Graphical abstract
Keywords: Exercise, Modifiable Lifestyle Factors, Alzheimer’s Disease, Modularity, Resting-State Functional MRI
Highlights
-
•
Actigraphy estimated physical activity and sleep are associated with functional connectivity.
-
•
Physical activity and sleep are not associated with amyloid positive status.
-
•
Amyloid positive status modifies associations between sleep and functional connectivity.
Abstract
Greater physical activity and better sleep are associated with reduced risk of cognitive decline and dementia among older adults, but little is known about their combined associations with measures of brain function and neuropathology. This study investigated potential independent and interactive cross-sectional relationships between actigraphy-estimated total volume of physical activity (TVPA) and sleep patterns [i.e., total sleep time (TST), sleep efficiency (SE)] with resting-state functional magnetic resonance imaging (rs-fMRI) measures of large scale network connectivity and positron emission tomography (PET) measures of amyloid-β. Participants were 135 non-demented older adults from the BIOCARD study (116 cognitively normal and 19 with mild cognitive impairment; mean age = 70.0 years). Using multiple linear regression analyses, we assessed the association between TVPA, TST, and SE with connectivity within the default-mode, salience, and fronto-parietal control networks, and with network modularity, a measure of network segregation. Higher TVPA and SE were independently associated with greater network modularity, although the positive relationship of SE with modularity was only present in amyloid-negative individuals. Additionally, higher TVPA was associated with greater connectivity within the default-mode network, while greater SE was related to greater connectivity within the salience network. In contrast, longer TST was associated with lower network modularity, particularly among amyloid-positive individuals, suggesting a relationship between longer sleep duration and greater network disorganization. Physical activity and sleep measures were not associated with amyloid positivity. These data suggest that greater physical activity levels and more efficient sleep may promote more segregated and potentially resilient functional networks and increase functional connectivity within specific large-scale networks and that the relationship between sleep and functional networks connectivity may depend on amyloid status.
1. Introduction
Greater engagement in physical activity (Blondell et al., 2014, Nuzum et al., 2020, Sewell et al., 2023a) and adequate sleep quality and duration (Sabia et al., 2021, Shi et al., 2018) are associated with a reduced rate of cognitive decline and a lower risk of dementia. Furthermore, there is evidence that physical activity and sleep may provide additive benefits for cognitive function (Callow et al., 2024, Falck et al., 2018, Wei et al., 2021) or work synergistically to reduce the incidence of dementia among older adults (Bloomberg et al., 2023, Huang et al., 2022). However, the mechanisms through which physical activity and sleep might affect the brain, either alone or in combination, to improve cognitive and clinical outcomes remain elusive.
It has been suggested that higher levels of physical activity and healthier sleep patterns may confer resilience against Alzheimer's Disease (AD) and other age-related neuropathologies by delaying the onset of, or reducing the rate of, pathology accumulation (Memel et al., 2021). However, evidence for an association between physical activity and AD pathology are mixed. A few cross-sectional studies among cognitively normal (Brown et al., 2013, Liang et al., 2010, Okonkwo et al., 2014) and non-demented older adults, including individuals with mild cognitive impairment, (MCI) (Stillman et al., 2017, Treyer et al., 2021), have reported that greater self-reported physical activity is associated with less abnormal levels of amyloid beta (Aβ) in the brain or in blood, one of the proteins that accumulates in the brain in AD. However, other studies using objectively measured physical activity have observed no association between physical activity and Aβ levels (De Souto Barreto et al., 2015, Pedrero-Chamizo et al., 2021, Pedrini et al., 2022, Sohn et al., 2022), suggesting that physical activity may influence brain health through mechanisms other than amyloid accumulation (Frederiksen et al., 2019, Rodriguez-Ayllon et al., 2023).
Meanwhile, both animal (Xie et al., 2013) and human studies (Ooms et al., 2014) provide evidence that sleep plays a crucial role in modulating Aβ concentrations in the brain, with poorer objectively and subjectively measured sleep efficiency and duration showing an association with higher brain Aβ burden, quantified with positron emission tomography (PET) in cognitively normal (Ju et al., 2013) and non-demented older adults (Lim et al., 2013a; Spira et al., 2013). However, the relationship between sleep dysfunction and AD pathology is complex and may be bi-directional in nature, with AD pathology contributing to sleep disturbances and vice versa (Ju et al., 2014, Lucey, 2020). Moreover, a recent cross-sectional study found that self-reported physical activity attenuated the negative relationship between suboptimal sleep and greater PET-Aβ burden in cognitively normal older adults (Sewell et al., 2023b), highlighting the importance of considering both physical activity and sleep when evaluating their associations with AD pathology.
Although physical activity and sleep may reduce accumulation of AD pathology, they may also influence cognitive outcomes by altering other aspects of brain function and structure, including those that protect against the negative impact of pathology on cognition (Arenaza-Urquijo and Vemuri, 2018, Memel et al., 2021). For example, resting-state network functional connectivity, including connectivity within large-scale brain networks and functional network segregation (or modularity), may enhance resilience to AD pathology, as they are associated with better cognitive performance in the presence of amyloid and tau (Ewers et al., 2021, Soldan et al., 2021). Network segregation reflects high connectivity within networks (or modules) and low connectivity between networks (see Fig. 1A for a graphical representation) and is associated with better cognitive performance across a variety of tasks (Chan et al., 2014, Cohen and D’Esposito, 2016, Kong et al., 2020, Wang et al., 2021, Zhang et al., 2023). Consistent with this perspective, a few observational studies among older cognitively normal and non-demented individuals have reported links between greater levels of physical activity (Soldan et al., 2022, Soldan et al., 2021) or physical fitness (Voss et al., 2016) with greater connectivity in the default-mode network and with greater network segregation. Similarly, exercise interventions among non-demented older adults have demonstrated exercise-related enhancements of within-network connectivity in functional networks (Meng et al., 2022, Menon, 2011), including the default mode, salience, and frontoparietal networks (Dion et al., 2021, Porto et al., 2018, Won et al., 2021, Won et al., 2023). Self-reported sleep quality has also been shown to have a positive association with default mode and attentional network connectivity in cognitively normal older adults, whereas subjective sleep disturbances and disorders in those with MCI are associated with reduced default mode connectivity (Luo et al., 2022, McKinnon et al., 2017). Notably, amyloid burden may influence functional network connectivity in non-demented older adults (Lin et al., 2020, Magalhães et al., 2021, Sintini et al., 2021), underscoring the need to consider amyloid burden when evaluating links among physical activity, sleep, and functional network connectivity.
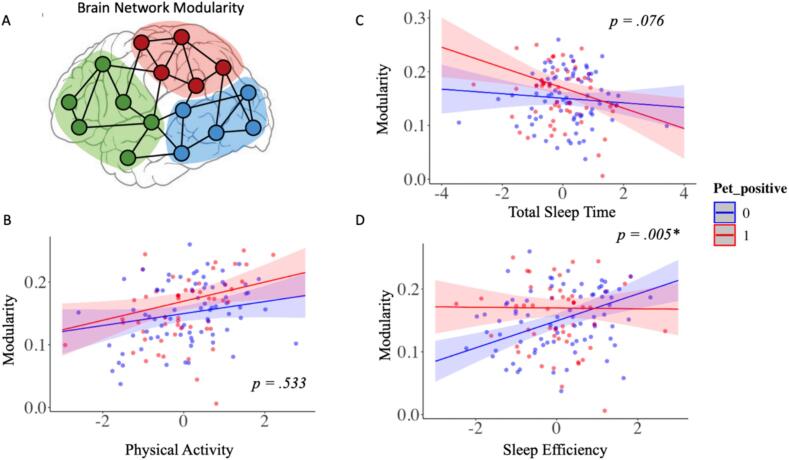
Fig. 1.
(A) Graphical depiction of brain network modularity. Interaction of amyloid PET status with (B) total volume of physical activity, (C) total sleep time, and (D) sleep efficiency, in the 10 most active hours in relation to resting-state fMRI network segregation (modularity).
This study builds on prior research that examined the separate associations of physical activity or sleep with amyloid burden or functional connectivity by examining both physical activity and sleep within the same individuals to determine whether they have independent or synergistic relationships with functional connectivity, network segregation, and amyloid PET burden. Additionally, we examined whether brain amyloid levels modified the relationships between physical activity and sleep with functional network connectivity and segregation. Both physical activity and sleep were measured using actigraphy among well-characterized older individuals with normal cognition or MCI.
2. Methods
Analyses were preformed using data from the ongoing prospective, longitudinal Biomarkers for Older Controls as Risk of Dementia (BIOCARD) study (https://www.biocard-se.org), which was started in 1995 at the National Institute of Health (NIH). At enrollment, participants were primarily middle aged with approximately 75 % having a family history of dementia. The study was stopped in 2005 for administrative reasons and was re-established in 2009 at the Johns Hopkins University (JHU) School of Medicine. Annual visits included clinical and cognitive assessments during both the NIH and JHU phases of the study. Written informed consent was provided by all participants in the study. At JHU, MRI scans and amyloid PET scans have been collected bi-annually since 2015; wrist actigraphy data collection began in 2016.
This study reports cross-sectional data from 135 non-demented participants who had both valid actigraphy data for ≥ 3 days and rs-fMRI data and amyloid PET collected between 2015–2023. To maximize our sample size, we used actigraphy data from each participants’ first study visit with actigraphy data and used rsfMRI data from the scan performed closest to that date. The mean interval between actigraphy data collection and the rs-fMRI scan was 1.0 years (range = -1.3 to 1.5 years). Details regarding the temporal stability of the actigraphy measures can be found below.
2.1. Clinical Assessment
Clinical evaluations and cognitive assessments were conducted at each annual visit, which included a battery of neuropsychological tests (see Albert et al., 2014 for details) and a semi-structured interview based on the Clinical Dementia Rating (CDR) scale (Morris, 1993). The JHU BIOCARD Clinical Core staff generates a consensus diagnosis annually using procedures comparable to those established by the National Institute on Aging (NIA) Alzheimer’s Disease Centers program. First, a syndromic diagnosis is generated based on the following data: (1) clinical information pertaining to the medical, neurological, and psychiatric status of the individual; (2) reports of cognitive changes by the individual and collateral source based on the CDR interview; and (3) cognitive test scores relative to age-matched published norms and prior performance. Syndromic diagnostic categories included: (1) cognitively normal (referred to as normal throughout the manuscript), (2) MCI, (3) impaired not MCI, and (4) dementia. When a participant was believed to be cognitively impaired, a decision regarding the probable etiology of the syndrome was determined based on the clinical information provided at each visit, including the individual’s medical history. Multiple etiologies for a single participant were possible. The consensus diagnostic procedures are consistent with the recommendations of the NIA/Alzheimer’s Association working groups for a diagnosis of MCI (Albert et al., 2011) and dementia (McKhann et al., 2011). An “impaired not MCI” diagnosis is given in the case of contrasting information from the CDR interview and cognitive test scores (i.e., participants or collateral source reported concerns for cognitive changes in daily life, but the cognitive testing did not show changes, or vice versa). Because participants who are “impaired not MCI” do not meet MCI criteria, they were included with the group of normal participants, consistent with prior publications (see Albert et al., 2014).
