Abstract
Reovirus infection induces apoptosis in cultured cells and in vivo. To identify host cell factors that mediate this response, we investigated whether reovirus infection alters the activation state of the transcription factor nuclear factor kappa B (NF-κB). As determined in electrophoretic mobility shift assays, reovirus infection of HeLa cells leads to nuclear translocation of NF-κB complexes containing Rel family members p50 and p65. Reovirus-induced activation of NF-κB DNA-binding activity correlated with the onset of NF-κB-directed transcription in reporter gene assays. Three independent lines of evidence indicate that this functional form of NF-κB is required for reovirus-induced apoptosis. First, treatment of reovirus-infected HeLa cells with a proteasome inhibitor prevents NF-κB activation following infection and substantially diminishes reovirus-induced apoptosis. Second, transient expression of a dominant-negative form of IκB that constitutively represses NF-κB activation significantly reduces levels of apoptosis triggered by reovirus infection. Third, mutant cell lines deficient for either the p50 or p65 subunits of NF-κB are resistant to reovirus-induced apoptosis compared with cells expressing an intact NF-κB signaling pathway. These findings indicate that NF-κB plays a significant role in the mechanism by which reovirus induces apoptosis in susceptible host cells.
Many viruses are capable of inducing programmed cell death, which results in apoptosis of infected cells (43, 45, 52, 60). Apoptotic cell death is characterized by cell shrinkage, membrane blebbing, condensation of nuclear chromatin, and activation of endogenous endonucleases. These changes occur according to developmental programs or in response to certain environmental stimuli (2, 43, 52, 71). In some cases, apoptosis triggered by virus infection appears to serve as a host defense mechanism to limit viral replication or spread. This defense mechanism is mediated either directly by self-destruction of the host cell prior to completion of viral replication or indirectly through recognition of the infected cell by cytotoxic T lymphocytes (43, 52). In other cases, induction of apoptosis may enhance viral infection by facilitating virus spread or allowing the virus to evade host inflammatory or immune responses (20, 43, 60). For some viruses, cellular factors operant during apoptosis may function to increase the production of viral progeny (45, 52).
Mammalian reoviruses have served as useful models for studies of viral pathogenesis. Reoviruses are nonenveloped icosahedral viruses with a genome consisting of 10 double-stranded RNA gene segments (reviewed in reference 41). After infection of newborn mice, reoviruses are highly virulent, inducing injury to a variety of host organs including the central nervous system, heart, and liver (reviewed in reference 62). In both cultured cells (46, 63) and the murine central nervous system (42) and heart (R. DeBiasi, B. Sherry, and K. Tyler, Abstr. Am. Soc. Virol. 18th Annu. Meet., abstr. 52-1, p. 152, 1999), reoviruses induce the morphological and biochemical features of apoptosis.
Insight into the mechanisms by which reoviruses trigger apoptosis has emerged from studies of viral prototype strains that vary in their capacity to elicit this cellular response. Reovirus strains type 3 Abney and type 3 Dearing (T3D) induce apoptosis in cultured cells to a substantially greater extent than does strain type 1 Lang (46, 63). Differences in the capacity of these strains to induce apoptosis are determined by the viral S1 gene (46, 63), which encodes two proteins, attachment protein ς1 and nonstructural protein ς1s (25, 31, 50). Reovirus ς1s-null mutant T3C84-MA induces apoptosis with an efficiency equivalent to its ς1s-expressing parental strain, T3C84 (47), which indicates that the ς1 protein is the S1 gene product responsible for mediating differences in the efficiency with which reovirus strains induce apoptosis. Therefore, these studies suggest that apoptosis induced by reovirus is triggered by a signaling pathway initiated by early steps in the virus replication cycle.
The nuclear factor kappa B (NF-κB) family of transcription factors plays a key role in the regulation of cell growth and survival. The prototypical form of NF-κB exists as a heterodimer of proteins p50 and p65 (RelA) (4, 27). In quiescent cells, NF-κB is sequestered in the cytoplasm by the IκB family of inhibitory proteins (3, 66). Following exposure of cells to a variety of stimuli (including tumor necrosis factor alpha [TNF-α], interleukin-1, and lipopolysaccharide), activation of NF-κB is accomplished by a mechanism involving site-specific phosphorylation, ubiquitination, and proteasomal degradation of IκB (11, 12, 17, 61). Release of IκB reveals a nuclear localization signal on NF-κB, which allows NF-κB to translocate to the nucleus (7), where it serves as a transcriptional regulator (reviewed in references 38 and 66). In systems in which NF-κB is activated during induction of apoptosis, NF-κB can either prevent (6, 35, 65, 69) or potentiate (1, 29, 32, 34) apoptosis.
We conducted experiments to investigate the role of NF-κB in reovirus-induced apoptosis. We show that NF-κB complexes are activated in HeLa cells in response to reovirus infection and that these complexes contain both p50 and p65. Using two independent methods to block NF-κB activation following reovirus infection, we demonstrate that reovirus-induced apoptosis requires NF-κB. Furthermore, reovirus-induced apoptosis is inhibited in cells deficient in expression of either p50 or p65. These results provide strong evidence that reovirus activates NF-κB and that NF-κB activation is required for apoptosis induced by reovirus infection.
MATERIALS AND METHODS
Cells and viruses.
Murine L929 (L) cells were maintained as previously described (47). p50+/+, p50−/−, p65+/+, and p65−/− immortalized fibroblasts were obtained from David Baltimore, California Institute of Technology, Pasadena, Calif. HeLa, p50+/+, p50−/−, p65+/+, and p65−/− cells were grown in Dulbecco's modified Eagle's medium (Gibco BRL, Gaithersburg, Md.) supplemented to contain 10% fetal bovine serum (Intergen, Purchase, N.Y.), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml (Sigma Chemical Co., St. Louis, Mo.), and 0.25 μg of amphotericin B per ml (Irvine Scientific, Santa Ana, Calif.).
Reovirus strain T3D is a laboratory stock. Purified virion preparations were made as previously described using second-passage L-cell lysate stocks of twice-plaque-purified reovirus (26). Concentrations of virions in purified preparations were determined from the equivalence 1 optical density at 260 nm unit = 2.1 × 1012 virions per ml (54).
Quantitation of reovirus growth.
Cells grown in 24-well tissue culture plates (Costar, Cambridge, Mass.) were infected with T3D at a multiplicity of infection (MOI) of 1 PFU per cell. After viral adsorption for 1 h, the inoculum was removed, 1.0 ml of fresh medium was added, and the cells were incubated at 37°C for various intervals. After incubation, cells and culture medium were frozen (−70°C) and thawed twice, and viral titers in cell lysates were determined by plaque assay using L-cell monolayers (67).
Quantitation of apoptosis by acridine orange staining.
Cells grown in 24-well tissue culture plates were treated with 20 ng of human recombinant TNF-α (Sigma) per ml or infected with T3D at an MOI of 100 PFU per cell. This MOI was chosen to produce a synchronous infection and to ensure maximum levels of apoptosis. The percentage of apoptotic cells was determined using acridine orange staining as previously described (23, 46, 63). The cell culture medium was removed, and the cells were incubated with trypsin-EDTA (Irvine Scientific). The cell culture medium and trypsinized cells were combined and centrifuged. The cell pellet was resuspended in 200 μl of phosphate-buffered saline and stained using 10 μl of a solution containing 100 μg of acridine orange (Sigma) per ml and 100 μg of ethidium bromide (Sigma) per ml. For each experiment, 200 to 300 cells were counted and the percentage of cells exhibiting condensed chromatin was determined by epi-illumination fluorescence microscopy using a fluorescein filter set (Photomicroscope III; Zeiss, Oberköchen, Germany).
