Abstract
Background
Three Amino acid Loop Extension (TALE) belongs to the homeobox group of genes that are important constituents of plant systems. The TALE gene family is instrumental not only in growth and development but also plays an essential role in regulating plant response to environmental adversaries.
Results
In the present study, we isolated 21 CsTALE genes from the cucumber (Cucumis sativus L.) genome database. Bioinformatics tools were put in place to understand the structural and functional components of the CsTALE gene family. The evolutionary analysis dissected them into seven subclades (KNOX-I, KNOX-II, and BELL-I to BELL-V). The cis-acting elements in the promoter region of CsTALE genes disclosed that they are key regulators of hormonal and stress-related processes. Additionally, the STRING database advocated the concerting role of CsTALE proteins with other key transcription factors potent in plant developmental biology. The CsmiR319 and CsmiR167a-3p targeting the CsTALE15 and CsTALE16, respectively, further assert the importance of the CsTALE gene family posttranscriptional-related processes. Tissue-specific gene expression unfolded the fundamental involvement of CsTALE genes as they were expressed throughout the developmental stages. Under waterlogging stress, the CsTALE17 expressed significantly higher values in WL, WL-NAA, and WL-ETH but not in WL-MeJA-treated samples.
Conclusions
The present study reveals the evolution and functions of the CsTALE gene family in cucumber. Our work will provide a platform that will help future researchers address the issue of waterlogging stress in the Yangtze River Delta.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05274-3.
Keywords: Cucumber, TALE, Waterlogging, Hormones, Bioinformatics
Introduction
TALE, a class of transcription factors, is found in eukaryotes fine-tuning key regulatory processes [1]. An exceptionally conserved sequence, later dubbed the homeobox, was identified by Gehring et al. (1987) [2] by other researchers as a result of gene mutations leading to a similar phenotype. The family gene contains KNOX and BLH/BELL domain-encoded proteins [3], consisting of physically and functionally similar heterodimers. TALE family genes encode 63-amino-acid homeobox domains. This family is known as the homeobox protein superfamily because of the three-amino acid loop extension that connects the homeodomain’s first and second helices [4]. The Arabidopsis thaliana (AtTALE) transcription factors are classified into two subfamilies: the BELL and the KNOX [4]. Specific interactions between these two subfamilies govern downstream genes for various biological roles [5]. The four domains that make up the KNOX subfamily—KNOX1, KNOX2, ELK, and Homeobox KN—are found in plants [6].
The initial homeobox in plants was identified as Kn-1 (Knotted-1) by [7]. SAM production was shown to be significantly inhibited by a mutation in the STM (SHOOTMERISTEMLESS) [3]. KNAT7 played a role in secondary cell wall biosynthesis [8, 9]. Cotton fiber synthesis is controlled by GhKNL1, a member of the TALE family [10]. Flowers, xylem differentiation, and hormone effects may be regulated by Arabidopsis KNOX Class I family genes (STM, KNAT2, BREVIPEDICELLUS (BP)/ KNAT1, and KNAT6) [10]. The roots, stems, leaves, and flowers are the locations where the Class II KNOX genes are expressed among the angiosperms. This set of genes’ primary function is to control the organ differentiation process in plants [3, 10]. Gibberellic acid (GA), cytokinin (CK), and lignin are likewise regulated by KNOX proteins [11]. Floral development and metamorphosis are regulated in significant ways by members of the BELL family. Different from one another, overexpression of the BLH3 gene causes flowers to open earlier, and overexpression of the BLH6 gene causes delayed flowering [12]. Selectively combining KNOX and BELL forms heterodimer heteromers that bind to target sequences. Various pairings of two subfamilies can control distinct sets of genes to carry out their roles in plants [5]. A key enzyme in the GA biochemical pathway, ga20ox1, was inhibited in its expression by the BELL/KNOX dimer StBEL5 / POTH1 [3]. ATH1 and PENNYWISE (PNY), both BELL family members, regulated plant meristem development similar to STM [8]. A. thaliana BLH1 and KNAT3 proteins combine to produce heterodimers that impact seed germination and development as well as the expression level of genes responsive to ABA [13, 14]. The development of pistil marginal tissue is regulated by KNAT1 through interactions with RPL, FUL, and AP [10].
C. sativus is the third most popular vegetable crop, growing on 1.98 million hectares and producing 93 million tons. China accounts for 78% of the world’s production, with 72 million tons out of a total of 93 million tons [15, 16]. Flooding is the biggest threat to agriculture and food security worldwide. Flood losses in low- and lower-middle-income nations were USD 21 billion between 2008 and 2018, 19% of all agricultural losses [17–19]. Ethylene pretreatments increased hypoxia tolerance, rosette diameters, and root tip re-grow after recovery in A. thaliana [20–22]. Other hormones, such as auxin and methyl jasmonate, were also reported to regulate plant response to waterlogging stress [21, 23–26]. Thus far, not a great deal of literature is available on the involvement of the TALE gene family in parthenocarpy and waterlogging.
In this work, we have conducted a detailed analysis of the CsTALE gene family using numerous bioinformatic tools. Applying phytohormones (IAA, CK, and GA) induces parthenocarpy in cucumber. We have analyzed the expression of CsTALE genes in response to IAA, CK, and GA. Additionally, the expression of CsTALE genes was analyzed in different tissues and under waterlogging stress. Our conducted study will be of great interest to future research work, particularly in waterlogging.
Results
Identification of TALE genes in the cucumber genome
To isolate the TALE proteins from the cucumber genome database (CGD), we utilized the BLASTP program. The retrieved proteins were further investigated for the Homeobox_KNOX (HB_KN) domain. Proteins lacking the HB_KN domain were discarded, and following that, we were left with 21 CsTALE members. The chromosomal position was investigated in the CGD. The ExPASy online server was used for the number of amino acids, molecular weight, and isoelectric point. Finally, the Subcellular analysis was performed, and we observed that all the CsTALE proteins reside in the nucleus (Table 1).
Table 1.