2.2. Actigraphy measures
Participants were provided an actigraph (Actiwatch-2, Philips Respironics, Bend, OR) which was worn on their non-dominant wrist for seven consecutive days. Participants completed daily logs to document the removal of the actigraph, travel across time zones, naps, and time of sleep. Actigraphs were returned via mail, at which point the data were downloaded using Actiware Software (v. 6.0.9) and processed without knowledge of the participants’ clinical diagnoses. Actigraphy data collected during participant-reported travel periods across time zones, illness, medical procedures using anesthesia, non-wear time, and device malfunction were excluded from analyses.
2.3. Sleep assessment
Two standard nighttime sleep parameters were extracted from valid nights, utilizing a widely used algorithm (Kushida et al., 2001): (1) total sleep time (TST; the number of minutes slept while in bed), and (2) sleep efficiency (SE; the proportion of time in bed asleep, %). These standard sleep parameters were averaged across valid nights and analyzed as continuous variables.
2.4. Physical activity assessment
Thirty-second epochs of actigraph recorded movement (i.e., acceleration) were recorded and aggregated into activity counts, a unitless measure of movement. As described previously (Callow et al., 2024) participants had to have at least three 24-hour daily intervals of valid data to be included in the analysis. A valid 24-hour daily interval was defined as having < 5 % of missing data. For each participant, missing epoch-level activity counts were imputed using the average value of activity counts across the remaining valid days at the same time of day. Activity counts were log-transformed to correct for skewness by applying log (1 + activity counts per minute), and then summed over two consecutive (30-sec) epochs to generate minute-level activity counts. Minute-level data were summed on an hourly basis. The number of activity counts during the 10 most active hours of each 24-hour interval were averaged across all valid 24-hour intervals and used to measure the total volume of physical activity (TVPA) (Varma et al., 2017). TVPA has previously been linked to aspects of functional and structural brain network connectivity in BIOCARD (Soldan et al., 2022). The 10 most active hours of physical activity (PA) for our TVPA measure were used to minimize overlap between actigraphic sampling for our PA and sleep parameters.
2.5. Temporal stability of physical activity and sleep measures
Given the time lag between MRI and actigraphy collection, we evaluated the temporal stability of the physical activity and sleep measures using intraclass correlation coefficients (ICC) for the subset of participants with a follow-up actigraphy visit (n = 116, 2–6 years apart). The ICC between the first and second visit were 0.8 (p < 0.001) for physical activity and greater than 0.6 (p < 0.001) for both sleep parameters, suggesting both actigraphy measures were relatively stable over time and reasonably representative of general sleep and physical activity patterns at the time of the MRI scan.
2.6. MRI acquisition
MRI scans were conducted on a 3 T Phillips Achieva scanner (Eindhoven, The Netherlands). The multi-modal imaging protocol encompassed a magnetization-prepared rapid gradient echo (MPRAGE) scan, which served as anatomical references and for image registration purposes. These scans were acquired with the following parameters: TR = 6.7 ms, TE = 3.1 ms, shot interval of 3000 ms, flip angle of 8°, FOV = 240x256 mm2, consisting of 170 slices with voxel dimensions of 1x1x1.2 mm3, and a scan duration of 5 min and 59 s. Resting state BOLD data were acquired using an echo-planar imaging (EPI) sequence with the following parameters: 48 slices, field of view (FOV) = 212 x 212 mm2, voxel size = 3.3 x 3.3 x 3.3 mm3, TR = 3000 ms, TE = 30 ms, and a flip angle of 75 degrees. Each scan session lasted 420 s, comprising 140 functional volumes. During scanning, participants received instructions to relax, keep their eyes closed, and maintain stillness.
2.7. Resting-state fMRI Processing and Functional Network Generation
The BOLD data underwent a standard preprocessing pipeline, which involved slice timing correction, realignment, normalization to Montreal Neurologic Institute (MNI) 152 volumetric space using the MPRAGE image, and spatial smoothing via a Gaussian filter with a full-width half-maximum of 4 mm. The BOLD image series then underwent detrending and bandpass filtering, retaining components within the 0.01 – 0.1 Hz frequency range to isolate low-frequency fluctuations. Temporal filtering was performed by flagging frames contaminated by motion artifacts, which were subsequently excluded from correlation matrix calculations. Frame-wise displacement (FD), calculated as the sum of absolute differentials of the six rigid-body head motion parameters, was used to identify and scrub volumes with FD ≥ 0.5 mm (Power et al., 2014). Following the motion-scrubbing step, we excluded participants with fewer than 80 frames of usable data from the fMRI analysis. Regression analysis was used to remove nuisance signals, including global, white matter, and CSF signals, along with the six rigid-body head motion parameters. Data were then parcellated into 114 regions of interest (ROIs), as defined in the MNI 152 volumetric space, based on the parcellation method developed by Yeo et al. (Thomas Yeo et al., 2011).
Cross-correlation coefficients were computed between each pair of ROIs and transformed into z-scores using Fisher-z transformation. This process resulted in a 114 x 114 matrix of z-transformed values. To characterize functional connectivity at the network level, the z-transformed values were reduced to a 7 x 7 matrix by averaging values within the same network (Yeo et al., 2011). The present analyses focused on within-network connectivity of 3 of the 7 networks identified in this approach: the default mode (DMN), fronto-parietal executive control (FPN), and salience/ventral attention (SAL), as these networks have been shown to change with aging and AD (Meng et al., 2022, Menon, 2011), are associated with measures of physical activity and/or sleep (Amorim et al., 2018, Won et al., 2021), and are related to cognition among older adults (Soldan et al., 2021). Additional networks were not examined to limit the number of comparisons while maintaining the focus of the manuscript.
To compute functional-network modularity (a measure of network segregation, see Fig. 2A) for each subject, graph theory was employed utilizing the Brain Connectivity Toolbox (version 2019–03-03, (Rubinov & Sporns, 2009)) in conjunction with custom MATLAB scripts. The modularity measure assesses the extent to which a network can be partitioned into distinct subnetworks (or modules) that exhibit internal cohesion (i.e., high within-network connectivity) while remaining segregated from one another (i.e., low between-network connectivity) (Betzel et al., 2014). Higher modularity values indicate greater separation between networks and enhanced connectivity within individual networks. Notably, modularity was strongly correlated with the related measure of network segregation (r = 0.97, p < 0.0001; (Wig, 2017)); and thus, we present results exclusively for network modularity in this report. For further details, please consult Soldan et al., 2022.
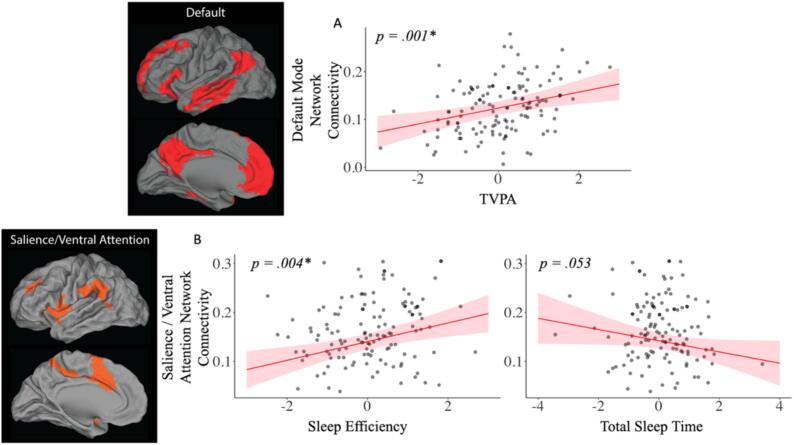
Fig. 2.
Combined associations of total volume of physical activity (TVPA) with A) Default Mode Network Connectivity (top); and of B) sleep efficiency (SE) and total sleep time (TST) with Salience/Ventral Attention network connectivity (bottom).
2.8. PiB PET image acquisition and processing
All participants underwent PET imaging, utilizing the 11C-labeled Pittsburgh compound B (PiB) tracer, conducted on an Advance PET scanner from GE Healthcare. These scans were performed immediately after an intravenous bolus injection. Distribution volume ratio (DVR) images were computed within the native space of each PET image, employing a simplified reference tissue model with cerebellar gray matter serving as the reference region (Zhou et al., 2003). Anatomical regions were delineated based on the structural MRI scans of each participant, utilizing MRICloud (Mori et al., 2016), and subsequently registered to the native space of each PET image. The mean cortical DVR (cDVR) was determined by averaging cDVR values across cortical regions, following established methods previously described (Bilgel et al., 2018, Walker et al., 2020). Participants exhibiting a mean cDVR value exceeding 1.06 were classified as PiB positive. This threshold was derived in a prior study, employing a 2-class Gaussian mixture model fitted to cDVR data (Bilgel et al., 2016). Due to disruptions in testing during the COVID-19 pandemic and to maximize our available sample, PET scans performed as long as 3 years before or after the rs-fMRI scan were used for analyses. Additionally, we included PET scans obtained more than 3 years following the rs-fMRI scan if identified as amyloid negative (based on the above-mentioned cutoff, as these scans can be assumed to be negative at the time of the fMRI scan) and more than 3 years prior if identified as already being amyloid positive (as they would be positive at the time of the fMRI scan). The average PET scan occurred 1.6 years (−3.8 to 6.0 years) apart from the fMRI scan. Sensitivity analyses examined whether exclusion of PET scans more than 2 years before or after the fMRI scan altered the results.
2.9. Vascular risk factors
The vascular risk score was based on the medical history of the participant. This score, which has been previously validated (Gottesman et al., 2017), is the sum of five binary vascular risk factors, where a value of 1 signifies the presence of the risk factor (recent/remote), and a value of 0 indicates its absence. These factors encompass hypertension, hypercholesterolemia, current smoking within the last 30 days, obesity (i.e., measured body mass index of 30 kg/m2 or higher), and diabetes. The vascular risk summary score has been shown to predict dementia incidence (Gottesman et al., 2017) and been used in a number of prior studies (Newton et al., 2023, Pettigrew et al., 2020, Soldan et al., 2020). Data for vascular risk factors were obtained at the same visit as the MRI scan.
