Abstract
Neutralizing antibodies were assessed before and after intravenous challenge with pathogenic SIVsmE660 in rhesus macaques that had been immunized with recombinant modified vaccinia virus Ankara expressing one or more simian immunodeficiency virus gene products (MVA-SIV). Animals received either MVA-gag-pol, MVA-env, MVA-gag-pol-env, or nonrecombinant MVA. Although no animals were completely protected from infection with SIV, animals immunized with recombinant MVA-SIV vaccines had lower virus loads and prolonged survival relative to control animals that received nonrecombinant MVA (I. Ourmanov et al., J. Virol. 74:2740–2751, 2000). Titers of neutralizing antibodies measured with the vaccine strain SIVsmH-4 were low in the MVA-env and MVA-gag-pol-env groups of animals and were undetectable in the MVA-gag-pol and nonrecombinant MVA groups of animals on the day of challenge (4 weeks after final immunization). Titers of SIVsmH-4-neutralizing antibodies remained unchanged 1 week later but increased approximately 100-fold 2 weeks postchallenge in the MVA-env and MVA-gag-pol-env groups while the titers remained low or undetectable in the MVA-gag-pol and nonrecombinant MVA groups. This anamnestic neutralizing antibody response was also detected with T-cell-line-adapted stocks of SIVmac251 and SIV/DeltaB670 but not with SIVmac239, as this latter virus resisted neutralization. Most animals in each group had high titers of SIVsmH-4-neutralizing antibodies 8 weeks postchallenge. Titers of neutralizing antibodies were low or undetectable until about 12 weeks of infection in all groups of animals and showed little or no evidence of an anamnestic response when measured with SIVsmE660. The results indicate that recombinant MVA is a promising vector to use to prime for an anamnestic neutralizing antibody response following infection with primate lentiviruses that cause AIDS. However, the Env component of the present vaccine needs improvement in order to target a broad spectrum of viral variants, including those that resemble primary isolates.
Efforts to develop an AIDS vaccine have included the use of recombinant poxvirus vectors that are engineered to express one or more gene products of human immunodeficiency virus type 1 (HIV-1) (12, 15, 27). Vectors such as these have the potential to generate virus-specific CD8+ cytotoxic T lymphocytes (CTL) and neutralizing antibodies (7) as two immune responses considered important for HIV-1 vaccine efficacy (14). Studies in macaques have shown that recombinant vaccinia virus vectors containing the Env glycoproteins of simian immunodeficiency virus (SIV) prime B cells to produce low levels of SIV-specific neutralizing antibodies and that subsequent boosting with subunit protein can dramatically elevate the levels of those antibodies (20, 21). A similar priming and boosting effect for neutralizing antibody production has been observed in phase I clinical trials of candidate HIV-1 vaccines consisting of recombinant vaccinia or canarypox virus vectors followed by Env glycoprotein inoculation (1, 5, 6, 41). These results suggest that recombinant poxviruses might prime for a similar secondary (anamnestic) neutralizing antibody response following virus infection. Hu et al. showed that a recombinant vaccinia virus vector containing HIV-1 gp160 (strain LAV) primed for anamnestic neutralizing antibody production in chimpanzees following challenge with homologous virus (22). Although it is currently unknown whether an accelerated neutralizing antibody response would provide a clinical benefit in HIV-1-infected individuals, the fact that many months are needed for neutralizing antibodies to rise to detectable levels following initial infection (24, 34, 40, 42) leaves open the possibility that it will.
We sought to determine whether prior inoculation with a recombinant attenuated poxvirus known as modified vaccinia virus Ankara (MVA) and containing the Env glycoproteins of SIV would prime B cells for an anamnestic neutralizing antibody response in rhesus macaques (Macaca mulatta) after intravenous challenge with pathogenic SIVsmE660 in cases where complete protection was not achieved. Four groups of six macaques received four inoculations intramuscularly with 108 PFU of either MVA, MVA-gag-pol, MVA-env, or MVA-gag-pol-env. Each of these poxvirus vectors has been described previously (17, 39, 45) and contained SIV components derived from nonpathogenic, molecularly cloned SIVsmH-4 (18). The animals were challenged intravenously with the related uncloned, highly pathogenic SIVsmE660 (19, 23) 4 weeks after final boosting. Although all animals became infected, those that received MVA-gag-pol, MVA-env, and MVA-gag-pol-env had lower plasma viral RNA (P = 0.0016) and prolonged survival relative to animals that received nonrecombinant MVA (39). There were no significant differences in the levels of plasma viremia between the three groups of animals receiving recombinant MVAs. Plasma samples were obtained prior to vaccination, on the day of challenge, and at multiple times for up to 28 weeks postchallenge. Neutralizing activity against SIV was assessed in a CEMx174-cell-killing assay as described previously (32). Unless indicated otherwise, virus stocks were produced in either H9 cells (SIVsmH-4, SIVmac251, and SIV/DeltaB670), CEMx174 cells (SIVsmE660), or rhesus peripheral blood mononuclear cells (PBMC) (SIVmac239). An exception was one set of neutralization assays that was performed with the original animal challenge stock of SIVsmE660 grown in rhesus PBMC.
