NK has received invited speaker fees from Bristol Myers Squibb, Bayer, Eli Lilly, MSD, Ono Pharmaceutical, Merck Biopharma, and Eisai, and advisory board fees from Ono Pharmaceutical and Adlai Nortye. SB has received invited speaker fees from MSD, ONO Pharmaceutical, Bristol Myers Squibb, Taiho Pharmaceutical, and Chugai Pharmaceutical. YI has received invited speaker fees from Daiichi Sankyo and MSD. NS has received invited speaker fees from Chugai Pharmaceutical, Kyowa Kirin, Pfizer, Daiichi Sankyo, Eisai, Yakult Honsha, Taiho Pharmaceutical, Takeda Pharmaceutical, Novartis, AstraZeneca, Merck Biopharma, Bayer, Eli Lilly, Ono Pharmaceutical, Nippon Kayaku, and Medicon, and advisory board fees from Kyowa Kirin and Daiichi Sankyo. HSa’s institution reports receiving research funding from Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Sanofi, and Asahi Kasei; HSa has received invited speaker fees from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eli Lilly, Merck Biopharma, MSD, Ono Pharmaceutical, Sanofi, Taiho Pharmaceutical, Takeda, and Yakult Honsha. KT has reported receiving invited speaker fees from AstraZeneca, Merck Biopharma, Eisai, Bristol Myers Squibb, Ono Pharmaceutical, MSD, Chugai Pharmaceutical, Takeda Pharmaceutical, Taiho Pharmaceutical, and Novartis. HH’s institution has received research funding from Guardant Health Japan, IQVIA, Eisai, Syneos Health, EP-CRSU, EPS, Shionogi, Nippon Kayaku, Otsuka Pharmaceutical, Takeda Pharmaceutical, GlaxoSmithKline, MSD, Sanofi, Amgen, Chugai Pharmaceutical, Taiho Pharmaceutical, Nippon Boehringer Ingelheim, Bristol Myers Squibb, SRL Medisearch, Janssen Pharmaceutical, PRA Health Sciences, CMIC, Astellas Pharma, Pfizer R&D Japan G.K., Ascent Development Services, Labcorp Development Japan, Eisai, Kobayashi Pharmaceutical, Bayer, and Pfizer Japan; HH has received invited speaker fees from Ono Pharmaceutical, Merck Biopharma, Daiichi Sankyo, 3H Clinical Trial, AstraZeneca, Novartis, Chugai Pharmaceutical, Bristol Myers Squibb, Eli Lilly, Amgen, MSD, Sysmex, Pfizer Japan, Takeda Pharmaceutical, and Nippon Boehringer Ingelheim. TO’s department has reported receiving grants from MSD and Merck Biopharma; TO has received invited speaker fees from Eisai, Chugai Pharmaceutical, Taiho Pharmaceutical, and Bristol Myers Squibb, and advisory board fees from Eisai. YN has reported receiving personal honoraria as an invited speaker from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, and Eli Lilly. HSh has reported receiving an invited speaker fee from Rakuten Medical. HM’s institution has reported receiving grants from Abbvie, Asahi-Kasei Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Chugai, Eisai, Daiichi-Sankyo, DaiNippon Sumitomo, GSK, Insight, Kyowa-Kirin, Eli Lilly, Merck Biopharma, MSD, Nihon-Kayaku, Nihon-Shinyaku, Shionogi, Novartis, Sanofi, Teijin Pharma, Taiho, Ono Pharmaceutical, Otsuka Pharmaceutical, Sumitomo Pharma, Takeda, Tsumura, and Yakult; HM has received personal honoraria as an invited speaker from Abbvie, Asahi-Kasei Pharma, Bayer, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi-Sankyo, Daiichi-Sankyo Espha, Eisai, Kyowa-Kirin, Eli Lilly, Meiji Seika Pharma, Merck Biopharma, Nihon Servier, Novartis, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Sanofi, Shionogi, Taiho, and Takeda. All other authors have declared no conflicts of interest.
Supplementary data
Supplemental Figure S1.
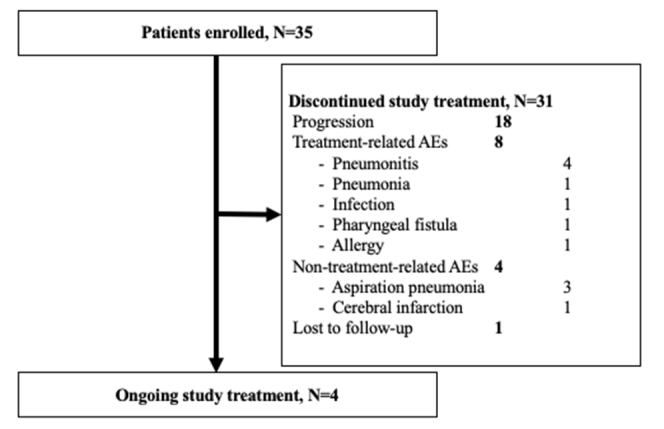
Flow diagram (N=35), Flow diagram of patient disposition and discontinuation details at the cut-off date.