EMSA.
Cells grown in 75-cm2 tissue culture flasks (Costar) were either treated with 20 ng of TNF-α per ml or adsorbed with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for various intervals, nuclear extracts were prepared by washing cells in phosphate-buffered saline and incubating them in hypotonic lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail [Boehringer Mannheim, Indianapolis, Ind.]) at 4°C for 15 min. Then 1/20 volume of 10% Nonidet P-40 was added to the cell lysate, and the sample was vortexed for 10 s and centrifuged at 10,000 × g for 5 min. The nuclear pellet was washed once in hypotonic buffer, resuspended in high-salt buffer (25% glycerol, 20 mM HEPES [pH 7.9], 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail), and incubated at 4°C for 2 to 3 h. Samples were centrifuged at 10,000 × g for 10 min, and the supernatant was used as the nuclear extract.
Nuclear extracts were assayed for NF-κB activation by an electrophoretic mobility shift assay (EMSA) using a 32P-labeled oligonucleotide consisting of the NF-κB consensus binding sequence (Santa Cruz Biotechnology, Santa Cruz, Calif.). Nuclear extracts (5 to 10 μg of total protein) were incubated at 4°C for 20 min with a binding-reaction buffer containing 2 μg of poly(dI-dC) (Sigma) in 20 mM HEPES (pH 7.9)–60 mM KCl–1 mM EDTA–1 mM dithiothreitol–5% glycerol. Radiolabeled NF-κB consensus oligonucleotide (0.1 to 1.0 ng) was added, and the mixture was incubated at room temperature for 20 min. For competition experiments, a 10-fold excess of unlabeled consensus oligonucleotide or of an oligonucleotide containing a point mutation in the NF-κB consensus site (Santa Cruz Biotechnology) was added to reaction mixtures. For supershift experiments, 1 μl of rabbit polyclonal antiserum raised against either human p50 or p65 (Santa Cruz Biotechnology) or a control antibody raised against reovirus nonstructural protein ς1s (47) was added to binding-reaction mixtures and incubated at 4°C for 30 min prior to the addition of radiolabeled oligonucleotide. Nucleoprotein complexes were subjected to electrophoresis on native 5% polyacrylamide gels at 180 V, dried under vacuum, and exposed to Biomax MR film (Kodak, Rochester, N.Y.).
Luciferase gene reporter assay.
The NF-κB-dependent luciferase reporter construct was a gift from Lucy Ghoda. The construct is composed of pGL2-Basic (Promega, Madison, Wis.) and three NF-κB-binding sites from the major histocompatibility complex class I promoter. HeLa cells (1.5 × 105) in six-well tissue culture plates (Costar) were incubated for 24 h and then transfected with 10 μg of the luciferase reporter construct and 2 μg of a cytomegalovirus (CMV)–β-galactosidase reporter construct (Clontech, Palo Alto, Calif.) using LipofectAMINE (Gibco BRL). After an additional 24-h incubation, cells were either mock infected or infected with T3D at an MOI of 100 PFU per cell and incubated at 37°C for various intervals. The cells were resuspended in 1 ml of sonication buffer (91 mM dithiothreitol, 0.91 M K2HPO4 [pH 7.8]), centrifuged at 2,000 × g for 10 min, and resuspended in 100 μl of sonication buffer. The cells were then vortexed, frozen (−20°C) and thawed three times, and centrifuged at 14,000 × g for 10 min. Samples (10 μl) were assessed for luciferase activity after addition of 350 μl of luciferase assay buffer (85 mM dithiothreitol, 0.85 M K2HPO4 [pH 7.8], 50 mM ATP, 15 mM MgSO4) by determining the optical density in a luminometer (Monolight 2010; Analytical Luminescence Laboratory). Samples were assayed for β-galactosidase activity using standard procedures (49) to normalize for transfection efficiency.
Proteasome inhibitor treatment.
The proteasome inhibitor Z-L3VS was obtained from Hidde Ploegh (10). HeLa cells were incubated at 37°C for 1 h with medium containing 5 μM Z-L3VS. TNF-α at 20 ng per ml was added, and the cells were incubated at 37°C for 18 h, or the medium was removed and the cells were adsorbed at 4°C for 1 h with T3D at an MOI of 100 PFU per cell in gelatin saline containing 5 μM Z-L3VS. Following adsorption, medium containing the proteasome inhibitor was added and the cells were incubated at 37°C for various intervals. Cells were harvested for EMSA or acridine orange staining assays.
Transient transfection of HeLa cells.
The coding sequence of FLAG epitope-tagged human IκBα lacking amino acids 1 to 36 (IκBα-ΔN) (11) was inserted into the multiple-cloning site of pHook-2 (Invitrogen, Carlsbad, Calif.) to generate pHook-2/IκBα-ΔN. HeLa cells (7 × 105) in 60-mm dishes (Corning, Corning, N.Y.) were incubated at 37°C for 24 h and then transfected with either 5 μg of pHook-2/lacZ (Invitrogen) or 5 μg of pHook-2/IκBα-ΔN using LipofectAMINE PLUS reagent (Gibco BRL). Transfected cells were selected using Capture-Tec magnetic beads (Invitrogen) 24 h following infection and plated in 24-well plates for use in acridine orange staining assays.
Statistical analysis.
Acridine orange staining data were tested using parametric statistical analysis with a two-sample t test. Statistical analysis was performed using Minitab statistical software (Addison-Wesley, Reading, Mass.).
RESULTS
Reovirus replicates efficiently and induces apoptosis in HeLa cells.
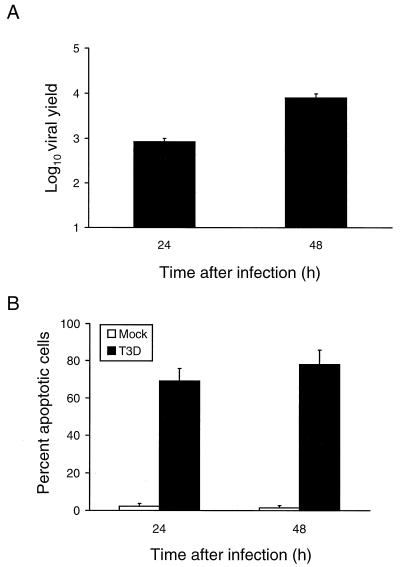
To determine whether reovirus is capable of productively infecting HeLa cells, reovirus strain T3D was adsorbed to cells at an MOI of 1 PFU per cell and viral yields were determined 24 and 48 h after infection (Fig. 1A). T3D replicated efficiently in HeLa cells, producing yields of approximately 800 and 8,000 progeny virions per input 24 and 48 h following infection, respectively. To determine whether reovirus induces apoptosis of HeLa cells, T3D was adsorbed to cells at an MOI of 100 PFU per cell and apoptosis was assessed by acridine orange staining 24 and 48 h after infection (Fig. 1B). In previous work, we showed that cell death detected using acridine orange staining of infected L cells and Madin-Darby canine kidney (MDCK) epithelial cells correlates with ultrastructural changes characteristic of apoptosis and formation of oligonucleosome-length DNA ladders (46, 63). T3D infection of HeLa cells induced chromatin condensation and the morphological changes of apoptosis in approximately 70% of cells 24 h after infection and 80% of cells 48 h after infection. These results indicate that reovirus grows efficiently in HeLa cells and induces the death of these cells by apoptosis.