Physiochemical properties of CsTALE proteins
| Locus ID | Gene | Chr. | Start | End | AA | MW (kDa) | PI | SL |
|---|---|---|---|---|---|---|---|---|
| Csa1G004870 | CsTALE1 | 1 | 839,762 | 845,531 | 314 | 35207.52 | 5.38 | Nucleus |
| Csa1G118890 | CsTALE2 | 1 | 9,012,090 | 9,017,334 | 820 | 91560.96 | 6.11 | Nucleus |
| Csa2G034560 | CsTALE3 | 2 | 3,335,281 | 3,338,884 | 327 | 37369.31 | 5.04 | Nucleus |
| Csa2G249860 | CsTALE4 | 2 | 12,228,795 | 12,231,645 | 687 | 75512.65 | 5.98 | Nucleus |
| Csa2G287110 | CsTALE5 | 2 | 13,696,177 | 13,702,147 | 661 | 72975.29 | 5.79 | Nucleus |
| Csa2G348930 | CsTALE6 | 2 | 15,770,430 | 15,774,065 | 169 | 19521.10 | 7.09 | Nucleus |
| Csa2G351760 | CsTALE7 | 2 | 16,208,915 | 16,210,622 | 492 | 55228.72 | 5.76 | Nucleus |
| Csa2G352430 | CsTALE8 | 2 | 16,306,379 | 16,310,046 | 406 | 46520.65 | 6.11 | Nucleus |
| Csa3G733340 | CsTALE9 | 3 | 27,883,158 | 27,886,801 | 486 | 54935.44 | 6.81 | Nucleus |
| Csa3G782620 | CsTALE10 | 3 | 30,260,873 | 30,264,668 | 722 | 79328.86 | 6.66 | Nucleus |
| Csa4G297540 | CsTALE11 | 4 | 12,242,105 | 12,246,510 | 467 | 53319.24 | 6.62 | Nucleus |
| Csa4G645830 | CsTALE12 | 4 | 21,808,266 | 21,812,207 | 375 | 43152.31 | 5.91 | Nucleus |
| Csa4G652730 | CsTALE13 | 4 | 22,778,556 | 22,782,578 | 476 | 52996.85 | 5.98 | Nucleus |
| Csa5G152860 | CsTALE14 | 5 | 4,854,760 | 4,858,291 | 612 | 67568.45 | 5.98 | Nucleus |
| Csa5G156150 | CsTALE15 | 5 | 5,425,321 | 5,428,901 | 307 | 34445.81 | 6.06 | Nucleus |
| Csa5G600390 | CsTALE16 | 5 | 21,925,448 | 21,932,133 | 330 | 37407.15 | 5.01 | Nucleus |
| Csa6G426360 | CsTALE17 | 6 | 20,030,373 | 20,034,057 | 704 | 76970.39 | 6.97 | Nucleus |
| Csa6G487770 | CsTALE18 | 6 | 22,977,392 | 22,982,614 | 702 | 76464.81 | 6.46 | Nucleus |
| Csa6G513430 | CsTALE19 | 6 | 26,530,396 | 26,533,379 | 357 | 39895.86 | 5.55 | Nucleus |
| Csa7G041370 | CsTALE20 | 7 | 2,231,073 | 2,235,700 | 356 | 40080.13 | 6.44 | Nucleus |
| Csa7G432650 | CsTALE21 | 7 | 17,316,741 | 17,319,618 | 554 | 61892.46 | 5.64 | Nucleus |
Chromosome: Chr., Amino acid: AA, Molecular Weight: MW, Isoelectric point: PI, Subcellular location: SL
CsTALE Gene family conserved domain analysis
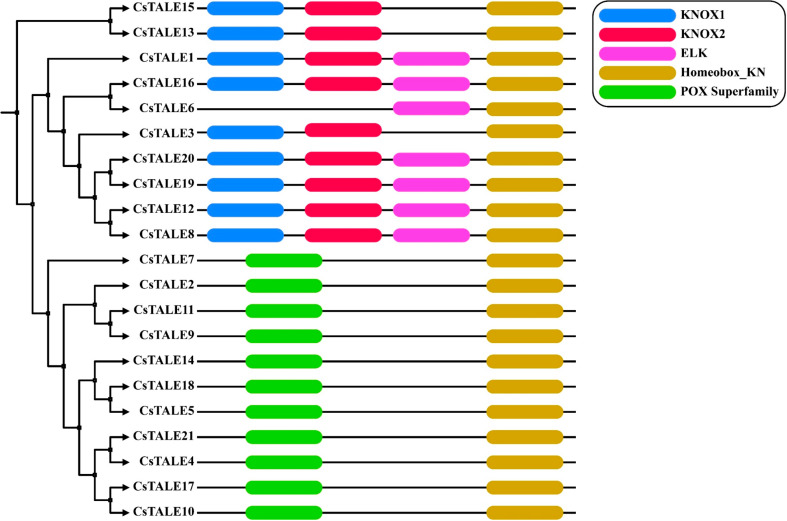
In order to gain a deep insight into the diversity of CsTALE gene functions, we used the MEME online server (http://meme-suite.org/tools/meme) to predict conserved motifs in CsTALE proteins. A total of five different motifs were found in the CsTALE proteins. Four members of the CsTALE gene family (CsTALE1, CsTALE8, CsTALE12, CsTALE16, CsTALE19, CsTALE20) were found with the highest number of KNOX1, KNOX2, ELK, Homeobox_KN and POX Superfamily domains. Furthermore, only CsTALE proteins such as CsTALE3, CsTALE13, and CsTALE15 possess KNOX1, KNOX2, and Homeobox_KN domains, and the CsTALE6 with ELK and Homeobox_KN domains. Finally, the highest number of CsTALE proteins was found with the Homeobox_KN and POX Superfamily domains (Fig. 1); the A. thaliana conserved domains of the two subfamilies (KNOX, BELL) are attached in the supplementary data Figure S1.
Fig. 1.
Schematic representation of the conserved Domains of CsTALE genes in C. sativus. The conserved domains were identified using the NCBI conserved domain search
Evolutionary analysis of CsTALE genes
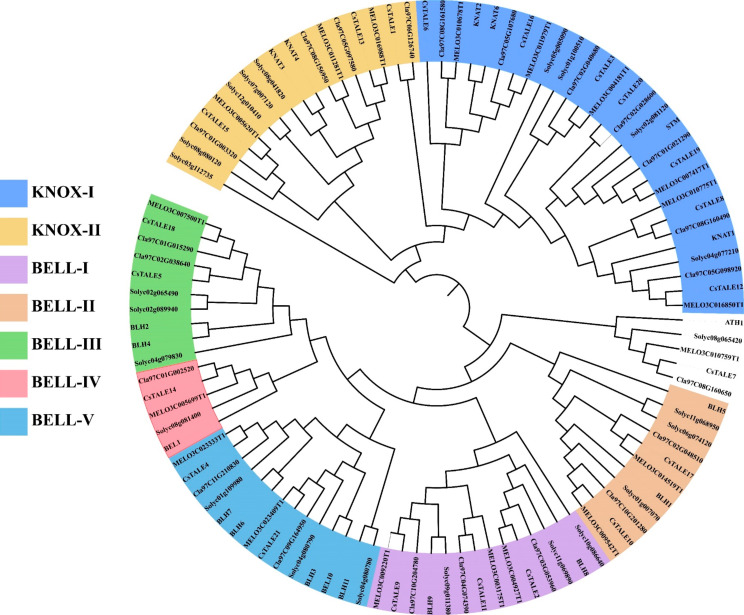
The phylogeny analysis was conducted to examine the evolutionary characteristics of CsTALE with other species, including A. thaliana, Cucumis melo, Citrullus lanatus, and Solanum lycopersicum. The highest number of TALE genes were attributed to KNOX-1, followed by KNOX-II (Fig. 2). The BELL group of subfamilies consists of 5 additional subfamilies (BELL-I to BELL-V). The BELL-I and BELL-V contain the highest number of TALE genes, whereas the BELL-II, BELL-III, and BELL IV exhibit a decent amount of TALE genes
Fig. 2.
Phylogenetic analysis of the CsTALE genes family. The phylogenetic tree was generated using the amino-acid sequences via ML methods. All CsTALE were classified into seven groups, and the final tree was displayed using ITOL.
Synteny analysis among CsTALE genes
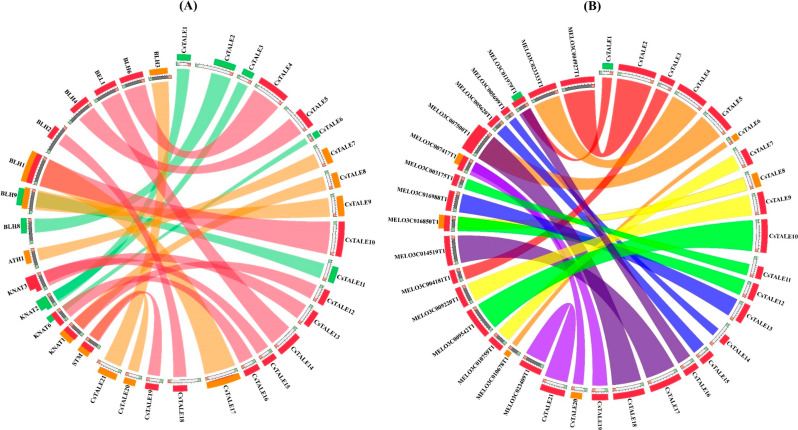
Synteny links between the cucumber, melon, and Arabidopsis genomes were also examined to determine the likely roles of the CsTALE genes. In both the Arabidopsis (~ 50%) and cucumber (~ 86%) genomes, all of the CsTALE genes had synteny links, as shown in (Fig. 3A). Similarly, high syntenic links between cucumber (~ 70%) and melon (~ 55%) were also observed (Fig. 3B). During genome evolution, these wide synteny relations at the gene level can demonstrate the close evolutionary links and extensive rearrangement events of the cucumber chromosomes.
Fig. 3.
