Abstract
Background and purpose:
Losing independence is a main concern for hip fracture patients, and particularly not being able to return home. Given the large impact on quality of life by loss of independence and the high risk for institutionalization after hip fracture, it is of importance to identify modifiable risk factors for such negative outcomes. This study aimed to investigate the association between two such factors, that is, lean body mass and 4 months post-discharge walking capacity, and the risk of institutionalization in previously independent living older people who suffer a hip fracture.
Patients and methods:
A retrospective cohort study was conducted using Swedish national-based population registers. Patients ⩾60 years with a hip fracture during 2008–2017 were included from the Swedish National Registry for Hip Fractures. Risk of institutionalization over the 1-year period following a hip fracture was analyzed using logistic regression analyses adjusted for potential predictors and characteristics.
Results:
In total, 11,265 patients were included. Over the first year, 8% (95% CI: 8–9) of the patients with a hip fracture had lost independence, increasing to 15% (95% CI: 14–16) after 5 years. Poor recovery of post-discharge walking ability was associated with a higher odds ratio of losing independence compared with good recovery (OR 12.0; 95% CI: 7.8–18.4; p < 0.001). Having higher estimated lean body mass than 45 kg at index was associated with lower odds of losing independence.
Conclusion:
Maintaining lean body mass and mobility after a hip fracture is likely important from an individual as well as public health perspective.
Keywords: Hip fracture, institutionalization, walking capacity, lean body mass
What do we already know about this topic?
Patients who experience good and moderate recovery in 4 months post-discharge walking ability are less likely to lose independence following hip fracture, compared with patients with poor recovery.
How does your research contribute to the field?
Lean Body Mass (LBM) is scarcely investigated as a risk factor for institutionalization in older patients suffering hip fractures.
What are your research’s implications toward theory, practice, or policy?
Maintaining LBM and mobility after a hip fracture is likely important from an individual as well as public health perspective.
Introduction
Hip fractures are a major cause of morbidity and mortality. More than 20% of patients die within the first year of the fracture1,2 and survivors are often affected by a decrease in quality of life and reduced function. 3 Less than half of the patients regain the level of function they had prior to the hip fracture,4,5 compromising an independent lifestyle. Losing independence is a main concern for hip fracture patients, and particularly not being able to return home.6–8 Between 10% and 20% of the survivors move into nursing homes in the year after the hip fracture. 9
Many of the risk factors for nursing home admission after hip fracture are shared with those for increased risk of mortality such as higher age, having more comorbid conditions and cognitive impairment.10,11 In addition, the combination of reduced muscle mass and physical function, that is, sarcopenia,12,13 is a risk factor observed in at least 30%–40% of older people sustaining a hip fracture, 14 a condition that is further aggravated after the fracture.15,16
Given the large impact on quality of life by loss of independence and the high risk for institutionalization after hip fracture, it is of importance to identify potentially modifiable risk factors, both pre- and post-fracture, for such nonbeneficial outcomes.
This study aimed to investigate the association between two potentially modifiable factors indicative of sarcopenia; that is, estimated LBM and 4 months post-discharge walking ability, and loss of independence, as defined as a major change in residential housing status5,17,18 for previously independent living older people who suffer a hip fracture.
Patients and methods
Study design and study population
A retrospective population-based cohort study was conducted using Swedish national-based population quality registers. Patients ⩾60 years with a hip fracture during the period of 1 January, 2008 to 31 December, 2017 were included from the Swedish National Registry of Hip Fractures (SHR). SHR is a National Quality Register collecting data on hip fracture patients and cover over 80% of all hip fracture patients in Sweden. 19 The index date was defined as the date of the first hip fracture recorded in SHR 2008–2017. Information on demographic and clinical characteristics were gathered at index date or in the 5-year period prior to the index date. The data extraction was carried out in 2019 and at that time complete data were available until the end of 2017. Updating with more recent years would require repeating the whole data extraction process which was not within the scope of the study.
Data regarding lead times, comorbidities, diagnosis, and surgical treatments in the SHR questionnaire 20 are obtained from the medical records and are routinely validated by the registry and lead times, fracture classification and type of surgery have recently been validated against the National Patient Register. 19
The information about walking ability and function in the SHR is self-reported and gathered by interviews which is optimal, in first person or by proxy, if the hip fracture patient is unable to be interviewed. 21 The follow-up interviews are carried out at a follow-up visit, over the phone or, if that is not possible, through a questionnaire sent home. The follow-up at 4 months post-fracture is set to cover a complete measure of recovery and is used by 10 other different hip fracture registers worldwide, to enable comparability. 22 The questionnaires used at baseline and at 4 months include standardized questions adapted for nonclinical follow-up of fractured patients and are included in a minimum common dataset developed by the Fragility Fracture Network and are being used for registry-based data collections in several countries. 23
Additional data were retrieved from four national registers and linked together by the Swedish National Board of Health and Welfare using unique personal identifiers.24–27 Information on hospitalizations and outpatient specialist visits were collected from the National Patient Register and the Prescription Drug Register provided data on all prescriptions filled at pharmacies for the complete observable period for each patient. Confirmed dates of death were provided by the Cause of Death Register. The Social Services Register provided information on permanent living in special forms of housing. These national registers are mandatory to report to and are associated with a close to 100% completeness and coverage for the whole Swedish population.24,25 There is no risk of loss to follow-up for the study subjects except for the event of death or a move to another country over the observation period (2008–2017). Thus, the research data set includes all patients in Sweden available in the underlying data sources. For this reason, no sample size calculations were required.
