Abstract
Introduction
Corilagin possesses a diverse range of pharmacologic bioactivities. However, the specific protective effects and mechanisms of action of corilagin in the context of atherosclerosis remain unclear. In this study, we investigated the impact of corilagin on the toll-like receptor (TLR)4 signaling pathway in a mouse vascular smooth muscle cell line (MOVAS) stimulated by oxidized low-density lipoprotein (ox-LDL). Additionally, we examined the effects of corilagin in Sprague–Dawley rats experiencing atherosclerosis.
Methods
The cytotoxicity of corilagin was assessed using the CCK8 assay. MOVAS cells, pre-incubated with ox-LDL, underwent treatment with varying concentrations of corilagin. TLR4 expression was modulated by either downregulation through small interfering (si)RNA or upregulation via lentivirus transfection. Molecular expression within the TLR4 signaling pathway was analyzed using real-time polymerase chain reaction (PCR) and Western blotting. The proliferation capacity of MOVAS cells was determined through cell counting. In a rat model, atherosclerosis was induced in femoral arteries using an improved guidewire injury method, and TLR4 expression in plaque areas was assessed using immunofluorescence. Pathological changes were examined through hematoxylin and eosin staining, as well as Oil-Red-O staining.
Results
Corilagin demonstrated inhibitory effects on the TLR4 signaling pathway in MOVAS cells pre-stimulated with ox-LDL, consequently impeding the proliferative impact of ox-LDL. The modulation of TLR4 expression, either through downregulation or upregulation, similarly influenced the expression of downstream molecules. In an in vivo context, corilagin exhibited the ability to suppress TLR4 and MyD88 expression in the plaque lesion areas of rat femoral arteries, thereby alleviating the formation of atherosclerotic plaques.
Conclusion
Corilagin can inhibit the TLR4 signaling pathway in VSMCs, possibly by downregulating TLR4 expression and, consequently, relieving atherosclerosis.
Keywords: corilagin, atherosclerosis, vascular smooth muscle cell, toll-like receptor 4, ox-LDL
Introduction
Lower limb atherosclerosis stands as the prevailing and challenging form of peripheral arterial disease (PAD). It results in intermittent claudication, chronic limb-threatening ischemia, and tissue damage. 1 Its intricate pathogenesis, implicating monocytes/macrophages, lymphocytes, dendritic cells, endothelial cells, and vascular smooth muscle cells (VSMCs), undermines the effectiveness of current treatments like endovascular interventions, surgery, and pharmacotherapy.2,3
Recent research has underscored the significance of VSMCs throughout the progression of atherosclerosis. Endothelial injury initiates the transportation of lipids and cholesterol across the endothelial barrier, triggering a cascade of inflammatory reactions within the vessel wall. This, in turn, can influence the migration, proliferation, and characteristics of medial VSMCs. Notably, VSMCs exhibit reduced expression of typical smooth muscle cell (SMC) markers like SMC myosin heavy chain and smooth muscle α-actin, while displaying substantial synthetic capabilities. 4 These traits empower VSMCs to secrete more pro-inflammatory cytokines and extracellular matrix, potentially fostering the development of atherosclerosis. 5 Intriguingly, VSMCs transitioning phenotypically into macrophage-like cells may also adopt macrophage markers and attributes, including elevated expression of toll-like receptors (TLRs). In atherosclerotic plaques, cells exhibiting macrophage markers may not necessarily derive from bone marrow-derived macrophages. Dual positivity for SMCs and macrophage markers in a large fraction of foam cells suggests that these cells originate from VSMCs. 6
Chronic inflammation has been recognized as a hallmark of atherosclerosis and several studies have highlighted the pathogenic role of TLRs. For instance, atherosclerosis is attenuated in TLR4-deficient mice. 7 Oxidized low-density lipoprotein (ox-LDL), a ligand and enhancer of endogenous TLR4, contributes to the initiation and progression of atherosclerosis by inducing the secretion of pro-inflammatory cytokines in VSMCs.8,9 In addition, Lee et al. demonstrated that TLR4 can accelerate VSMC migration by activating p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK)1/2 signaling. 10 TLR4 ligands can lead to the prompt activation of multiple transcription factors, such as the Nuclear Factor-kappa B (NF-κB) and interferon regulatory factor (IRF) families. Consequently, numerous genes amplifying the initial inflammatory response are swiftly induced, contributing to atherogenesis. 11 This evidence implies that modulation of the TLR4 signaling pathway could impede VSMC function and, consequently, alleviate atherosclerosis.
Corilagin (beta-1-O-galloyl-3,6-(R)-hexa hydroxy diphenoyl-d-glucose, Supplemental Figure 1) was extracted from Phyllanthus urinaria and P. niruri. It has been found to have broad-spectrum pharmacological activity. 12 Corilagin has protective effects against cholangiocarcinoma, breast cancer, esophageal cancer, and hepatocellular carcinoma.13–16 Notably, in breast cancer, the microenvironment is anti-inflammatory, which favors cancer and corilagin-regulated reactive oxygen species (ROS) release, thereby inhibiting cancer cell proliferation. 16 Xu et al. found corilagin could induce inhibition of cell growth, induce the apoptosis of gastric cancer cells, trigger autophagy, increase intracellular ROS production. These were powerful evidence that corilagin may be developed as a potential therapeutic drug for gastric cancer. 17 Furthermore, our research team has documented the pharmacological role of corilagin in the protection against hepatic fibrosis induced by schistosome eggs, its anti-inflammatory and antioxidative actions in cholestasis, and its regulation of the immune response in herpes simplex encephalitis.18–22 Some of these biological effects are thought to be closely associated with TLRs, particularly TLR2 and TLR4, suggesting that corilagin may have an anti-atherosclerotic effect.
In our previous investigation, we observed that corilagin dampened the activity of pro-inflammatory factors such as IFN-γ, IL-1β, IL-6, and TNF-α via the TLR4 signaling pathway in macrophages. 23 Given the significant contribution of macrophage-like VSMCs to atherosclerosis progression, we sought to examine whether corilagin could similarly impede the TLR4 signaling pathway in VSMCs, thereby mitigating the pro-atherogenic impact of TLR4 in vivo and in vitro.
Materials and methods
Reagents
Corilagin (purity >99%) for cell experiments was sourced from the China National Institute for the Control of Pharmaceutical and Biological Products in Beijing, China. Professor Rong-Zeng Huang, from the School of Pharmacy at Hubei University of Chinese Medicine in Hubei, China, provided bioactives for animal experiments (purity >90%).
Fetal bovine serum (FBS, Cat No. 10,099,141C) and Dulbecco’s modified Eagle’s medium (DMEM, Cat No. 11,965,092) were obtained from Gibco (Grand Island, NY, USA). Phosphate-buffered saline (PBS, Cat No. GNM20012) was purchased from Genom Biotechnology (Hangzhou, China). Dimethyl sulfoxide (DMSO, Cat No. C6164) was sourced from Sigma–Aldrich (Shanghai, China). Cell Counting kit-8 (CCK8, Cat No. CK04) was obtained from Dojindo WST (Tokyo, Japan). Ox-LDL (Cat No. YB-002) was obtained from Yiyuan Biotech (Guangzhou, China).
Cell culture
The MOVAS cell lines, obtained from the American Type Culture Collection (Manassas, VA, USA), were utilized for experiments at passages 3-5 subsequent to their purchase. The cells were cultured in DMEM supplemented with 10% FBS under standard conditions at 37°C in a fully humidified cell culture incubator with 5% CO2.
Cytotoxic effect of corilagin and observation of cell morphology
The cytotoxic effects of corilagin were assessed using the CCK8 assay. MOVAS cells were seeded in 96-well plates at a density of 10,000 cells per milliliter and exposed to varying concentrations of corilagin (12.5–1600 μg/mL) for 24 h. Positive and negative controls were established with MOVAS cells in culture medium and culture medium alone, respectively. Cell morphology was examined using a microscope (IX2; Olympus, Tokyo, Japan). Subsequently, 10 μL of CCK8 was introduced to each well, followed by a 2-h incubation at 37°C. The absorbance at 450 nm was then quantified using a microplate reader.
Intervention for establishment of a cellular model
To ascertain the optimal concentration of ox-LDL (6.25–100 μg/mL) for inducing changes in mRNA expression within the TLR4 signaling pathway, MOVAS cells were subjected to stimulation. The presence of LPS contamination was examined through Limulus assays, yielding negative results (<0.01 EU/ml). Subsequently, cells were exposed to ox-LDL for varying durations (6–72 h) to identify the most suitable time frame for establishing a cellular model, employing the same methodology.