Synteny analysis of TALE genes between (A) C. sativus and A. thaliana and (B) C. sativus and C. melo. The chromosomes of C. sativus, A. thaliana, and C. melo are arranged as a circle. Colored lines represent syntenic occurrences of TALE genes
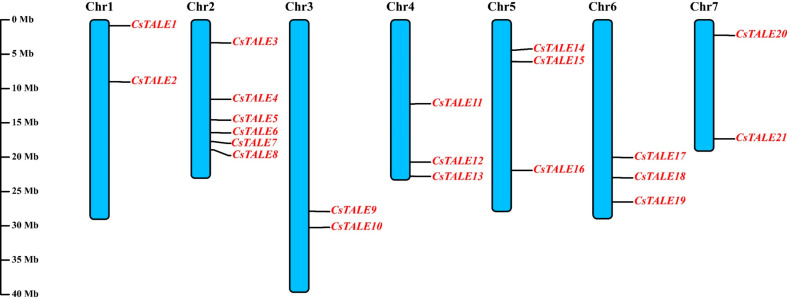
Fig. 4.
Localization of CsTALE genes on chromosomes. There are 7 chromosomes in cucumber, and the number of chromosomes is indicated at the top of each chromosome. The relative positions of CsTALE genes are marked on the chromosomes. The schematic representation was made using TBtools-II (Version 1.098765)
Chromosomal localization of CsTALE genes
Chromosomal localization is performed to visualize the physical location of CsTALE genes. The cucumber genome contains 7 chromosomes, and the CsTALE genes were distributed in random positions (Fig. 4). Chromosome 2 possesses the highest number of genes (six genes), followed by chromosomes 4, 5, and 6 (3 genes each). Chromosomes 1, 3, and 7 only accounted for 2 CsTALE genes each.
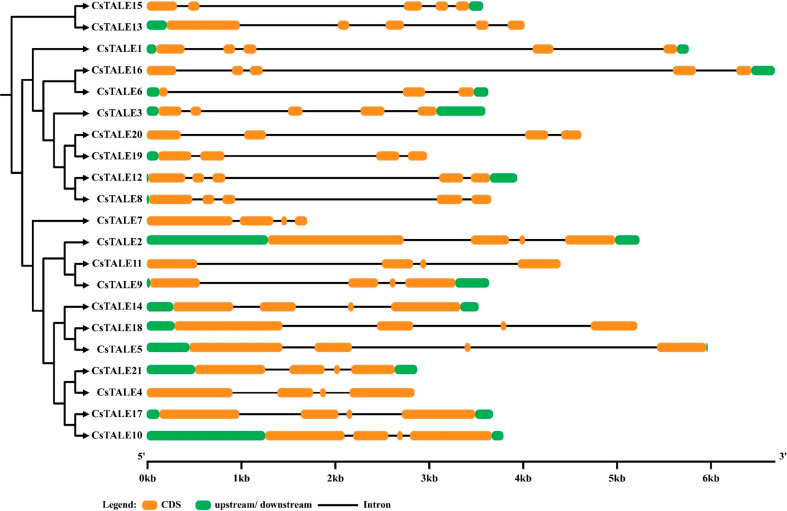
Gene structure and motif analysis
The gene structure analysis is vital to understanding the evolutionary composition of a certain gene or family. The CsTALE gene’s structure was drawn using genomic and CDS sequences. The exons (CDS) and introns were randomly distributed as disclosed by structural analysis (Fig. 5). For instance, the majority of the CsTALE genes possess 4–5 exons in their structure. On the other hand, 2–3 introns were recorded in the bulk of the CsTALE genes.
Fig. 5.
Schematic representation of gene structure. The gene structure analysis was obtained from the gene display structure
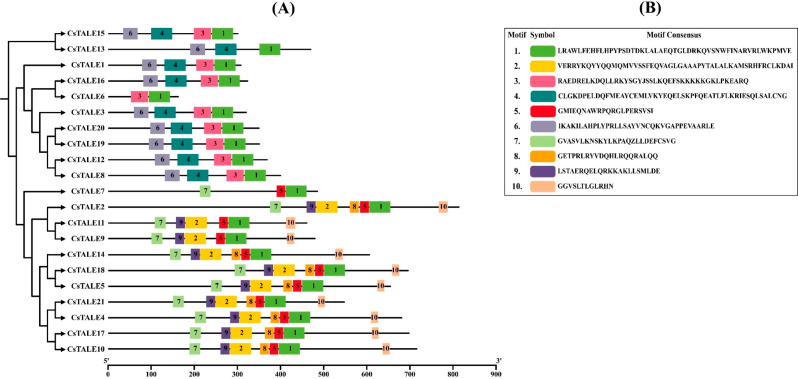
The MEME online server predicted a total of 4 motifs in the protein sequence of CsTALE (Fig. 6). The highly conserved motif 1 (Homeobox) was presented in all the CsTALE proteins, whereas motif 2 (POX) was found missing in several. The motif 3 (KNOX II) was recorded in predominant numbers, whereas the motif 4 (KNOX I) was absent in many CsTALE proteins.
Fig. 6.
Schematic representation of motifs distribution in CsTALE gene family. The distribution of the motifs was obtained from the MEME online server
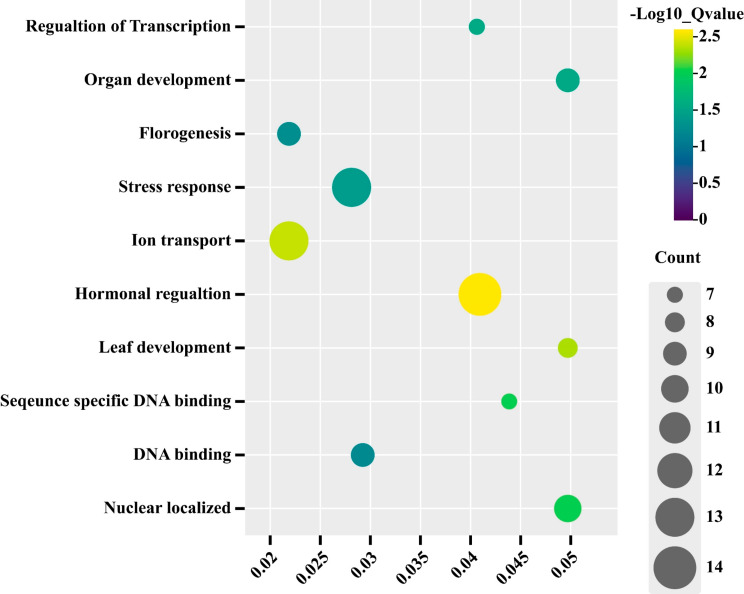
Gene ontology analysis of CsTALE genes
The gene ontology (GO) analysis comprised three categories: biological functions, molecular functions, and cellular components. The BF predicted that CsTALE genes are, by and large, involved in flowering, hormonal regulation, organ development, and stress response (Fig. 7). The CsTALE genes exhibit DNA binding ability as suggested by the molecular function. According to CC, all the CsTALE genes reside in the nucleus.
Fig. 7.
GO enrichment analysis of CsTALE genes in cucumber. The data represented biological processes, molecular functions, cellular components, and their localization proportions
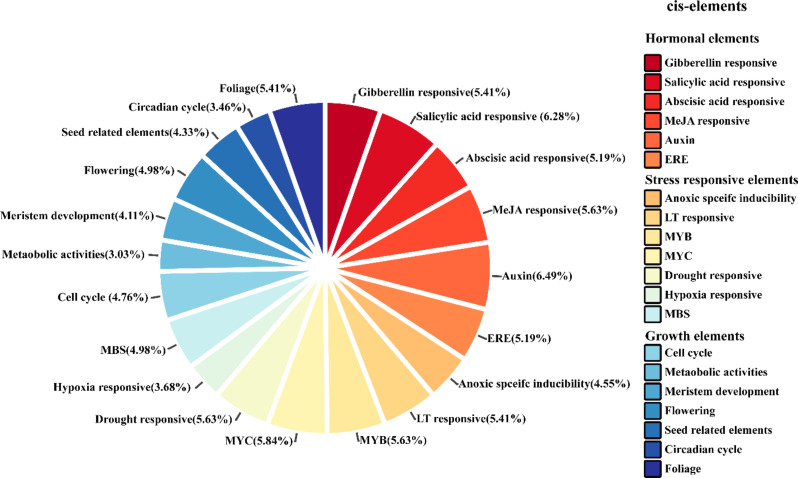
Promoter analysis of CsTALE genes
The 1.5 kb promoter region upstream of CsTALE genes was retrieved from the cucumber genome database, and the predicted cis-elements were searched by the PlantCARE database. The predicted cis-acting elements were categorized into different groups (hormonal, stress, and growth). The hormonal-related elements contain cis-elements for almost all the major hormones, including GA, SA, ABA, MeJA, auxin, and ERE (ethylene-responsive elements). The CsTALE genes could be responsive to a variety of environmental stresses. For instance, anoxia, low temperature, drought, and hypoxia. The drought-responsive element MBS is also presented in the upstream region of the CsTALE gene (Fig. 8). Could be of a versatile nature, the CsTALE genes tailor key growth processes such as cell cycle, metabolic activities, flowering, circadian cycle, and foliage formation.