FIG. 1.
(A) Growth of reovirus in HeLa cells. Cells (1 × 105) were infected with reovirus strain T3D at an MOI of 1 PFU per cell. After adsorption for 1 h, the inoculum was removed, fresh medium was added, and the cells were incubated at 37°C for 0, 24, or 48 h. The cells were frozen and thawed twice, and viral titers were determined by a plaque assay. The results are expressed as the mean viral yields, calculated by dividing the viral titer at 24 or 48 h by the viral titer at 0 h, for three independent experiments. Error bars indicate standard error of the mean. (B) Apoptosis induced by reovirus infection of HeLa cells. Cells (5 × 104) were either mock infected or infected with reovirus strain T3D at an MOI of 100 PFU per cell. After adsorption for 1 h, the cells were incubated at 37°C for 24 or 48 h and stained with acridine orange. The results are expressed as the mean percentage of cells undergoing apoptosis in three independent experiments. Error bars indicate standard error of the mean.
NF-κB is activated by reovirus.
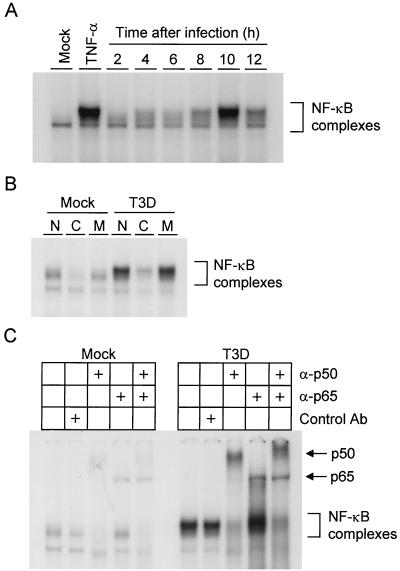
To determine whether NF-κB is activated following reovirus infection of HeLa cells, we used EMSAs to detect NF-κB in nuclear extracts prepared from reovirus-infected cells. HeLa cells were either mock infected or infected with reovirus strain T3D, and nuclear extracts were prepared at various times after viral adsorption. Extracts were incubated with a 32P-labeled oligonucleotide consisting of the NF-κB consensus binding sequence and resolved in a nondenaturing polyacrylamide gel (Fig. 2A). Following infection with reovirus, proteins capable of shifting the radiolabeled oligonucleotide to a higher relative molecular mass were increased in nuclear extracts. NF-κB activation was first detected at 4 h postinfection, peaked at 10 h postinfection, and was diminishing by 12 h postinfection. Activated complexes could not be detected in mock-infected cultures at any time point (data not shown). As assessed by EMSA, NF-κB was activated in L cells and MDCK cells with similar kinetics following reovirus infection (data not shown).
FIG. 2.
(A) Time course of NF-κB gel shift activity in nuclear extracts prepared from reovirus-infected HeLa cells. Cells (5 × 106) were either mock infected or infected with T3D at an MOI of 100 PFU per cell and incubated at 37°C for the times shown. Uninfected cells also were treated with 20 ng of TNF-α per ml for 1 h. Nuclear extracts were prepared and incubated with a 32P-labeled oligonucleotide consisting of the NF-κB consensus binding sequence. Incubation mixtures were resolved by acrylamide gel electrophoresis, dried, and exposed to film. NF-κB-containing complexes are indicated. (B) Specificity of NF-κB gel shift activity. Nuclear extracts were prepared as in panel A 10 h after viral adsorption. Extracts were incubated with 32P-labeled NF-κB consensus oligonucleotide alone (lanes N), a 10-fold excess of unlabeled consensus probe (lanes C), or a 10-fold excess of unlabeled mutant probe consisting of the NF-κB consensus sequence with a point mutation that abolishes NF-κB binding (lanes M). NF-κB-containing complexes are indicated. (C) Identification of NF-κB family members activated by reovirus infection. Nuclear extracts were prepared as in panel A 10 h after viral adsorption. Extracts were incubated with no antibody, a control antibody (Ab), p50-specific antiserum, p65-specific antiserum, or both p50- and p65-specific antisera. NF-κB complexes not shifted by antibody and supershifted complexes containing p50 or p65 are indicated.
To confirm the specificity of NF-κB DNA-binding activity in these experiments, HeLa cells were either mock infected or infected with T3D at an MOI of 100 PFU per cell and nuclear extracts were prepared 10 h after adsorption. Nuclear extracts were incubated with a 32P-labeled NF-κB consensus oligonucleotide in the presence of a 10-fold excess of either unlabeled consensus oligonucleotide or unlabeled mutant oligonucleotide (Fig. 2B). The mutant oligonucleotide consists of the NF-κB consensus sequence with a single point mutation that abolishes NF-κB binding. Binding of the radiolabeled probe was competed with unlabeled consensus oligonucleotide but not with mutant oligonucleotide. We conclude that the gel shift activity detected following reovirus infection is specific for sequences that are bound by NF-κB.
To identify NF-κB family members present in complexes activated following reovirus infection, nuclear extracts were prepared from mock-infected cells and cells infected with T3D 10 h after viral adsorption. The nuclear extracts were incubated with a p50-specific antiserum, a p65-specific antiserum, or both antisera prior to addition of the 32P-labeled NF-κB consensus oligonucleotide (Fig. 2C). The addition of anti-p50 or anti-p65 antiserum or both antisera resulted in bands of higher relative molecular mass, indicating that both p50 and p65 are present in complexes activated following reovirus infection. These findings indicate that reovirus infection of HeLa cells results in nuclear translocation of NF-κB complexes and that these complexes contain NF-κB family members p50 and p65.
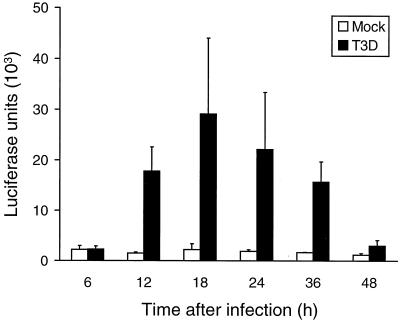
To determine whether NF-κB is capable of stimulating transcription following reovirus infection, we used a reporter gene construct containing NF-κB-binding sites to direct the expression of luciferase. Following transfection of HeLa cells with this construct, cells were either mock infected or infected with T3D. After incubation for various intervals, cell extracts were prepared and assayed for luciferase activity (Fig. 3). T3D infection was associated with substantial NF-κB-dependent luciferase expression in comparison to mock-infected cells. Luciferase activity in infected cells was first detected between 6 and 12 h postinfection and was greatest 18 h postinfection, after which it declined and was nearly undetectable by 48 h postinfection. Transfection efficiencies were normalized by cotransfection of a β-galactosidase reporter construct driven by the CMV promoter. β-Galactosidase expression from the CMV reporter was not altered by reovirus infection (data not shown). These results indicate that reovirus infection of HeLa cells activates NF-κB-dependent transcription, which is consistent with the results obtained from biochemical experiments.
FIG. 3.
NF-κB-dependent luciferase expression in reovirus-infected HeLa cells. Cells (1.5 × 105) were transfected with 10 μg of a luciferase reporter construct containing NF-κB binding sites. After 24 h, the cells were either mock infected or infected with T3D at an MOI of 100 PFU per cell and incubated at 37°C for the times shown. Cell extracts were prepared, and luciferase activity was determined. The results are expressed as the mean luciferase units in two independent experiments. Error bars indicate standard error of the mean.