Lentivirus transfection in MOVAS cells
To overexpress TLR4 expression, GV492-TLR4 a NC-enhanced green fluorescent protein-puromycin resistance gene (PuroR) lentivirus was transfected into MOVAS cells. After preliminary experiments confirmed the multiplicity of infection (MOI), we cultured MOVAS cells with lentivirus at a MOI of 40 in an enhanced infection solution (ENi.S., GeneChem, Shanghai, China) for 72 h. ENi.S. was a serum-free medium similar to minimum Eagle’s medium, and minimal growth factors and trace elements were added. As a filter, puromycin (Cat No. 540222, Sigma–Aldrich, Saint Louis, MO, USA) was used to screen untransfected cells to leave stable upregulated cells that emitted bright green fluorescence. Following the confirmation of upregulation efficiency through observation under a fluorescence microscope (BX-51; Olympus, Tokyo, Japan) and validation using real-time PCR, the remaining cells were harvested and maintained for subsequent experimental procedures.
Small interfering (si) RNA transfection in MOVAS cells
MOVAS cells were transfected with siRNA (Ribobio, Guangzhou, China) to knock down TLR4 expression. The sequence was GAAAUGAGCUGGUAAAGAATT (sense). The transfection mixture included 100 pmol of siRNA, 5 μL of Lipofectamine™ 2000 (Cat No. 11668500, Invitrogen, Carlsbad, CA, USA), FBS, and DMEM, ensuring a final siRNA concentration of 0.02 nmol/μL. The interference persisted for 6 h, followed by a 48-h cultivation period. Subsequent treatments were administered to the transfected cells after confirming the efficiency of downregulation in gene expression through real-time PCR.
Corilagin treatment after ox-LDL stimulation
Utilizing the findings from the CCK8 assay, MOVAS cells were initially subjected to varying concentrations of corilagin, extending up to the maximum non-toxic concentration range, following 24 h of ox-LDL stimulation. Subsequently, the expression of TLR4 was assessed through Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) at optimal experimental concentration (Supplemental Figure 2).
MOVAS cells were seeded in six-well plates. With the exception of the control group, all other groups underwent overnight stimulation with ox-LDL (25 μg/mL) as part of the experimental atherosclerotic model. The control, siRNA-TLR4 group, Lentivirus-TLR4 group (if required), and experimental atherosclerotic model groups were treated with PBS only. The corilagin group was treated with 100 μg/mL, 50 μg/mL, or 25 μg/mL corilagin. Aspirin, a widely used medication in clinical practice for atherosclerosis treatment and known to inhibit TLR4 expression.24,25 Therefore, the positive control group in this study was treated with 10 mmol/L aspirin according to a previous study. 24
To assess the impact of corilagin on cell proliferation, MOVAS cells were seeded at a concentration of 1 × 103 cells/ml in 12-well plates. After culturing with ox-LDL and Corilagin, MOVAS cells were trypsinized and counted every 3 days.
Establishment of an animal model and specimen collection
Sprague–Dawley rats, aged 3 weeks and weighing between 100–150 g, were procured from the Experimental Animal Centre of Tongji Medical College. They were then randomly allocated into seven groups. As described by other researchers,26,27 guidewire aspiration was performed to injure vascular walls. With the exception of the control and sham-operation groups, an improved method for guidewire-induced injuries was used. Briefly, we separated the femoral artery and temporarily occluded both the ends. The femoral artery was then filled with sterile water (instead of inserting a straight spring wire) per centimeter of the separated artery and keep filling for 10 min to ensure the destruction of endothelial cells caused by extracellular hypoosmosis. Following the closure of the incision with 5-0 absorbable sutures, rats underwent a 90-day period of high-fat and cholesterol-rich diet (D12109 C; HFKbios, Beijing, China) post-surgical intervention. Control and sham groups were maintained on a standard diet. Rats in the corilagin groups received oral gavage (i.g.) of 40 mg/kg/, 20 mg/kg/d, or 10 mg/kg/d concentrations of corilagin for 1 month, as predetermined in a prior study. 28 The positive control group received aspirin (20 mg/kg/day). Physiological (0.9%) saline was administered to the control group, sham operation group, and the experimental atherosclerotic model groups. The femoral artery on the affected side was separated and collected for hematoxylin and eosin (HE) staining, Oil Red O staining, and immunohistochemical analyses.
Oil-red-O and HE staining
For pathological examination, femoral artery samples were sectioned into frozen tissue slices measuring 5-10 μm in thickness. These sections were then subjected to staining with a 1% Oil Red O solution in isopropanol for 10 min, followed by rinsing in 60% isopropanol and water. Hematoxylin and eosin (H&E) staining was conducted according to established protocols as described previously. 29
Immunofluorescence for detection of TLR4 and myeloid differentiation factor 88 (MyD88) expression in vascular walls
Cross-sections of the femoral artery measuring 5 µm in thickness were fixed using 4% paraformaldehyde and then blocked with 5% bovine serum albumin/0.5% Triton X-100 in PBS for a duration of 30 min. Afterwards, the cells were subjected to a 2-h incubation at 37°C with either primary anti-TLR4 antibody (1:200 dilution; Cat No. BA1717; Boster Biological Technology, Pleasanton, CA, USA) or anti-MyD88 antibody (1:400 dilution; Cat No. PB9148; Boster Biological Technology, Pleasanton, CA, USA). Following this incubation, the cells were rinsed three times with PBS and then incubated with the secondary antibody (goat anti-rabbit immunoglobulin-G conjugated to Alexa 594) for an additional 2 h at 37°C. Sections were mounted utilizing ProLong™ Antifade mountant with 4′,6-diamidino-2-phenylindole. Subsequently, fluorescence was examined using a fluorescence microscope (BX-51; Olympus, Tokyo, Japan) and analyzed using ImageJ 1.8.0 (NIH).
Quantitative real-time polymerase chain reaction
The quantitative real-time polymerase chain reaction (qRT-PCR) was employed to assess the mRNA expression levels of key molecules in the TLR4 signaling pathway. The targeted molecules included toll-interleukin one receptor domain-containing adaptor protein (TIRAP), MyD88, tumor necrosis factor receptor-associated factor 6 (TRAF6), NF-κB essential modulator (NEMO), mitogen-activated protein kinase p38 (p38), and interferon regulatory factor 5 (IRF5). Glyceraldehyde-3-phosphate dehydrogenase served as the reference gene in this analysis. The specific primer sequences utilized can be found in Table 1.
Table 1.
Primers of qRT-PCR.
| Forword | Reverse | |
|---|---|---|
| TLR-4 | TCGAATCCTGAGCAAACAGC | CCCGGTAAGGTCCATGCTAT |
| TIRAP | GTGGCCGCTGGAGCAAAGAC | TTGCCTCTGCCATCCACATA |
| MyD88 | AAGAAAGTGAGTCTCCCCTC | TCCCATGAAACCTCTAACAC |
| TRAF6 | GCACAAGTGCCCAGTTGACAATGA | AGTGTCGTGCCAAGTGATTCCTCT |
| p38 | ATGCTACTGTCTGCGCCTCTCT | CAGCTTCTTAACTGCCACACGA |
| NEMO | GGTGGAGAGACTGAGCTTGG | CTAAAGCTTGCCGATCCTTG |
| IRF5 | AATACCCCACCACCTTTTGA | TTGAGATCCGGGTTTGAGAT |
| GAPDH | GACCCGCTTCATGCCTGG | GGTGATGGTGTCCATCTGGAC |
Surgery was performed according to a protocol published previously. 30 First, we extracted total RNA from MOVAS cells using RNAiso™ Plus (Cat No. 9108, TaKaRa Biotechnology, Dalian, China) and reverse-transcribed it to complementary DNA (c) using the PrimeScript™ RT Reagent kit (Cat No. RR047 A, TaKaRa Biotechnology). In accordance with the SYBR™ Premix Ex Taq kit instructions (Cat No. RR036 A, TaKaRa Biotechnology), qRT-PC amplification was conducted. The thermal cycling conditions involved an initial denaturation at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. A final step consisted of denaturation at 95°C for 15 s, annealing at 60°C for 1 min, and a final denaturation at 95°C for 15 s. The qRT-PCR was executed using a StepOne™ Plus device (Applied Biosystems, Foster City, CA, USA). Data analysis was performed employing the 2−ΔΔCT method.