Fig. 8.
The cis-acting elements of CsTALE genes in cucumber. The data represented hormonal regulation, stress-responsive, and growth-related elements
Interactive protein network analysis
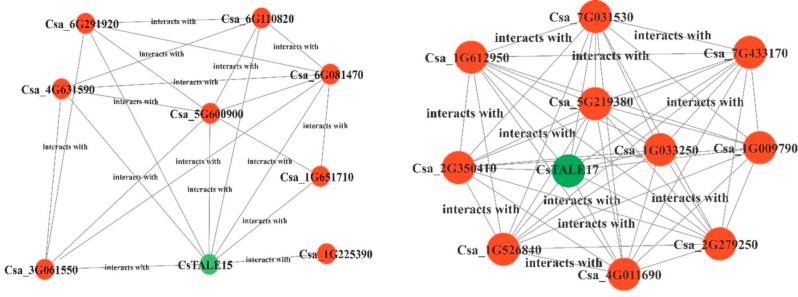
The interactive protein analysis via the STRING online database is a key tool for identifying the functional partners. Herein, we referred to two CsTALE proteins (CsTALE15 and CsTALE17) to recognize their functional predictors (Fig. 9). For CsTALE15, an array of key proteins was found in the interaction. Amongst them, Csa1G225390 (Receptor-like protein kinase), Csa3G061550 (Scarecrow-like 1 transcription factor), Csa1G651710 (Flowering locus T-like 2) and Csa5G600900 (KELCH DOMAIN-CONTAINING F-BOX PROTEIN). The CsTALE17 was also found in interaction with several key transcription factors. The Csa2G350410 (Trihelix transcription factor GT-1), Csa1G526840 (UDP-glycosyltransferase 1), Csa7G031530 (CONSTANS-like 9), and Csa1G033250 (Dof zinc finger protein).
Fig. 9.
The predictive functional partners of CsTALE-proteins. The CsTALE15 and CsTALE17 were used as a reference in the String online tool for functional predictor analysis
10 targeted miRNA of CsTALE genes
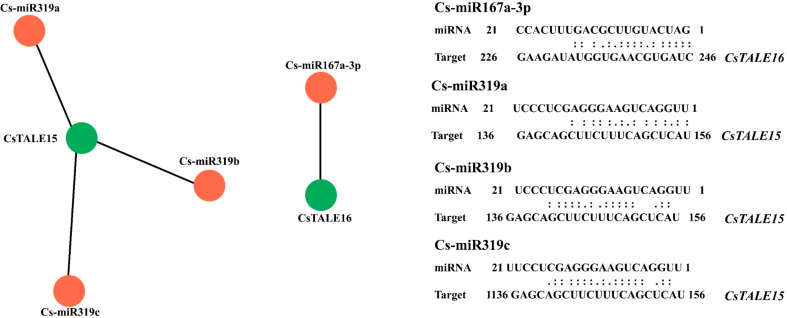
The miRNA prediction is vital to understand the posttranscriptional regulatory mechanism of CsTALE genes. We used the CDS sequences of CsTALE genes to identify their sponged miRNAs. The analysis revealed that the miRNA319 family (Cs-miR319a, CsmiR319b, and Cs-miR319c) targeted the CsTALE15 gene (Fig. 10). Additionally, the Cs-miR167a-3p targets the CsTALE16 gene, as can be seen from the outcome of the predictor analysis.
Fig. 10.
Schematic representation of (A) miRNA network interaction with CsTALE genes. The miRNA network was presented through Cytoscape (version 3.9.1)
11 tissue-specific expression analysis of CsTALE genes
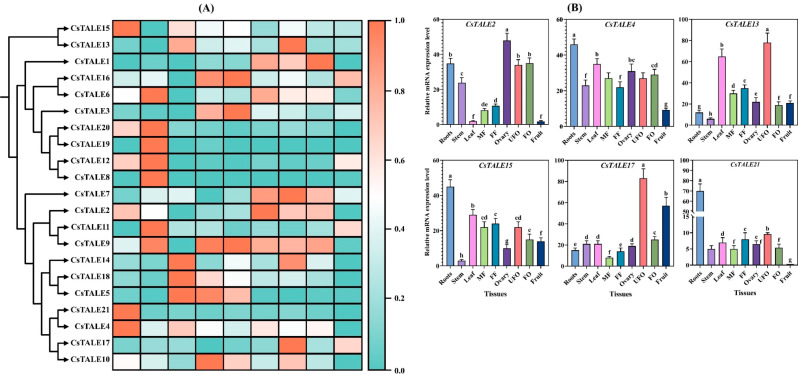
The expression of CsTALE genes was investigated in different cucumber tissues to map their general regulatory role in growth. The mRNA abundance data of different tissues was retrieved from the cucumber genome database (Fig. 11A). Varied expression of CsTALE genes was observed in different tissues. For instance, in roots, CsTALE2, CsTALE4, CsTALE12, CsTALE15, CsTALE20 and CsTALE21 were expressed dominantly. In stem, the CsTALE6, CsTALE8, CsTALE9, CsTALE11, CsTALE12 and CsTALE20 displayed high mRNA level. The CsTALE5, CsTALE14, and CsTALE18 were among the highly expressed genes in the leaf. The CsTALE3, CsTALE5, CsTALE9, CsTALE10, and CsTALE16 showed expression abundance in the male flower (MF) and female flower (FF). In the reproductive tissue (ovary, fertilized ovary [FO] and unfertilized ovary [UO]), the CsTALE1, CsTALE2, CsTALE6, CsTALE7, and CsTALE9 were highly expressed. Only CsTALE17 displayed a high level of expression in fruit tissue (Fig. 11A).
Fig. 11.
Organs-specific expression analysis of CsTALE genes family. (A) Heatmap representation of CsTALE genes in developmental stages. The log2 transformation method normalized and converted the RPKM values. (B) qRT-PCR expression analysis of selected CsTALE genes in different tissues
Further, we selected six genes from the heatmap based on their expression to validate the data by qRT-PCR analysis. The qRT-PCR expression analysis revealed an almost similar expression trend to that with the heatmap (Fig. 11B).
12 CsTALE genes expression under waterlogging stress and hormonal applications
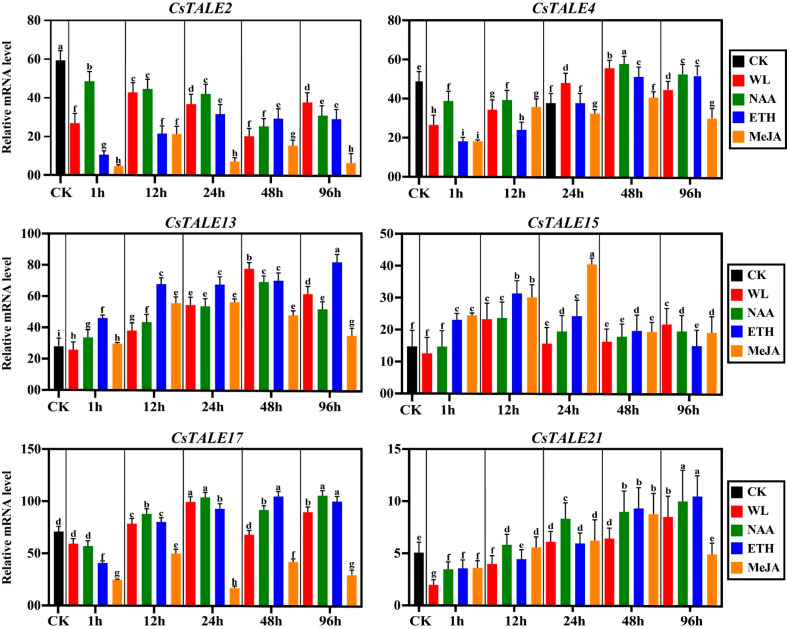
The CsTALE gene expression under waterlogging in combination with hormones was analyzed. The expression of the CsTALE1 gene showed lower expression at 1 h after waterlogging (WL), WL-NAA, WL-ETH, and WL-MeJA than the CK (control). At 12 h, the expression was higher under WL and WL-NAA but suppressed again under WL-ETH and WL-MeJA. At 24 h, 48 h, and 96 h, the transcription level of the CsTALE1 gene was induced under WL, WL-NAA, and WL-ETH compared to WL-MeJA. The CsTALE4, in a similar fashion, expressed more in CK than WL, WL-NAA, WL-ETH, and WL-MeJA at 1 h. However, under 12 h, 24 h, 48 h, and 96 h, its mRNA level was sharply induced under WL, NAA, and ETH but reduced under MeJA (Fig. 12). The CsTALE13 showed similar expressions in CK and WL at 1 h but were stimulated in response to WL-NAA and WL-ETH. The CsTALE17 displayed the most significant expression in response to WL, WL-NAA, and WL-ETH. The CsTALE17 was upregulated at all the time points in the WL, WL-NAA, and ETH treated, whereas substantial suppression was observed in the treated cucumber. There was no significant difference in the expression of CsTALE15 and CsTALE21 among the treatments.