2.10. APOE Genotype
APOE genotyping was conducted by the digestion of polymerase chain reaction-amplified genomic DNA using restriction endonucleases (carried out by Athena Diagnostic, Worcester, MA). Individuals carrying at least one ε4 allele were designated as ε4 carriers; those without an ε4 allele were classified as non-carriers.
2.11. Statistical analysis
Differences between diagnostic groups (normal vs. MCI) were tested using Wilcoxon signed-rank tests for continuous variables and chi-square tests for categorical variables. Bivariate Pearson correlation analyses were used to determine simple associations between the objectively measured PA and sleep parameter variables while controlling for age. Based on significant Shapiro Wilk tests for non-normal distributions, SE and TVPA were log transformed to correct for skewness.
Primary analyses were performed using multivariable linear and logistic regression analysis. Minimally adjusted models (Model 1) accounted for age, sex, education, and diagnosis. Fully adjusted models (Model 2) additionally included PiB-PET positive status, vascular risk summary score, and APOE ε4 status (which has been reported to influence rs-fMRI networks) (Sintini et al., 2021)). To determine the combined associations of PA and sleep with rsFC, Models 1 and 2 regressed all three predictors of interest (i.e., TVPA, TST, and SE) in the same model and the covariates on rsFC, using separate models for each rsFC measures (e.g., rsFC within the DMN, SAL, and FPN networks, and modularity). To assess the combined associations of sleep and PA with amyloid burden, measured by amyloid positive status, Model 1 (minimally adjusted) and Model 2 (fully-adjusted, excluding amyloid) used logistic regression analysis with amyloid status (dichotomous variable) as the outcome. Based on previous reports suggesting ε4 carrier status can moderate associations between sleep and AD pathology levels (Fenton et al., 2023; Lim et al., 2013b), we additionally assessed whether the presence of the ε4 allele modified associations between physical activity and sleep with amyloid status in fully adjusted models. We further tested for potential 2-way interactions between PA x sleep parameters in relationships with both rsFC and amyloid status, although, non-significant interaction terms are not reported. Interaction terms were tested in separate models, no 3-way or multiple interactions terms were tested in a single model.
Finally, Model 3 (fully adjusted) tested whether the independent association of TVPA, TST, and SE with rsFC were moderated by PET amyloid status (which can influence relationships among sleep, physical activity, and resting-state connectivity (Kim et al., 2023, Lin et al., 2020, Pruzin et al., 2022)), by additionally including an interaction term PET amyloid status x [PA or sleep measure]) in the fully adjusted model. Non-significant interaction terms between diagnosis and PET amyloid status with PA and sleep metrics are not reported. A sensitivity analysis was also conducted to determine whether the primary results from Models 1, 2, and 3 were the same when excluding participants with MCI, as well as participants with a diagnosis of Impaired not MCI. Finally, based on previous reports of a U-shaped relationship between sleep duration and cognition (Ma et al., 2020), an additional sensitivity analysis was conducted with TST as a categorical predictor.
All multiple linear regression models were tested for data points with abnormal leverage (hat value > 3 times average), influence (Cook's D > 0.5), and discrepancy (studentized residuals greater > 3). Furthermore, collinearity between covariates and predictors in all models were checked for a high variance inflation factor (VIF > 5). No additional data were removed from further analyses based on an exclusionary criterion of violating more than one of these three heuristics. Statistical significance was set based on a two-tailed alpha < 0.05 and Bonferroni family wise error rate (FWER) correction for multiple comparisons of individual within network connectivity analysis (i.e. 3 comparisons). Multiple comparison correction was not applied for modularity or amyloid PET status as they were distinct a priori tests of whole brain connectivity and AD pathology.
3. Results
Demographic characteristics of the sample and a comparison between the groups shown in Table 1. There were no significant differences in demographics, sleep, PA, and rs-fMRI measures between the MCI and cognitively normal groups. As expected, the MCI group showed lower MMSE scores compared to those who were cognitively normal.
Table 1.
Participant characteristics by diagnosis. Values reflect mean (SD) unless otherwise indicated.
| Participant Characteristics | Total Sample (n = 135) | Cognitively Normal (n = 116) | MCI (n = 19) | p-value |
|---|---|---|---|---|
| Sex, N (%) | 94F (69.4 %) | 80F (69 %) | 14F (74 %) | 0.683 |
| Age (yrs) | 70.0 (7.9) | 69.7 (7.6) | 72.8 (8.6) | 0.163 |
| Education (yrs) | 17.1 (2.3) | 17.3 (2.3) | 16.1 (2.5) | 0.056 |
| Vascular Risk | 1.5 (1.1) | 1.4 (1.1) | 1.8 (1.0) | 0.113 |
| Mini Mental State Exam | 29.0 (1.2) | 29.2 (1.0) | 27.5 (1.4) | <0.001*** |
| APOE- ε4 Carriers, N | 37 (27 %) | 31 (27 %) | 6 (32 %) | 0.664 |
| PET Amyloid positive | 53 (39 %) | 45 (39 %) | 8 (42 %) | 0.984 |
| Total Volume of Physical Activity | 12.0 (0.3) | 12.1 (0.3) | 12.0 (0.3) | 0.381 |
| Total Sleep Time (min) | 415.0 (53.1) | 417.2 (50.3) | 405.4 (68.6) | 0.955 |
| Sleep Efficiency (%) | 86.4 (5.4) | 86.6 (5.4) | 85.5 (5.8) | 0.361 |
| Modularity | 0.15 (0.05) | 0.15 (0.05) | 0.14 (0.04) | 0.183 |
| DMN Connectivity | 0.13 (0.05) | 0.13 (0.06) | 0.11 (0.04) | 0.374 |
| SAL Network Connectivity | 0.16 (0.06) | 0.16 (0.07) | 0.16 (0.05) | 0.937 |
| FPN Connectivity | 0.12 (0.05) | 0.12 (0.06) | 0.14 (0.05) | 0.416 |
Notes: MCI = Mild Cognitive Impairment. Sex: F = Female. Total Volume of Physical Activity = average log transformed total activity epoch counts from 10 most active hours. DMN = Default Mode Network. SAL = Salience Network. FPN = Frontoparietal Network. *** p<0.001
3.1. Correlations between TVPA and Sleep actigraphy measures
Across the total sample, there was a significant negative association between TVPA and SE (r = -0.26, p = 0.003), and a non-significant negative association between TVPA and TST (r = -0.05, p = 0.527).
3.2. Association of physical activity and sleep with resting state functional connectivity
In Model 1 (minimally adjusted), TVPA (β = 0.018, p < 0.001) and SE (β = 0.014, p = 0.002) were each positively associated with modularity, while TST (β = -0.011, p = 0.013) was negatively associated with modularity in the total sample (Table 2). Results were the same in Model 2 (fully adjusted models) (See Table 2). Additionally, in fully adjusted models, TVPA (β = 0.017, p = 0.001), but neither sleep parameter, was positively associated with DMN connectivity. In contrast, higher SE (β = 0.019, p = 0.001) but not TST (β = -0.011, p = 0.053) was associated with higher SAL network connectivity measures in fully adjusted models (Fig. 2). Neither TVPA nor the sleep measures were associated with FPN connectivity measures, see Table 2 for model results for the total sample. No significant interactions between TVPA and SE or TVPA and TST were found with modularity or any of the individual network measures (all p’s > 0.100), and thus, they are not reported in our final models.
Table 2.
Combined associations of physical activity and sleep parameters with resting-state functional network connectivity and amyloid burden.
| Model 1 Minimally adjusted | Model 2 Fully adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| rs-fMRI / PET Measure | Actigraphy Measure | Estimate | SE | p-value | R2adj | Estimate | SE | p-value | R2adj |
| Modularity | TVPA | 0.018 | 0.004 | <0.001 | 0.247 | 0.012 | 0.004 | 0.006** | 0.299 |
| TST | −0.011 | 0.005 | 0.013* | −0.010 | 0.004 | 0.027* | |||
| Sleep Efficiency | 0.014 | 0.005 | 0.002** | 0.014 | 0.004 | 0.002* | |||
| Default Mode Connectivity | TVPA | 0.019 | 0.005 | <0.001** | 0.181 | 0.017 | 0.005 | 0.001*** | 0.176 |
| TST | −0.006 | 0.005 | 0.207 | −0.006 | 0.005 | 0.215 | |||
| Sleep Efficiency | 0.008 | 0.005 | 0.090 | 0.007 | 0.005 | 0.163 | |||
| Salience Ventral Attention Connectivity | TVPA | 0.012 | 0.006 | 0.037 | 0.111 | 0.005 | 0.006 | 0.381 | 0.221 |
| TST | −0.015 | 0.006 | 0.015* | −0.011 | 0.006 | 0.053 | |||
| Sleep Efficiency | 0.020 | 0.006 | 0.001** | 0.019 | 0.006 | 0.001** | |||
| Frontoparietal Network Connectivity | TVPA | <-0.001 | 0.006 | 0.957 | 0.009 | −0.003 | 0.006 | 0.639 | 0.118 |
| TST | −0.004 | 0.005 | 0.512 | −0.002 | 0.006 | 0.764 | |||
| Sleep Efficiency | 0.002 | 0.005 | 0.752 | 0.002 | 0.006 | 0.674 | |||
| PET Amyloid Positive | TVPA | 0.030 | 0.201 | 0.881 | 0.015 | 0.321 | 0.251 | 0.200 | 0.106 |
| TST | −0.253 | 0.219 | 0.248 | −0.485 | 0.256 | 0.650 | |||
| Sleep Efficiency | −0.009 | 0.215 | 0.966 | 0.116 | 0.262 | 0.064 | |||
Note. TVPA = Total volume of physical activity. SE = Standard Error. TST = Total Sleep Time. PET = Amyloid Positron Emission Tomography Status. Both multiple linear and logistic regressions controlled for age, sex, education, and diagnosis in Model 1 and additionally PiB-PET positive status, vascular risk summary score, and APOE ε4 status in Model 2 (excluding PiB-PET status in the logistic regression for cortical Amyloid PET burden). * p-value < 0.05; ** p-value < 0.01, *** p-value < 0.001. Statistical significance was set based on a two-tailed alpha < 0.05 and Bonferroni family wise error rate (FWER) correction for multiple comparisons of within individual network connectivity analysis. R2adj = adjusted R-squared model values.