Outcome and exposure variables
Institutionalization was defined as the change from living in own housing to living permanently in special forms of housing. Patients who live in special forms of housing already before the fracture were excluded from the analysis, as the investigated level of independence cannot decrease further for these patients.
Main explanatory variables were walking ability recovery and estimated LBM. Walking ability recovery was defined as a combined categorical measure (Poor, Moderate, Good) of the two variables independent walking and use of walking aids as reported in SHR at 4 months post-fracture, as used previously in the literature (which also included walking speed). 28
LBM, as a proxy for muscle mass, is an important consideration for many physiologic processes and is key for the definition of sarcopenia, that is, the combined loss of muscle strength and muscle mass.12,13,29–31 LBM is ideally estimated with magnetic resonance imaging (MRI), or alternatively by dual-energy X-ray absorptiometry (DXA) which can estimate appendicular lean soft tissue as a surrogate for skeletal muscle mass. 32 As variables on muscle mass based on MRI or DXA was missing from the present study, predictive models were used to calculate estimated LBM based on anthropometric assessments. Anthropometry is often used in large population-based studies as an inexpensive and accessible body composition assessment, validated by previous literature.33–35 The equation to estimate LBM was based on height, weight, and gender as collected in SHR before surgery (for full definition see Supplemental material Appendix).36–44
Potential predictors included demographics (age, gender), comorbidities (including cognitive status), lifestyle factors, days from fracture to surgery, and functional status prior to hip fracture.
Statistical analysis
Risk of loss of independence over the 1-year period following a hip fracture was analyzed using a logistic regression adjusted for the potential predictors.
As a supportive analysis, loss of independence was also measured continuously over time, until death or end of data availability, using a time-to-event analysis with institutionalization as failure event, adjusted for potential predictors. A proportional hazard regression model with the time to institutionalization as the event adjusted to the index independence status and potential predictors was performed. In addition, a sensitivity analysis excluding those patients that died during the first year was carried out.
Walking ability is measured at 4 months after fracture and to some extent overlaps institutionalization that can occur any time during the year. Recovery is something that occurs progressively over time, and even though walking ability is measured at 4 months, it should be a good measure for the whole recovery process that start soon after the hip fracture. To address this chronological overlap of the exposure and the outcome variable, a sensitivity analysis excluding patients being institutionalized prior 4 months was performed.
Analyses were also conducted over a subgroup of patients who had not been diagnosed with dementia at index.
A sensitivity analysis was performed by adding a variable with information on cohabitation to test for differences in risk of institutionalization over patients living alone or with spouse/partner. Another sensitivity analysis was carried out where all patients with poor pre-fracture walking ability were excluded.
The reference for each categorical variable was mostly based on considerations of the most common group in the population. All tests were two-tailed and an alpha value of 0.05 was employed as the cutoff for statistical significance. RStudio version 1.3 (RStudio Inc., Boston, MA) and a licensed STATA version 16 (StataCorp LLC, College Station, Texas) was used for data management and statistical analysis.
Ethical approval
Ethical approval was obtained from the Regional Stockholm Research Ethics Committee at the Karolinska Institutet (reference number: 2018/887-31) on 16 May, 2018. Individual patient informed consent is not required for register studies on retrospective data in Sweden and was therefore not collected.
Results
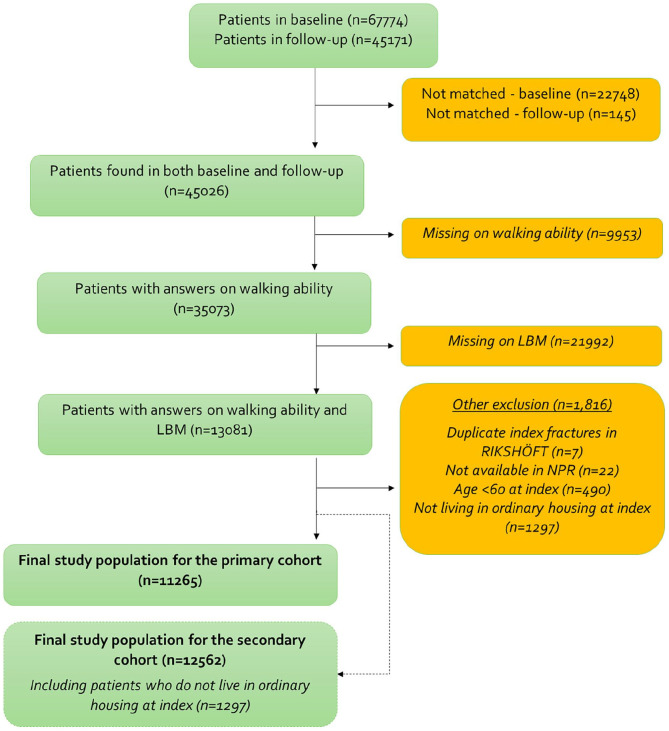
In total, 67,774 patients were identified in the RIKSHÖFT register. After exclusion criteria, 11,265 patients were included in the final study population for the primary objective (Figure 1).