Proteasome inhibitor treatment inhibits reovirus-induced apoptosis.
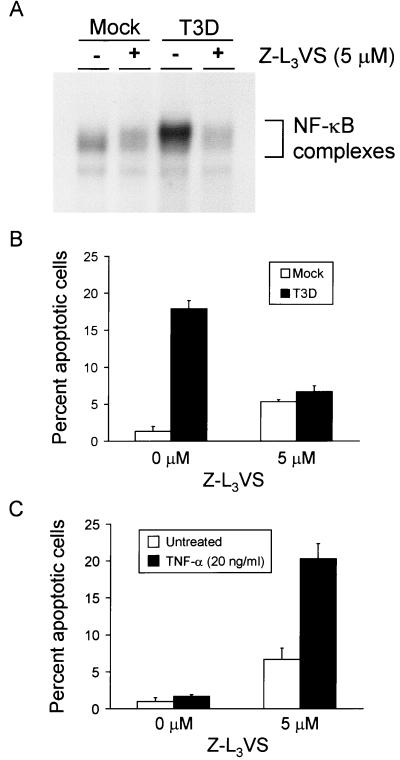
To determine the role of NF-κB activation in reovirus-induced apoptosis, HeLa cells were treated with Z-L3VS, a synthetic inhibitor of proteasome function (10). Previous studies of other proteasome inhibitors have demonstrated that NF-κB activation induced by a variety of stimuli, including TNF-α, lipopolysaccharide, and phorbol esters, is blocked by treatment of cells with inhibitors of proteasome catalytic activity (36, 44). Since degradation of IκB and the subsequent release of NF-κB require proteasome activity (17), proteasome inhibitors lead to sequestration of NF-κB in the cytoplasm. To determine whether Z-L3VS is capable of blocking NF-κB activation following reovirus infection, cells were cultured in the absence or presence of 5 μM Z-L3VS and then either mock infected or infected with T3D at an MOI of 100 PFU per cell. Nuclear extracts were prepared 10 h following infection and used in an EMSA (Fig. 4A). Treatment of HeLa cells with Z-L3VS abolished NF-κB activation following reovirus infection, which confirms that inhibition of proteasome function blocks nuclear translocation of NF-κB.
FIG. 4.
(A) NF-κB gel shift activity in reovirus-infected cells cultured in the presence of Z-L3VS. Cells (5 × 106) were either mock infected or infected with T3D at an MOI of 100 PFU per cell and cultured in the absence or presence of 5 μM Z-L3VS. After incubation at 37°C for 10 h, nuclear extracts were prepared and incubated with a 32P-labeled oligonucleotide consisting of the NF-κB consensus sequence. Incubation mixtures were resolved by acrylamide gel electrophoresis, dried, and exposed to film. NF-κB-containing complexes are indicated. (B) Quantitation of apoptosis in reovirus-infected HeLa cells cultured in the presence of Z-L3VS. Cells (5 × 104) were either mock infected or infected with T3D at an MOI of 100 PFU per cell and cultured in the absence or presence of 5 μM Z-L3VS. After incubation at 37°C for 18 h, the cells were stained with acridine orange. (C) Quantitation of apoptosis in TNF-α-treated HeLa cells cultured in the presence of Z-L3VS. Cells (5 × 104) were either untreated or treated with 20 ng of TNF-α per ml and cultured in the absence or presence of 5 μM Z-L3VS. After incubation at 37°C for 18 h, the cells were stained with acridine orange. The results of the experiments in panels B and C are expressed as the mean percentage of cells undergoing apoptosis in three independent experiments. Error bars indicate standard error of the mean.
To determine the effect of blockade of NF-κB activation on reovirus-induced apoptosis, HeLa cells were cultured in the absence or presence of 5 μM Z-L3VS and then either mock infected or infected with T3D at an MOI of 100 PFU per cell. Apoptosis was assessed using acridine orange staining 18 h after infection (Fig. 4B). This time point was chosen because more prolonged incubation with the proteasome inhibitor resulted in cytotoxicity. Approximately 20% of reovirus-infected cells cultured in the absence of the proteasome inhibitor were apoptotic 18 h after infection; however, only 6% of infected cells cultured in the presence of the proteasome inhibitor were apoptotic (P = 0.005). The level of apoptosis detected in infected cells cultured with Z-L3VS did not significantly differ from that observed in uninfected cells cultured with the proteasome inhibitor (P = 0.25). As a control for the blockade of NF-κB using Z-L3VS, cells also were treated with TNF-α (Fig. 4C), which has been shown to increase the levels of apoptosis in cells lacking functional NF-κB (6, 35, 65, 69). Apoptosis was increased in TNF-α-treated cells cultured in the presence of Z-L3VS, suggesting that alterations in apoptosis induction mediated by the proteasome inhibitor are due to blockade of NF-κB. These results provide evidence that interference with signal-dependent degradation of IκB prevents reovirus-induced apoptosis.
Expression of a transdominant inhibitor of NF-κB inhibits reovirus-induced apoptosis.
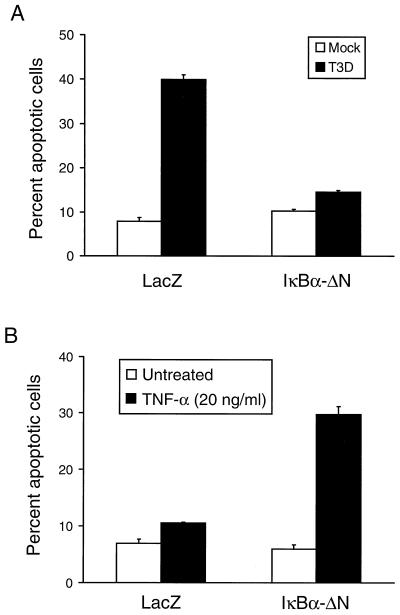
To exclude the possibility that exposure of cells to a proteasome inhibitor results in the blockade of apoptosis by inhibiting viral replication or by exerting other nonspecific effects, we tested whether transient transfection of a transdominant inhibitor of NF-κB, IκBα-ΔN, alters reovirus-induced apoptosis. HeLa cells were transfected with the pHook-2 plasmid containing human IκBα-ΔN appended at the amino terminus with a FLAG epitope. IκBα-ΔN is a 36-amino-acid amino-terminal truncation of IκBα that lacks the two serine residues required for IκBα degradation (12, 51, 61). IκBα-ΔN cannot be targeted for proteasome-mediated degradation and thus functions as a trans-dominant inhibitor of NF-κB. The pHook-2 plasmid allows the selection of transfected cells from a population of cells by virtue of coexpression of a cell surface marker that allows subsequent isolation using magnetic beads (19). Cells selected following transfection of either pHook-2/IκBα-ΔN or a control plasmid, pHook-2/lacZ, were either mock infected or infected with T3D at an MOI of 100 PFU per cell. Apoptosis was assessed using acridine orange staining 24 h after infection (Fig. 5A). Approximately 40% of pHook-2/lacZ-transfected cells were apoptotic following infection with reovirus. In sharp contrast, only 15% of pHook-2/IκBα-ΔN-transfected cells were apoptotic following reovirus infection (P = 0.002). This low level of apoptosis in the pHook-2/IκBα-ΔN-transfected cells was similar to the level of apoptosis in mock-infected cultures (approximately 10%). The percentage of apoptotic cells in mock-infected cultures was higher than routinely observed for untransfected cells, which is probably due to transfection and selection conditions. As a control, transfected cells also were treated with TNF-α (Fig. 5B), which has been shown to increase levels of apoptosis in cells lacking p65 (6) and in cells expressing mutant forms of IκBα (65, 69). Levels of apoptosis were increased following TNF-α treatment of cells transfected with pHook-2/IκBα-ΔN in comparison to cells transfected with pHook-2/lacZ. Therefore, it is likely that transfection with pHook-2/IκBα-ΔN effectively blocks NF-κB activation. Stable expression of mutant forms of IκB in L cells and MDCK cells also inhibits both NF-κB activation and apoptosis following reovirus infection (data not shown). These results demonstrate that expression of an NF-κB trans-dominant inhibitor blocks reovirus-induced apoptosis and further supports the hypothesis that NF-κB activation is required for apoptosis induced by reovirus infection.