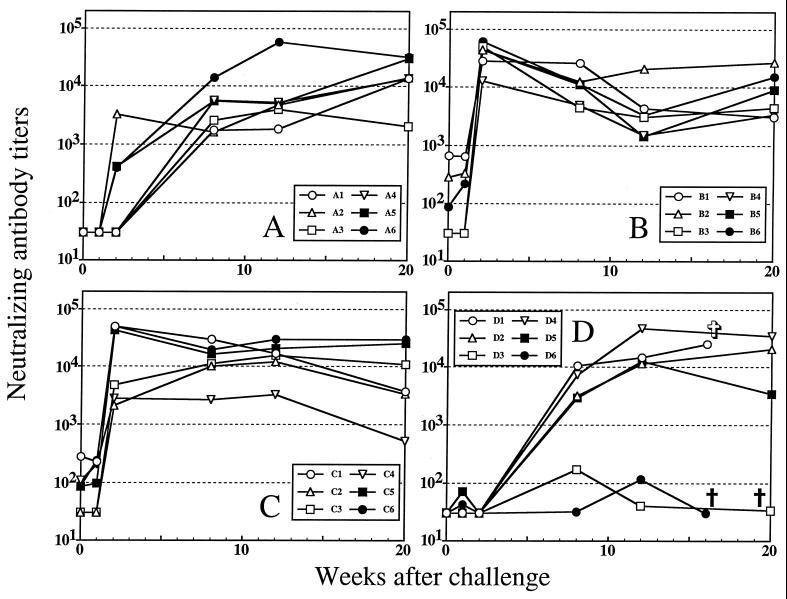
Neutralizing antibodies were first assessed with the vaccine strain of the virus, SIVsmH-4. The results are shown in Fig. 1. No SIVsmH-4-neutralizing antibodies were detected on the day of challenge in animals that received nonrecombinant MVA or MVA-gag-pol, which is consistent with the absence of env in these vaccines. Low titers of SIVsmH-4-neutralizing antibodies were detected on the day of challenge in three recipients of MVA-env (titers of 86 to 663) and four recipients of MVA-gag-pol-env (titers of 85 to 274). The titers remained essentially unchanged 1 week later for all animals. Titers of SIVsmH-4-neutralizing antibodies increased dramatically 2 weeks postchallenge in the MVA-env (average titer, 39,848) and MVA-gag-pol-env (average titer, 25,160) and remained low or undetectable in the MVA-gag-pol and nonrecombinant MVA groups at this time. These results suggest that MVA-env and MVA-gag-pol-env primed B cells sufficiently to permit a rapid and dramatic anamnestic neutralizing antibody response between 1 and 2 weeks postchallenge. A similar anamnestic antibody response was detected by SIVsmH-4 gp130 enzyme-linked immunosorbent assay (39). Nearly all animals had high titers of SIVsmH-4-neutralizing antibodies 8 weeks postchallenge (Fig. 1). Exceptions at 8 weeks were two animals in the nonrecombinant MVA group, whose neutralization titers were extremely low (animals D3 and D6). These two animals progressed to AIDS very rapidly (39). Early onset of virus-induced immune suppression in the two rapid progressors could account for their poor antibody-neutralizing response.
FIG. 1.
Time course of SIVsmH-4-neutralizing antibody production following intravenous inoculation of vaccinated macaques with SIVsmE660. SIVsmH-4-neutralizing antibodies were measured in plasma samples obtained on the day of challenge and at multiple time points for 12 weeks postchallenge. Titers of neutralizing antibodies are the reciprocal plasma dilution at which 50% of CEMx174 cells were protected from virus-induced cell killing. Undetectable neutralization was given a value of 30, which was the lowest reciprocal plasma dilution tested. Panel A, MVA-gag-pol; panel B, MVA-env; panel C, MVA-gag-pol-env; panel D, MVA. †, animals sacrificed because of clinical manifestations of AIDS; ✞, animal that died of causes unrelated to AIDS.