Figure 1.
Patient attrition from raw data to final study population for the primary objective.
Mean age at time of hip fracture was 81 years and 70% of the patients were female (Table 1). Before fracture, the majority of patients walked alone outdoors and without aids. The share of patients who experienced good, moderate, or poor recovery in walking ability from hip fracture to 4 months after the fracture was evenly distributed (30%, 35%, and 35%, respectively). The mean LBM was higher among patients with good walking ability recovery compared to moderate or poor recovery (Table 2).
Table 1.
Characteristics of patients.
| Variable | Primary objective (n = 11,265) |
|---|---|
| Age (years) at index | |
| Mean (SD) | 80.6 (8.5) |
| Sex, n (%) | |
| Males | 3473 (31) |
| Females | 7792 (69) |
| Length of follow-up (days) | |
| Mean (SD) | 774 (477) |
| Time from fracture to surgery (days) | |
| Mean (SD) | 1.1 (1.3) |
| Elixhauser comorbidity index | |
| Mean (SD) | 1.1 (1.2) |
| Change in walking ability from hip fracture to 4 months post-fracture, n (%) | |
| Good recovery | 3382 (30) |
| Moderate recovery | 3994 (35) |
| Poor recovery | 3889 (35) |
| Pre-fracture independent walking, n (%) | |
| Walked alone outdoors | 8693 (77) |
| Walked only with company outdoors | 751 (7) |
| Walked alone but only indoors | 1423 (13) |
| Walked only with company indoors | 247 (2) |
| Could not walk | 151 (1) |
| Pre-fracture use of walking aids, n (%) | |
| Walked without aids | 6389 (57) |
| One aid (cane/crutch) | 768 (7) |
| Two aids (canes/crutches) | 250 (2) |
| Rolling walker/walking frame | 3654 (32) |
| Wheel chair/bed bound | 204 (2) |
| Estimated LBM, n (%) | |
| <45 | 4969 (44.1) |
| 45–49 | 2498 (22.2) |
| 50–54 | 1743 (15.5) |
| 55–59 | 1246 (11.1) |
| 60+ | 809 (7.2) |
| Dementia, n (%) | 572 (5) |
| Cancer, n (%) | 2081 (18) |
Table 2.
Mean estimated lean body mass by walking ability category.
| Walking ability | Mean estimated LBM | 95% CI |
|---|---|---|
| Good recovery | 48.9 | (48.6–49.2) |
| Moderate recovery | 46.8 | (46.5–47) |
| Poor recovery | 46.1 | (45.9–46.3) |
LBM: lean body mass.
Over the first year, 8% (95% CI: 8–9) of the patients with a hip fracture had lost independence, that is, they had moved into a special form of housing, increasing to 15% (95% CI: 14–16) after 5 years. The proportion who lost independence from index to 60 months after index was significantly higher in patients with poor recovery in walking ability, compared with those with good or moderate recovery (Figure 2). After 5 years, 29% (95% CI: 28–31) of patients who experienced poor recovery in walking ability had lost independence. The corresponding proportions for those who experience good and moderate recovery were 3% (95% CI: 3–5) and 14% (95% CI: 12–16), respectively. The restricted mean survival was 53 months (95% CI: 52.7–53.4) for all patients disregarding change in walking ability.
Figure 2.
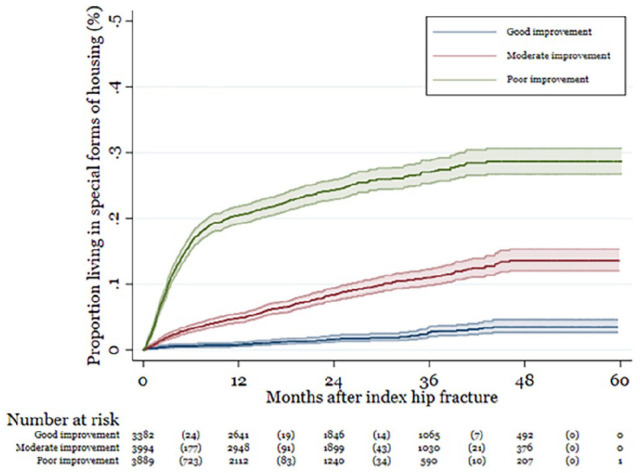
Proportion of who has lost independence by change in walking ability (number in parenthesis show the number of events during the time interval.