Western blotting
Protein concentrations were quantified by western blotting, following established protocols. 28 Briefly, MOVAS cells lysed in RIPA buffer supplemented with a cocktail of protease inhibitors were chilled on ice and subsequently rinsed with PBS to extract total protein. The protein concentration was assessed utilizing a bicinchoninic acid protein assay kit. Next, protein samples were combined with an equal volume of sodium dodecyl sulfate (SDS) loading buffer and subjected to denaturation at 95°C for 10 min. The proteins were separated through sodium dodecyl sulfate-polyacrylamide gel electrophoresis for approximately 90 min and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. A blocking step was performed using 5% non-fat milk in Tris-buffered saline containing Tween 20 (TBST) for 1 h at room temperature. Following an overnight incubation of the PVDF membranes with primary antibodies at 4°C, the target proteins underwent further incubation with species-specific secondary antibodies conjugated to horseradish peroxidase for 1 h at room temperature. The primary antibodies used were rabbit anti-mouse TLR4 1:1000 (Cat No. 30400-1-AP, Proteintech, Beijing, China), rabbit anti-mouse MyD88 1:800 (Cat No. A0980, ABclonal), rabbit anti-mouse TIRAP 1:800 (Cat No. A12606, ABclonal), rabbit anti-mouse TRAF6 1:1000 (Cat No. 12809-1-AP, Proteintech), rabbit anti-mouse NEMO 1:1000 (Cat No. 18474-1-AP, Proteintech), rabbit anti-mouse p38MAPK 1:1000 (Cat No. 14064-1-AP, Proteintech), and rabbit anti-mouse IRF5 1:1000 (Cat No. 10547-1-AP, Proteintech). Western blot band density was analyzed using ImageJ 1.8.0 (NIH).
Enzyme-linked immunosorbent assays
The concentrations of interleukin-6 (IL-6) and Monocyte Chemotactic Protein-1 (MCP-1) in the cell culture supernatant were quantified using ELISAs. ELISA kits specific for IL-6 (Cat No. E-EL-M0044c) and MCP-1 (Cat No. E-EL-M3001) were obtained from ElabScience (Wuhan, China). The assays were performed in accordance with the manufacturer’s instructions, and sterile PBS was utilized as a control during the procedures.
Annexin V staining for measurement apoptosis ratio
To analyze apoptosis, the Annexin V-FITC/PI Apoptosis Detection Kit (Cat No. KGA1101-10, KeyGen Biotechnology, China) was employed following the manufacturer’s guidelines. Each sample was subjected to flow cytometry analysis using a FACSCalibur flow cytometer (BD Biosciences).
Statistical analyses
The results are expressed as the mean ± standard deviation (SD). A significance level of p < .05 was considered statistically significant. The differences in significance were evaluated using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls q-test. Statistical analysis was conducted using SPSS version 24.0 (IBM, Armonk, NY, USA).
Ethical approval and experimental procedures
The animal experiments and procedures adhered to the Guidelines for the Care and Use of Laboratory Animals set forth by the National Institutes of Health (NIH) in Bethesda, MD, USA. Approval for the study protocol was obtained from the Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), with an assigned IACUC number: S2314. The principle of randomized grouping is adhered to in our animal experiments. The experimental process can be referred to the flow chart (Supplemental Figure 3).
Results
Cytotoxicity and corilagin’s impact on the TLR4 signaling pathway in MOVAS cells
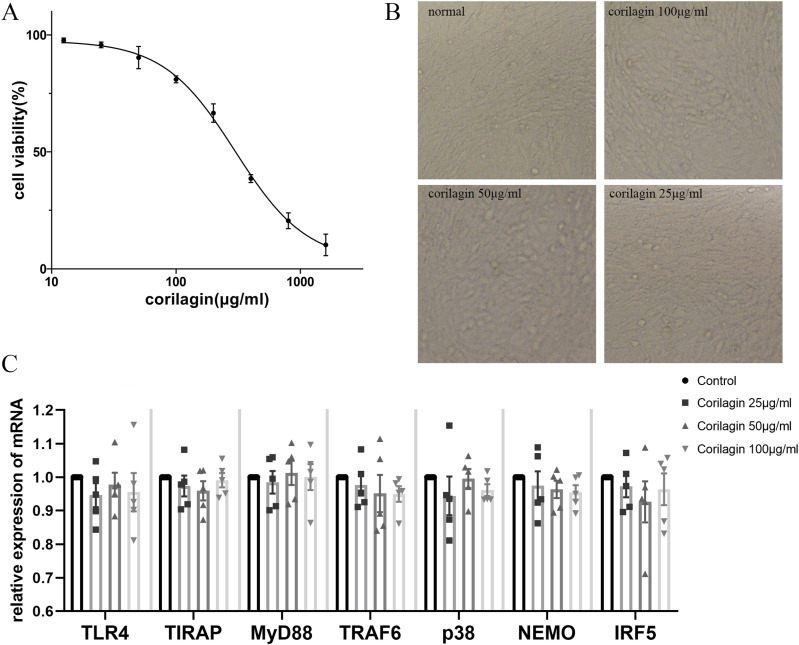
The CCK8 assay showed that the half-maximal inhibitory concentration (IC50) was 298.7 μg/mL (95% confidence interval, 261.7–352.4). To ensure that cell viability was ≥70%, we cultured MOVAS cells with corilagin (100, 50, 25 μg/mL) tentatively for 24 h according to the simulation equation of the curve (Figure 1(a)). No significant interference was observed with regard to the cell viability or morphological characteristics (Figure 1(b)). There were no significant differences in the expression levels of TLR4, TIRAP, MyD88, TRAF6, p38, NEMO, or IRF5 (Figure 1(c)). Therefore, this concentration gradient was used in subsequent experiments.
Figure 1.
Cytotoxicity of corilagin (a) determined using the CCK8 assay for MOVAS cell viability after treatment with various concentrations of corilagin for 24 h. The equation for the dose–effect curve was Y = (94.54/(1 + 10^ (log X − 2.475))) + 3.035 (IC50 = 298.7 μg/mL. (b) Morphology of MOVAS cells after corilagin treatment for 24 h. (c) mRNA expression was measured by qRT-PCR. Data are presented as mean ± SEM. No significant difference was found between groups, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5).
Concentration and duration of ox-LDL in MOVAS cells
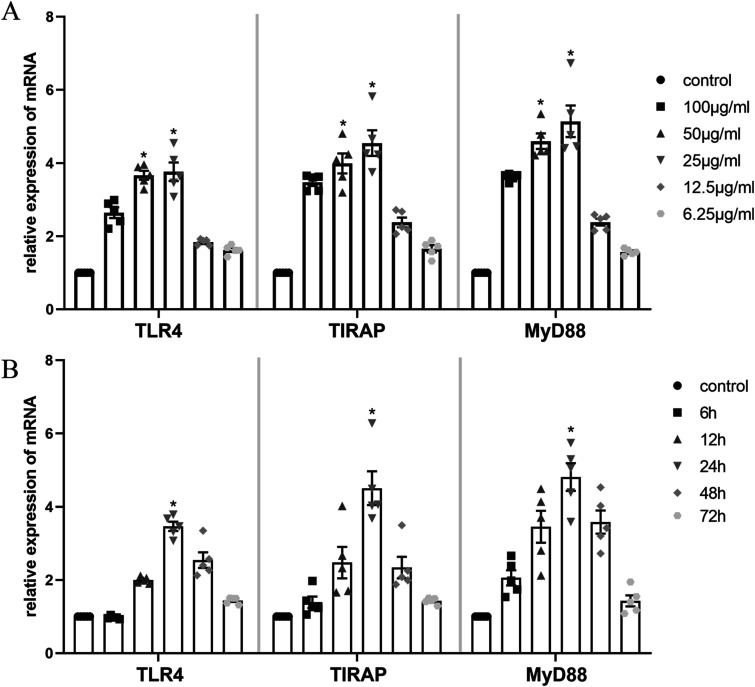
To determine the optimal stimulation conditions, MOVAS cells were exposed to various concentrations and durations of ox-LDL, guided by existing literature.31–33 Analysis revealed that mRNA expression levels of TLR4, TIRAP, and MyD88 were significantly elevated upon stimulation with ox-LDL at concentrations of 25 and 50 μg/mL compared to other concentrations (p < .05, n = 5) (Figure 2(a)). Notably, although not statistically significant, mRNA expression at 25 μg/mL ox-LDL exceeded that at 50 μg/mL ox-LDL. Furthermore, the highest expression of these genes was observed after 24 h of ox-LDL stimulation, followed by a decline (p < .05, n = 5) (Figure 2(b)). Consequently, MOVAS cells were stimulated with 25 μg/mL ox-LDL for 24 h in subsequent experiments.
Figure 2.
Effect of ox-LDL on the TLR4 signaling pathway in MOVAS cells. (a) mRNA expression of TLR4, TIRAP and MyD88 was measured by qRT-PCR. *p < .05 compared with other concentrations of ox-LDL groups or control group as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (b) *p < .05 compared with either time point or control groups determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5).