3.3. Sensitivity analysis
The exclusion of participants with MCI (n = 19) did not significantly alter the associations between TVPA, TST, or SEFF and network modularity (see Table 2 and Supplementary Table 1). However, when participants with MCI were excluded, a significant association between higher TST and lower rs-fMRI measures within the DMN and SAL networks emerged. The pattern of results was also the same when additionally excluding participants with a diagnosis of Impaired not MCI (data not shown). Furthermore, results from analyses using TST as a categorical variable with previously published cutoffs (e.g., <6h vs. 6–8 h vs. > 8 h (Bloomberg et al., 2023)) were similar, suggesting there were no non-linear relationships between TST and the brain measures (data not shown).
3.4. Moderating effect of amyloid PET status on associations between TVPA and sleep with rs-fMRI networks
Amyloid PET status (i.e., negative vs. positive) significantly moderated the association between SE and network modularity (β = 0.014, p = 0.005) such that SE was positively associated with modularity in those who were PiB negative, but not associated with modularity in those classified as PiB positive (Fig. 1D). PET amyloid status also showed a marginally significant interaction with TST (β = 0.007, p = 0.076), such that TST was negatively associated with modularity in PiB-positive participants, but not PiB negative participants (Fig. 1C). Aβ status did not modify the relationship between TVPA and modularity (β = 0.001, p = 0.533) (Fig. 1B). Furthermore, Aβ PET burden did not moderate the association between TVPA, TST, or SE on rsFC within the DMN, SAL, or FPN network (all p’s > 0.200). In an additional sensitivity analysis, we also found that limiting PET measures to within 2 years of the MRI scan (n = 116) or to only before or after the resting state scan (n = 130 and 137, respectively) did not significantly alter our findings (data not shown).
3.5. Association of TVPA and sleep with cortical amyloid
Neither TVPA, TST, or SE were significantly associated with cortical amyloid burden (all p’s > 0.060, see Table 2). Furthermore, no APOE- ε4 x [PA or Sleep] and no PA x Sleep interactions were found in relation to cortical amyloid burden (both p > 0.100, data not shown). Similarly, limiting PET measures to those within 2 years of MRI scans or only before or after the resting-state scan did not significantly alter our findings (data not shown).
4. Discussion
This cross-sectional study of individuals with normal cognition and MCI investigated the association between objectively measured physical activity and sleep with functional connectivity in large-scale brain networks obtained from resting-state fMRI and brain amyloid burden quantified using PiB PET imaging. Results showed that greater physical activity was associated with greater functional network modularity and greater connectivity within the default-mode network (DMN), independent of amyloid status, sleep efficiency (SE) and total sleep duration (TST). In addition, independent of physical activity levels and TST, greater SE was also associated with greater functional network modularity, as well as with greater connectivity within the salience network, particularly among participants without significant amyloid burden. In contrast, longer TST was related to lower network modularity and lower connectivity within the SAL network, primarily among participants with high brain amyloid levels. Finally, there was little evidence that combined physical activity levels, SE, and TST were directly related to brain amyloid status. Taken together, these findings suggest that higher levels of physical activity and higher SE make independent and additive contributions to a more resilient functional network structure and increased connectivity within specific networks. These findings bolster the notion that greater physical activity and better sleep quality may be linked to better cognitive and clinical outcomes among older adults by altering aspects of functional connectivity, rather than by directly influencing amyloid accumulation.
4.1. Physical activity and resting-state functional connectivity
Analysis of the current data also revealed a positive association between total volume of physical activity and rs-fMRI modularity—a graph theory-based measure of network segregation and a potential neuroimaging marker of cognitive resilience (Adams et al., 2023, Ewers et al., 2021). This is consistent with our prior findings of a similar relationship between physical activity and functional network modularity in a subset of this study population (Soldan et al., 2022), and extends these findings by showing that this association is independent of amyloid status, as well as of SE and sleep duration. These findings are also consistent with a previous study linking objectively measured physical activity to greater within network functional connectivity across several large-scale networks (Pruzin et al., 2022), which would result in greater network modularity, though this was not directly assessed. Prior studies further suggest that greater structural and functional network segregation and connectivity mediate the positive associations between cardiorespiratory fitness, which is reflective of greater lifetime physical activity, and cognition in both younger and older adults (Callow and Smith, 2023, Kawagoe et al., 2017, Won et al., 2021). Taken together, these findings as well as prior studies suggest that physical activity may promote functional network modularity, thereby enhancing resilience against both age and pathology-related cognitive decline.
These results also demonstrate that greater total volume of physical activity in particular was associated with enhanced connectivity within the DMN. The DMN is a large-scale brain network that operates during wakeful rest, and reduced connectivity within it has consistently been associated with age-related and pathological cognitive decline (Buckner et al., 2008, Magalhães et al., 2021, Staffaroni et al., 2018). Consistent with these findings, prior studies have reported cross-sectional associations between greater self-reported (Boraxbekk et al., 2016, Soldan et al., 2021) and objectively measured physical activity (Dion et al., 2021, Gogniat et al., 2022, Pruzin et al., 2022, Soldan et al., 2022) and greater DMN connectivity in non-demented older adults. However, a recent longitudinal study measuring physical activity via self-report failed to identify a link with DMN connectivity in cognitively normal older adults (Dorsman et al., 2020), possibly because self-reported physical activity measures tend to be more weakly related to measures of brain health compared to more objective physical activity measures (Callow and Smith, 2023, Logan et al., 2013, Parker et al., 2008). Aβ burden did not moderate the relationship between physical activity and DMN connectivity, consistent with a study using self-reported physical activity (Soldan et al., 2021). Taken together, the finding reported here suggest that physical activity may promote more modular and resilient functional networks in nondemented older adults and increase connectivity within the DMN.
4.2. Sleep and resting-state functional connectivity
Independently of physical activity levels, higher SE was also associated with greater network modularity in those without significant amyloid burden. This suggests that greater SE is associated with more segregated and potentially more resilient functional networks in those who are amyloid negative. It also suggests that interventions focused on improving sleep may have beneficial consequences for global functional network organization, which in turn may improve cognitive outcomes of older adults. Consistent with this interpretation, several prospective investigations have demonstrated links between subjective and objective sleep measures, such as SE and TST, with cognitive performance both at baseline and over a 5-year follow-up period (Ma et al., 2020, McSorley et al., 2019). Moreover, a prospective cohort study demonstrated that enhanced objective sleep consolidation mitigated the impact of the APOE-e4 allele on the rate of cognitive decline and the incidence of AD dementia (Lim, Yu, et al., 2013). It is unclear, however, why SE was not related to functional network segregation among participants with significant amyloid burden. One possibility is that amyloid alters functional network segregation, as reported previously (Adams et al., 2022, Zhang et al., 2023), making associations between SE and segregation potentially more difficult to detect.
The finding that the association between SE and modularity persisted after accounting for the effects of physical activity is consistent with reports suggesting self-reported physical activity and sleep measures provide independent and additive associations with cognition (Falck et al., 2018) and cognitive decline in nondemented older adults (Bloomberg et al., 2023). Therefore, these findings indicate that physical activity and sleep may offer complementary benefits for functional network segregation, which may afford cognitive resilience in both healthy and pathological aging.
The finding that longer TST was associated with lower network modularity, primarily in those with significant amyloid burden suggests that, in the presence of amyloid, extended sleep duration, which may be more frequently disrupted, could lead to lower functional network modularity. This interpretation is consistent with the view that longer sleep duration, above and beyond what is typically observed among older adults, particularly when accounting for SE, may be detrimental to brain and cognitive health (Chen & Wang, 2022). Another possibility for the negative association between TST and network modularity relates to the finding that amyloid accumulation appears to be associated with increases in functional network modularity (Adams et al., 2022), possibly reflecting a compensatory response of the brain to maintain function in the presence of pathology and/or amyloid-related inflammation. Thus, observing shorter sleep duration being related to greater network modularity, particularly in amyloid positive individuals, could be indicative of these individuals requiring additional neural resources to maintain function in the presence of significant amyloid burden (Cabeza et al., 2018, Davis et al., 2018). However, this interpretation is speculative and requires future longitudinal assessments of sleep, functional connectivity, and amyloid accumulation.
We additionally observed that greater SE and shorter sleep duration were associated with higher connectivity in the salience network. The salience network is thought to facilitate individuals’ ability to identify information of importance and attend to it (Menon, 2015). Little is known about the relationship between sleep and salience network connectivity among cognitively normal or non-demented older adults, as most prior research on sleep and resting-state network connectivity focused on the DMN. Considering that the association of sleep and salience network connectivity was independent of amyloid status, results suggest that shorter but more efficient sleep may help promote the development of healthier salience functional networks in cognitively normal older adults, irrespective of AD pathology. Interestingly, loss of coherence within the salience network has been shown to mediate age-related declines in cognition (Touroutoglou et al., 2018), suggesting that more efficient sleep may protect against cognitive decline by increasing salience network connectivity, in addition to network modularity. Additionally, the observation that shorter sleep duration was associated with greater salience network connectivity may be consistent with findings indicating that insomnia symptoms (which are reflective of reduced sleep duration or quality) are associated with higher insula connectivity, a critical part of the salience network (Chen et al., 2014, Cheng et al., 2022). It is also in line with a recent investigation among individuals across the AD spectrum that found that subjective sleep disturbances were associated with hyperconnectivity within the salience network in individuals who were amyloid positive vs. negative (Kim et al., 2023).
Previous research on sleep and resting-state network connectivity has largely focused on the DMN and employed subjective measures of sleep, i.e. questionnaires that can be subject to recall bias and may have limited accuracy for participants with MCI (Dinapoli et al., 2017, Van Den Berg et al., 2008). Overall, these studies have produced mixed results, with some linking poorer subjective sleep quality to lower connectivity in the DMN among participants without dementia (Amorim et al., 2018) and participants with MCI (Luo et al., 2022, McKinnon et al., 2017) and others not finding this association (Lysen et al., 2020). In this study, we only found a weak relationship between greater sleep duration and lower DMN connectivity was observed among participants with normal cognition, independent of SE, which was not related to DMN connectivity. This may suggest that associations between sleep parameters and DMN connectivity are subtle, and that sleep is more strongly related to connectivity in the salience network.
4.3. Relationship of sleep and physical activity to brain amyloid burden
The current study did not demonstrate a significant association between total volume of physical activity and sleep parameters with amyloid positivity. This aligns with many prior studies that have failed to establish a direct link between physical activity and amyloid levels measured by PET, particularly studies using objective measures of physical activity (for reviews, see Brown et al., 2019, Frederiksen et al., 2019, Rodriguez-Ayllon et al., 2023). Most previous studies reporting associations of physical activity with PET amyloid levels relied on subjective physical activity assessments (i.e., questionnaires) (Brown et al., 2013, Okonkwo et al., 2014), which may be less accurate than actigraphy-based measures, as they rely on participants’ memory and are influenced by social desirability biases. Our results support findings from a recent meta-analysis of observational studies that did not find a significant association between physical activity and Aβ measured by PET or in blood among middle-aged and older adults with normal cognition or MCI (Rodriguez-Ayllon et al., 2023).