Odds and hazard ratio between risk factors and loss of independence
After adjusting for several patient characteristics including pre-fracture walking, the recovery in walking ability had a clear impact on the risk of becoming institutionalized over 1 year (Table 3). Poor recovery was associated with a higher odds ratio of losing independence compared with good recovery (OR 12.0; 95% CI: 7.8–18.4; p < 0.001) and the same tendency was seen for moderate recovery compared with good recovery (OR 3.5; 95% CI: 2.3–5.5; p < 0.001). Having an estimated LBM of at least 45 kg was numerically associated with lower risk of losing independence compared with LBM lower than 45 kg for all LBM categories (OR between 0.52 and 0.85 depending on LBM category), but only statistically significant for LBM categories 45–49 and 55–59 (p < 0.001).
Table 3.
Risk factors and loss of independence after hip fracture: odds ratio (first year), hazard ratio (continuously).
| Variable name | Loss of independence over 1 year | Loss of independence continuously over time | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Change in walking ability from index to 4 months after fracture (ref: Good recovery) | ||||||
| Moderate recovery | 3.52 | (2.27–5.46) | <0.001 | 2.60 | (1.97–3.42) | <0.001 |
| Poor recovery | 12.01 | (7.82–18.44) | <0.001 | 5.95 | (4.53–7.82) | <0.001 |
| Estimated LBM, kg (ref: <45) | ||||||
| 45–49 | 0.67 | (0.54–0.83) | <0.001 | 0.69 | (0.59–0.82) | <0.001 |
| 50–54 | 0.73 | (0.56–0.97) | 0.027 | 0.68 | (0.55–0.84) | <0.001 |
| 55–59 | 0.52 | (0.37–0.74) | <0.001 | 0.50 | (0.38–0.66) | <0.001 |
| 60+ | 0.85 | (0.57––1.27) | 0.430 | 0.62 | (0.45–0.85) | 0.003 |
| Male (ref: female) | 1.25 | (0.98–1.61) | 0.075 | 1.47 | (1.22–1.78) | <0.001 |
| Age group, years (ref: 85+) | ||||||
| 60–64 | 0.12 | (0.02–0.86) | 0.035 | 0.37 | (0.15–0.93) | 0.035 |
| 65–69 | 0.65 | (0.34–1.24) | 0.188 | 0.63 | (0.37–1.06) | 0.083 |
| 70–74 | 1.71 | (1.16–2.53) | 0.007 | 1.62 | (1.18–2.21) | 0.003 |
| 75–79 | 1.98 | (1.38–2.85) | <0.001 | 2.02 | (1.51–2.71) | <0.001 |
| 80–84 | 2.83 | (2–4.01) | <0.001 | 2.95 | (2.23–3.9) | <0.001 |
| Days from fracture to surgery (ref: 0 days) | ||||||
| 1–2 days | 0.82 | (0.69–0.97) | 0.019 | 0.89 | (0.78–1.01) | 0.074 |
| 3–7 days | 0.64 | (0.44–0.94) | 0.024 | 0.74 | (0.55–1) | 0.050 |
| >1 week | 0.73 | (0.32–1.67) | 0.450 | 0.87 | (0.48–1.59) | 0.657 |
| Pre-fracture independent walking (ref: could not walk/walked only with company indoors) | ||||||
| Walked alone outdoors | 0.46 | (0.35–0.62) | <0.001 | 0.57 | (0.45–0.71) | <0.001 |
| Walked only with company outdoors, or walked alone but only indoors | 0.83 | (0.63–1.1) | 0.203 | 0.89 | (0.72–1.11) | 0.313 |
| Pre-fracture use of walking aids (ref: rolling walker/wheelchair/bed bound) | ||||||
| No aids | 0.75 | (0.63–0.89) | 0.001 | 0.77 | (0.67–0.88) | <0.001 |
| One or two aid(s) (cane/crutch) | 0.96 | (0.74–1.24) | 0.736 | 0.93 | (0.76–1.13) | 0.473 |
| Elixhauser comorbidity index (continuous) | 1.03 | (0.96–1.1) | 0.417 | 1.04 | (0.99–1.1) | 0.097 |
| Specific comorbidities (ref groups: not having the comorbidity) | ||||||
| Dementia | 2.79 | (2.24–3.47) | <0.001 | 2.40 | (2.04–2.82) | <0.001 |
| Rheumatoid arthritis | 0.78 | (0.38–1.59) | 0.493 | 0.83 | (0.49–1.41) | 0.489 |
| Osteoporosis treatment experience | 0.90 | (0.75–1.08) | 0.243 | 0.96 | (0.84–1.1) | 0.541 |
| Glucocorticoid use | 0.74 | (0.57–0.97) | 0.028 | 0.83 | (0.68–1.01) | 0.066 |
| Secondary osteoporosis | 0.74 | (0.54–1.01) | 0.057 | 0.84 | (0.65–1.07) | 0.157 |
| Exposure to drugs that increase the risk of falls | 1.13 | (0.82–1.55) | 0.452 | 1.18 | (0.93–1.51) | 0.177 |
| Lifestyle factors (ref groups: not having the lifestyle factors) | ||||||
| Current smoking | 1.91 | (0.76–4.78) | 0.167 | 1.29 | (0.68–2.45) | 0.436 |
| Alcohol use | 0.51 | (0.18–1.42) | 0.194 | 0.73 | (0.35–1.54) | 0.411 |
| Parent hip fracture | 0.81 | (0.44–1.48) | 0.490 | 0.82 | (0.5–1.34) | 0.428 |
| Constant | 0.17 | (0.07–0.39) | <0.001 | 0.17 | (0.07–0.39) | <0.001 |
LBM: lean body mass.