Impact of corilagin on the TLR4 signaling pathway in MOVAS cells under ox-LDL stimulation
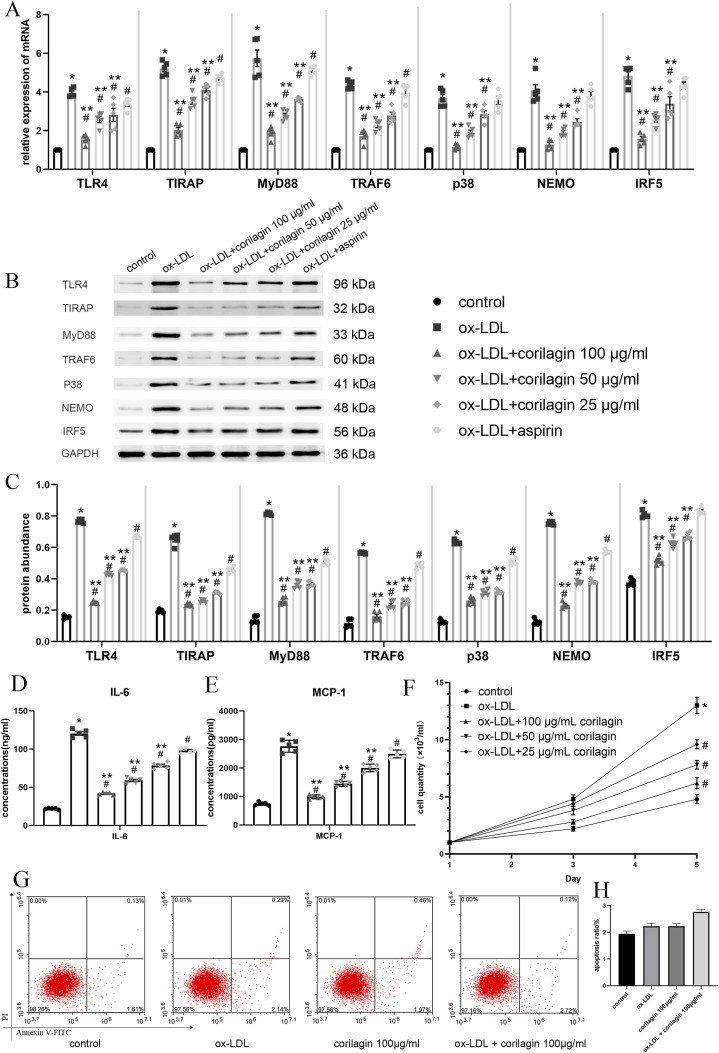
After establishing an experimental atherosclerotic model using ox-LDL, MOVAS cells were treated with corilagin (100, 50, and 25 μg/mL) and aspirin in the positive control group. In Figure 3(a), the mRNA expression levels of molecules implicated in the TLR4 signaling pathway are depicted. Notably, all corilagin groups exhibited a discernible decrease in expression compared to both the model group and aspirin group (p < .05, n = 5). No statistically significant differences were observed for p38, NEMO, and IRF5 when comparing the aspirin and model groups. The corresponding protein expression data are illustrated in Figure 3(b) and (c). In comparison to both the model group and the aspirin group, a significant reduction in protein expression was observed in all three corilagin groups (p < .05, n = 5). Aspirin did not effectively inhibit IRF5 expression. Importantly, this inhibitory effect was augmented with increasing the corilagin concentration. ELISA assays revealed elevated concentrations of IL-6 and MCP-1 in the cell culture supernatant (Figure 3(d) and (e)). Moreover, when compared to the experimental atherosclerotic model group, a conspicuous decrease was noted in all three corilagin groups (p < .05, n = 5).
Figure 3.
Effect of corilagin on the TLR4 signaling pathway in MOVAS cells stimulated by ox-LDL. (a) mRNA expression of TLR4, TIRAP, MyD88, TRAF6, p38, NEMO and IRF5 was measured by qRT-PCR. *p < .05 compared with the control group, #p < .05 compared with the ox-LDL group, **p < .05 compared with the aspirin group as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (B. C) Protein abundance was measured by western blotting. *p < .05 compared with the control group, #p < .05 compared with the ox-LDL group, **p < .05 compared with the aspirin group, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (D, E) Abundance of IL-6 and MCP-1 in cell culture supernatant was measured by ELISAs. *p < .05 compared with the control group, #p < .05 compared with the ox-LDL group, **p < .05 compared with the aspirin group as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (F) Effect of corilagin on the MOVAS cells proliferation. *p < .05 compared with the control group, #p < .05 compared with the ox-LDL group determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (G, H) apoptosis ratio of MOVAS were detected by Annexin V staining. No significant difference was found among four groups determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5).
In contrast, the enumeration of MOVAS cells following culture with ox-LDL and corilagin revealed that ox-LDL promoted MOVAS cell proliferation, while corilagin effectively diminished cell numbers compared to the experimental atherosclerotic model group (p < .05, n = 5) (Figure 3(f)). The apoptotic ratio of MOVAS cells was determined using Annexin V staining. As shown in Figure 3(g) and (h), there were no significant differences among the control, ox-LDL, corilagin, and ox-LDL + corilagin groups.
Impact of corilagin on upregulation of TLR4 expression in MOVAS cells under ox-LDL stimulation
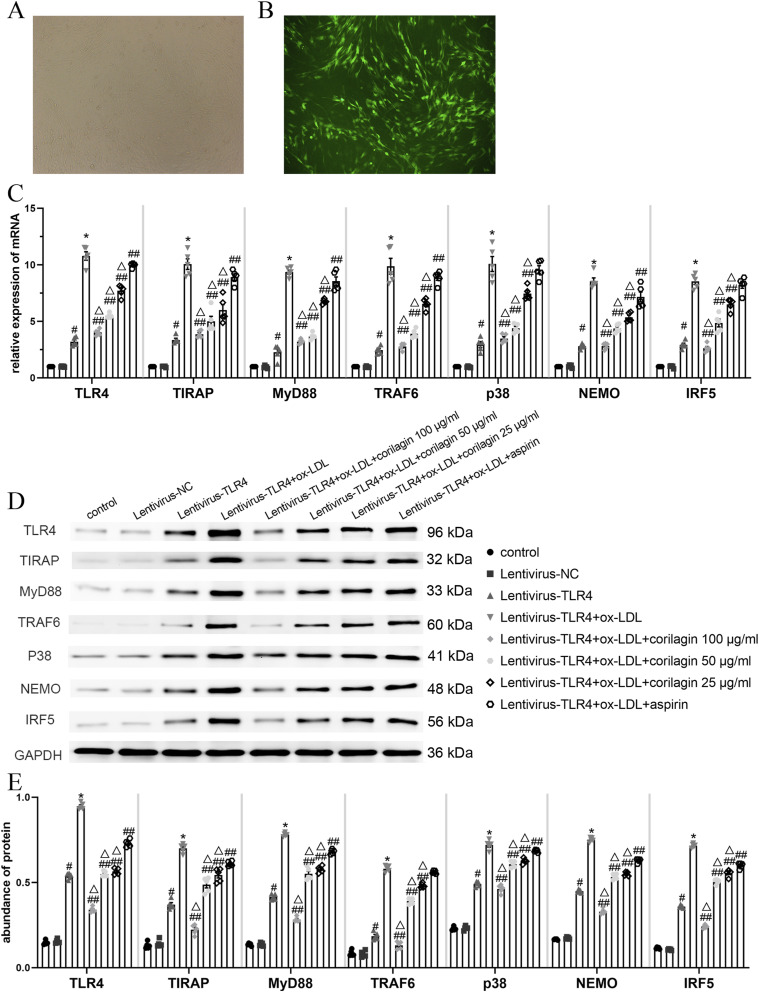
For further investigation of corilagin’s effects, we transfected MOVAS cells with the TLR4 lentiviral vector GV492 to induce upregulation of TLR4 expression. Seventy-2 hours post-transfection, and following 5 days of selection with puromycin, we assessed transfection efficiency by observing green fluorescence in MOVAS cells under a fluorescence microscope. (Figure 4(a) and (b)). We then stimulated MOVAS cells transfected with ox-LDL prior to treatment with corilagin or aspirin. Finally, we measured mRNA and protein expression levels (Figure 4(c)–(e)).
Figure 4.