Consistent with our findings, a smaller study investigating objective sleep measures in relation to global amyloid burden in older adults failed to identify significant associations (Spira et al., 2021). One possibility why we did not observe a direct relationship between amyloid PET burden and SE and TST in this study is our use of objective sleep measures, as previous studies linking sleep and amyloid have predominantly used subjective sleep measures (for a review, see Lucey, 2020). Supporting this conclusion, a recent study found amyloid burden led to a mismatch in perceived vs objective sleep quality in older adults, with amyloid burden successfully predicting worse self-reported sleep quality compared to objective sleep measures (Winer et al., 2021). Furthermore, previous research suggests that the relationship between subjective sleep duration and Aβ accumulation may be specific to certain brain regions in cognitively normal adults (Insel et al., 2021), while we focused on amyloid positivity based on measures across the cortex. Finally, the findings reported here are limited by the temporal discrepancy between some of our PET measurements and actigraphy data collection. It is worth noting, however, that limiting the analysis of PET measurements within a shorter time frame and to either those obtained before or after the actigraphy assessments did not alter the results. Future research should prioritize longitudinal studies that examine the connection between objective sleep with changes in multiple neuroimaging-based measures of AD pathology, including region-specific Aβ and Tau PET markers. This would provide a more comprehensive understanding of the impact of physical activity and sleep on resilience against AD pathological accumulation in older adults.
4.4. Potential Mechanisms Relating Sleep and Physical Activity to rsFC
Cross-sectional studies have revealed that fitness and physical activity are correlated with the development of more efficient cortical neurite density and white matter connection efficiency across the lifespan (Callow et al., 2022, Callow and Smith, 2023). These structural effects may facilitate functional network connectivity, as suggested by some reports linking better white matter structural connectivity to better functional connectivity within networks (Neudorf et al., 2022, Rieck et al., 2020). Additionally, exercise interventions in older cognitively normal adults and those with MCI have demonstrated exercise-related alterations in resting-state connectivity within the SAL and DMN (Won et al., 2023) that are accompanied by exercise-related gray matter neurite density changes within the same networks. (Callow et al., 2021). Taken together this supports the view that one avenue by which physical activity influences rs-fMRI networks (and thereby potentially cognition) may be through its effects on neurite density and white matter connectivity. Additionally, neuropathological evidence suggests that objectively measured late-life physical activity levels are associated with greater synaptic density and reduced microglial inflammatory activity in post-mortem brain tissue, which in turn mediated the positive association between physical activity and global cognition (Casaletto et al., 2021, Casaletto et al., 2022). These findings collectively suggest that physical activity may promote cognitive resilience by facilitating the development of healthy white matter tracts and synaptic and dendritic tissue, thereby potentially enhancing the efficiency of functional networks.
Only a few neuroimaging studies provide insight into the impact of sleep on brain health in aging, although there are several recent reports linking sleep and structural network connectivity. For example, objective measures of healthy sleep were found to be associated with better structural network integrity in healthy aging (Altendahl et al., 2020), while healthy self-reported sleep throughout middle and older age has been found to be predictive of better white matter integrity and neurite density at a 5–20 year follow-up in nondemented older adults (Tsiknia et al., 2023). Therefore, complementing physical activity, healthy sleep may increase the density of white matter connections and thus structural network integrity, which may in turn promote more resilient functional networks. Of note, emerging evidence indicates that a multidomain approach to lifestyle factors provides the most significant benefits for promoting healthy neurocognitive aging and dementia prevention (Montero-Odasso et al., 2022). Additional studies of the effects of objectively measured sleep on neuroimaging measures of brain health in older adults are needed to elucidate the mechanisms by which sleep may influence the organization of functional networks.
5. Limitations
It is important to acknowledge the limitations of this study. The participants in the BIOCARD cohort are predominantly white, highly educated, and have a strong family history of AD, which may limit the generalizability of the findings to more diverse populations. Additionally, the study design employed here was cross-sectional in nature, which limits making any causal inferences regarding sleep, physical activity, and brain health. The MCI group was small, which limits the ability to detect differences by diagnostic status. Furthermore, the calculation of total volume of physical activity was based on the 10 most active hours of the day. It is conceivable that categorizing activity across various time ranges of the day or breaking down physical activity into different intensities could yield different results. Additionally, we focused predominantly on within network and modularity-based network measures of connectivity and did not explore associations with additional network measures focusing on between network measures of connectivity. Future longitudinal studies in larger, more diverse samples are needed to confirm some of these findings regarding relationships and mechanisms through which physical activity and sleep may contribute to functional network organization and ultimately cognitive resilience in aging and AD.
Funding
This work was supported by the National Institutes of Health [grant numbers U19-AG033655, P30-AG005146, R01-AG050507, and T32-AG027668)].
CRediT authorship contribution statement
Daniel D. Callow: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Data curation, Conceptualization. Adam P. Spira: Writing – review & editing, Resources, Methodology, Formal analysis, Conceptualization. Vadim Zipunnikov: Writing – review & editing, Formal analysis, Data curation. Hanzhang Lu: Methodology, Formal analysis, Data curation. Sarah K. Wanigatunga: Writing – review & editing, Methodology, Data curation. Jill A. Rabinowitz: Writing – review & editing, Methodology, Data curation. Marilyn Albert: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition. Arnold Bakker: Writing – review & editing, Supervision, Formal analysis. Anja Soldan: Writing – review & editing, Writing – original draft, Visualization, Validation, Resources, Project administration, Investigation, Formal analysis, Data curation, Conceptualization.
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers U19-AG033655, P30-AG005146]. The BIOCARD Study consists of 7 Cores and two Projects with the following members: (1) the Administrative Core (Marilyn Albert); (2) the Clinical Core (Marilyn Albert, Anja Soldan, Corinne Pettigrew, Greg Pontone, Leonie Farrington, Jules Gilles, Nicole Johnson, Maura Grega, Gay Rudow, Scott Rudow); (3) the Imaging Core (Michael Miller, Susumu Mori, Tilak Ratnanather, Andrea Faria, Anthony Kolasny, Kenichi Oishi, Laurent Younes, Hanzhang Lu, Peter vanZijl); (4) the Biospecimen Core (Abhay Moghekar, Alexandria Lewis, Megha Patel, Sara Ho); (5) the Informatics Core (Ann Ervin, David Shade, Jennifer Jones, Hamadou Coulibaly, Tara Foley); (6) the Biostatistics Core (Mei-Cheng Wang, Yuxin (Daisy) Zhu, Jiangxia Wang); (7) the Neuropathology Core (Juan Troncoso, David Nauen, Javier Redding, Roberta Knox); (8) Project 1 (Paul Worley, Jeremy Walston), and (9) Project 2 (Mei-Cheng Wang, Yifei Sun). The authors are grateful to the members of the BIOCARD Scientific Advisory Board who provide continued oversight and guidance regarding the conduct of the study including: Drs. David Holtzman, William Jagust, David Knopman, Walter Kukull, and Kevin Grimm, and Drs. John Hsiao and Laurie Ryan, who provide oversight on behalf of the National Institute on Aging. The authors thank the members of the BIOCARD Resource Allocation Committee who provide ongoing guidance regarding the use of the biospecimens collected as part of the study, including: Drs. Constantine Lyketsos, Carlos Pardo, Gerard Schellenberg, Leslie Shaw, Madhav Thambisetty, and John Trojanowski. The authors acknowledge the contributions of the Geriatric Psychiatry Branch of the intramural program of NIMH who initiated the study (Principal investigator: Dr. Trey Sunderland). The authors are indebted to Dr. Karen Putnam, who provided documentation of the Geriatric Psychiatry Branch study procedures and the data files received from NIMH.
Conflicts of Interest.
Vadim Zipunnikov consults with Takeda Pharmaceuticals. The details of the contracts are disclosed through the Johns Hopkins University eDisclose system and have no direct or apparent relationship with the current paper. Adam Spira received payment for serving as a consultant for Merck, received honoraria from Springer Nature Switzerland AG for guest editing special issues of Current Sleep Medicine Reports, and is a paid consultant to Sequoia Neurovitality and BellSant, Inc. These roles role has no direct or apparent relationship with the current paper. Arnold Bakker is a paid consultant to AgeneBio, Inc. Arnold Bakker’s role in the current study was in compliance with the conflict of interest policies of the Johns Hopkins School of Medicine. His consulting role has no direct or apparent relationship with the current paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103621.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data used in these analyses are available through standard application procedures described on the BIOCARD website (www.biocard-se.org). Additional information or materials relating to the analysis are available from the corresponding author DC upon reasonable request.