When measured continuously over time, the risk of losing independence was higher in patients with poor recovery compared with good recovery (HR 6.0; 95% CI: 4.5–7.8) and moderate recovery compared with good recovery (HR 2.6; 95% CI: 2.0–3.4) (Table 3). The risk of losing independence was lower in patients with higher estimated LBM at index as LBM of at least 45 kg was significantly associated with lower risk of losing independence compared with LBM lower than 45 kg (HR 0.69–0.50 depending on LBM category).
Subgroups and sensitivity analysis
Patients with a diagnosis of dementia had a higher risk of being institutionalized after hip fracture. To test the robustness of the predicators, the main analysis was also run for patients without dementia (95% of the population). For the subgroup analysis, the associations for walking ability recovery and estimated LBM were similar as for the overall study population (Supplemental material Appendix, Table 4).
Results from the sensitivity analysis that included the variable cohabitation indicate that living alone increases the odds of institutionalization compared with living with a spouse or partner.
Overall, the strength of association between the outcome and the exposure variables (walking ability recovery and estimated LBM) was similar in the sensitivity analyses compared to the main analysis (Table 5 in Supplemental material Appendix). However, in the analysis estimating the risk of institutionalization between 4 and 12 months after fracture, the odds of institutionalization was markedly higher for moderate and poor recovery.
Discussion
This study found that 8% of patients with a hip fracture lose their independence and moved from own living to special forms of housing within 1 year. After 5 years, 15% of the surviving patients were institutionalized. Rates of institutionalization in patients with hip fracture reported from previous studies varies and is dependent on studied age group. For example, in a study of 278 hip fracture patients at 7 Swedish hospitals, the rate of patients living in special housing 1 year after the fracture was reported at 5%. 45 A study of 1503 patients with hip fractures in England observed a 20% rate of discharge to nonhome location. 46 However, this included short-term residences and rehabilitation facilities; therefore, the share of patients who permanently lived in special housing after the fracture may have been lower. The annual proportion of individuals who transition to special forms of housing in the general Swedish population is approximately 0.5% and 2% for a 65- and 85-year-old individual, respectively. 47 This indicates that the risk of losing independence is higher in patients who sustain hip fracture compared with the general population.
Patients who experience good and moderate recovery in walking ability are less likely to lose independence following hip fracture, compared with patients with poor recovery. Mobility difficulties have been found to predict further disability and loss of independence in older people. 6 The current findings add to this evidence. In addition, higher LBM at index was significantly associated with lower risk of losing independence, supporting previous data on mobility limitations in sarcopenic hip fracture patients 1 year after surgery. 48 LBM is not extensively investigated as a risk factor for institutionalization in older patients suffering hip fractures. Our findings corroborate observations of increased risk of nursing home admission when sarcopenia occurs. 48 In our study, the impact of LBM remained both in size and statistical significance when body mass index was added as covariate (not shown), indicating that the estimation of LBM added information beyond that of weight and height alone for the risk of institutionalization. Treatment of sarcopenia usually includes resistance exercise and protein supplementation, a combination that was shown to counteract sarcopenia in a 3-month intervention study in Swedish nursing home residents. 49 An Australian multidomain treatment study in postsurgical hip fracture patients reported strikingly reduced 1-year risk of nursing home admission. 50
Both having a cognitive impairment 51 and solitary living 52 have previously been shown to affect rates of institutionalization after hip fracture. Our study demonstrated the robustness of walking ability recovery and LBM as predictors for institutionalization by showing similar rates of associations in subgroup (patients without dementia) and sensitivity analyses (including variable on cohabitation).
One unexpected finding was the reduced risk of institutionalization with glucocorticoid use (OR: 0.74). We do not have any clear rationale for this, but some speculative explanations could perhaps be that the invigorating effect of glucocorticoids could lead to that some patients can return to home instead of going to long-term care. Another explanation could be that glucocorticoids are usually less prescribed in patients with dementia and these patients are institutionalized to a larger extent.
Two recent studies from the United Kingdom have studied predictors of returning to own residence after a hip fracture. Hawley et al. 53 found that patient factors such as cognitive impairment, malnutrition, delay to surgery due to medication, and service factors such as surgery delays due to logistical reasons and early morning admission were associated with a lower likelihood of returning to home. Nerve block prior arrival to the operating theater was associated with higher likelihood of being discharged to home.
Patel et al. 54 studied hospital organizational factors associated with recovery of mobility and change in patient residence after hip fracture. They found that 19 factors independently predicted residence on hospital discharge. For example, if anesthetic lead for hip fracture had time allocated in their job plan and if hip fracture service clinical governance meetings were attended by an orthopedic surgeon return to own residence was more likely.
Both studies mainly analyzed modifiable factors related to the hip fracture admission and hospital discharge and not the relation of patient recovery and long-term risk of institutionalization as in our study. However, the factors studied by Patel et al. and Hawley et al. are likely to correlate with patient recovery and thus support the findings in our study.