Neutralizing antibodies were next assessed with SIVsmE660. This virus is an uncloned quasispecies of the same parental strain from which SIVsmH-4 was derived (18) and, therefore, is closely related to SIVsmH-4 genetically. As shown in Fig. 2, all animals were negative for SIVsmE660-neutralizing antibodies on the day of challenge. Also, no neutralization of this virus was detected 2 weeks postchallenge, and little neutralization was detected 8 weeks postchallenge despite the fact that most animals had very high SIVsmH-4 neutralization titers at one or both of these time points. It is important to note that preferential neutralization of SIVsmH-4 by week-8 plasma samples was seen for all groups of animals, including those that received recombinant MVAs lacking Env (i.e., MVA-gag-pol and nonrecombinant MVA). This outcome indicates that the inability to neutralize SIVsmE660 was not related to vaccine-induced immune interference associated with SIVsmH-4 Env priming (38). The outcome is more likely explained by epitopes shared by both viruses that, although they are highly immunogenic in infected macaques, are not adequately exposed for antibody binding on the native SIVsmE660 Env complex relative to their exposure on the native SIVsmH-4 Env complex. On the basis of immunophenotype and implied differences in native envelope glycoprotein structure, SIVsmH-4 resembles a T-cell-line-adapted (TCLA) strain, whereas SIVsmE660 resembles primary isolates of HIV-1 (3, 35, 49).
FIG. 2.
Time course of challenge-strain-neutralizing antibody production following intravenous inoculation of vaccinated macaques with SIVsmE660. SIVsmE660-neutralizing antibodies were measured in plasma samples obtained on the day of challenge and at multiple time points for 28 weeks postchallenge. Titers of neutralizing antibodies are the reciprocal plasma dilution at which 50% of CEMx174 cells were protected from virus-induced cell killing. Undetectable neutralization was given a value of 30, which was the lowest reciprocal plasma dilution tested. Panel A, MVA-gag-pol; panel B, MVA-env; panel C, MVA-gag-pol-env; panel D, MVA. †, animals sacrificed because of clinical manifestations of AIDS; ✞, animal that died of causes unrelated to AIDS.
Neutralization of SIVsmE660 was first detected 12 weeks postchallenge with plasmas from a subset of animals in each group, where the titers either peaked at this time or continued to rise for at least 20 to 28 weeks. Peak titers of SIVsmE660-neutralizing antibodies never reached the levels observed with SIVsmH-4 and, in fact, were always >10-fold lower in magnitude, again indicating that SIVsmE660 is much less sensitive to antibody-mediated neutralization than SIVsmH-4. It should be noted that the rapid progressors in the MVA group that developed low levels of neutralizing antibody to SIVsmH-4 also produced no antibodies that neutralized SIVsmE660.
The difficulty by which SIVsmE660 was neutralized relative to SIVsmH-4 suggests that detection of an anamnestic neutralizing antibody response targeting SIVsmE660 would be delayed relative to the response measured with SIVsmH-4. The MVA-gag-pol-env group of animals was the only case where an anamnestic neutralizing antibody response might have been detected with SIVsmE660. For example, two of six animals in this group had low titers of SIVsmE660-neutralizing antibodies 8 weeks postchallenge. By comparison, all animals in the remaining groups were negative (<30) at this time. In addition, five of six animals in the MVA-gag-pol-env group had titers of SIVsmE660-neutralizing antibodies that surpassed those in the MVA-gag-pol and MVA-env groups of animals 12 weeks postchallenge. However, because the magnitude of neutralization in the MVA-gag-pol-env animals was not much different from the nonrecombinant MVA group, any anamnestic response targeting SIVsmE660 was probably weak.