Fig. 12.
Expression analysis of CsTALE genes in response to WL and hormones. The data displays CsTALE gene expression under CK, WL, NAA, ETH, and MeJA at 1 h, 12 h, 24 h, 48 h, and 96 h. The histogram bars indicate expression, and the error bars show means ± SEM. The log2 transformation method normalized and converted the RPKM values displayed
CsTALE genes expression hormonal applications, NaCl, and silica stress
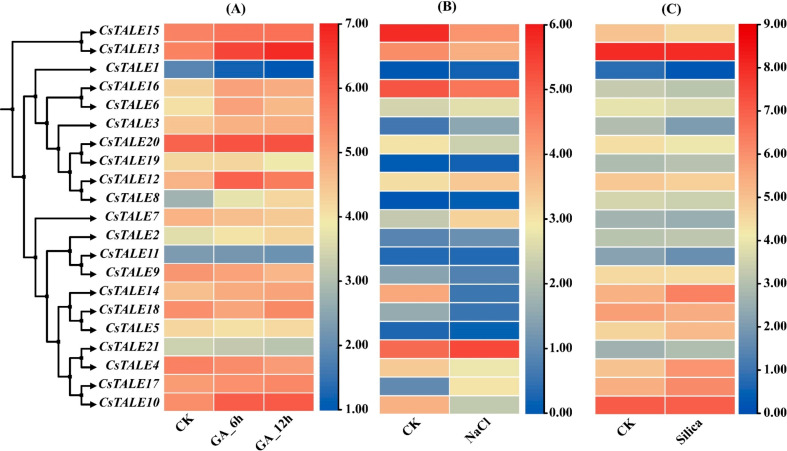
To further elucidate the potential role of the CsTALE gene family role in regulating the response of C. sativus in response to silica stress, NaCl stress, and the GA hormone treatment. We retrieved the expression data from the cucumber genome database and presented it in the form of a heatmap; the heatmap color from blue to red represents the expression percentages (Fig. 12). Under silica stress, only two genes, CsTALE13 and CsTALE10, displayed elevated expression. Several other genes, including CsTALE4, CsTALE7, and CsTALE14, were observed with moderate expression, whereas the rest of the CsTALE family genes were observed with lowest to no expressions (Fig. 13C).
Fig. 13.
Expression analysis of CsTALE genes in response to (a) GA treatment, (b) NaCl Stress, and (c) Silica stress. The expression data was retrieved from the cucumber genome database. The Log2 fold normalized data was presented in the form of a heatmap using TBtools-II software (Version 2.027)
CsTALE gene family members also observed a similar expression trend in response to NaCl stress. In comparison with the control group, fewer genes were highly expressed in response to NaCl stress, such as CsTALE21 (Fig. 13B). Numerous other genes were also found with moderate expressions, such as CsTALE13, CsALE15, and CsTALE16. Other members of the CsTALE gene family were found to have the lowest or no transcription. Finally, under the GA treatment, the CsTALE gene family members showed similar expression trends in both 6 h and 12 h treatments in comparison with the control (Fig. 13A). A single gene, CsTALE13, showed the dominant expression in both 6 h and 12 h, followed by CsALE10, CsTALE12, CsTALE15, and CsTALE20 with moderate expression. The CsTALE11 gene was observed with the lowest expression, and the rest of the CsTALE gene family members were found to the moderate to lowest expression, suggesting that the GA-induced CsTALE gene family is crucial in regulating the response of C. sativa to stressful conditions.
Discussion
TALE genes are widely distributed across the genome
Research on TALE, a widely dispersed gene family in eukaryotes, accelerated in the early 20th century [27]. Herein, the isolation of 21 CsTALE genes from the cucumber genome database was put forward for comprehensive bioinformatic analysis [28]. As expected, they are all predicted to be located in the nucleus (Table 1) and distributed randomly across 7 chromosomes (Fig. 4).
We built an unrooted phylogenetic tree to investigate the evolutionary relationships of the proteins encoded by the 21 genes. In contrast to earlier research, we categorized these proteins into seven distinct groups [29, 30]. The KNOX genes are categorized into two groups, whereas the BELL genes are further classified into five groups (Fig. 2). The annotations of the SMART database and the ML-phylogenetic tree constructed using the best model also support our classification results [20, 31, 32]. Each of the classes is represented by specific domains or domain combinations.
Gene structure study revealed that every CsTALE gene has an intron. Genomic architectures are highly conserved throughout members of the same family. Exon lengths are also quite similar among closely related members of the phylogenetic tree (Fig. 5). Consistent with earlier research on the poplar TALE family, protein motif analysis and annotation showed that members of the same class have identical protein motifs [33].
The cis-elements bind transcription factors selectively at the gene promoter region, regulating gene transcription. Several cis-elements associated with hormonal response and abiotic stress were discovered in the CsTALE promoter sequence, including methyl jasmonate, abscisic acid, and gibberellin (Fig. 8), which is comparable to previous research [11, 34, 35]. Findings suggested a conservative element in the CsTALE gene promoter. Research has linked ABRE to ABA induction, drought, and extreme salt stress in plants [36–38]. Several stress-related components are also present, including ARE, MBS, and LTR. According to the findings, the CsTALE gene family is involved in the abiotic stress that cucumbers experience. According to gene function prediction and protein-protein network analysis, the CsTALE family is key in controlling ovule and inflorescence development. Similarly to the previous study, gene functional prediction and protein-protein network analysis demonstrated that CsTALE15 and CsTALE17 interact with a variety of floral organ proteins [11].
TALE genes are crucial for growth and development
We investigated the tissue-specific expression of CsTALE genes because of the extensive literature demonstrating the TALE gene family’s critical function in plant development and growth. Differences in gene expression patterns among the three organs are clear. Of the 21 CsTALE genes, 7 are expressed more in stems than in other tissues (Fig. 11A). To guarantee accurate regulation, TALE expression is closely controlled by upstream genes and other internal and external variables. Many upstream regulators of TALE have been found to have a detrimental effect. The YABBY gene is a negative regulator of the TALE gene that inhibits the expression of class I KNOX genes (STM, BP/KNAT1, and KNAT2). Mutations in this gene cause improper lateral meristem development and the loss of lateral meristem polarity [39]. AS1 controls A. thaliana and Chinese cabbage (Brassica rapa) leaf dorsoventral polarity [39]. Multiple KNOX genes (GhKNL2/3/4) that are similar to AtKNAT1 were markedly elevated in GhAS1 or GhAS2 silenced cotton (Gossypium hirsutum) [39]. One negative regulator of blooming transition in tobacco (Nicotiana tabacum), the SHORT VEGETATIVE PHASE (SVP), controls pedicel length by directly inhibiting the KNAT1 gene NtBPL [39]. The gynoecium developmental program encourages the proper patterning of future fruit once it is established. TALE transcription factors are required for the formation of a number of distinct tissues [40]. At stage 6, the gynoecium creates a ridge of elevated cells around a central cleft and develops replum, valve borders, and valves. In the transverse plane, the adaxial inner side of the replum has a typical meristematic layered structure [40] and accordingly expresses the meristematic genes STM, CLV1/2, and CRN [41]. Only four genes were expressed at a high level in fruit tissue, suggesting their role in fruit development. The study by Jia et al. (2021) advocated the role of MdTALE genes in the fruit organ development of apple (Malus domestica) [42].