References
- Adams J.N., Chappel-Farley M.G., Yaros J.L., Taylor L., Harris A.L., Mikhail A., McMillan L., Keator D.B., Yassa M.A. Functional network modularity and efficiency supports episodic memory in older adults with amyloid-beta pathology. Alzheimer’s & Dementia. 2022;18(S5):e062561. [Google Scholar]
- Adams J.N., Chappel-Farley M.G., Yaros J.L., Taylor L., Harris A.L., Mikhail A., McMillan L., Keator D.B., Yassa M.A. Functional network structure supports resilience to memory deficits in cognitively normal older adults with amyloid-β pathology. Sci. Rep. 2023;13(1):13953. doi: 10.1038/S41598-023-40092-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert, M., Soldan, A., Gottesman, R., McKhann, G., Sacktor, N., Farrington, L., Grega, M., Turner, R., Lu, Y., Li, S., Wang, M.-C., Selnes, O., & Team, the B. R. (2014). Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Current Alzheimer Research, 11(8), 773. https://doi.org/10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed]
- Altendahl M., Cotter D.L., Staffaroni A.M., Wolf A., Mumford P., Cobigo Y., Casaletto K., Elahi F., Ruoff L., Javed S., Bettcher B.M., Fox E., You M., Saloner R., Neylan T.C., Kramer J.H., Walshid C.M. REM sleep is associated with white matter integrity in cognitively healthy, older adults. PLoS One. 2020;15(7) doi: 10.1371/JOURNAL.PONE.0235395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim L., Magalhães R., Coelho A., Moreira P.S., Portugal-Nunes C., Castanho T.C., Marques P., Sousa N., Santos N.C. Poor Sleep Quality Associates With Decreased Functional and Structural Brain Connectivity in Normative Aging: A MRI Multimodal Approach. Front. Aging Neurosci. 2018;10:375. doi: 10.3389/FNAGI.2018.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenaza-Urquijo E.M., Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology. 2018;90(15):695. doi: 10.1212/WNL.0000000000005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R.F., Byrge L., He Y., Goñi J., Zuo X.N., Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(P2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Bilgel M., An Y., Zhou Y., Wong D.F., Prince J.L., Ferrucci L., Resnick S.M. Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association. 2016;12(4):373–379. doi: 10.1016/J.JALZ.2015.08.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgel M., An Y., Helphrey J., Elkins W., Gomez G., Wong D.F., Davatzikos C., Ferrucci L., Resnick S.M. Effects of amyloid pathology and neurodegeneration on cognitive change in cognitively normal adults. Brain. 2018;141(8):2475. doi: 10.1093/BRAIN/AWY150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondell, S. J., Hammersley-Mather, R., & Veerman, J. L. (2014). Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. In BMC Public Health (Vol. 14, Issue 1, p. 510). BioMed Central Ltd. https://doi.org/10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed]
- Bloomberg M., Brocklebank L., Hamer M., Steptoe A. Joint associations of physical activity and sleep duration with cognitive ageing: longitudinal analysis of an English cohort study. The Lancet Healthy Longevity. 2023;4(7):e345–e353. doi: 10.1016/S2666-7568(23)00083-1. [DOI] [PubMed] [Google Scholar]
- Boraxbekk C.J., Salami A., Wåhlin A., Nyberg L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network—A multimodal approach. Neuroimage. 2016;131:133–141. doi: 10.1016/J.NEUROIMAGE.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Brown B.M., Peiffer J.J., Taddei K., Lui J.K., Laws S.M., Gupta V.B., Taddei T., Ward V.K., Rodrigues M.A., Burnham S., Rainey-Smith S.R., Villemagne V.L., Bush A., Ellis K.A., Masters C.L., Ames D., MacAulay S.L., Szoeke C., Rowe C.C., Martins R.N. Physical activity and amyloid-β plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol. Psychiatry. 2013;18(8):875–881. doi: 10.1038/MP.2012.107. [DOI] [PubMed] [Google Scholar]
- Brown B.M., Peiffer J., Rainey-Smith S.R. Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review. Ageing Res. Rev. 2019;50:9–18. doi: 10.1016/J.ARR.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The Brain’s Default Network. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., Lindenberger, U., Nyberg, L., Park, D. C., Reuter-Lorenz, P. A., Rugg, M. D., Steffener, J., & Rajah, M. N. (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. In Nature Reviews Neuroscience (Vol. 19, Issue 11, pp. 701–710). Nature Publishing Group. https://doi.org/10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed]
- Callow D.D., Purcell J.J., Won J., Smith J.C. Neurite dispersion and density mediates the relationship between cardiorespiratory fitness and cognition in healthy younger adults. Neuropsychologia. 2022;169 doi: 10.1016/J.NEUROPSYCHOLOGIA.2022.108207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow D.D., Smith J.C. Physical fitness, cognition, and structural network efficiency of brain connections across the lifespan. Neuropsychologia. 2023;182 doi: 10.1016/j.neuropsychologia.2023.108527. [DOI] [PubMed] [Google Scholar]
- Callow D.D., Won J., Pena G.S., Jordan L.S., Arnold-Nedimala N.A., Kommula Y., Nielson K.A., Smith J.C. Exercise Training-Related Changes in Cortical Gray Matter Diffusivity and Cognitive Function in Mild Cognitive Impairment and Healthy Older Adults. Front. Aging Neurosci. 2021;13:164. doi: 10.3389/fnagi.2021.645258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow D.D., Zipunnikov V., Spira A.P., Wanigatunga S.K., Pettigrew C., Albert M., Soldan A. Actigraphy estimated sleep moderates the relationship between physical activity and cognition in older adults. Ment. Health Phys. Act. 2024;26 doi: 10.1016/J.MHPA.2023.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto K., Lindbergh C.A., VandeBunte A., Neuhaus J., Schneider J.A., Buchman A.S., Honer W.G., Bennett D.A. Microglial correlates of late life physical activity: Relationship with synaptic and cognitive aging in older adults. J. Neurosci. 2021 doi: 10.1523/JNEUROSCI.1483-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto K., Ramos-Miguel A., Ba A.V., Memel M., Buchman A., Bennett D., Honer W. Late-life physical activity relates to brain tissue synaptic integrity markers in older adults. Alzheimer’s & Dementia. 2022 doi: 10.1002/ALZ.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, M. Y., Park, D. C., Savalia, N. K., Petersen, S. E., & Wig, G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111(46), E4997–E5006. https://doi.org/10.1073/PNAS.1415122111/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed]
- Chen M.C., Chang C., Glover G.H., Gotlib I.H. Increased insula coactivation with salience networks in insomnia. Biol. Psychol. 2014;97(1):1–8. doi: 10.1016/J.BIOPSYCHO.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.C., Wang X.Y. Longitudinal associations between sleep duration and cognitive impairment in Chinese elderly. Front. Aging Neurosci. 2022;14:1037650. doi: 10.3389/FNAGI.2022.1037650/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Xue T., Dong F., Hu Y., Zhou M., Li X., Huang R., Lu X., Yuan K., Yu D. Abnormal functional connectivity of the salience network in insomnia. Brain Imaging Behav. 2022;16(2):930–938. doi: 10.1007/S11682-021-00567-9. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., D’Esposito M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016;36(48):12083. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.W., Szymanski A., Boms H., Fink T., Cabeza R. Cooperative contributions of structural and functional connectivity to successful memory in aging. Network Neurosci. 2018;3(1):173–194. doi: 10.1162/netn_a_00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souto Barreto P., Andrieu S., Payoux P., Demougeot L., Rolland Y., Vellas B. Physical Activity and Amyloid-β Brain Levels in Elderly Adults with Intact Cognition and Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2015;63(8):1634–1639. doi: 10.1111/JGS.13530. [DOI] [PubMed] [Google Scholar]
- Dinapoli E.A., Gebara M.A., Kho T., Butters M.A., Gildengers A.G., Albert S.M., Dew M.A., Erickson K.I., Reynolds C.F., Karp J.F. Subjective-Objective Sleep Discrepancy in Older Adults With MCI and Subsyndromal Depression. 2017;30(6):316–323. doi: 10.1177/0891988717731827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion C., Tanner J.J., Crowley S.J., Wiggins M.E., Mareci T., Ding M., Price C.C., Manini T.M. Functional connectivity of key resting state networks and objectively measured physical activity in older adults with joint pain: A pilot study. Exp. Gerontol. 2021;153 doi: 10.1016/J.EXGER.2021.111470. [DOI] [PubMed] [Google Scholar]
- Dorsman K.A., Weiner-Light S., Staffaroni A.M., Brown J.A., Wolf A., Cobigo Y., Walters S., Kramer J.H., Casaletto K.B. Get Moving! Increases in Physical Activity Are Associated With Increasing Functional Connectivity Trajectories in Typically Aging Adults. Front. Aging Neurosci. 2020;12 doi: 10.3389/FNAGI.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M., Luan Y., Frontzkowski L., Neitzel J., Rubinski A., Dichgans M., Hassenstab J., Gordon B.A., Chhatwal J.P., Levin J., Schofield P., Benzinger T.L.S., Morris J.C., Goate A., Karch C.M., Fagan A.M., McDade E., Allegri R., Berman S., Franzmeier N. Segregation of functional networks is associated with cognitive resilience in Alzheimer’s disease. Brain. 2021;144(7):2176. doi: 10.1093/BRAIN/AWAB112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck R.S., Best J.R., Davis J.C., Liu-Ambrose T. The Independent Associations of Physical Activity and Sleep with Cognitive Function in Older Adults. Journal of Alzheimer’s Disease. 2018;63(4):1469–1484. doi: 10.3233/JAD-170936. [DOI] [PubMed] [Google Scholar]
- Fenton L., Isenberg A.L., Aslanyan V., Albrecht D., Contreras J.A., Stradford J., Monreal T., Pa J. Variability in objective sleep is associated with Alzheimer’s pathology and cognition. Brain. Communications. 2023;5(2) doi: 10.1093/BRAINCOMMS/FCAD031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen K.S., Gjerum L., Waldemar G., Hasselbalch S.G. Physical Activity as a Moderator of Alzheimer Pathology: A Systematic Review of Observational Studies. Curr. Alzheimer Res. 2019;16(4):362–378. doi: 10.2174/1567205016666190315095151. [DOI] [PubMed] [Google Scholar]