Effect of corilagin on the TLR4 signaling pathway after upregulation of TL4 expression in MOVAS cells stimulated by ox-LDL. (A. B) 72 hours after transfection of the lentiviral vector, >85% of MOVAS cells expressed green fluorescent protein as observed with a fluorescence microscope. (C) mRNA expression was measured by qRT-PCR. #p < .05 compared with the control group, *p < .05 compared with the lentivirus-TLR4 group, ##p < .05 compared with the model group (lentivirus-TLR4 + ox-LDL), △p < .05 compared with the aspirin group, as determined by one-way ANOVA and Student–Newman–Keuls q-test (n = 5). (D. E) Protein abundance was measured by western blotting. #p < .05 compared with the control group, *p < .05 compared with the lentivirus-TLR4 group, ##p < .05 compared with the model group, △p < .05 compared with the aspirin group, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5).
In comparison to the control group, the lentiviral-TLR4 group exhibited a more than threefold increase in TLR4 mRNA and protein expression (p < .05, n = 5). The expression levels of TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5 were also significantly elevated. No noticeable differences were observed in the lentiviral NC group. Furthermore, in comparison to the lentiviral-TLR4 group, both mRNA and protein expression of molecules within the TLR4 signaling pathway were further increased in the ox-LDL group (p < .05, n = 5). Conversely, the corilagin groups displayed significantly lower expression levels of TLR4, TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5 compared to the model group and aspirin group (p < .05, n = 5). Notably, aspirin did not exert a statistically significant effect on the mRNA expression of p38 and IRF5 or the protein expression of TRAF6.
Downregulation of TLR4 expression in MOVAS cells
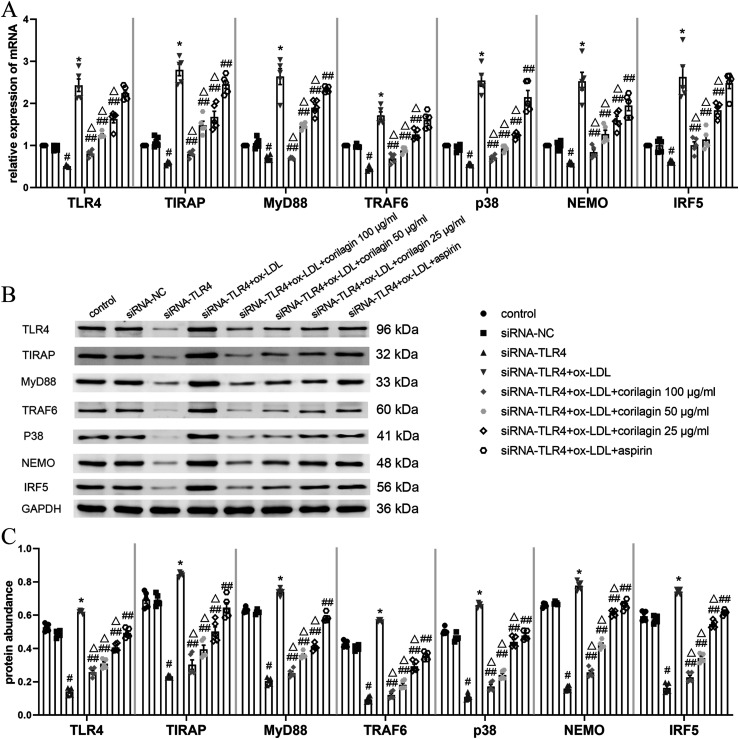
To validate the impact of TLR4 on downstream molecules, we employed siRNA to downregulate TLR4 expression in MOVAS cells. In the siRNA-TLR4 group, TLR4 expression decreased by 50% compared to the control group (p < .05, n = 5) (Figure 5(a)). No significant difference was noted between the siRNA-negative control (siRNA-NC) group and the control groups. Downstream molecules' expression was diminished in the siRNA-TLR4 group (p < .05, n = 5). MOVAS cells in the model group, stimulated with ox-LDL, exhibited significantly higher expression of genes in the TLR4 signaling pathway compared to the siRNA-TLR4 group (p < .05, n = 5). Notably, corilagin treatment led to a significant reduction in expression (p < .05, n = 5). Aspirin, however, did not exhibit an inhibitory effect on TLR4, TRAF6, or IRF5. The abundance of TLR4 protein in the siRNA-TLR4 group decreased by approximately 70% compared to the control group (p < .05, n = 5) (Figure 5(b) and (c)). Protein expression levels of TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5 were also downregulated (p < .05, n = 5). The expression of these proteins in the corilagin groups was significantly lower than that in the model group and aspirin group (p < .05, n = 5).
Figure 5.
Effect of corilagin on the TLR4 signaling pathway after downregulation of TLR4 expression in MOVAS cells stimulated by ox-LDL. (A) mRNA expression of molecules in the TLR4 signaling pathway was measured by qRT-PCR. #p < .05 compared with the control group, *p < .05 compared with the siRNA-TLR4 group, ##p < .05 compared with the model group (siRNA-TLR4 + ox-LDL), △p < .05 compared with the aspirin group, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). (B. C) Protein abundance was measured by western blotting. #p < .05 compared with the control group, *p < .05 compared with the siRNA-TLR4 group, ##p < .05 compared with the model group, △p < .05 compared with the aspirin group, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 5). siRNA-TLR4: TLR4 was knocked down in MOVAS cells by siRNA. siRNA-NC: siRNA-negative control group.
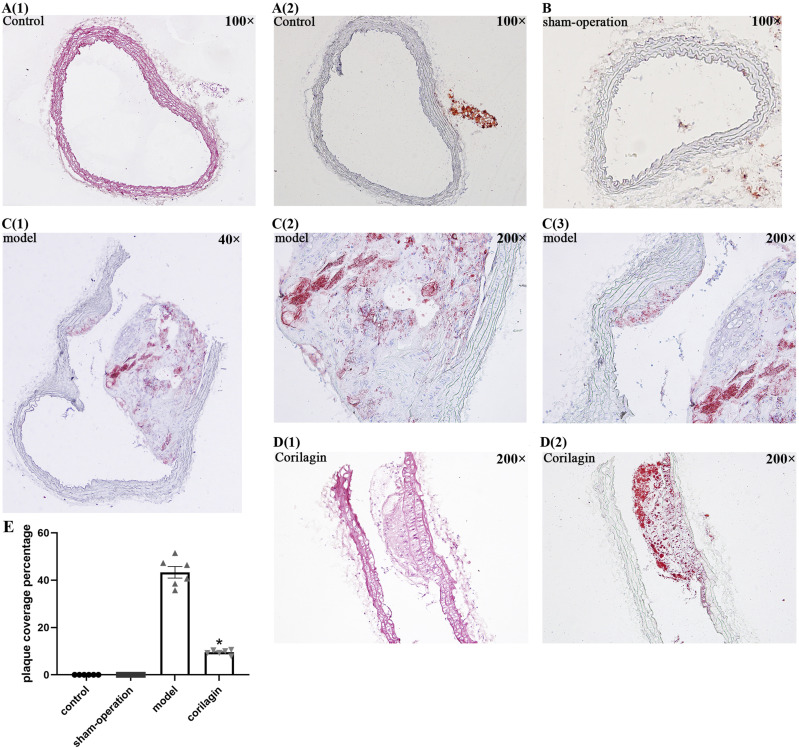
Impact of corilagin on a model of atherosclerosis in rats
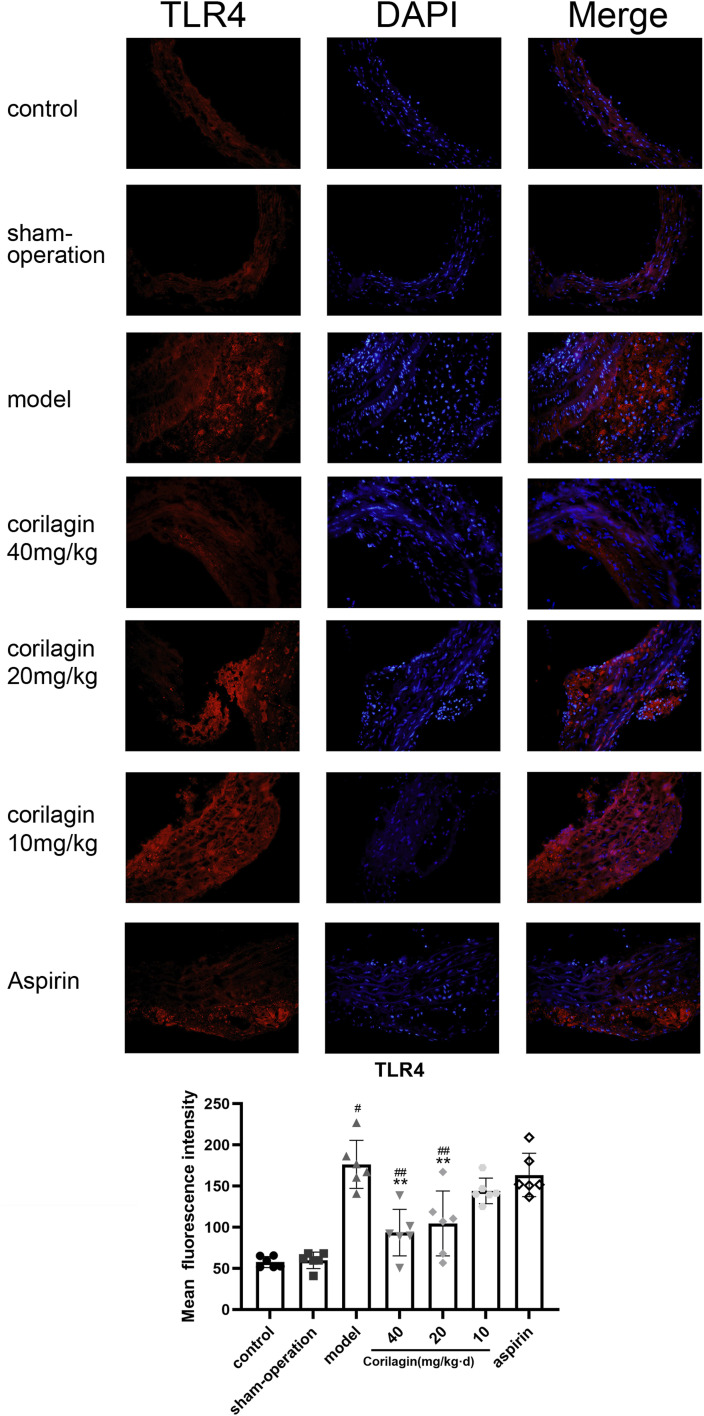
As per the findings from HE and Oil Red O staining in Figure 6, neither the control group nor the sham operation group exhibited atheromatous plaque. Conversely, conspicuous atherosclerotic plaque lesions, foam cells, and lipid accumulation were evident in the experimental groups. Notably, the corilagin group displayed a nearly 30% reduction in plaque coverage percentage compared to the model group. (p < .05, n = 6). TLR4 expression in atherosclerotic lesions was detected using immunofluorescence (Figure 7). Foam cells (derived from macrophages and VSMCs), VSMCs, and a few other inflammatory cells are primary components of this area. Compared to the model group and aspirin group, the mean fluorescence intensity decreased significantly and statistically gradually with an increase in corilagin concentration (p < .05, n = 6). The changes in MyD88 expression were consistent with those observed for TLR4 (Supplemental Figure 4).
Figure 6.
(A1-2) HE and O-R-O staining of one femoral artery in the control group viewed at 100x magnification; (B) O-R-O staining of one femoral artery in the sham-operation group viewed at 100x magnification; (C1-3) O-R-O staining of one femoral artery in the model group. C1 was viewed at 40x magnification and C2 and C3 were viewed at 200x magnification. (D1-2) HE and O-R-O staining of one femoral artery in the corilagin group viewed at 200 times magnification.
Figure 7.
Effect of corilagin on TLR4 in atheromatous plaque in the femoral arteries of rats. #p < .05 compared with the control group, **p < .05 compared with the model group, ##p < .05 compared with the aspirin group, as determined by one-way ANOVA and subsequent Student–Newman–Keuls q-test (n = 6).
Discussion