FIG. 5.
Quantitation of apoptosis in HeLa cells transiently expressing IκBα-ΔN. Cells (1 × 106) were either transfected with 5 μg of pHook-2/IκBα-ΔN or 5 μg of pHook-2/lacZ. After 24 h, transfected cells were isolated using Capture-Tec magnetic beads and plated in 24-well plates. (A) Transfected cells (2 × 104) were either mock infected or infected with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for 24 h, the cells were stained with acridine orange. (B) Transfected cells (2 × 104) were either not treated or treated with 20 ng of TNF-α per ml. After incubation at 37°C for 24 h, the cells were stained with acridine orange. The results of the experiments in both panels are expressed as the mean percentage of cells undergoing apoptosis in three independent experiments. Error bars indicate standard error of the mean.
Reovirus-induced apoptosis is inhibited in cell lines deficient for p50 or p65.
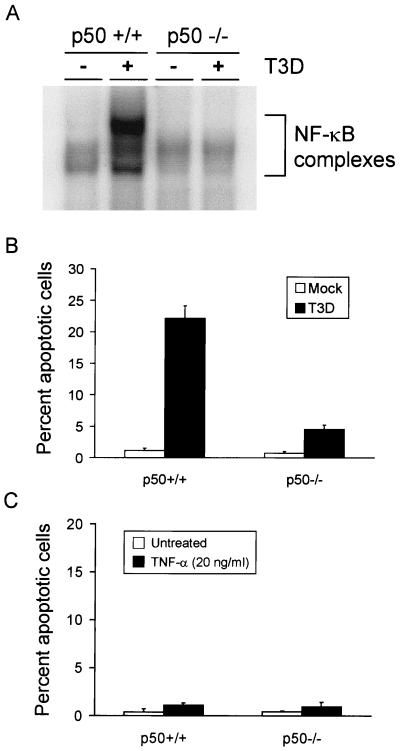
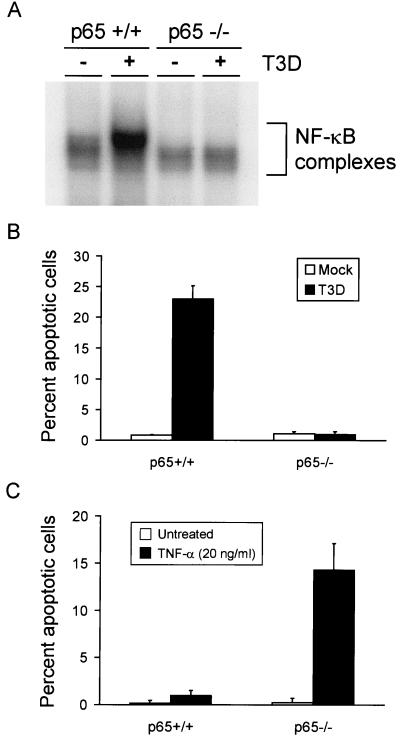
Since both p50 and p65 are present in NF-κB complexes activated following reovirus infection, we performed experiments to specifically determine whether p50 or p65 is required for reovirus-induced apoptosis. Immortalized embryonic fibroblasts containing a null mutation in the gene encoding either the p50 or p65 subunit of NF-κB were infected with reovirus and assayed for NF-κB activation and apoptosis induction. To determine whether NF-κB complexes are activated following reovirus infection of the null cell lines, the p50−/− and p65−/− cell lines and their respective p50+/+ and p65+/+ littermate control cell lines were either mock infected or infected with T3D at an MOI of 100 PFU per cell. Nuclear extracts were prepared 6 h (p50+/+ and p50−/−) or 8 h (p65+/+ and p65−/−) following infection and used in EMSAs (Fig. 6A and 7A). The results demonstrate that NF-κB complexes are not activated in the p50−/− and p65−/− cell lines following infection. This result was anticipated based on the biochemical results with HeLa cells, which demonstrated that p50 and p65 are the primary constituents of NF-κB complexes activated by reovirus.
FIG. 6.
(A) NF-κB gel shift activity in reovirus-infected p50+/+ and p50−/− immortalized fibroblast cells. Cells (5 × 106) were either mock infected or infected with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for 6 h, nuclear extracts were prepared and incubated with a 32P-labeled DNA probe consisting of the NF-κB consensus sequence. Incubation mixtures were resolved by acrylamide gel electrophoresis, dried, and exposed to film. NF-κB-containing complexes are indicated. (B) Quantitation of apoptosis in reovirus-infected p50+/+ and p50−/− cells. Cells (2.5 × 104) were either mock infected or infected with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for 48 h, the cells were stained with acridine orange. (C) Quantitation of apoptosis in TNF-α-treated p50+/+ and p50−/− cells. Cells (2.5 × 104) were either untreated or treated with 20 ng of TNF-α per ml. After incubation at 37°C for 24 h, the cells were stained with acridine orange. The results of the experiments in panels B and C are expressed as the mean percentage of cells undergoing apoptosis in three independent experiments. Error bars indicate standard error of the mean.
FIG. 7.
(A) NF-κB gel shift activity in reovirus-infected p65+/+ and p65−/− immortalized fibroblast cells. Cells (5 × 106) were either mock infected or infected with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for 8 h, nuclear extracts were prepared and incubated with a 32P-labeled DNA probe consisting of the NF-κB consensus sequence. Incubation mixtures were resolved by acrylamide gel electrophoresis, dried, and exposed to film. NF-κB-containing complexes are indicated. (B) Quantitation of apoptosis in reovirus-infected p65+/+ and p65−/− cells. Cells (2.5 × 104) were either mock infected or infected with T3D at an MOI of 100 PFU per cell. After incubation at 37°C for 48 h, the cells were stained with acridine orange. (C) Quantitation of apoptosis in TNF-α-treated p65+/+ and p65−/− cells. Cells (2.5 × 104) were either untreated or treated with 20 ng of TNF-α per ml. After incubation at 37°C for 24 h, the cells were stained with acridine orange. The results of the experiments in panels B and C are expressed as the mean percentage of cells undergoing apoptosis in three independent experiments. Error bars indicate standard error of the mean.
To determine whether reovirus is capable of inducing apoptosis in the mutant cell lines, p50+/+, p50−/−, p65+/+, and p65−/− cells were either mock infected or infected with T3D at an MOI of 100 PFU per cell. Apoptosis was assessed using acridine orange staining 48 h after infection (Fig. 6B and 7B). Reovirus infection of both p50+/+ and p65+/+ cell lines resulted in apoptosis of approximately 25% of cells. However, only 5% of p50−/− cells and 1% of p65−/− cells were apoptotic following infection. Although apoptosis was not abolished in p50-deficient cells, the levels of apoptosis were significantly reduced in comparison to those in p50-expressing control cells (P = 0.013). This result suggests that p50 serves as an enhancer of reovirus-induced apoptosis but is not absolutely required for this effect. Reovirus infection of p65-deficient cells resulted in levels of apoptosis indistinguishable from those of mock-infected cells (P = 0.78), which indicates a strict requirement for p65 in the signaling pathway that results in apoptosis following reovirus infection. Differences in p65+/+ and p65−/− cell apoptosis induced by reovirus were highly statistically significant (P = 0.01).