References
- 1.Colevas A.D. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24(17):2644–2652. doi: 10.1200/JCO.2005.05.3348. [DOI] [PubMed] [Google Scholar]
- 2.Ferris R.L., Blumenschein G., Jr., Fayette J., et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen E.E.W., Soulieres D., Le Tourneau C., et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 4.Burtness B., Harrington K.J., Greil R., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 5.Oka H., Nagashima K., Nishi H., et al. Clinical outcomes in patients with recurrent or metastatic head and neck squamous cell carcinoma after failure of platinum and nivolumab: a multicenter retrospective study. Ann Oncol. 2022;33(7):S853–S854. [Google Scholar]
- 6.Saleh K., Daste A., Martin N., et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123–129. doi: 10.1016/j.ejca.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Park S.E., Lee S.H., Ahn J.S., et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(1):106–111. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Cabezas-Camarero S., Cabrera-Martin M.N., Merino-Menendez S., et al. Safety and efficacy of cetuximab-based salvage chemotherapy after checkpoint inhibitors in head and neck cancer. Oncologist. 2021;26(6):e1018–e1035. doi: 10.1002/onco.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrera Gomez R.G., Saleh K., Auclin E., et al. 942P Response to salvage chemotherapy with paclitaxel +/- cetuximab after progression on immune checkpoint inhibitors in platinum-refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) patients (CeTax study) Ann Oncol. 2023;34:S592. [Google Scholar]
- 10.Tabernero J., Pfeiffer P., Cervantes A. Administration of cetuximab every 2 weeks in the treatment of metastatic colorectal cancer: an effective, more convenient alternative to weekly administration? Oncologist. 2008;13(2):113–119. doi: 10.1634/theoncologist.2007-0201. [DOI] [PubMed] [Google Scholar]
- 11.Parikh A.R., Gonzalez-Gugel E., Smolyakova N., et al. Efficacy and safety of cetuximab dosing (biweekly vs weekly) in patients with KRAS wild-type metastatic colorectal cancer: a meta-analysis. Oncologist. 2022;27(5):371–379. doi: 10.1093/oncolo/oyab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossi P., Kornek G., Lanzetta G., et al. Safety and feasibility of every-other-week maintenance cetuximab after first-line chemotherapy in patients with recurrent or metastatic head and neck squamous cell cancer. Head Neck. 2013;35(10):1471–1474. doi: 10.1002/hed.23170. [DOI] [PubMed] [Google Scholar]
- 13.Addeo R., Montella L., Mastella A., et al. Maintenance therapy with biweekly cetuximab: optimizing schedule can preserve activity and improves compliance in advanced head and neck cancer. Oncology. 2018;95(6):353–359. doi: 10.1159/000492153. [DOI] [PubMed] [Google Scholar]
- 14.Tahara M., Minami H., Hasegawa Y., et al. Weekly paclitaxel in patients with recurrent or metastatic head and neck cancer. Cancer Chemother Pharmacol. 2011;68(3):769–776. doi: 10.1007/s00280-010-1550-3. [DOI] [PubMed] [Google Scholar]
- 15.Knoedler M., Gauler T.C., Gruenwald V., et al. Phase II study of cetuximab in combination with docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck after platinum-containing therapy: a multicenter study of the Arbeitsgemeinschaft Internistische Onkologie. Oncology. 2013;84(5):284–289. doi: 10.1159/000345453. [DOI] [PubMed] [Google Scholar]
- 16.Hitt R., Irigoyen A., Cortes-Funes H., et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. 2012;23(4):1016–1022. doi: 10.1093/annonc/mdr367. [DOI] [PubMed] [Google Scholar]
- 17.Morillo E.D., Mesía R., Klain J.C.A., et al. Phase II study of panitumumab and paclitaxel as first-line treatment in recurrent or metastatic head and neck cancer. TTCC-2009-03/VECTITAX study. Oral Oncol. 2016;62:54–59. doi: 10.1016/j.oraloncology.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Rubio-Casadevall J., Cirauqui Cirauqui B., Martinez Trufero J., et al. TTCC-2019-02: real-world evidence of first-line cetuximab plus paclitaxel in recurrent or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1226939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevalier T., Daste A., Saada-Bouzid E., et al. Cetuximab combined with paclitaxel or paclitaxel alone for patients with recurrent or metastatic head and neck squamous cell carcinoma progressing after EXTREME. Cancer Med. 2021;10(12):3952–3963. doi: 10.1002/cam4.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez B., Trigo J.M., Pajares B.I., et al. Efficacy and safety of weekly paclitaxel combined with cetuximab in the treatment of pretreated recurrent/metastatic head and neck cancer patients. Oral Oncol. 2013;49(2):182–185. doi: 10.1016/j.oraloncology.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Yasumatsu R., Shimizu Y., Hanai N., et al. Outcomes of long-term nivolumab and subsequent chemotherapy in Japanese patients with head and neck cancer: 2-year follow-up from a multicenter real-world study. Int J Clin Oncol. 2022;27(1):95–104. doi: 10.1007/s10147-021-02047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pestana R.C., Becnel M., Rubin M.L., et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 2020;101 doi: 10.1016/j.oraloncology.2019.104523. [DOI] [PubMed] [Google Scholar]