Ox-LDL contributes to atherosclerotic lesions by causing VSMC dysfunction and instability of atherosclerotic plaques. 34 TLR4 expression is upregulated by ox-LDL treatment. 35 MOVAS cells were stimulated with ox-LDL to generate an atherosclerosis cell model. We demonstrated that not only was the expression of TLR4 elevated, but also that of downstream molecules such as TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5. These data were in accordance with those reported by Chen et al. 36
The TLR4 signaling pathway holds significant implications in atherosclerosis. Previous research has highlighted that a deficiency in MyD88 can mitigate atherosclerosis.37,38 Furthermore, studies have suggested that inhibiting the TRAF6/p38/NF-κB signaling pathway can attenuate the inflammatory response.39,40 Overexpression of IRF-5, a downstream component of the TLR-MyD88 signaling pathway, has been shown to enhance the production of interleukin (IL)-6 and IL-12 by inducing interferon gene expression. 41 Based on these studies, we endeavored to pinpoint a novel medication capable of addressing this pathway.
Previously, we showed that the expression of TLR4, MyD88, TIR-domain-containing adaptor-inducing interferon-β (TRIF), and TRAF6 can be effectively controlled by corilagin in a cellular model of sepsis. 42 Furthermore, in our recent investigation, we observed that corilagin effectively suppressed the TLR4 signaling pathway in macrophages, leading to the amelioration of atherosclerosis. 23 Parallelly, some research also tried to explain the possible mechanism in macrophage that how corilagin ameliorates atherosclerosis.43–45 Yet, attributing the anti-atherosclerotic effect of corilagin to individual target cells poses a challenge.
Our research aimed to shift our focus towards a different category of cells that are prevalent in atherosclerosis and possess secretory properties along with pro-inflammatory functions. Hence, in the present study, we innovatively investigated the impact of corilagin on the TLR4 signaling pathway in atherosclerotic VSMCs. We demonstrated that corilagin exerts an inhibitory effect resulting in a notable reduction in the mRNA and protein levels of TLR4, TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5. Additionally, corilagin notably suppressed the secretion of IL-6 and MCP-1, two significant gene products responsive to TLR4. Being the prevalent anti-inflammatory and anti-atherogenic agent, aspirin has been associated with pharmacological inhibition of the TLR4/NF-κB signaling pathway.46,47 We showcased corilagin’s distinct advantage in this capacity through a comparison with aspirin’s effects. Meanwhile, Bo He et al. have similar interests to ours. 45 They investigated corilagin’s anti-atherosclerotic effects by inhibiting the LOX-1 signaling pathway in VSMCs. Given the partial overlap in signal transduction between these pathways, Bo He’s research findings provide highly favorable evidence and complement our own study.
Building upon these current findings, more research should be endeavored to delve deep into understanding the mechanism behind the effect of corilagin on TLR4. In order to achieve this, utilize high throughput single-cell RNA sequencing and bioinformatics analysis might be the logical foundation for further investigations. The aim is to elucidate how corilagin influences TLR4 at the DNA and RNA level.
Moreover, corilagin inhibited the TLR4 signaling pathway and the proliferative effect of ox-LDL on MOVAS cells. As depicted in Figure 3, corilagin could hinder proliferation without triggering apoptosis in MOVAS cells. This inhibitory effect displayed a concentration-dependent pattern. Such observations may elucidate how corilagin alleviates atherosclerotic plaque formation in vivo.
We observed that the signaling pathway was further augmented by ox-LDL in MOVAS cells with up-regulated TLR4 expression. Conversely, in MOVAS cells with downregulated TLR4, activation by ox-LDL persisted, possibly due to residual TLR4. However, corilagin demonstrated potent inhibitory effects on the TLR4 signaling pathway in both types of MOVAS cells upon ox-LDL stimulation. Importantly, there was no regulatory effect observed in MOVAS cells without ox-LDL stimulation. When MOVAS were transfected with lentivirus, High TLR4 expression in the lentiviral-TLR4 group was caused by the Ubi promoter and exogenous TLR4 sequence. This type of overexpression was stable and could not be reversed by corilagin. Moreover, ox-LDL induced further overexpression of TLR4, which was reversed by corilagin treatment. Therefore, we have grounds to assert that corilagin solely impeded the pathological elevation of TLR4.
In this study, the atherosclerotic plaques in the corilagin group exhibited a significant reduction in size compared to those in the model group, aligning with findings from previous studies. 23 Furthermore, low expression of TLR4 and MyD88 was found in plaque lesions and in the basal zone (where the main components were VSMCs and a few inflammatory cells). Relating this phenomenon with the molecular mechanisms of pharmacological action demonstrated in vitro, we postulated that corilagin inhibits the proliferation of VSMCs or other pro-atherogenic functions by suppressing the TLR4 signaling pathway, and thus, opposes atherosclerosis.
Although corilagin shows minor adverse effects and toxic effects in our research, similar to previous study, we still cannot ignore the possible impairment of liver and kidney function because corilagin is metabolized through the them. In addition, current research is primarily focused on cell and animal experiments, lacking sufficient clinical data to determine the potential distance from the therapeutic target or the occurrence of new lesions with long-term application. This highlights the need for more extensive research on corilagin. By conducting numerous studies, we can evaluate both the therapeutic effects and adverse effects of corilagin, aiming to minimize toxicity and side effects while maintaining therapeutic efficacy. Consequently, it becomes essential to expedite the application of corilagin in clinical settings.