A previous study of the p50−/− and p65−/− cell lines demonstrated that p65 but not p50 is required to inhibit apoptosis induced by TNF-α (6), the opposite effect observed with reovirus infection. To confirm that p65−/− cells but not p50−/− cells are more sensitive to TNF-α-induced cell death, the null and control cell lines were either not treated or treated with TNF-α (Fig. 6C and 7C). TNF-α treatment induced apoptosis of p65−/− cells but did not alter the viability of p50−/− or control cell lines. These results provide strong genetic evidence that both p50 and p65 are critical for mediating the apoptotic response triggered by reovirus infection and support the idea that reovirus and TNF-α engage NF-κB in fundamentally different ways to influence stimulus-induced cell death.
Growth of reovirus is diminished in cell lines deficient for p50 and p65.
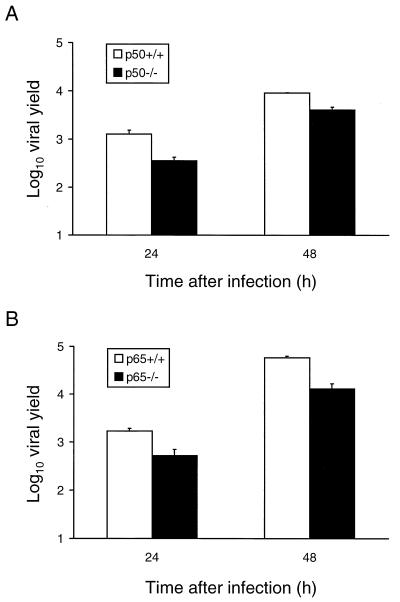
For some viruses, induction of apoptosis may lead to the activation of cellular signaling molecules required to render a cell fully permissive for virus replication. Apoptosis may also facilitate virus release and dissemination from infected cells, resulting in an increase in viral progeny. To determine whether NF-κB family members are required for maximal viral replication in cultured cells, yields of reovirus were determined after viral growth in the p50−/− and p65−/− cell lines and their respective p50+/+ and p65+/+ littermate control cell lines (Fig. 8). Cells were infected with T3D at an MOI of 1 PFU per cell, and viral yields were determined 24 and 48 h after infection. T3D replicated efficiently in all four cell lines; however, viral yields in the control cell lines were two- to fivefold greater, after 24 or 48 h of viral growth, than were the yields in their respective null cell lines. In p50+/+ cells, T3D produced yields of approximately 1,300 and 9,100 progeny virions per input 24 and 48 h following infection, respectively. However, in p50−/− cells, the yields of T3D were reduced to approximately 350 and 3,900 progeny per input 24 and 48 h following infection, respectively. Similarly, in p65+/+ cells, T3D produced yields of approximately 1,700 and 57,500 progeny per input 24 and 48 h following infection, respectively. However, in p65−/− cells, the yields of T3D were reduced to approximately 500 and 12,900 progeny per input 24 and 48 h following infection, respectively. Similar two- to fivefold reductions in viral yields were observed in the p50−/− and p65−/− cell lines relative to the control cell lines when cells were infected at an MOI of 100 PFU per cell (data not shown). These results suggest that expression of p50 and p65 confers a modest viral growth advantage. The capacity of p50+/+ and p65+/+ cells to undergo apoptosis following reovirus infection may directly enhance viral replication, or expression of p50 and p65 may allow a more permissive cellular environment to achieve maximal viral growth.
FIG. 8.
Growth of reovirus in p50−/− and p65−/− immortalized fibroblast cells. p50+/+ and p50−/− cells (A) or p65+/+ and p65−/− cells (B) (2.5 × 104 cells per experiment) were infected with T3D at an MOI of 1 PFU per cell. After adsorption for 1 h, the inoculum was removed, fresh medium was added, and the cells were incubated at 37°C for 0, 24, or 48 h. The cells were frozen and thawed twice, and viral titers were determined by a plaque assay. The results are presented as the mean viral yields (viral titer at 24 or 48 h divided by viral titer at 0 h) in three independent experiments. Error bars indicate standard error of the mean.
DISCUSSION
Mammalian reoviruses have served as a useful experimental system for studies of viral pathogenesis, and studies of these viruses have provided important insights into how viruses interact with host cells (68). In this study, we demonstrate that NF-κB is activated following infection of cultured cells with reovirus. This conclusion is supported by two lines of evidence. First, reovirus infection of HeLa cells and immortalized cell lines derived from murine embryonic fibroblasts leads to nuclear translocation of NF-κB complexes containing the p50 and p65 subunits. Second, reovirus infection of HeLa cells induces NF-κB-directed expression of a luciferase reporter gene. Maximal luciferase activity follows the peak of NF-κB gel shift activity, as would be expected to allow NF-κB-directed gene expression. Thus, reovirus infection is capable of functional activation of NF-κB.
Our results using proteasome inhibitor Z-L3VS and transient expression of mutant forms of IκBα suggest that reovirus-induced activation of NF-κB involves targeted degradation of IκBα by the 26S proteasome. However, the precise mechanism by which reovirus activates NF-κB remains unknown. We consider it unlikely that NF-κB activation is triggered solely by attachment of the virus to its cognate cellular receptor, because peak NF-κB activity follows reovirus adsorption by several hours. Activation of NF-κB in response to physiologic receptor-ligand interactions occurs with more rapid kinetics (66). Therefore, we suspect that reovirus transcription or translation is a prerequisite for access to the host NF-κB pathway, which is more consistent with the delayed response observed in our studies. It is also possible that reovirus infection induces a soluble factor that mediates activation of NF-κB, which also would account for the delay in NF-κB activation.
Prior studies have firmly established that NF-κB activation can be achieved by a broad spectrum of biochemical inducing cues, resulting in either enhancement (1, 29, 32) or inhibition (6, 8, 65) of programmed cell death (reviewed in reference 55). Using a proteasome inhibitor and a trans-dominant inhibitor of NF-κB, we demonstrated that interference with the NF-κB pathway leads to inhibition of reovirus-induced apoptosis. These findings strongly suggest that NF-κB enhances apoptosis in response to reovirus infection. In support of this contention, cell lines deficient for either p50 or p65, the primary constituents of NF-κB complexes activated by reovirus, are significantly more resistant to reovirus-induced apoptosis than are control cell lines. In fact, fibroblasts deficient in p65 expression do not undergo apoptosis in response to reovirus infection over an observation period of 48 h. These results provide compelling evidence that NF-κB plays an essential role in the mechanism by which reovirus triggers an apoptotic program in infected cells.
In contrast to our finding that NF-κB functions as a proapoptotic factor during reovirus infection of cultured cells, NF-κB plays an antiapoptotic role in cells treated with TNF-α (6, 35, 65, 69) (Fig. 4 to 7). Engagement of the TNF receptor by TNF-α induces protein-protein interactions that lead directly to the activation of NF-κB (30), which results in inhibition of apoptosis (6, 35, 65, 69). Since activation of NF-κB by reovirus is not likely to occur directly following receptor ligation, it is possible that the mechanism that promotes activation of NF-κB by reovirus explains its proapoptotic effects. Alternatively, reovirus infection and TNF-α receptor engagement may induce different auxiliary factors that influence the effects of NF-κB within a given cell type.