TALE genes regulate plant response to waterlogging stress
One significant phenotypic response to waterlogging stress is the formation of adventitious root (AR) in cucumber. The formation of AR is greatly influenced by the local production and movement of the plant hormone auxin [21]. Endogenous auxin in the hypocotyl rose 72 h post-waterlogging (WL) stress. The use of 10 mg/L of 1-naphthylacetic acid (NAA) improved AR formation [17]. Further investigation showed that auxin treatment elevated ethylene biosynthesis genes (CsACS1, CsACS2, CsACO5) and ROS signaling genes (CsRBOHB and CsRBOHF3) under WL stress. Treatment with 1-naphthylphthalamic acid (NPA), on the other hand, greatly reduced AR development [20]. Although exogenous NAA enhanced the production of AR, no elongation was seen. As the research showed, sugar treatment has been successful in achieving elongation of the AR [17]. A decrease in AR development and elongation was seen upon shoot removal. By dramatically increasing the expression of CsPIN1, CsPIN1b, CsPIN8, CsARF5, CsARF6, and CsSAUR29, sugar treatment (300 µL) positively influenced AR formation and elongation [17]. On the other hand, jasmonate, also known as MeJA, is shown to be a negative regulator of WL stress [20]. In our study, the CsTALE13 and CsTALE17 were significantly upregulated under WL, NAA, and ethylene (ETH) but not MeJA at all the timepoints (Fig. 12). We speculate that CsTALE13 and CsTALE17 could be potential candidates in enhancing cucumber tolerance to WL.
Conclusion
A total of 21 CsTALE genes were identified in the cucumber genome and categorized into 7 groups according to their evolutionary relationship. The presence of hormonal and stress-responsive cis-acting elements makes them key regulators of growth and immunity trade-off. The sponging of miR167 and miR319 to the CsTALE16 and CsTALE15 advocates the involvement of CsTALE genes in posttranscriptional regulation of growth and stress-related processes. The significant upregulation of CsTALE17 under WL and hormones makes it a possible candidate for functional studies to generate stress-resilient cucumber lines.
Materials and methods
Identification of TALE genes in Cucumis sativus
The entire set of sequence information came from the cucumber genome database (http://cucurbitgenomics.org/organism/2). 19 AtTALE proteins sequences from Arabidopsis were obtained from the Arabidopsis Information Resource (TAIR) website. The CsTALE genes in cucumbers were located using a two-stage BLAST search. For the first step, Arabidopsis TALE was utilized in order to look for potential cucumber TALE [43]. Following this, we used BLASTP (e-value, 1e-5) from the National Center for Biotechnology (NCBI; https://www.ncbi.nlm.nih.gov/) to further identify all potential cucumber TALE. Lastly, the SMART (http://smart.embl.de/) and Pfam (http://pfam.xfam.org/) databases were used to corroborate the candidate proteins [44, 45]. Additionally, the physiochemical properties of the TALE proteins in the studied plants were discovered using the ExPASy online server (http://web.expasy.org/protparam/).
Phylogenetic tree, conserved motifs, gene structure, and cis-acting elements analysis
Phylogenetic analysis was conducted using TALE protein sequences from the following plants: 21 CsTALE (C. sativus), 19 CmTALE (Cucumis melo), 23 SlTALE (Solanum lycopersicum), and 19 AtTALE (A. thaliana). The neighbor-joining (NJ) approach was used to create the phylogenetic tree in MEGAX software v.10.1.8 (https://www.megasoftware.net/). A bootstrap test with 1000 iterations was then performed. Utilizing the Evolview V3 (https://www.evolgenius.info//evolview/#login) display format, the results were prepared for presentation [46, 47]. Using TBtools-II software (Version 2.027), we were able to determine the structure of the cucumber CsTALE genes [48]. For the purpose of predicting conserved motifs of the CsTALE, the online application MEME, version 5.0.5 (http://meme-suite.org/tools/meme), was utilized. Also, using Gene Structure Display Server 2.0 (GSDS) (http://gsds.cbi.pku.edu.cn), we compared the gene sequences to the predicted coding sequences in order to conduct the gene structure analysis, which included exon and intron analysis [49, 50]. Finally, the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was utilized to examine the cis-acting elements in the 1.5 kb promoter upstream region of CsTALE genes with start codon ATG [20].
Chromosomal localization and prediction of miRNA target genes
Using TBtools-II, we were able to obtain the physical information of the CsTALE genes on the chromosomes [48]. A final step involved blasting the CDS sequence of 21 CsTALE genes using the online psRNATarget service (http://plantgrn.noble.org/psRNATarget/) in order to identify the cleaved miR319 and miR167 genes. The online server string (https://string-db.org) identified interaction proteins with CsTALE proteins [51].
Plant material and hormonal + waterlogging treatment
C. sativus seeds of the ‘CCMC’ variety (Chinese long) were used for the investigation. All fully developed plants were cultivated in the greenhouses of the Jiangsu Academy of Agriculture Science under natural photoperiod circumstances. For tissue-specific expression analysis, we used forceps to collect mature plants’ roots, stems, leaves, male flowers, and fruits from the field and then placed them in liquid nitrogen. Throughout the experiment, the seedlings were placed in 7 cm diameter pots that contained peat, vermiculite, and perlite in a 3:1:1 ratio (volume/volume). The pots were then placed in growth chambers that were maintained at a constant day temperature of 26 ± 2°C and a night temperature of 18°C, with a relative humidity ranging from 70 to 85%. The 100 µmol/L MeJA, 100 µmol/L NAA, and 100µmol/L ETH (Solarbio, Beijing, China) were used for treatment on the three-week-old seedling stage. For waterlogging stress the C. sativus was grown in plastic pots containing a 1:3:1 (v/v/v) mixture of peat, vermiculite and perlite. The pots were placed in a growth chamber with an interior humidity of 80% ± 5%, temperature of 28°C/20°C (14 h light/10 h dark) and light intensity of 300 µmol m− 2 sec− 1. For waterlogging treatment, the potted seedlings at 21 days after germination (with three true leaves) were moved into plastic cups filled with water to the top of the hypocotyls (approx. 4 cm above the soil surface). The volume of water was kept constant throughout the experiment. Plants were placed in the same cups for control treatments without adding extra water following the same procedure of Xu et al (2023) [52]. All samples were immediately frozen in liquid nitrogen and stored at -80°C until use.
Quantitative real-time PCR (qRT-PCR)
Using a plant total RNA purification kit from Tiangen (Beijing, China), we followed the manufacturer’s instructions to isolate the total RNA. Gene expression was measured using SYBR® Premix Ex Taq™ II (TaKaRa, China) and an iQ™ 5 Multicolor real-time PCR detection system (Bio-Rad, USA). Using the 2−ΔΔCT technique [53], the relative expression of genes was determined. In keeping with our prior research, we employed three biologically separate replicates for each treatment and normalized the mRNA abundance of each sample using the internal reference gene (Actin) [54].
Statistical analysis
The SPSS software (version 25.0, SPSS Inc., USA) was used for statistical analysis (ANOVA) and to determine statistical significance (P 0.05). Means with standard deviations (SDs) from three biologically independent replicates were used to express the results for all assessed parameters. According to Sharif et al. (2021) [55], the graphical representation was created using GraphPad Prism (version 9.4.1) from GraphPad Software, Inc. in La Jolla, CA, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R176) King Saud University, Riyadh, Saud Arabia.