We next examined whether an anamnestic neutralizing antibody response could be detected with other strains of SIV. For this, plasma samples from three animals in each group were assessed for their ability to neutralize highly neutralization-sensitive, TCLA stocks of SIV/DeltaB670 (50) and SIVmac251 (25, 30), and a neutralization-insensitive stock of molecularly cloned SIVmac239/nef-open (30, 32). Because the anamnestic responses detected with SIVsmH-4 were fairly uniform, we selected plasma samples randomly from a subset of animals in each group for these assessments. The results shown in Tables 1 and 2 include titers measured with SIVsmH-4 and SIVsmE660 derived from Fig. 1 and 2 for comparison. Table 1 shows that an anamnestic neutralizing antibody response was detectable with SIV/DeltaB670 and, to a lesser extent, with SIVmac251 2 weeks postchallenge in the MVA-env and MVA-gag-pol-env groups of animals. Table 2 shows that the anamnestic response was no longer evident 8 weeks postchallenge, which was similar to the results obtained with SIVsmH-4. Table 2 also shows that all 8-week plasma samples failed to neutralize SIVmac239. We conclude that the neutralizing activity of the antibodies produced during the anamnestic phase, and shortly thereafter, were highly specific for SIVsmH-4 and heterologous TCLA strains of SIV.
TABLE 1.
Magnitude and cross-reactivity of neutralizing antibodies detected 2 weeks post-virus challenge
| Vaccine group and animal no.a | Nab titer to SIV ofb:
|
||
|---|---|---|---|
| smH-4 | DeltaB670 | mac251 | |
| MVA-SIV-gag-pol | |||
| A4 | <30 | <20 | <20 |
| A5 | 420 | 31 | <20 |
| A6 | 396 | 30 | <20 |
| MVA-SIV-env | |||
| B1 | 28,478 | 2,116 | 45 |
| B5 | 43,680 | 5,947 | 831 |
| B6 | 60,309 | 3,483 | 37 |
| MVA-SIV-gag-pol-env | |||
| C1 | 50,072 | 6,034 | 969 |
| C5 | 42,297 | 3,700 | 958 |
| C6 | 48,938 | 2,561 | 48 |
| MVA | |||
| D1 | <20 | <20 | <20 |
| D2 | <20 | <20 | <20 |
| D4 | <20 | <20 | 44 |
Plasma samples from three animals in each of four immunization groups were obtained 2 weeks post-intravenous challenge with SIVsmE660.
Plasma samples were assessed for antibodies neutralizing SIVsmH-4, SIV/DeltaB670, and SIVmac251 in CEMx174 cells as described in the text. Titers of neutralizing antibodies are the reciprocal plasma dilution at which 50% of cells were protected from virus-induced killing.
TABLE 2.
Magnitude and cross-reactivity of neutralizing antibodies detected 8 weeks post-virus challenge
| Vaccine group and animal no.a | Nab titer to SIV ofb:
|
||||
|---|---|---|---|---|---|
| smH-4 | E660 | DeltaB670 | mac251 | mac239 | |
| MVA-SIV-gag-pol | |||||
| A4 | 5,788 | <30 | 2,011 | 476 | <20 |
| A5 | 5,539 | <30 | 1,978 | 343 | <20 |
| A6 | 14,168 | <30 | 3,713 | 503 | <20 |
| MVA-SIV-env | |||||
| B1 | 26,347 | <30 | 1,968 | 162 | <20 |
| B5 | 11,279 | <30 | 1,599 | 756 | <20 |
| B6 | 11,546 | <30 | 506 | <20 | <20 |
| MVA-SIV-gag-pol-env | |||||
| C1 | 29,281 | <30 | 4,127 | 858 | <20 |
| C5 | 16,310 | 180 | 2,950 | 1,015 | <20 |
| C6 | 19,422 | <30 | 2,494 | <20 | <20 |
| MVA | |||||
| D1 | 10,633 | <30 | 4,689 | 661 | <20 |
| D2 | 3,206 | <30 | 1,118 | 345 | <20 |
| D4 | 7,405 | <30 | 2,465 | 337 | <20 |
Plasma samples from three animals in each of four immunization groups were obtained 8 weeks post-intravenous challenge with SIVsmE660.
Plasma samples were assessed for antibodies neutralizing SIVsmH-4, SIVsmE660, SIV/DeltaB670, SIVmac251, and molecularly cloned SIVmac239 in CEMx174 cells as described in the text. Titers of neutralizing antibodies are the reciprocal plasma dilution at which 50% of cells were protected from virus-induced killing.