The requirement for NF-κB activation in reovirus-induced apoptosis suggests that NF-κB functions to increase the expression of proapoptotic genes. Several genes encoding proteins involved in mediating apoptosis induced by a variety of stimuli are regulated by NF-κB and contain NF-κB response elements in their promoters. Such NF-κB-responsive proapoptotic proteins include p53 (70), caspase-1 (14), and FasL (58). Activation of NF-κB following reovirus infection may induce the expression of one or more of these genes or other proapoptotic genes, which include death receptors and their ligands, such as DR4, DR5, and TRAIL; effector or initiator caspases, such as caspase-3 and caspase-9; and prodeath Bcl-2 family members, such as Bax, Bik, and Bad. In a previous study, we demonstrated that inhibitors of calpain, a calcium-dependent papain-like cysteine protease, block reovirus-induced apoptosis (21). NF-κB may function to upregulate the expression of calpain activator proteins (22, 48, 53) or growth factors (15, 40), which have been shown to increase calpain activity. NF-κB also may be involved in regulating genes that control cellular calcium flux, which is required for calpain activation (57). It is also possible that NF-κB induces the expression of transcription factors that in turn augment the transcription of proapoptotic genes not directly under the control of NF-κB.
Why would reovirus activate NF-κB? One possibility is that induction of apoptosis of infected cells would reduce host inflammatory responses, potentially leading to increased dissemination of the virus. Thus, viruses capable of this response would have a clear selective advantage. Given the well-established role of NF-κB in signal-induced cell growth pathways (reviewed in reference 66), a second possibility is that activation of NF-κB produces a cellular environment that is more permissive for reovirus replication. Reovirus yields are substantially higher in rapidly dividing or transformed cells (24, 56, 59), which suggests that cellular factors associated with cell growth augment viral replication. Reovirus-induced NF-κB activation might lead to expression of growth-associated cellular genes that promote more efficient viral nucleic acid or protein synthesis, intracellular transport of viral proteins, or assembly and release of progeny virions. In support of this idea, we found that reovirus yields are decreased in both p50−/− and p65−/− cells relative to control cells.
Other viruses induce NF-κB activation (13, 34, 37, 64, 72), and in some cases NF-κB is required for maximal viral replication. For instance, the long terminal repeat of human immunodeficiency virus contains κB response elements, and activation of NF-κB directly stimulates viral gene expression (16, 18). The human T-cell leukemia virus Tax protein induces NF-κB activation (9, 72), which in turn induces the expression of cellular genes that promote human T-cell leukemia virus replication (5, 28, 33, 39). Thus, the NF-κB signaling pathway may be a common pathway by which viruses confer an optimum environment to achieve their replication.
The results reported here establish that NF-κB is activated following reovirus infection and demonstrate that activation of NF-κB is required for reovirus-induced apoptosis. Most RNA-containing viruses, such as reovirus, are thought to replicate independently of the nucleus. Our results, however, clearly show that infection with an RNA-containing cytoplasmic virus triggers a signal transduction pathway involving nuclear components, which leads to cellular gene expression. Activation of this signaling pathway is critical for cell death caused by reovirus and probably contributes to reovirus-induced pathology (42; DeBiasi et al., Abstr. Am. Soc. Virol. 18th Annu. Meet. 1999). Understanding the signaling pathways used by reovirus to induce cellular gene expression and apoptosis will contribute important new information about mechanisms by which viruses produce cell death and disease.
ACKNOWLEDGMENTS
We thank David Baltimore for the NF-κB null and control cell lines and Hidde Ploegh for the proteasome inhibitor. We are grateful to Zhi-Liang Chu, David Scherer, and Alexander Hoffman for expert advice. We thank Erik Barton, Jim Chappell, and Tibor Valyi-Nagy for careful review of the manuscript.
This work was supported by Public Health Service awards T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical Scientist Training Program (S.E.R.), AI33839 from the National Institutes of Allergy and Infectious Diseases (D.W.B.), AG14071 from the National Institute on Aging (K.L.T.), and AI38296 from the National Institute of Allergy and Infectious Diseases (J.L.C. and T.S.D.); Department of Veterans Affairs Merit and REAP Awards (K.L.T.); U.S. Army grant DAMD17-98-1-8614 (K.L.T.); and the Elizabeth B. Lamb Center for Pediatric Research (J.L.C. and T.S.D.). Additional support was provided by Public Health Service awards CA68485 for the Vanderbilt Cancer Center and DK20593 for the Vanderbilt Diabetes Research and Training Center.
REFERENCES
- 1.Abbadie C, Kabrun N, Bouali F, Vandenbunder B, Enrietto P. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell. 1993;75:899–912. doi: 10.1016/0092-8674(93)90534-w. [DOI] [PubMed] [Google Scholar]
- 2.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P, Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle P, Baltimore D. A 65-kD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes Dev. 1989;3:1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 5.Ballard D W, Bohnlein E, Lowenthal J W, Wano Y, Franza B R, Greene W C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988;241:1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- 6.Beg A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 7.Beg A A, Ruben S M, Scheinman R I, Haskill S, Rosen C A, Baldwin A J. IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 8.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 9.Beraud C, Greene W C. Interaction of HTLV-1 Tax with human proteasome: implications for NF-kappa B induction. J Acquired Immunodefic Syndr. 1996;13(Suppl. 1):S76–S84. doi: 10.1097/00042560-199600001-00014. [DOI] [PubMed] [Google Scholar]
- 10.Bogyo M, McMaster J, Gaczynska M, Tortorella D, Goldberg A, Ploegh H. Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 13.Bussfeld D, Bacher M, Mortiz A, Gemsa D, Sprenger H. Expression of transcription factor genes after influenza A virus infection. Immunobiology. 1997;198:291–298. doi: 10.1016/S0171-2985(97)80049-6. [DOI] [PubMed] [Google Scholar]
- 14.Casano F, Rolando A, Mudgett J, Molineaux S. The structure and complete nucleotide sequence of the murine gene encoding interleukin-1 beta converting enzyme (ICE) Genomics. 1994;20:474–481. doi: 10.1006/geno.1994.1203. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti A K, Neuberger T, Russell T, Banik N L, DeVries G H. Immunolocalization of cytoplasmic and myelin mCalpain in transfected Schwann cells. II. Effect of withdrawal of growth factors. J Neurosci Res. 1997;47:609–616. doi: 10.1002/(sici)1097-4547(19970315)47:6<609::aid-jnr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Chen B K, Feinberg M B, Baltimore D. The kappaB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–5504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1585–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 18.Chene L, Nugeyre M T, Barre-Sinoussi F, Israel N. High-level replication of human immunodeficiency virus in thymocytes requires NF-kappaB activation through interaction with thymic epithelial cells. J Virol. 1999;73:2064–2073. doi: 10.1128/jvi.73.3.2064-2073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesnut J, Baytan A, Russell M, Chang M, Bernard A, Maxwell I, Hoeffler J. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- 21.DeBiasi R L, Squier M K T, Pike B, Wynes M, Dermody T S, Cohen J J, Tyler K L. Reovirus-induced apoptosis is preceded by increased cellular calpain activity and is blocked by calpain inhibitors. J Virol. 1999;73:695–701. doi: 10.1128/jvi.73.1.695-701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMartino G N, Blumenthal D K. Identification and partial purification of a factor that stimulates calcium-dependent proteases. Biochemistry. 1982;21:4297–4303. doi: 10.1021/bi00261a019. [DOI] [PubMed] [Google Scholar]