Author contributions
Sheraz Ahmad and Naveed Ahmad Writing-original draft, Data analysis, Formal analysis. Khushboo Khan; Ibrahim A. Saleh: Data analysis, Investigation. Sheraz Ahmad: Writing-review & editing. Mohammad K. Okla, Ibrahim A. Alaraidh, Hamada AbdElgawad: Data analysis Muhammad Naeem: Writing-review & editing. Shah Fahad: Conceived and designed the experiments. Naveed Ahmad: supervision.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R176) King Saud University, Riyadh, Saud Arabia.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable. Study protocol must comply with relevant institutional, national, and international guidelines and legislation. Our experiment follows the with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Our experiment follows the with relevant institutional, national, and international guidelines and legislation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sheraz Ahmad, Email: dh20010@yzu.edu.cn.
Naveed Ahmad, Email: naveedjlau@gmail.com.
Shah Fahad, Email: shah_fahad80@yahoo.com.
References
- 1.Bürglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–80. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gehring WJ. Homeo boxes in the study of development. Science. 1987;236(4806):1245–52. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- 3.Jia P, Sharif R, Li Y, Sun T, Li S, Zhang X, Dong Q, Luan H, Guo S, Ren X, et al. The BELL1-like homeobox gene MdBLH14 from apple controls flowering and plant height via repression of MdGA20ox3. Int J Biol Macromol. 2023;242:124790. doi: 10.1016/j.ijbiomac.2023.124790. [DOI] [PubMed] [Google Scholar]
- 4.Hamant O, Pautot V. Plant development: a TALE story. CR Biol. 2010;333(4):371–81. doi: 10.1016/j.crvi.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Smith HMS, Hake S. The Interaction of Two Homeobox Genes, BREVIPEDICELLUS and PENNYWISE, regulates Internode Patterning in the Arabidopsis inflorescence. Plant Cell. 2003;15(8):1717–27. doi: 10.1105/tpc.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, et al. The pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2015;44(D1):D279–85. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350(6315):241–3. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Wang N, Hao P, Sun H, Wang C, Ma L, Wang H, Zhang X, Wei H, Yu S. Genome-wide identification and characterization of TALE superfamily genes in cotton reveals their functions in regulating secondary cell wall biosynthesis. BMC Plant Biol. 2019;19(1):432. doi: 10.1186/s12870-019-2026-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Sun F, Ahmad N, Jin L, Xinyue Z, Xintong M, Hoang NQ, Mallano AI, Wang N, Zhuoda Y, Liu X. Genome-wide investigation of Hydroxycinnamoyl CoA: Shikimate Hydroxycinnamoyl Transferase (HCT) gene family in Carthamus tinctorius L. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2021;49(3):12489. doi: 10.15835/nbha49312489. [DOI] [Google Scholar]
- 10.Guo C, Quan S, Zhang Z, Kang C, Liu J, Niu J. Genome-wide identification, characterization and expression profile of TALE gene family in (Juglans regia L) Sci Hort. 2022;297:110945. doi: 10.1016/j.scienta.2022.110945. [DOI] [Google Scholar]
- 11.Jia P, Sharif R, Li Y, Sun T, Li S, Zhang X, Dong Q, Luan H, Guo S, Ren X et al. The BELL1-like homeobox gene MdBLH14 from apple controls flowering and plant height via repression of MdGA20ox3. Int J Biol Macromol 2023:124790. [DOI] [PubMed]
- 12.Zhang L, Zhang X, Ju H, Chen J, Wang S, Wang H, Zhao Y, Chang Y. Ovate family protein1 interaction with BLH3 regulates transition timing from vegetative to reproductive phase in Arabidopsis. Biochem Biophys Res Commun. 2016;470(3):492–7. doi: 10.1016/j.bbrc.2016.01.135. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Cho YH, Ryu H, Kim Y, Kim TH, Hwang I. BLH1 and KNAT3 modulate ABA responses during germination and early seedling development in Arabidopsis. Plant Journal: Cell Mol Biology. 2013;75(5):755–66. doi: 10.1111/tpj.12236. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Liu X, Ahmad N, Wang Y, Ge H, Wang Y, Liu W, Li X, Wang N, Wang F. CePP2C19 confers tolerance to drought by regulating the ABA sensitivity in Cyperus esculentus. BMC Plant Biol. 2023;23(1):524. doi: 10.1186/s12870-023-04522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statista . In. Statista Inc. 3 World Trade Center 175 Greenwich Street; 36th floor New York, NY 10007. United States: Statista; 2023. [Google Scholar]
- 16.Zhao Z, Qi Y, Yang Z, Cheng L, Sharif R, Raza A, Chen P, Hou D, Li Y. Exploring the Agrobacterium-mediated transformation with CRISPR/Cas9 in cucumber (Cucumis sativus L) Mol Biol Rep. 2022;49(12):11481–90. doi: 10.1007/s11033-022-07558-z. [DOI] [PubMed] [Google Scholar]
- 17.Sharif R, Su L, Chen X, Qi X. Involvement of auxin in growth and stress response of cucumber. Vegetable Res 2022:1–9.
- 18.Raza A, Mubarik MS, Sharif R, Habib M, Jabeen W, Zhang C, Chen H, Chen Z-H, Siddique KHM, Zhuang W, et al. Developing drought-smart, ready-to-grow future crops. Plant Genome. 2023;16(1):e20279. doi: 10.1002/tpg2.20279. [DOI] [PubMed] [Google Scholar]
- 19.Raza A, Charagh S, Zahid Z, Mubarik MS, Javed R, Siddiqui MH, Hasanuzzaman M. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep 2020:1–29. [DOI] [PubMed]
- 20.Pan J, Tu J, Sharif R, Qi X, Xu X, Chen X. Study of JASMONATE ZIM-Domain gene family to waterlogging stress in Cucumis sativus L. Vegetable Res. 2021;1(1):1–12. doi: 10.48130/VR-2021-0003. [DOI] [Google Scholar]
- 21.Pan J, Sharif R, Xu X, Chen X. Waterlogging Response mechanisms in plants: Research Progress and prospects. Front Plant Sci. 2020;11:2319. doi: 10.3389/fpls.2020.627331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou Y, Wang Y, Liu X, Ahmad N, Wang N, Jin L, Yao N, Liu X. A cinnamate 4-HYDROXYLASE1 from Safflower promotes flavonoids Accumulation and stimulates antioxidant Defense System in Arabidopsis. Int J Mol Sci. 2023;24(6):5393. doi: 10.3390/ijms24065393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raza A, Charagh S, Zahid Z, Mubarik MS, Javed R, Siddiqui MH, Hasanuzzaman M. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep 2020. [DOI] [PubMed]
- 24.Raza A. Metabolomics: a systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep 2020:1–23. [DOI] [PubMed]
- 25.Zhang X, Ahmad N, Zhang Q, Wakeel Umar A, Wang N, Zhao X, Zhou K, Yao N, Liu X. Safflower Flavonoid 3′ 5′ hydroxylase promotes Methyl Jasmonate-Induced Anthocyanin Accumulation in transgenic plants. Molecules. 2023;28(7):3205. doi: 10.3390/molecules28073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N, Jianyu L, Xu T, Noman M, Jameel A, Na Y, Yuanyuan D, Nan W, Xiaowei L, Fawei W. Overexpression of a novel cytochrome P450 promotes flavonoid biosynthesis and osmotic stress tolerance in transgenic Arabidopsis. Genes. 2019;10(10):756. doi: 10.3390/genes10100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S, Chen Y, Shah AZ, Wang H, Xi C, Zhu H, Ge L. The homeodomain-leucine Zipper genes Family regulates the Jinggangmycin Mediated Immune Response of Oryza sativa to Nilaparvata lugens, and Laodelphax striatellus. Bioengineering. 2022;9(8):398. doi: 10.3390/bioengineering9080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Y, Wu S, Bai Y, Sun H, Jiao C, Guo S, Zhao K, Blanca J, Zhang Z, Huang S. Cucurbit Genomics Database (CuGenDB): a central portal for comparative and functional genomics of cucurbit crops. Nucleic Acids Res. 2018;47(D1):D1128–36. doi: 10.1093/nar/gky944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Zhang L, Yan L, Xiong X, Wang W, Zhang X-H, Min D-H. Genome-wide analysis of TALE superfamily in Triticum aestivum reveals TaKNOX11-A is involved in abiotic stress response. BMC Genomics. 2022;23(1):89. doi: 10.1186/s12864-022-08324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zhao P, Cheng B, Zhang Y, Shen Y, Wang X, Zhang Q, Lou Q, Zhang S, Wang B et al. Identification of TALE Transcription Factor Family and expression patterns related to Fruit Chloroplast Development in Tomato (Solanum lycopersicum L). Int J Mol Sci 2022, 23(9). [DOI] [PMC free article] [PubMed]