The fact that passively administered neutralizing antibodies have proven effective against AIDS viruses in macaques (4, 8, 13, 28, 33, 46) and hu-PBL-SCID mice (9) suggests that neutralizing antibody induction would benefit an HIV-1 vaccine. How much of a benefit the antibodies provide may depend on their ability to neutralize diverse genetic variants of the virus, including primary isolates (9, 33, 46). Antibodies produced during the anamnestic phase in our vaccinated animals did not neutralize SIVsmE660, making it uncertain that the antibodies contributed to the partial efficacy observed (39). The fact that equal levels of efficacy were achieved regardless of whether env was present in the recombinant MVA vaccine (39) is further evidence that neutralizing antibodies probably contributed little. Nonetheless, it was possible that our assay stock of SIVsmE660 produced in CEMx174 cells was less sensitive to neutralization than the animal challenge stock produced in rhesus PBMC and, therefore, underestimated neutralization potency. To address this possibility, we performed neutralization assays with the animal challenge stock of SIVsmE660 without further passage. Because this stock was limited in supply and is valuable for animal challenges, our assessments were made on a subset of plasma samples. We selected five samples obtained during the anamnestic phase (2 weeks postchallenge) that contained high titers of SIVsmH-4-neutralizing antibodies (animals B1, B5, B6, C1, and C3), and three samples obtained 28 weeks postchallenge that neutralized our assay stock of SIVsmE660. As can be seen in Table 3, titers of neutralizing antibodies obtained with the 28-week-postchallenge samples were similar for both stocks of virus in two of three cases. Also, we showed that 50% protection from virus-induced cell killing corresponds very closely to a 90% reduction in p27 Gag antigen synthesis in these assays (Table 3). Although the titer was approximately 4 times higher with the animal challenge stock in one case (animal B6), we do not consider this to be a major difference with respect to occasional assay-to-assay variation. No neutralization of the challenge stock of SIVsmE660 was detected with plasmas obtained during the anamnestic phase, which agreed with results obtained with our assay stock of the virus.
TABLE 3.
Comparative neutralization of the assay and animal challenge stocks of SIVsmE660
| Animal no.a | Nab titer measured with SIVsmE660b:
|
||
|---|---|---|---|
| Cell viability
|
Reduction in p27
|
||
| Assay stock | Challenge stock | Challenge stock | |
| B5 | 1,082 | 1,340 | 2,118 |
| B6 | 1,841 | 7,502 | 7,405 |
| C1 | 470 | 384 | 181 |
Plasma samples were obtained 28 weeks post-intravenous inoculation with SIVsmE660.
Neutralization was measured with the assay stock of SIVsmE660 grown in CEMx174 cells and the animal challenge stock of the same virus grown in macaque PBMC. Assays were performed with CEMx174 cells as described in the text with one modification: cells exposed to the animal challenge stock of virus were washed three times with growth medium 1 day after the addition of cells in order to remove the virus inoculum and residual anti-p27 antibodies. Culture supernatants were harvested for p27 quantification at the time cells were stained for viability. Neutralization titers are the reciprocal plasma dilution where either 50% of cells were protected from virus-induced killing or p27 synthesis was reduced 90% relative to virus control wells (no plasma sample). The average amount of p27 present in the virus control wells was 95 ng/ml.
We conclude that SIVsmH-4 Env did not prime for a secondary neutralizing antibody response to SIVsmE660 in these studies. However, there are a number of cases where antibodies lacking detectable neutralizing activity in vitro were nonetheless capable of preventing infection by other viruses (10, 26, 29, 36, 37, 43, 44), and at least one example exists in the SIV-macaque model (48). With this in mind, we do not wish to exclude the possibility that nonneutralizing antibodies, particularly those involved in antibody-dependent cytotoxicity (47) and immune complex clearance (31), played a role in our studies.
The ability to prime for a more-broadly cross-reactive neutralizing antibody response with this vaccine candidate will most likely depend on the nature of the immunogen(s) incorporated into the vector. One example would be to use multiple recombinant MVA vectors, each containing the Env glycoproteins from a different strain of virus as a single vaccination modality. An alternate strategy would be to incorporate the Env glycoproteins from a single strain of virus after modifying them to present conserved neutralization epitopes in a highly immunogenic configuration. An important first step will be to determine whether greater efficacy can be achieved in the present model by priming for a neutralizing antibody response that is capable of targeting the challenge virus. This can be tested by incorporating the Env glycoproteins from a strain of virus that is closely matched to the challenge virus in terms of antigenicity and quasispecies complexity. We are in the process of pursuing this goal by using the highly pathogenic, molecularly cloned SIVsmE543-3 as both the vaccine and challenge strain. This virus exhibits a neutralization-resistant phenotype reminiscent of primary HIV-1 isolates (16).