- 23.Duke R C, Cohen J J. Morphological and biochemical assays of apoptosis. In: Coligan J E, editor. Current Protocols in Immunology. New York, N.Y: Wiley & Sons; 1992. pp. 17.1–17.16. [Google Scholar]
- 24.Duncan M R, Stanish S M, Cox D C. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol. 1978;28:444–449. doi: 10.1128/jvi.28.2.444-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst H, Shatkin A J. Reovirus hemagglutinin mRNA codes for two polypeptides in overlapping reading frames. Proc Natl Acad Sci USA. 1985;82:48–52. doi: 10.1073/pnas.82.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furlong D B, Nibert M L, Fields B N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghosh S, Gifford A, Riviere L, Tempst P, Nolan G, Baltimore D. Cloning of the p50 DNA binding subunit of NF-κB: homology to rel and dorsal. Cell. 1990;62:1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- 28.Greene W C, Bohnlein E, Ballard D W. HIV-1, HTLV-1 and normal T-cell growth: transcriptional strategies and surprises. Immunol Today. 1989;10:272–278. doi: 10.1016/0167-5699(89)90141-2. [DOI] [PubMed] [Google Scholar]
- 29.Grimm S, Bauer M K A, Baeuerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell. 1995;82:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs B L, Samuel C E. Biosynthesis of reovirus-specified polypeptides: the reovirus S1 mRNA encodes two primary translation products. Virology. 1985;143:63–74. doi: 10.1016/0042-6822(85)90097-2. [DOI] [PubMed] [Google Scholar]
- 32.Jung M, Zhang Y, Lee S, Dritschilo A. Correction of radiation sensitivity in ataxia telangiectasia cells by a truncated IκB-α. Science. 1995;268:1619–1621. doi: 10.1126/science.7777860. [DOI] [PubMed] [Google Scholar]
- 33.Leung K, Nabel G J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988;333:776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- 34.Lin K I, Lee S H, Narayanan R, Baraban J, Hardwick J, Ratan R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kappa B. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z-G, Hsu H, Goeddel D, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 36.Lum R, Kerwar S, Meyer S, Nelson M, Schow S, Shiffman D, Wick M, Joly A. A new structural class of proteasome inhibitors that prevent NF-kappa B activation. Biochem Pharmacol. 1998;55:1391–1397. doi: 10.1016/s0006-2952(97)00655-2. [DOI] [PubMed] [Google Scholar]
- 37.Marianneau P, Cardona A, Edelman L, Deubel V, Despres P. Dengue virus replication in human hepatoma cells activates NF-κB, which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May M J, Ghosh S. Rel/NF-kappa B and I kappa B proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 39.Mesnard J M, Devaux C. Multiple control levels of cell proliferation by human T-cell leukemia virus type 1 Tax protein. Virology. 1999;257:277–284. doi: 10.1006/viro.1999.9685. [DOI] [PubMed] [Google Scholar]
- 40.Neuberger T, Chakrabarti A K, Russell T, DeVreis G H, Hogan E L, Banik N L. Immunolocalization of cytoplasmic and myelin mCalpain in transfected Schwann cells. I. Effect of treatment with growth factors. J Neurosci Res. 1997;47:521–530. [PubMed] [Google Scholar]
- 41.Nibert M L, Schiff L A, Fields B N. Reoviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1557–1596. [Google Scholar]
- 42.Oberhaus S M, Smith R L, Clayton G H, Dermody T S, Tyler K L. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J Virol. 1997;71:2100–2106. doi: 10.1128/jvi.71.3.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien V. Viruses and apoptosis. J Gen Virol. 1998;79:1833–1845. doi: 10.1099/0022-1317-79-8-1833. [DOI] [PubMed] [Google Scholar]
- 44.Palombella V, Rando O, Goldberg A, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 45.Razvi E S, Welsh R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 46.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers S E, Connolly J L, Chappell J D, Dermody T S. Reovirus growth in cell culture does not require the full complement of viral proteins: identification of a ς1s-null mutant. J Virol. 1998;72:8597–8604. doi: 10.1128/jvi.72.11.8597-8604.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salamino F, DeTullio R, Mengotti P, Viotti P L, Melloni E, Pontremoli S. Site-directed activation of calpain is promoted by a membrane-associated natural activator protein. Biochem J. 1993;290:191–197. doi: 10.1042/bj2900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 50.Sarkar G, Pelletier J, Bassel-Duby R, Jayasuriya A, Fields B N, Sonenberg N. Identification of a new polypeptide coded by reovirus gene S1. J Virol. 1985;54:720–725. doi: 10.1128/jvi.54.3.720-725.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 53.Shiba R, Ariyoshi H, Yano Y, Kawasaki T, Sakon M, Kambayashi J, Mori T. Purification and characterization of a calpain activator from human platelets. Biochem Biophys Res Commun. 1992;182:461–465. doi: 10.1016/0006-291x(92)91754-e. [DOI] [PubMed] [Google Scholar]
- 54.Smith R E, Zweerink H J, Joklik W K. Polypeptide components of virions, top components and cores of reovirus type 3. Virology. 1969;39:791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- 55.Sonenshein G E. Rel/NF-κB transcription factors and the control of apoptosis. Semin Cancer Biol. 1997;8:113–119. doi: 10.1006/scbi.1997.0062. [DOI] [PubMed] [Google Scholar]
- 56.Strong J E, Lee P W. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70:612–616. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K, Saido T C, Hirai S. Modulation of cellular signals by calpain. Ann N Y Acad Sci. 1992;674:218–227. doi: 10.1111/j.1749-6632.1992.tb27490.x. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 59.Taterka J, Sugcliffe M, Rubin D H. Selective reovirus infection of murine hepatocarcinoma cells during cell division. A model of viral liver infection. J Clin Investig. 1994;94:353–360. doi: 10.1172/JCI117329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyler K L, Fields B N. Reoviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1597–1623. [Google Scholar]
- 63.Tyler K L, Squier M K T, Rodgers S E, Schneider B E, Oberhaus S M, Grdina T A, Cohen J J, Dermody T S. Differences in the capacity of reovirus strains to induce apoptosis are determined by viral attachment protein ς1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umansky V, Shatrov V A, Lehmann V, Schirrmacher V. Induction of NO synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor-kappa B. Int Immunol. 1996;8:491–498. doi: 10.1093/intimm/8.4.491. [DOI] [PubMed] [Google Scholar]
- 65.Van Antwerp D, Martin S, Kafri T, Green D, Verma I. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 66.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and disassociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 67.Virgin H W, IV, Bassel-Duby R, Fields B N, Tyler K L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Virgin H W, Tyler K L, Dermody T S. Reovirus. In: Nathanson N, editor. Viral pathogenesis. New York, N.Y: Lippincott-Raven; 1997. pp. 669–699. [Google Scholar]
- 69.Wang C-Y, Mayo M, Baldwin A. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 70.Wu H, Lozano G. NF-kappa B activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem. 1994;269:20067–20074. [PubMed] [Google Scholar]
- 71.Wyllie A H. Apoptosis: an overview. Br Med Bull. 1997;53:451–465. doi: 10.1093/oxfordjournals.bmb.a011623. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida M. Molecular biology of HTLV-I: recent progress. J Acquired Immune Defic Syndr. 1996;13(Suppl. 1):S63–S68. doi: 10.1097/00042560-199600001-00012. [DOI] [PubMed] [Google Scholar]