- 31.Ullah U, Shalmani A, Ilyas M, Raza A, Ahmad S, Shah AZ, Khan FU, AzizUd D, Bibi A, Rehman SU, et al. BZR proteins: identification, evolutionary and expression analysis under various exogenous growth regulators in plants. Mol Biol Rep. 2022;49(12):12039–53. doi: 10.1007/s11033-022-07814-2. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y, Lv Y, Zhang J, Ahmad N, Li X, Yao N, Liu X, Li H. The safflower MBW complex regulates HYSA accumulation through degradation by the E3 ligase CtBB1. J Integr Plant Biol 2023. [DOI] [PubMed]
- 33.Zhao K, Zhang X, Cheng Z, Yao W, Li R, Jiang T, Zhou B. Comprehensive analysis of the three-amino-acid-loop-extension gene family and its tissue-differential expression in response to salt stress in poplar. Plant Physiol Biochem. 2019;136:1–12. doi: 10.1016/j.plaphy.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Zhao Y, Yan M, Zhao H, Zhang X, Yuan Z. Genome-wide identification and expression analysis of TALE Gene Family in Pomegranate (Punica granatum L) Agronomy. 2020;10(6):829. doi: 10.3390/agronomy10060829. [DOI] [Google Scholar]
- 35.Hong Y, Ahmad N, Zhang J, Lv Y, Zhang X, Ma X, Xiuming L, Na YJMG. Genomics: genome-wide analysis and transcriptional reprogrammings of MYB superfamily revealed positive insights into abiotic stress responses and anthocyanin accumulation in Carthamus tinctorius L. 2022:1–21. [DOI] [PubMed]
- 36.Muhammad I, Jing X-Q, Shalmani A, Ali M, Yi S, Gan P-F, Li W-Q, Liu W-T, Chen K-M. Comparative in Silico Analysis of Ferric Reduction Oxidase (FRO) genes expression patterns in response to Abiotic stresses, metal and hormone applications. Molecules. 2018;23(5):1163. doi: 10.3390/molecules23051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalmani A, Fan S, Jia P, Li G, Muhammad I, Li Y, Sharif R, Dong F, Zuo X, Li K. Genome Identification of B-BOX Gene Family members in seven Rosaceae species and their expression analysis in response to Flower Induction in Malus domestica. Molecules. 2018;23(7):1763. doi: 10.3390/molecules23071763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing X-Q, Shalmani A, Zhou M-R, Shi P-T, Muhammad I, Shi Y, Sharif R, Li W-Q, Liu W-T, Chen K-M. Genome-wide identification of malectin/malectin-like domain containing protein family genes in rice and their expression regulation under various hormones, abiotic stresses, and heavy metal treatments. J Plant Growth Regul 2019:1–15.
- 39.Jia P, Wang Y, Sharif R, Dong Q-l, Liu Y, Luan H-a. Zhang X-m, Guo S-p, Qi G-h: KNOTTED1-like homeobox (KNOX) transcription factors - hubs in a plethora of networks: a review. Int J Biol Macromol. 2023;253:126878. doi: 10.1016/j.ijbiomac.2023.126878. [DOI] [PubMed] [Google Scholar]
- 40.Arnaud N, Pautot V. Ring the BELL and tie the KNOX: roles for TALEs in gynoecium development. Front Plant Sci 2014, 5. [DOI] [PMC free article] [PubMed]
- 41.Jia P, Xing L, Zhang C, Chen H, Li Y, Zhang D, Ma J, Zhao C, Han M, Ren X, et al. MdKNOX15, a class I knotted-like transcription factor of apple, controls flowering and plant height by regulating GA levels through promoting the MdGA2ox7 transcription. Environ Exp Bot. 2021;185:104411. doi: 10.1016/j.envexpbot.2021.104411. [DOI] [Google Scholar]
- 42.Jia P, Xing L, Zhang C, Zhang D, Ma J, Zhao C, Han M, Ren X, An N. MdKNOX19, a class II knotted-like transcription factor of apple, plays roles in ABA signalling/sensitivity by targeting ABI5 during organ development. Plant Sci. 2021;302:110701. doi: 10.1016/j.plantsci.2020.110701. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad S, Ali S, Shah AZ, Khan A, Faria S. Chalcone synthase (CHS) family genes regulate the growth and response of cucumber (Cucumis sativus L.) to Botrytis Cinerea and abiotic stresses. Plant Stress. 2023;8:100159. doi: 10.1016/j.stress.2023.100159. [DOI] [Google Scholar]
- 44.Ahmad S, Zhang J, Wang H, Zhu H, Dong Q, Zong S, Wang T, Chen Y, Ge L. The Phosphoserine phosphatase alters the free amino acid compositions and fecundity in Cyrtorhinus lividipennis Reuter. Int J Mol Sci. 2022;23(23):15283. doi: 10.3390/ijms232315283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Li L, ShangGuan G, Jia C, Deng S, Noman M, Liu Y, Guo Y, Han L, Zhang X. Genome-wide identification and expression analysis of bZIP gene family in Carthamus tinctorius L. Sci Rep. 2020;10(1):1–15. doi: 10.1038/s41598-020-72390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian B, Gao S, Lercher MJ, Hu S, Chen W-H. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):W270–5. doi: 10.1093/nar/gkz357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad N, Tian R, Lu J, Li G, Sun J, Lin R, Zhao C, Zhou C, Chang H, Zhao S, et al. DNA fingerprinting and genetic diversity analysis in Asparagus officinalis L. cultivars using microsatellite molecular markers. Genet Resour Crop Evol. 2023;70(4):1163–77. doi: 10.1007/s10722-022-01493-5. [DOI] [Google Scholar]
- 48.Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, et al. TBtools-II: a one for all, all for one bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–42. doi: 10.1016/j.molp.2023.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2014;31(8):1296–7. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad N, Li T, Liu Y, Hoang NQV, Ma X, Zhang X, Liu J, Yao N, Liu X, Li H. Molecular and biochemical rhythms in dihydroflavonol 4-reductase- mediated regulation of leucoanthocyanidin biosynthesis in Carthamus tinctorius L. Ind Crops Prod. 2020;156:112838. doi: 10.1016/j.indcrop.2020.112838. [DOI] [Google Scholar]
- 51.Ahmad S, Ali S, Shah AZ, Khan A, Faria S. Chalcone synthase (CHS) family genes regulate the growth and response of cucumber (Cucumis sativus L.) to Botrytis Cinerea and abiotic stresses. Plant Stress 2023:100159.
- 52.Xu X, Liu M, Hu Q, Yan W, Pan J, Yan Y, Chen X. A CsEIL3-CsARN6.1 module promotes waterlogging-triggered adventitious root formation in cucumber by activating the expression of CsPrx5. Plant J. 2023;114(4):824–35. doi: 10.1111/tpj.16172. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad N, Zhang K, Ma J, Yuan M, Zhao S, Wang M, Deng L, Ren L, Gangurde SS, Pan J, et al. Transcriptional networks orchestrating red and pink testa color in peanut. BMC Plant Biol. 2023;23(1):44. doi: 10.1186/s12870-023-04041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaman QU, Hussain MA, Khan LU, Hui L, Khan D, Khokhar AA, Cui J, Raza A, Wang H-F. Genome-wide identification and expression profiling of APX gene family under multifactorial stress combinations and melatonin-mediated tolerance in pitaya. Sci Hort. 2023;321:112312. doi: 10.1016/j.scienta.2023.112312. [DOI] [Google Scholar]
- 55.Sharif R, Liu P, Wang D, Jin Z, Uzair U, Yadav V, Mujtaba M, Chen P, Li Y. Genome-wide characterisation and expression analysis of cellulose synthase genes superfamily under various environmental stresses in Cucumis sativus L. New Zeal J Crop Hort. 2021;49(2–3):127–50. doi: 10.1080/01140671.2021.1926291. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.