- Gogniat M.A., Robinson T.L., Jean K.R., Miller L.S. Physical activity moderates the association between executive function and functional connectivity in older adults. Aging Brain. 2022;2 doi: 10.1016/J.NBAS.2022.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman R.F., Schneider A.L.C., Zhou Y., Coresh J., Green E., Gupta N., Knopman D.S., Mintz A., Rahmim A., Sharrett A.R., Wagenknecht L.E., Wong D.F., Mosley T.H. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317(14):1443–1450. doi: 10.1001/JAMA.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.Y., Li Y.Z., Zhang Y.R., Huang Y.Y., Wu B.S., Zhang W., Deng Y.T., Chen S.D., He X.Y., Chen S.F., Dong Q., Zhang C., Chen R.J., Suckling J., Rolls E.T., Feng J.F., Cheng W., Yu J.T. Sleep, physical activity, sedentary behavior, and risk of incident dementia: a prospective cohort study of 431,924 UK Biobank participants. Mol. Psychiatry. 2022;27(10):4343–4354. doi: 10.1038/s41380-022-01655-y. [DOI] [PubMed] [Google Scholar]
- Insel P.S., Mohlenhoff B.S., Neylan T.C., Krystal A.D., Mackin R.S. Association of Sleep and β-Amyloid Pathology Among Older Cognitively Unimpaired Adults. JAMA Netw. Open. 2021;4(7):e2117573–e. doi: 10.1001/JAMANETWORKOPEN.2021.17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.E.S., McLeland J.S., Toedebusch C.D., Xiong C., Fagan A.M., Duntley S.P., Morris J.C., Holtzman D.M. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587–593. doi: 10.1001/JAMANEUROL.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.E.S., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat. Rev. Neurol. 2014;10(2):115. doi: 10.1038/NRNEUROL.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe T., Onoda K., Yamaguchi S. Associations among executive function, cardiorespiratory fitness, and brain network properties in older adults. Sci. Rep. 2017;7 doi: 10.1038/srep40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Zhu X., Zhao Y., Bell S., Gehrman P., Cohen D., Devanand D., Goldberg T., Lee S. Resting-State Functional Connectivity Changes in Older Adults with Sleep Disturbance and the Role of Amyloid Burden. Research Square. 2023 doi: 10.21203/RS.3.RS-2547880/V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T.S., Gratton C., Low K.A., Tan C.H., Chiarelli A., Fletcher M.A., Zimmerman B., Maclin E.L., Sutton B.P., Gratton G., Fabiani M. Age-related differences in functional brain network segregation are consistent with a cascade of cerebrovascular, structural, and cognitive effects. Network Neurosci. 2020;4(1):89. doi: 10.1162/NETN_A_00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushida C.A., Chang A., Gadkary C., Guilleminault C., Carrillo O., Dement W.C. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/S1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Liang K.Y., Mintun M.A., Fagan A.M., Goate A.M., Bugg J.M., Holtzman D.M., Morris J.C., Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010;68(3):311–318. doi: 10.1002/ANA.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.S.P., Kowgier M., Yu L., Buchman A.S., Bennett D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36(7):1027. doi: 10.5665/SLEEP.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A.S.P., Yu L., Kowgier M., Schneider J.A., Buchman A.S., Bennett D.A. Sleep Modifies the Relation of APOE to the Risk of Alzheimer Disease and Neurofibrillary Tangle Pathology. JAMA Neurol. 2013;70(12):1544–1551. doi: 10.1001/JAMANEUROL.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Ly M., Karim H.T., Wei W., Snitz B.E., Klunk W.E., Aizenstein H.J. The effect of amyloid deposition on longitudinal resting-state functional connectivity in cognitively normal older adults. Alzheimer’s Research and Therapy. 2020;12(1):1–10. doi: 10.1186/S13195-019-0573-1/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S.L., Gottlieb B.H., Maitl S.B., Meegan D., Spriet L.L. The physical activity scale for the elderly (PASE) questionnaire; Does it predict physical health? Int. J. Environ. Res. Public Health. 2013;10(9):3967–3986. doi: 10.3390/ijerph10093967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey B.P. It’s Complicated: The Relationship Between Sleep and Alzheimer’s Disease in Humans. Neurobiol. Dis. 2020;144 doi: 10.1016/J.NBD.2020.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Qiao M., Liang Y., Chen C., Zeng L., Wang L., Wu W. Functional Brain Connectivity in Mild Cognitive Impairment With Sleep Disorders: A Study Based on Resting-State Functional Magnetic Resonance Imaging. Front. Aging Neurosci. 2022;14 doi: 10.3389/FNAGI.2022.812664/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysen T.S., Zonneveld H.I., Muetzel R.L., Ikram M.A., Luik A.I., Vernooij M.W., Tiemeier H. Sleep and resting-state functional magnetic resonance imaging connectivity in middle-aged adults and the elderly: A population-based study. J. Sleep Res. 2020;29(5):e12999. doi: 10.1111/jsr.12999. [DOI] [PubMed] [Google Scholar]
- Ma Y., Liang L., Zheng F., Shi L., Zhong B., Xie W. Association Between Sleep Duration and Cognitive Decline. JAMA Netw. Open. 2020;3(9):e2013573–e. doi: 10.1001/JAMANETWORKOPEN.2020.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães T.N.C., Gerbelli C.L.B., Pimentel-Silva L.R., de Campos B.M., de Rezende T.J.R., Rizzi L., Joaquim H.P.G., Talib L.L., Forlenza O.V., Cendes F., Balthazar M.L.F. Differences in structural and functional default mode network connectivity in amyloid positive mild cognitive impairment: a longitudinal study. Neuroradiology. 2021;2021(1):1–10. doi: 10.1007/S00234-021-02760-5. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : the Journal of the Alzheimer’s Association. 2011;7(3):263–269. doi: 10.1016/J.JALZ.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon A.C., Duffy S.L., Cross N.E., Terpening Z., Grunstein R.R., Lagopoulos J., Batchelor J., Hickie I.B., Lewis S.J.G., Shine J.M., Naismith S.L. Functional Connectivity in the Default Mode Network is Reduced in Association with Nocturnal Awakening in Mild Cognitive Impairment. Journal of Alzheimer’s Disease. 2017;56(4):1373–1384. doi: 10.3233/JAD-160922. [DOI] [PubMed] [Google Scholar]
- McSorley V.E., Bin Y.S., Lauderdale D.S. Associations of Sleep Characteristics With Cognitive Function and Decline Among Older Adults. Am. J. Epidemiol. 2019;188(6):1066–1075. doi: 10.1093/AJE/KWZ037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memel, M., Buchman, A. S., Bennett, D. A., & Casaletto, K. (2021). Relationship between objectively measured physical activity on neuropathology and cognitive outcomes in older adults: Resistance versus resilience? Alzheimer’s & Dementia (Amsterdam, Netherlands), 13(1). https://doi.org/10.1002/DAD2.12245. [DOI] [PMC free article] [PubMed]
- Meng X., Wu Y., Liang Y., Zhang D., Xu Z., Yang X., Meng L. A Triple-Network Dynamic Connection Study in Alzheimer’s Disease. Front. Psych. 2022;13 doi: 10.3389/FPSYT.2022.862958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/J.TICS.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V. Vol. 2. Elsevier Inc.; 2015. Salience Network. (Brain Mapping: an Encyclopedic Reference). [DOI] [Google Scholar]
- Montero-Odasso M., Zou G.Y., Kamkar N., Feldman H.H., Belleville S., Chertkow H., Nygaard H.B., Son S., Speechley M. Multidomain trials to prevent dementia: addressing methodological challenges. Alzheimer’s Research and Therapy. 2022;14(1):1–5. doi: 10.1186/S13195-022-01036-1/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wu D., Ceritoglu C., Li Y., Kolasny A., Vaillant M.A., Faria A.V., Oishi K., Miller M.I. MRICloud: Delivering high-throughput MRI neuroinformatics as cloud-based software as a service. Comput. Sci. Eng. 2016;18(5):21–35. doi: 10.1109/MCSE.2016.93. [DOI] [Google Scholar]
- Neudorf J., Kress S., Borowsky R. Structure can predict function in the human brain: a graph neural network deep learning model of functional connectivity and centrality based on structural connectivity. Brain Struct. Funct. 2022;227(1):331–343. doi: 10.1007/S00429-021-02403-8/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P., Tchounguen J., Pettigrew C., Lim C., Lin Z., Lu H., Moghekar A., Albert M., Soldan A. Regional White Matter Hyperintensities and Alzheimer’s Disease Biomarkers Among Older Adults with Normal Cognition and Mild Cognitive Impairment. Journal of Alzheimer’s Disease : JAD. 2023;92(1):323–339. doi: 10.3233/JAD-220846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzum H., Stickel A., Corona M., Zeller M., Melrose R.J., Wilkins S.S. Potential Benefits of Physical Activity in MCI and Dementia. Behav. Neurol. 2020;2020 doi: 10.1155/2020/7807856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo O.C., Schultz S.A., Oh J.M., Larson J., Edwards D., Cook D., Koscik R., Gallagher C.L., Dowling N.M., Carlsson C.M., Bendlin B.B., LaRue A., Rowley H.A., Christian B.T., Asthana S., Hermann B.P., Johnson S.C., Sager M.A. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753. doi: 10.1212/WNL.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms S., Overeem S., Besse K., Rikkert M.O., Verbeek M., Claassen J.A.H.R. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71(8):971–977. doi: 10.1001/JAMANEUROL.2014.1173. [DOI] [PubMed] [Google Scholar]
- Parker S.J., Strath S.J., Swartz A.M. Physical activity measurement in older adults: Relationships with mental health. J. Aging Phys. Act. 2008;16(4):369–380. doi: 10.1123/japa.16.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrero-Chamizo R., Szoeke C., Dennerstein L., Campbell S. Influence of Physical Activity Levels and Functional Capacity on Brain β-Amyloid Deposition in Older Women. Front. Aging Neurosci. 2021;13 doi: 10.3389/FNAGI.2021.697528/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini S., Chatterjee P., Nakamura A., Tegg M., Hone E., Rainey-Smith S.R., Rowe C.C., Dore V., Villemagne V.L., Ames D., Kaneko N., Gardener S.L., Taddei K., Fernando B., Martins I., Bharadwaj P., Sohrabi H.R., Masters C.L., Brown B., Martins R.N. The Association Between Alzheimer’s Disease-Related Markers and Physical Activity in Cognitively Normal Older Adults. Front. Aging Neurosci. 2022;14 doi: 10.3389/FNAGI.2022.771214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew C., Soldan A., Wang J., Wang M.C., Arthur K., Moghekar A., Gottesman R.F., Albert M. Association of midlife vascular risk and AD biomarkers with subsequent cognitive decline. Neurology. 2020;95(23):E3093–E3103. doi: 10.1212/WNL.0000000000010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto F.H.G., Coutinho A.M., de Souza Duran F.L., de Sá Pinto A.L., Gualano B., Buchpiguel C.A., Busatto G., Nitrini R., Brucki S.M.D. Aerobic training modulates salience network and default mode network metabolism in subjects with mild cognitive impairment. NeuroImage : Clinical. 2018;19:616. doi: 10.1016/J.NICL.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/J.NEUROIMAGE.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzin J.J., Klein H., Rabin J.S., Schultz A.P., Kirn D.R., Yang H.S., Buckley R.F., Scott M.R., Properzi M., Rentz D.M., Johnson K.A., Sperling R.A., Chhatwal J.P. Physical activity is associated with increased resting-state functional connectivity in networks predictive of cognitive decline in clinically unimpaired older adults. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2022;14(1):e12319. doi: 10.1002/dad2.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck J.R., Rodrigue K.M., Park D.C., Kennedy K.M. White Matter Microstructure Predicts Focal and Broad Functional Brain Dedifferentiation in Normal Aging. J. Cogn. Neurosci. 2020;32(8):1536. doi: 10.1162/JOCN_A_01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ayllon M., Solís-Urra P., Arroyo-Ávila C., Álvarez-Ortega M., Molina-García P., Molina-Hidalgo C., Gómez-Río M., Brown B., Erickson K.I., Esteban-Cornejo I. Physical activity and amyloid beta in middle-aged and older adults: A systematic review and meta-analysis. J. Sport Health Sci. 2023 doi: 10.1016/J.JSHS.2023.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. 2009 doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sabia, S., Fayosse, A., Dumurgier, J., van Hees, V. T., Paquet, C., Sommerlad, A., Kivimäki, M., Dugravot, A., & Singh-Manoux, A. (2021). Association of sleep duration in middle and old age with incidence of dementia. Nature Communications 2021 12:1, 12(1), 1–10. https://doi.org/10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed]