Prevention of loss of independence and institutionalization not only serves the interest of fracture patients. In view of the increasing numbers of frail older adults, the prevention of institutionalization is of great interest for policy makers and health care providers in general. 11 Institutionalization also implies a considerable financial burden on the individual, the family, and society. 55 A great financial benefit could be gained by preventing people from becoming dependent.
Strengths and limitations
Our study has some major strengths. This is a large study that relies on register-based data which are known to have a high degree of completeness. Reporting of certain variables used in this study is mandatory for the medical and care staff, thus, for health care visits and prescriptions all the necessary information can be expected to be present. The Swedish social security number allows following patients over time and allows data to be linked to other registers. Minimal exclusion criteria were applied; therefore, it could be argued that the study provides a more representative depiction of events in a real-world setting, compared with smaller, more selective studies, for example, that the study included patients with cognitive dysfunction which often is an exclusion criterion in prospective studies of hip fracture patients.
We acknowledge that the study has limitations. Attrition bias may be present due to potential differences between patients who did and did not answer the 4-month follow-up questionnaire in SHR. Diagnoses of comorbidities were identified using diagnosis and procedure codes, which are subject to potential miscoding. The health registers included in the study do, however, cover all diagnoses and procedures in the hospital setting irrespective of where they occurred. In the very unlikely event that a patient’s health care visit or prescription would not have been captured at all by the registers, this instance of missing data would not be possible to identify.
We did not have access to information about time of mobilization and rehabilitation proceedings, which are known to affect outcomes after hip fracture. However, low variance is expected as Swedish guidelines stipulate a fast mobilization after surgery.
Another limitation is that LBM was estimated using predictive models based on anthropometrical assessments, rather than by devices like DXA or MRI. However, it has previously been shown that models based on age, height, and weight, predict LBM with reasonably low prediction error 56 and may be a feasible option in epidemiological studies where muscle or LBM measurements are missing.
Conclusions
Low LBM and restricted recovery in post-discharge walking ability is associated with an increased risk of institutionalization after hip fracture in older patients living independently prior to the fracture. Thus, maintaining muscle mass and mobility by exercise and adequate nutrition in late life are likely key factors to avoid loss of independence post-fracture, which is important both for the individual and from a societal perspective.
Supplemental Material
Supplemental material, sj-pdf-1-smo-10.1177_20503121241258409 for Association between institutionalization by 4 months post-discharge walking capacity and lean body mass in elderly hip fracture patients: Evidence from a Swedish Registry Based Study by Sara Hallberg, Emma Söreskog, Fredrik Borgström, Tommy Cederholm and Margareta Hedström in SAGE Open Medicine
Supplemental material, sj-pdf-2-smo-10.1177_20503121241258409 for Association between institutionalization by 4 months post-discharge walking capacity and lean body mass in elderly hip fracture patients: Evidence from a Swedish Registry Based Study by Sara Hallberg, Emma Söreskog, Fredrik Borgström, Tommy Cederholm and Margareta Hedström in SAGE Open Medicine
Footnotes
Author contributions: All authors contributed to the study design and protocol. SH, ES, and FB retrieved the data. SH and ES contributed to the statistical analyses. SH wrote the initial manuscript, and all authors critically revised the manuscript.
Data availability statement: Access to research data is for ethical and legal reasons only available for this study’s researchers that have been approved by each data holder.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FB is employed and owns stock in Quantify Research, a contract research organization in real world evidence. SH was employed by Quantify Research when this study was conducted. MH and TC have no conflicts of interest to declare. Reference number 2018/887-31.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection and analysis was funded by an unrestricted research grant from Novartis to Quantify Research. No funding was obtained for writing of the article.
Study approval statement: The study protocol was approved and reviewed by the Regional Stockholm Research Ethics Committee at the Karolinska Institutet. The committee also decided that written patient consent was not required for this study, approval number 2018/887-31.
ORCID iD: Fredrik Borgström  https://orcid.org/0000-0002-5095-7148
https://orcid.org/0000-0002-5095-7148
Supplemental material: Supplemental material for this article is available online.