Numerous studies have consistently reported concurrent alterations in the expression of TLR4 and its downstream molecules. For instance, the expressions of TLR4, MyD88, and NF-κB p65 can be jointly regulated by a TLR4-specific inhibitor. 48 Similarly, atorvastatin has been shown to simultaneously suppress the expression of TLR4 and TRAF6. 49 IRF5 expression is altered in tandem with TLR4 expression following N-acetylcysteine treatment. 50 Our study demonstrated that both the downregulation and upregulation of TLR4 expression in MOVAS cells resulted in similar variations in the expression of TIRAP, MyD88, TRAF6, p38, NEMO, and IRF5. This observation suggests the existence of a potential positive feedback mechanism between TLR4 and its downstream molecules. Such findings lend support to the hypothesis that corilagin targets TLR4, thereby influencing the expression of downstream molecules. While this hypothesis is promising, additional experiments focusing on the molecular mechanisms are imperative to substantiate and further validate this proposed relationship.
In addition, previous studies predominantly focused on detecting atherosclerotic lesions in the aorta or coronary arteries when utilizing ApoE−/− mice to construct an atherosclerotic mouse model. However, consistent observation of atherosclerosis in the peripheral arteries, particularly the femoral artery, was not reliably achieved in this particular model. So we did some improvement on traditional guidewire-induced injury and tried to establish PAD animal model. Unfortunately, during our initial experiment, we discovered that the femoral artery in mice presented a challenge due to its thinness, making it impractical for surgical procedures or specimen collection beyond a 90-day period. Rats, if utilized, will not pose these issues. But it is inevitable that there will be species differences in vivo and in vitro experiments. Future studies are expected to address and resolve these. Another limitation of our animal experiments is the insufficient sample size, with a small number of animals employed in the study. According to current guidelines, a larger sample size is needed. 51
The present study demonstrates four primary advantages. Initially, we investigated, for the first time, corilagin’s inhibitory impact on the VSMCs’ TLR4 signaling pathway in vivo and in vitro. Secondly, this inhibition is exclusively targeted at pathological alterations. Thirdly, we verified that corilagin could hinder proliferation without triggering apoptosis in MOVAS cells. Fourthly, our results offer insights into corilagin’s molecular pharmacological mechanism against atherogenesis, indicating that TLR4 could be a target for corilagin.
The present study had some main limitations. First, the exact effect of corilagin on TLR4 has not been determined. Second, whether corilagin could regulate other signaling pathways in atherosclerosis was not tested. Third, the species differences between in vivo and in vitro experiments may affects the coherence of the experiment. Fourth, sample size of animal experiments is insufficient.
Conclusions
We demonstrated that corilagin could inhibit the TLR4 signaling pathway in VSMCs, possibly by downregulating TLR4 expression, consequently relieving atherosclerosis. The intention for our findings is to pave the way for subsequent human trials, in order to further validate the anti-atherosclerosis effects of corilagin.
Supplemental Material
Supplemental Material for Corilagin relieves atherosclerosis via the toll-like receptor 4 signaling pathway in vascular smooth muscle cells by Yujie Wang, Yiqing Li, Yunfei Chen, Jinqian Mao, Jingyu Ji, Shaojun Zhang, Pan Liu, Khrystyna Pronyuk, David Fisher Yiping Dang and Lei Zhao in International Journal of Immunopathology and Pharmacology
Author contributions: All authors contributed to the conception and design of the study. Material preparation, data collection and analysis were performed by Wang Yujie, Li Yiqing, Chen Yunfei, Mao Jinqian, Ji Jingyu, Zhang Shaojun and Liu Pan. The first draft of the manuscript was written by Wang Yujie and Li Yiqing, manuscript was reviewed and edited by Zhao Lei, Dang Yiping, Khrystyna Pronyuk and David Fisher, and all authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers: 81974530, 82100518, and 81600373) and Hubei International Sciencific and Technological Cooperation Project (grant numbers: 2022EHB039 and 2023EHA057).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Dang Yiping https://orcid.org/0009-0009-4950-529X
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because subsequent articles will be published based on these data but are available from the corresponding author upon reasonable request.
References
- 1.Hiramoto JS, Teraa M, de Borst GJ, et al. (2018) Interventions for lower extremity peripheral artery disease. Nat Rev Cardiol 15(6): 332–350. DOI: 10.1038/s41569-018-0005-0. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. (2000) Atherosclerosis. Nature 407(6801): 233–241. DOI: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reinecke H, Unrath M, Freisinger E, et al. (2015) Peripheral arterial disease and critical limb ischaemia: still poor outcomes and lack of guideline adherence. Eur Heart J 36(15): 932–938. DOI: 10.1093/eurheartj/ehv006. [DOI] [PubMed] [Google Scholar]
- 4.Owens GK, Kumar MS, Wamhoff BR. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84(3): 767–801. DOI: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 5.Alexander MR, Owens GK. (2012) Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 74: 13–40. DOI: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MR, Sinha S, Owens GK. (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118(4): 692–702. DOI: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoneveld AH, Hoefer I, Sluijter JP, et al. (2008) Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis 197(1): 95–104. DOI: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Howell KW, Meng X, Fullerton DA, et al. (2011) Toll-like receptor 4 mediates oxidized LDL-induced macrophage differentiation to foam cells. J Surg Res 171(1): e27–31. DOI: 10.1016/j.jss.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Yang K, Zhang XJ, Cao LJ, et al. (2014) Toll-like receptor 4 mediates inflammatory cytokine secretion in smooth muscle cells induced by oxidized low-density lipoprotein. PLoS One 9(4): e95935. DOI: 10.1371/journal.pone.0095935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee GL, Wu JY, Tsai CS, et al. (2016) TLR4-Activated MAPK-IL-6 Axis regulates vascular smooth muscle cell function. Int J Mol Sci 17(9): 40. DOI: 10.3390/ijms17091394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtiss LK, Tobias PS. (2009) Emerging role of Toll-like receptors in atherosclerosis. Journal of lipid research 50 Suppl(Suppl): S340–S345. DOI: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Deng Y, Zheng Z, et al. (2018) Corilagin, a promising medicinal herbal agent. Biomed Pharmacother 99: 43–50. DOI: 10.1016/j.biopha.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Xiao L, Ming Y, et al. (2016) Corilagin suppresses cholangiocarcinoma progression through Notch signaling pathway in vitro and in vivo. Int J Oncol 48(5): 1868–1876. DOI: 10.3892/ijo.2016.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ming Y, Zheng Z, Chen L, et al. (2013) Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol Int 37(10): 1046–1054. DOI: 10.1002/cbin.10132. [DOI] [PubMed] [Google Scholar]
- 15.Qiu F, Liu L, Lin Y, et al. (2019) Corilagin inhibits esophageal Squamous cell carcinoma by inducing DNA damage and down-regulation of RNF8. Anti Cancer Agents Med Chem 19(8): 1021–1028. DOI: 10.3390/molecules24183399. [DOI] [PubMed] [Google Scholar]
- 16.Tong Y, Zhang G, Li Y, et al. (2018) Corilagin inhibits breast cancer growth via reactive oxygen species-dependent apoptosis and autophagy. J Cell Mol Med. DOI: 10.1111/jcmm.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhang G, Tong Y, et al. (2019) Corilagin induces apoptosis, autophagy and ROS generation in gastric cancer cells in vitro. Int J Mol Med 43(2): 967–979. DOI: 10.3892/ijmm.2018.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo YJ, Luo T, Wu F, et al. (2015) Corilagin protects against HSV1 encephalitis through inhibiting the TLR2 signaling pathways in vivo and in vitro. Mol Neurobiol 52(3): 1547–1560. DOI: 10.1007/s12035-014-8947-7. [DOI] [PubMed] [Google Scholar]