- 23.Kacew A.J., Harris E.J., Lorch J.H., et al. Chemotherapy after immune checkpoint blockade in patients with recurrent, metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104676. [DOI] [PubMed] [Google Scholar]
- 24.Bauman J.E., Saba N.F., Roe D., et al. Randomized phase II trial of ficlatuzumab with or without cetuximab in pan-refractory, recurrent/metastatic head and neck cancer. J Clin Oncol. 2023;41(22):3851–3862. doi: 10.1200/JCO.22.01994. [DOI] [PubMed] [Google Scholar]
- 25.Fayette J., Licitra L.F.L., Harrington K.J., et al. INTERLINK-1: phase III study of cetuximab (CTX) ± monalizumab (M) in participants (pts) with recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) with disease progression on/after platinum chemotherapy (CT) and previously treated with an immune checkpoint inhibitor (ICI) Ann Oncol. 2023;34:S554–S555. [Google Scholar]
- 26.Sharma P., Hu-Lieskovan S., Wargo J.A., et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jie H.B., Srivastava R.M., Argiris A., et al. Increased PD-1(+) and TIM-3(+) TILs during cetuximab therapy inversely correlate with response in head and neck cancer patients. Cancer Immunol Res. 2017;5(5):408–416. doi: 10.1158/2326-6066.CIR-16-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris R.L., Lenz H.J., Trotta A.M., et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. 2018;63:48–60. doi: 10.1016/j.ctrv.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer J.R., Drake C.G., Wollner I., et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nose T., Funakoshi Y., Suto H., et al. Transition of the PD-1 occupancy of nivolumab on T cells after discontinuation and response of nivolumab re-challenge. Mol Clin Oncol. 2022;16(5):104. doi: 10.3892/mco.2022.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrington K.J., Burtness B., Greil R., et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 2023;41(4):790–802. doi: 10.1200/JCO.21.02508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco A.G., Chen R., Worden F.P., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22(6):883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Dermawan K., Jin M.L., et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129(2):219–229. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Osa A., Uenami T., Koyama S., et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight. 2018;3(19) doi: 10.1172/jci.insight.59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu N., Zheng Y.J., Zhu Y., et al. Selective impairment of CD4+CD25+Foxp3+ regulatory T cells by paclitaxel is explained by Bcl-2/Bax mediated apoptosis. Int Immunopharmacol. 2011;11(2):212–219. doi: 10.1016/j.intimp.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji A., Nakamura M., Watanabe T., et al. Phase II study of third-line panitumumab rechallenge in patients with metastatic wild-type colorectal cancer who obtained clinical benefit from first-line panitumumab-based chemotherapy: JACCRO CC-09. Target Oncol. 2021;16(6):753–760. doi: 10.1007/s11523-021-00845-y. [DOI] [PubMed] [Google Scholar]
- 37.Cremolini C., Rossini D., Dell’Aquila E., et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5(3):343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borel C., Regnier-Gavier O., Carinato H., et al. Interest to consider re-challenging by cetuximab and platinum containing regimen in recurrent head and neck cancer. Oncotarget. 2018;9(101):37581–37588. doi: 10.18632/oncotarget.26506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokota T., Ota Y., Fujii H., et al. Real-world clinical outcomes and prognostic factors in Japanese patients with recurrent or metastatic squamous cell carcinoma of head and neck treated with chemotherapy plus cetuximab: a prospective observation study (JROSG12-2) Int J Clin Oncol. 2021;26(2):316–325. doi: 10.1007/s10147-020-01817-4. [DOI] [PubMed] [Google Scholar]
- 40.Wakasaki T., Manako T., Yasumatsu R., et al. Effectiveness and safety of weekly paclitaxel and cetuximab as a salvage chemotherapy following immune checkpoint inhibitors for recurrent or metastatic head and neck squamous cell carcinoma: a multicenter clinical study. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0271907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo M., Yasumatsu R., Masuda M., et al. Drug-induced interstitial lung disease in recurrent and/or metastatic head and neck cancer patients treated with cetuximab and/or nivolumab. Oral Oncol. 2021;113 doi: 10.1016/j.oraloncology.2020.105129. [DOI] [PubMed] [Google Scholar]
- 42.Oshima Y., Tanimoto T., Yuji K., et al. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermorken J.B., Psyrri A., Mesia R., et al. Impact of tumor HPV status on outcome in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck receiving chemotherapy with or without cetuximab: retrospective analysis of the phase III EXTREME trial. Ann Oncol. 2014;25(4):801–807. doi: 10.1093/annonc/mdt574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S., Zheng M., Nie D., et al. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: a systematic review and meta-analysis. J Immunother Cancer. 2022;10(10) doi: 10.1136/jitc-2022-005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.