References
- 1. Mellner C, Hedström M, Hommel A, et al. The Sernbo score as a predictor of 1-year mortality after hip fracture: a registry study on 55,716 patients. Eur J Trauma Emerg Surg 2020; 47: 2043–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meyer AC, Ek S, Drefahl S, et al. Trends in hip fracture incidence, recurrence, and survival by education and comorbidity: a swedish register-based study. Epidemiology 2021; 32(3): 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peeters CMM, Visser E, Van de Ree CLP, et al. Quality of life after hip fracture in the elderly: a systematic literature review. Injury 2016; 47(7): 1369–1382. [DOI] [PubMed] [Google Scholar]
- 4. Melton LJ, 3rd. Adverse outcomes of osteoporotic fractures in the general population. J Bone Miner Res 2003; 18(6): 1139–1141. [DOI] [PubMed] [Google Scholar]
- 5. Marrinan S, Pearce MS, Jiang XY, et al. Admission for osteoporotic pelvic fractures and predictors of length of hospital stay, mortality and loss of independence. Age Ageing 2015; 44(2): 258–261. [DOI] [PubMed] [Google Scholar]
- 6. Rapp K, Rapp K, Büchele G, et al. Epidemiology of hip fractures: systematic literature review of German data and an overview of the international literature. Zeitschrift für Gerontologie und Geriatrie 2019; 52(1): 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson SM, Ní Bhuachalla B, Ní Mhaille B, et al. Home, please: a conjoint analysis of patient preferences after a bad hip fracture. Geriatr Gerontol Int 2015; 15(10): 1165–1170. [DOI] [PubMed] [Google Scholar]
- 8. Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ (Clinical research ed.) 2000; 320(7231): 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dyer SM, Crotty M, Fairhall N, et al. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatrics 2016; 16(1): 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cree M, Soskolne CL, Belseck E, et al. Mortality and institutionalization following hip fracture. J Am Geriatr Soc 2000; 48(3): 283–288. [DOI] [PubMed] [Google Scholar]
- 11. Schaller F, Sidelnikov E, Theiler R, et al. Mild to moderate cognitive impairment is a major risk factor for mortality and nursing home admission in the first year after hip fracture. Bone 2012; 51(3): 347–352. [DOI] [PubMed] [Google Scholar]
- 12. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48(1): 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019; 393(10191): 2636–2646. [DOI] [PubMed] [Google Scholar]
- 14. Cervera-Diaz MDC, Lopez-Gomez JJ, Garcia-Virto V, et al. Prevalence of sarcopenia in patients older than 75 years admitted for hip fracture. Endocrinol Diabetes Nutr (Engl Ed) 2023; 70(6): 396–407. [DOI] [PubMed] [Google Scholar]
- 15. Hedström M. Hip fracture patients, a group of frail elderly people with low bone mineral density, muscle mass and IGF-I levels. Acta Physiol Scand 1999; 167(4): 347–350. [DOI] [PubMed] [Google Scholar]
- 16. Hedström M, Ljungqvist O, Cederholm T. Metabolism and catabolism in hip fracture patients: nutritional and anabolic intervention – A review. Acta Orthop 2006; 77(5): 741–747. [DOI] [PubMed] [Google Scholar]
- 17. Donald GW, Ghaffarian AA, Isaac F, et al. Preoperative frailty assessment predicts loss of independence after vascular surgery. J Vasc Surg 2018; 68(5): 1382–1389. [DOI] [PubMed] [Google Scholar]
- 18. Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc 2000; 48(5): 493–498. [DOI] [PubMed] [Google Scholar]
- 19. Meyer AC, Hedstrom M, Modig K. The Swedish hip fracture register and national patient register were valuable for research on hip fractures: comparison of two registers. J Clin Epidemiol 2020; 125: 91–99. [DOI] [PubMed] [Google Scholar]
- 20. Variable list for Swedish hip fracture register. Available from: https://www.xn–rikshft-e1a.se/variabellista (2023, accessed 20 December 2023).
- 21. Lapin BR, Thompson NR, Schuster A, et al. The validity of proxy responses on patient-reported outcome measures: are proxies a reliable alternative to stroke patients’ self-report? Qual Life Res 2021; 30(6): 1735–1745. [DOI] [PubMed] [Google Scholar]
- 22. Johansen A, Ojeda-Thies C, Poacher AT, et al. Global Fragility Fracture Network Hip Fracture Audit Special Interest, Developing a minimum common dataset for hip fracture audit to help countries set up national audits that can support international comparisons. Bone Joint J 2022; 104-B(6): 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansen A, Hall AJ, Ojeda-Thies C, et al. Global Fragility Fracture Network Hip Fracture Audit Special Interest, Standardization of global hip fracture audit could facilitate learning, improve quality, and guide evidence-based practice. Bone Joint J 2023; 105-B(9): 1013–1019. [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson JF, Almqvist C, Bonamy A-KE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31(2): 125–136. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11(1): 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the swedish prescribed drug register – A systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol 2016; 119(5): 464–469. [DOI] [PubMed] [Google Scholar]
- 27. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register – Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16(7): 726–735. [DOI] [PubMed] [Google Scholar]
- 28. Ingemarsson AH, Frandin K, Mellstrom D, et al. Walking ability and activity level after hip fracture in the elderly – A follow-up. J Rehabil Med 2003; 35(2): 76–83. [DOI] [PubMed] [Google Scholar]
- 29. Rosenberg IH. Summary comments. Am J Clin Nutr 1989; 50(5): 1231–1233. [Google Scholar]