- Sewell K.R., Rainey-Smith S.R., Peiffer J., Sohrabi H.R., Taddei K., Ames D., Maruff P., Masters C.L., Rowe C.C., Martins R.N., Erickson K.I., Brown B.M. The relationship between objective physical activity and change in cognitive function. Alzheimer’s & Dementia. 2023 doi: 10.1002/ALZ.12950. [DOI] [PubMed] [Google Scholar]
- Sewell K.R., Rainey-Smith S.R., Villemagne V.L., Peiffer J., Sohrabi H.R., Taddei K., Ames D., Doré V., Maruff P., Laws S.M., Masters C.L., Rowe C.C., Martins R.N., Erickson K.I., Brown B.M. The interaction between physical activity and sleep on cognitive function and brain beta-amyloid in older adults. Behav. Brain Res. 2023;437 doi: 10.1016/J.BBR.2022.114108. [DOI] [PubMed] [Google Scholar]
- Shi L., Chen S.J., Ma M.Y., Bao Y.P., Han Y., Wang Y.M., Shi J., Vitiello M.V., Lu L. Sleep disturbances increase the risk of dementia: A systematic review and meta-analysis. Sleep Med. Rev. 2018;40:4–16. doi: 10.1016/J.SMRV.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Sintini, I., Graff-Radford, J., Jones, D. T., Botha, H., Martin, P. R., Machulda, M. M., Schwarz, C. G., Senjem, M. L., Gunter, J. L., Jack, C. R., Lowe, V. J., Josephs, K. A., & Whitwell, J. L. (2021). Tau and Amyloid Relationships with Resting-state Functional Connectivity in Atypical Alzheimer’s Disease. Cerebral Cortex (New York, N.Y. : 1991), 31(3), 1693–1706. https://doi.org/10.1093/CERCOR/BHAA319. [DOI] [PMC free article] [PubMed]
- Sohn B.K., Byun M.S., Yi D., Jeon S.Y., Lee J.H., Choe Y.M., Lee D.W., Lee J.Y., Kim Y.K., Sohn C.H., Lee D.Y. Late-Life Physical Activities Moderate the Relationship of Amyloid-β Pathology with Neurodegeneration in Individuals Without Dementia. Journal of Alzheimer’s Disease : JAD. 2022;86(1):441–450. doi: 10.3233/JAD-215258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan, A., Pettigrew, C., Zhu, Y., Wang, M. C., Bilgel, M., Hou, X., Lu, H., Miller, M. I., & Albert, M. (2021). Association of Lifestyle Activities with Functional Brain Connectivity and Relationship to Cognitive Decline among Older Adults. Cerebral Cortex (New York, N.Y. : 1991), 31(12), 5637–5651. https://doi.org/10.1093/CERCOR/BHAB187. [DOI] [PMC free article] [PubMed]
- Soldan, A., Alfini, A., Pettigrew, C., Faria, A., Hou, X., Lim, C., Lu, H., Spira, A. P., Zipunnikov, V., Albert, M., & Team, R. (2022). Actigraphy-estimated physical activity is associated with functional and structural brain connectivity among older adults. 116, 32–40. https://pubmed.ncbi.nlm.nih.gov/35551019/. [DOI] [PMC free article] [PubMed]
- Soldan A., Pettigrew C., Zhu Y., Wang M.C., Gottesman R.F., DeCarli C., Albert M. Cognitive reserve and midlife vascular risk: Cognitive and clinical outcomes. Ann. Clin. Transl. Neurol. 2020;7(8):1307–1317. doi: 10.1002/ACN3.51120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.P., Gamaldo A.A., An Y., Wu M.N., Simonsick E.M., Bilgel M., Zhou Y., Wong D.F., Ferrucci L., Resnick S.M. Self-Reported Sleep and β-Amyloid Deposition in Community-Dwelling Older Adults. JAMA Neurol. 2013;70(12):1537. doi: 10.1001/JAMANEUROL.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A.P., Zipunnikov V., Raman R., Choi J., Di J., Bai J., Carlsson C.M., Mintzer J.E., Marshall G.A., Porsteinsson A.P., Yaari R., Wanigatunga S.K., Kim J., Wu M.N., Aisen P.S., Sperling R.A., Rosenberg P.B. Brain amyloid burden, sleep, and 24-hour rest/activity rhythms: screening findings from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s and Longitudinal Evaluation of Amyloid Risk and Neurodegeneration Studies. Sleep Advances : A Journal of the Sleep Research Society. 2021;2(1) doi: 10.1093/SLEEPADVANCES/ZPAB015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffaroni A.M., Brown J.A., Casaletto K.B., Elahi F.M., Deng J., Neuhaus J., Cobigo Y., Mumford P.S., Walters S., Saloner R., Karydas A., Coppola G., Rosen H.J., Miller B.L., Seeley W.W., Kramer J.H. The Longitudinal Trajectory of Default Mode Network Connectivity in Healthy Older Adults Varies As a Function of Age and Is Associated with Changes in Episodic Memory and Processing Speed. J. Neurosci. 2018;38(11):2809–2817. doi: 10.1523/JNEUROSCI.3067-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman C.M., Lopez O.L., Becker J.T., Kuller L.H., Mehta P.D., Tracy R.P., Erickson K.I. Physical activity predicts reduced plasma β amyloid in the Cardiovascular Health Study. Ann. Clin. Transl. Neurol. 2017;4(5):284–291. doi: 10.1002/ACN3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fisch B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125. doi: 10.1152/JN.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Zhang J., Andreano J.M., Dickerson B.C., Barrett L.F. Dissociable Effects of Aging on Salience Subnetwork Connectivity Mediate Age-Related Changes in Executive Function and Affect. Front. Aging Neurosci. 2018;10 doi: 10.3389/FNAGI.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyer V., Meyer R.S., Buchmann A., Crameri G.A.G., Studer S., Saake A., Gruber E., Unschuld P.G., Nitsch R.M., Hock C., Gietl A.F. Physical activity is associated with lower cerebral beta-amyloid and cognitive function benefits from lifetime experience-a study in exceptional aging. PLoS One. 2021;16(2) doi: 10.1371/JOURNAL.PONE.0247225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiknia A.A., Parada H., Banks S.J., Reas E.T. Sleep quality and sleep duration predict brain microstructure among community-dwelling older adults. Neurobiol. Aging. 2023;125:90. doi: 10.1016/J.NEUROBIOLAGING.2023.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg J.F., Van Rooij F.J.A., Vos H., Tulen J.H.M., Hofman A., Miedema H.M.E., Neven A.K., Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons*. J. Sleep Res. 2008;17(3):295–302. doi: 10.1111/J.1365-2869.2008.00638.X. [DOI] [PubMed] [Google Scholar]
- Varma V.R., Dey D., Leroux A., Di J., Urbanek J., Xiao L., Zipunnikov V. Re-evaluating the effect of age on physical activity over the lifespan. Prev. Med. 2017;101:102. doi: 10.1016/J.YPMED.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Weng T.B., Burzynska A.Z., Wong C.N., Cooke G.E., Clark R., Fanning J., Awick E., Gothe N.P., Olson E.A., Mcauley E., Kramer A.F. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging HHS Public Access. Neuroimage. 2016;131:113–125. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K.A., Gross A.L., Moghekar A.R., Soldan A., Pettigrew C., Hou X., Lu H., Alfini A.J., Bilgel M., Miller M.I., Albert M.S., Walston J. Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of non-demented older adults. Brain Behav. Immun. 2020;87:388. doi: 10.1016/J.BBI.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Liu M., Cheng X., Wu Y., Hildebrandt A., Zhou C. Segregation, integration, and balance of large-scale resting brain networks configure different cognitive abilities. PNAS. 2021;118(23) doi: 10.1073/PNAS.2022288118/SUPPL_FILE/PNAS.2022288118.SD01.CSV. e2022288118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Hou R., Xie L., Chandrasekar E.K., Lu H., Wang T., Li C., Xu H. Sleep, sedentary activity, physical activity, and cognitive function among older adults: The National Health and Nutrition Examination Survey, 2011–2014. J. Sci. Med. Sport. 2021;24(2):189–194. doi: 10.1016/J.JSAMS.2020.09.013. [DOI] [PubMed] [Google Scholar]
- Wig G.S. Segregated Systems of Human Brain Networks. Trends Cogn. Sci. 2017;21(12):981–996. doi: 10.1016/J.TICS.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Winer J.R., Morehouse A., Fenton L., Harrison T.M., Ayangma L., Reed M., Kumar S., Baker S.L., Jagust W.J., Walker M.P. Tau and β-Amyloid Burden Predict Actigraphy-Measured and Self-Reported Impairment and Misperception of Human Sleep. J. Neurosci. 2021;41(36):7687. doi: 10.1523/JNEUROSCI.0353-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J., Callow D.D., Pena G.S., Gogniat M.A., Kommula Y., Arnold-Nedimala N.A., Jordan L.S., Smith J.C. Evidence for Exercise-Related Plasticity in Functional and Structural Neural Network Connectivity. Neurosci. Biobehav. Rev. 2021;131(October):923–940. doi: 10.1016/j.neubiorev.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J., Nielson K.A., Smith J.C. Large-Scale Network Connectivity and Cognitive Function Changes After Exercise Training in Older Adults with Intact Cognition and Mild Cognitive Impairment. Journal of Alzheimer’s Disease Reports. 2023;7(1):399. doi: 10.3233/ADR-220062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., Takano T., Deane R., Nedergaard M. Sleep Drives Metabolite Clearance from the Adult Brain. Science (New York, N.Y.) 2013;342(6156):373–377. doi: 10.1126/SCIENCE.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Chan, M. Y., Han, L., Carreno, C. A., Winter-Nelson, E., Wig, G. S., & (ADNI), for the A. D. N. I. (2023). Dissociable effects of Alzheimer’s Disease-related cognitive dysfunction and aging on functional brain network segregation. Journal of Neuroscience, JN-RM-0579-23. https://doi.org/10.1523/JNEUROSCI.0579-23.2023. [DOI] [PMC free article] [PubMed]
- Zhou Y., Endres C.J., Brašić J.R., Huang S.C., Wong D.F. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage. 2003;18(4):975–989. doi: 10.1016/S1053-8119(03)00017-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in these analyses are available through standard application procedures described on the BIOCARD website (www.biocard-se.org). Additional information or materials relating to the analysis are available from the corresponding author DC upon reasonable request.