With the majority of HIV-1 transmissions occurring in developing countries that have limited financial resources, the high cost of Env glycoprotein production, especially in the case of a polyvalent vaccine, will be a major economic challenge for global immunization (2). Recombinant vectors offer a cost-effective and feasible alternative. Although inoculation with recombinant poxviruses without Env glycoprotein boosting has not induced high levels of neutralizing antibodies, efficient B-cell priming by these vectors should facilitate an anamnestic neutralizing antibody response to infection. An appropriate anamnestic B-cell response might exert sufficient pressure on the virus during early stages of infection as the CTL response matures. One of the vectors used here (MVA-gag-pol) was previously shown to elicit potent SIV-specific CTL in macaques (45). Together, these immune responses might be capable of controlling virus replication to an extent that would limit immune suppression and virus transmission better than either response alone.
Acknowledgments
We thank Ronald C. Desrosiers for providing SIVmac251 and SIVmac239 and Michael Murphy-Corb for providing SIV/DeltaB670.
Partial support for these studies was provided by a grant from the NIH (AI-85343).
REFERENCES
- 1.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J-L, Duliege A-M, Tartaglia J, McNamara J, Kai-Lin H, Montefiori D, Weinhold K. Rapid induction of HIV-1 immune responses by canarypox (ALVAC) HIV-1 and gp120 SF2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Bloom B R. The highest attainable standard: ethical issues in AIDS vaccines. Science. 1998;279:186–188. doi: 10.1126/science.279.5348.186. [DOI] [PubMed] [Google Scholar]
- 3.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11(Suppl. A):S87–S98. [PubMed] [Google Scholar]
- 4.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements-Mann M L, Weinhold K, Matthews T J, Graham B S, Gorse G J, Keefer M C, McElrath M J, Hsieh R H, Mestecky J, Zolla-Pazner S, Mascola J, Scwartz D, Silicano R, Corey L, Wright P F, Belshe R, Dolin R, Jackson S, Xu S, Fast P, Walker M C, Stablein D, Excler J-L, Tartaglia J, Duliege A M, Sinangil F, Paoletti E. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. J Infect Dis. 1998;177:1230–1246. doi: 10.1086/515288. [DOI] [PubMed] [Google Scholar]
- 6.Cooney E L, McElrath M J, Corey L, Hu S-L, Collier A C, Arditti D, Hoffman M, Coombs R W, Smith G E, Greenberg P D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Excler J-L, Plotkin S. The prime-boost concept applied to HIV preventive vaccines. AIDS. 1997;11(Suppl. A):S127–S137. [PubMed] [Google Scholar]
- 8.Foresman L, Jia F, Li Z, Wang C, Stephens E B, Sahni M, Narayan O, Joag S V. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retrovir. 1998;14:1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 9.Gauduin M-C, Parren P W H I, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 10.Gould E A, Buckley A, Barret A D T, Cammack N. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J Gen Virol. 1986;67:591–595. doi: 10.1099/0022-1317-67-3-591. [DOI] [PubMed] [Google Scholar]
- 11.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, Corey L, Stablein D M, Esterlitz J R, Hu S-L, Smith G E, Fast P E, Koff W C. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naïve adults. J Infect Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 12.Graham B S, Wright P F. Candidate AIDS vaccines. J Engl J Med. 1995;333:1331–1339. doi: 10.1056/NEJM199511163332007. [DOI] [PubMed] [Google Scholar]
- 13.Haigwood N L, Watson A, Sutton W F, McClure J, Lewis A, Ranchalis J, Travis B, Voss G, Letvin N L, Hu S-L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 14.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 15.Heilman C A, Baltimore D. HIV vaccines—where are we going? Nat Med. 1998;4:532–534. doi: 10.1038/nm0598supp-532. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins W R, Montefiori D C. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741–3752. [DOI] [PMC free article] [PubMed]
- 18.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African non-human primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch V M, Zack P M, Vogel A P, Johnson P R. Simian immunodeficiency virus infection of macaques: pathogenesis of end-stage disease in characterized by high levels of proviral DNA in tissues. J Infect Dis. 1991;163:976–988. doi: 10.1093/infdis/163.5.976. [DOI] [PubMed] [Google Scholar]
- 20.Hu S-L. Recombinant subunit vaccines against primate lentiviruses. AIDS Res Hum Retrovir. 1996;12:451–453. doi: 10.1089/aid.1996.12.451. [DOI] [PubMed] [Google Scholar]