- 30. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997; 127(5): 990S–991S. [DOI] [PubMed] [Google Scholar]
- 31. Müller MJ, Bosy-Westphal A, Kutzner D, et al. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev 2002; 3(2): 113–122. [DOI] [PubMed] [Google Scholar]
- 32. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 2009; 4(9): e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doupe MB, Martin AD, Searle MS, et al. A new formula for population-based estimation of whole body muscle mass in males. Can J Appl Physiol 1997; 22(6): 598–608. [DOI] [PubMed] [Google Scholar]
- 34. Martin AD, Spenst LF, Drinkwater DT, et al. Anthropometric estimation of muscle mass in men. Med Sci Sports Exerc 1990; 22(5): 729–733. [DOI] [PubMed] [Google Scholar]
- 35. Matiegka J. The testing of physical efficiency. Am J Phys Anthropol 1921; 4: 223. [Google Scholar]
- 36. Hume R. Prediction of lean body mass from height and weight. J Clin Pathol 1966; 19(4): 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol 1984; 247(4 Pt 2): F632–F636. [DOI] [PubMed] [Google Scholar]
- 38. Garrow JS. Quetelet index as indicator of obesity. Lancet 1986; 1(8491): 1219. [DOI] [PubMed] [Google Scholar]
- 39. Heitmann BL. Evaluation of body fat estimated from body mass index, skinfolds and impedance. A comparative study. Eur J Clin Nutr 1990; 44(11): 831–837. [PubMed] [Google Scholar]
- 40. Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr 1991; 65(2): 105–114. [DOI] [PubMed] [Google Scholar]
- 41. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000; 39(3): 215–231. [DOI] [PubMed] [Google Scholar]
- 42. Bucaloiu ID, Wood GC, Norfolk ER, et al. Fat-free weight prediction in morbidly obese females. Int J Nephrol Renovasc Dis 2011; 4: 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Sallami HS, Goulding A, Grant A, et al. Prediction of fat-free mass in children. Clin Pharmacokinet 2015; 54(11): 1169–1178. [DOI] [PubMed] [Google Scholar]
- 44. James WPT, Waterlow JC. Research on obesity: a report of the DHSS/MRC Group. London: Her Majesty’s Stationery Office: UK Department of Health and Social Security/Medical Research Council Group on Obesity Research, 1976. [Google Scholar]
- 45. Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 2006; 17(5): 637–650. [DOI] [PubMed] [Google Scholar]
- 46. Nanjayan SK, John J, Swamy G, et al. Predictors of change in ‘discharge destination’ following treatment for fracture neck of femur. Injury 2014; 45(7): 1080–1084. [DOI] [PubMed] [Google Scholar]
- 47. Ström O, Borgström F, Kanis JA, et al. Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2011; 6: 59–155. [DOI] [PubMed] [Google Scholar]
- 48. Steihaug OM, Gjesdal CG, Bogen B, et al. Does sarcopenia predict change in mobility after hip fracture? a multicenter observational study with one-year follow-up. BMC Geriatr 2018; 18(1): 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karlsson ES, Gronstedt HK, Faxen-Irving G, et al. Response and adherence of nursing home residents to a nutrition/exercise intervention. J Am Med Dir Assoc 2021; 22(9): 1939.e3–1945.e3. [DOI] [PubMed] [Google Scholar]
- 50. Singh NA, Quine S, Clemson LM, et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc 2012; 13(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 51. Uriz-Otano F, Pla-Vidal J, López GT, et al. Factors associated to institutionalization and mortality over three years, in elderly people with a hip fracture. An observational study. Maturitas 2016; 89: 9–15. [DOI] [PubMed] [Google Scholar]
- 52. Jorgensen TSH, Meyer AC, Hedstrom M, et al. The importance of close next of kin for independent living and readmissions among older Swedish hip fracture patients. Health Soc Care Community 2022; 30(3): e727–e738. [DOI] [PubMed] [Google Scholar]
- 53. Hawley S, Inman D, Gregson CL, et al. Predictors of returning home after hip fracture: a prospective cohort study using the UK National Hip Fracture Database (NHFD). Age Ageing 2022; 51(8): afac131. [DOI] [PubMed] [Google Scholar]
- 54. Patel R, Judge A, Johansen A, et al. Patients’ recovery of mobility and return to original residence after hip fracture are associated with multiple modifiable components of hospital service organisation: the REDUCE record-linkage cohort study in England and Wales. BMC Geriatr 2023; 23(1): 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Katz PR. An international perspective on long term care: focus on nursing homes. J Am Med Directors Assoc 2011; 12(7): 487–492.e1. [DOI] [PubMed] [Google Scholar]
- 56. Kulkarni B, Kuper H, Taylor A, et al. Development and validation of anthropometric prediction equations for estimation of lean body mass and appendicular lean soft tissue in Indian men and women. J Appl Physiol (1985) 2013; 115(8): 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-smo-10.1177_20503121241258409 for Association between institutionalization by 4 months post-discharge walking capacity and lean body mass in elderly hip fracture patients: Evidence from a Swedish Registry Based Study by Sara Hallberg, Emma Söreskog, Fredrik Borgström, Tommy Cederholm and Margareta Hedström in SAGE Open Medicine
Supplemental material, sj-pdf-2-smo-10.1177_20503121241258409 for Association between institutionalization by 4 months post-discharge walking capacity and lean body mass in elderly hip fracture patients: Evidence from a Swedish Registry Based Study by Sara Hallberg, Emma Söreskog, Fredrik Borgström, Tommy Cederholm and Margareta Hedström in SAGE Open Medicine