- 19.Jin F, Cheng D, Tao JY, et al. (2013) Anti-inflammatory and anti-oxidative effects of corilagin in a rat model of acute cholestasis. BMC Gastroenterol 13: 79. DOI: 10.1186/1471-230x-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li LJ, Zhang SJ, Liu P, et al. (2019) Corilagin Interferes with toll-like receptor 3-mediated immune response in herpes simplex encephalitis. Front Mol Neurosci 12: 83. DOI: 10.3389/fnmol.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YQ, Chen YF, Dang YP, et al. (2017) Corilagin Counteracts IL-13Rα1 signaling pathway in macrophages to mitigate schistosome Egg-induced hepatic fibrosis. Front Cell Infect Microbiol 7: 443. DOI: 10.3389/fcimb.2017.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X, Xiong J, Lu S, et al. (2019) Inhibitory effect of corilagin on miR-21-regulated hepatic fibrosis signaling pathway. The American journal of Chinese medicine 47(7): 1541–1569. DOI: 10.1142/S0192415X19500794. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Wang Y, Chen Y, et al. (2020) Corilagin ameliorates atherosclerosis in peripheral artery disease via the toll-like receptor-4 signaling pathway in vitro and in vivo. Front Immunol 11: 1611. DOI: 10.3389/fimmu.2020.01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying J, Zhou H-Y, Liu P, et al. (2018) Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci 8: 1. DOI: 10.1186/s13578-017-0198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Nong L, Jia Y, et al. (2020) Aspirin alleviates hepatic fibrosis by suppressing hepatic stellate cells activation via the TLR4/NF-κB pathway. Aging 12(7): 6058–6066. DOI: 10.18632/aging.103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sata M, Maejima Y, Adachi F, et al. (2000) A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. Journal of molecular and cellular cardiology 32(11): 2097–2104. DOI: 10.1006/jmcc.2000.1238. [DOI] [PubMed] [Google Scholar]
- 27.Shawky NM, Pichavaram P, Shehatou GS, et al. (2016) Sulforaphane improves dysregulated metabolic profile and inhibits leptin-induced VSMC proliferation: implications toward suppression of neointima formation after arterial injury in western diet-fed obese mice. The Journal of nutritional biochemistry 32: 73–84. DOI: 10.1016/j.jnutbio.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang F, Wang Y, Li G, et al. (2018) Effects of corilagin on alleviating cholestasis via farnesoid X receptor-associated pathways in vitro and in vivo 175(5): 810–829. DOI: 10.1016/j.biopha.2018.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin F, Zhang R, Feng S, et al. (2015) Pathological features of transplanted tumor established by CD133 positive TJ905 glioblastoma stem-like cells. Cancer Cell Int 15: 60. DOI: 10.1186/s12935-015-0208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang F, Luo L, Zhu ZD, et al. (2017) Chlorogenic acid inhibits liver fibrosis by blocking the miR-21-regulated TGF-β1/Smad7 signaling pathway in vitro and in vivo. Front Pharmacol 8: 929. DOI: 10.3389/fphar.2017.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong XL, Ding Y, Chen ZL, et al. (2019) Emodin rescues intrahepatic cholestasis via stimulating FXR/BSEP pathway in promoting the Canalicular Export of accumulated bile. Front Pharmacol 10: 522. DOI: 10.3389/fphar.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei M, Liu Y, Zheng M, et al. (2019) Upregulation of protease-activated receptor 2 promotes proliferation and migration of human vascular smooth muscle cells (VSMCs). Med Sci Mon Int Med J Exp Clin Res : International Medical Journal of Experimental and Clinical Research 25: 8854–8862. DOI: 10.12659/MSM.917865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu CX, Xu L, Peng FZ, et al. (2019) MiR-647 promotes proliferation and migration of ox-LDL-treated vascular smooth muscle cells through regulating PTEN/PI3K/AKT pathway. Eur Rev Med Pharmacol Sci 23(16): 7110–7129. DOI: 10.26355/eurrev_201908_18756. [DOI] [PubMed] [Google Scholar]
- 34.Kattoor AJ, Kanuri SH, Mehta JL. (2019) Role of ox-LDL and LOX-1 in atherogenesis. Curr Med Chem 26(9): 1693–1700. DOI: 10.2174/0929867325666180508100950. [DOI] [PubMed] [Google Scholar]
- 35.Xu XH, Shah PK, Faure E, et al. (2001) Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104(25): 3103–3108. DOI: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Li W, Zhang Y, et al. (2018) MicroRNA-20a protects human aortic endothelial cells from Ox-LDL-induced inflammation through targeting TLR4 and TXNIP signaling. Biomed Pharmacother 103: 191–197. DOI: 10.1016/j.biopha.2018.03.129. [DOI] [PubMed] [Google Scholar]
- 37.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. (2004) Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature medicine 10(4): 416–421. DOI: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 38.Chen T, Luo W, Wu G, et al. (2019) A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages. Toxicol Appl Pharmacol 370: 44–55. DOI: 10.1016/j.taap.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Ou G, He Y, et al. (2019) Resveratrol attenuates the MSU crystal-induced inflammatory response through the inhibition of TAK1 activity. Int Immunopharm 67: 62–78. DOI: 10.1016/j.intimp.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Wei Q, Tu Y, Zuo L, et al. (2020) MiR-345-3p attenuates apoptosis and inflammation caused by oxidized low-density lipoprotein by targeting TRAF6 via TAK1/p38/NF-kB signaling in endothelial cells. Life Sci 241: 117142. DOI: 10.1016/j.lfs.2019.117142. [DOI] [PubMed] [Google Scholar]
- 41.Fan JH, Gao LB, Pan XM, et al. (2010) Association between IRF-5 polymorphisms and risk of acute coronary syndrome. DNA Cell Biol 29(1): 19–23. DOI: 10.1089/dna.2009.0929. [DOI] [PubMed] [Google Scholar]
- 42.Li HR, Liu J, Zhang SL, et al. (2017) Corilagin ameliorates the extreme inflammatory status in sepsis through TLR4 signaling pathways. BMC Compl Alternative Med 17(1): 18. DOI: 10.1186/s12906-016-1533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng D, Deng X, Wu Y, et al. (2023) Corilagin ameliorates macrophages inflammation in atherosclerosis through TLR4-NFκB/MAPK pathway. Heliyon 9(6): e16960. DOI: 10.1016/j.heliyon.2023.e16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tao Y, Zhang L, Yang R, et al. (2021) Corilagin ameliorates atherosclerosis by regulating MMP-1, -2, and -9 expression in vitro and in vivo. Eur J Pharmacol 906: 174200. DOI: 10.1016/j.ejphar.2021.174200. [DOI] [PubMed] [Google Scholar]
- 45.He B, Chen D, Zhang X, et al. (2022) Antiatherosclerotic effects of corilagin via suppression of the LOX-1/MyD88/NF-κB signaling pathway in vivo and in vitro. J Nat Med 76(2): 389–401. DOI: 10.1007/s11418-021-01594-y. [DOI] [PubMed] [Google Scholar]
- 46.Hally KE, La Flamme AC, Harding SA, et al. (2019) The effects of aspirin and ticagrelor on Toll-like receptor (TLR)-mediated platelet activation: results of a randomized, cross-over trial. Platelets 30(5): 599–607. DOI: 10.1080/09537104.2018.1479520. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Shen B, Sun D, et al. (2018) Aspirin ameliorates cerebral infarction through regulation of TLR4/NF‑κB‑mediated endoplasmic reticulum stress in mouse model. Mol Med Rep 17(1): 479–487. DOI: 10.3892/mmr.2017.7879. [DOI] [PubMed] [Google Scholar]
- 48.Su Q, Li L, Sun Y, et al. (2018) Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary Microembolization-induced Myocardial injury. Cell Physiol Biochem : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 47(4): 1497–1508. DOI: 10.1159/000490866. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Zhang X, Zhai L, et al. (2018) Atorvastatin attenuates cognitive deficits and neuroinflammation induced by Aβ1-42 involving modulation of TLR4/TRAF6/NF-κB pathway. Journal of molecular neuroscience : MN 64(3): 363–373. DOI: 10.1007/s12031-018-1032-3. [DOI] [PubMed] [Google Scholar]
- 50.Nasiri M, Saadat M, Karimi MH, et al. (2019) Evaluating mRNA expression levels of the TLR4/IRF5 signaling Axis during hepatic ischemia-Reperfusion injuries. Exp Clin Transplant Off J Middle East Soc Organ Transplant 17(5): 648–652. DOI: 10.6002/ect.2017.0007. [DOI] [PubMed] [Google Scholar]
- 51.Festing MFW, Altman DG. (2002) Guidelines for the design and statistical analysis of experiments using Laboratory animals. ILAR J 43(4): 244–258. DOI: 10.1093/ilar.43.4.244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Corilagin relieves atherosclerosis via the toll-like receptor 4 signaling pathway in vascular smooth muscle cells by Yujie Wang, Yiqing Li, Yunfei Chen, Jinqian Mao, Jingyu Ji, Shaojun Zhang, Pan Liu, Khrystyna Pronyuk, David Fisher Yiping Dang and Lei Zhao in International Journal of Immunopathology and Pharmacology
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because subsequent articles will be published based on these data but are available from the corresponding author upon reasonable request.