- 21.Hu S-L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 22.Hu S-L, Fultz P N, McClure H M, Eichberg J W, Thomas E K, Zarling J, Singhal M C, Kosowski S G, Swenson R B, Anderson D C, Todaro G. Effect of immunization with a vaccinia-HIV env recombinant on HIV infection of chimpanzees. Nature. 1987;328:721–723. doi: 10.1038/328721a0. [DOI] [PubMed] [Google Scholar]
- 23.Johnson P R, Montefiori D C, Goldstein S, Hamm T E, Zhou J, Kitov S, Haigwood N L, Misher L, London W T, Gerin J L, Allison A, Purcell R H, Chanock R M, Hirsch V M. Inactivated whole SIV vaccine in macaques: evaluation of protective efficacy against challenge with cell-free virus or infected cells. AIDS Res Hum Retrovir. 1992;8:1501–1505. doi: 10.1089/aid.1992.8.1501. [DOI] [PubMed] [Google Scholar]
- 24.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langlois A J, Desrosiers R C, Lewis M G, Kewalramani V N, Littman D R, Zhou J Y, Manson K, Bolognesi D P, Wyand M S, Montefiori D C. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J Virol. 1998;72:6950–6955. doi: 10.1128/jvi.72.8.6950-6955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefrancois L. Protection against lethal viral infection by neutralizing and nonneutralizing monoclonal antibodies: distinct mechanisms of action in vivo. J Virol. 1984;51:208–214. doi: 10.1128/jvi.51.1.208-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 28.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathews J H, Roehrig J T, Trent D W. Role of complement and the Fc portion of immunoglobulin G in immunity to Venezuelan equine encephalomyelitis virus infection with glycoprotein-specific monoclonal antibodies. J Virol. 1985;55:594–600. doi: 10.1128/jvi.55.3.594-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori D C. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin Immunopathol. 1997;18:371–390. doi: 10.1007/BF00813504. [DOI] [PubMed] [Google Scholar]
- 32.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239Δ3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 33.Montefiori, D. C., and T. G. Evans. Toward an HIV-1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res. Hum. Retrovir., in press. [DOI] [PubMed]
- 34.Moog C H, Fleury J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J P, Binley J. Envelope's letters boxed into shape. Nature. 1998;393:630–631. doi: 10.1038/31359. [DOI] [PubMed] [Google Scholar]
- 36.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–4355. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakanaga K, Yamanouchi K, Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986;59:168–171. doi: 10.1128/jvi.59.1.168-171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nara P L, Garrity R. Deceptive imprinting: a cosmopolitan strategy for complicating vaccination. Vaccine. 1998;16:1780–1787. doi: 10.1016/s0264-410x(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 39.Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in Macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J-L, Ragnaud J-M, Bernard N, Fleury H J A. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquir Immune Defic Syndr. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pialoux G, Excler J-L, Rivière Y, Gonzalez-Canali G, Feuillie V, Couland P, Gluckman J-C, Matthews T J, Meignier B, Kieny M-P, Gonnet P, Diaz I, Méric C, Paoletti E, Tartaglia J, Salomon H, Plotkin S. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI) AIDS Res Hum Retrovir. 1995;11:373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- 42.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 43.Pincus S H, Cole R, Ireland R, McAtee F, Fujisawa R, Portis J. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J Virol. 1995;69:7152–7158. doi: 10.1128/jvi.69.11.7152-7158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmaljohn A L, Johnson E D, Dalrymple J M, Cole G A. Nonneutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature (London) 1982;297:70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 45.Seth A, Ourmanov I, Kuroda M J, Schimtz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 47.Tyler D S, Lyerly H K, Weinhold K J. Anti-HIV-1 ADCC: a minireview. AIDS Res Hum Retrovir. 1989;5:557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- 48.Van Rompay K K A, Berardi C J, Dillard-Telm S, Tarara R P, Canfield D R, Valverde C R, Montefiori D C, Stefano-Cole K, Montelaro R C, Miller C J, Marthas M L. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 49.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J-Y, Martin L N, Watson E A, Montelaro R C, West M, Epstein L, Murphey-Corb M. Simian immunodeficiency virus/Delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1988;158:1277–1286. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]