Abstract
The quantitative relationship between the disruption of the blood-brain barrier (BBB) and the recruitment of glial cells was explored in a mouse model of endotoxemia. [18F]2-Fluoro-2-deoxy-sorbitol ([18F]FDS) PET imaging was used as a paracellular marker for quantitative monitoring of BBB permeability after i.v injection of increasing doses of lipopolysaccharide (LPS) or vehicle (saline, n = 5). The brain distribution of [18F]FDS (VT, mL.cm−3) was estimated using kinetic modeling. LPS dose-dependently increased the brain VT of [18F]FDS after injection of LPS 4 mg/kg (5.2 ± 2.4-fold, n = 4, p < 0.01) or 5 mg/kg (9.0 ± 9.1-fold, n = 4, p < 0.01) but not 3 mg/kg (p > 0.05, n = 7). In 12 individuals belonging to the different groups, changes in BBB permeability were compared with expression of markers of astrocyte (GFAP) and microglial cell (CD11b) using ex vivo immunohistochemistry. Increased expression of CD11b and GFAP expression was observed in mice injected with 3 mg/kg of LPS, which did not increase with higher LPS doses. Quantitative [18F]FDS PET imaging can capture different levels of BBB permeability in vivo. A biphasic effect was observed with the lowest dose of LPS that triggered neuroinflammation without disruptive changes in BBB permeability, and higher LPS doses that increased BBB permeability without additional recruitment of glial cells.
Keywords: Blood-brain barrier, neuroinflammation, neurovascular unit, permeability marker, positron emission tomography
Introduction
The blood-brain barrier (BBB) is composed of brain capillary endothelial cells sealed by tight junctions, which protect the brain parenchyma from potentially harmful substances in circulation. 1 Endothelial cells are central to the neurovascular unit (NVU), a complex module of functionally interconnected cells that control brain homeostasis and mediate the neuro-vascular coupling. 2 Within the NVU, the function of endothelial cells is regulated by adjacent cells including microglia, astrocytes, pericytes, and neurons. 3 Communication between these cells mediates the coupling between peripheral and CNS immunity and controls neuroimmune function.4,5 As a consequence, the NVU plays a key role in the development of neuroinflammation in many CNS diseases.6,7
Common features of neuroinflammation include BBB disruption, through dysregulation of tight-junctions, together with activation of endothelial cells and recruitment/activation of glial cells, including astrocytes and microglia.8,9 It is widely assumed that the BBB prevents immune cells and mediators of peripheral immunity from accessing the CNS and trigger neuroinflammation.10,11 However, endotoxemia induced by the presence of pro-inflammatory bacterial lipopolysaccharide (LPS) in circulation, ultimately leads to inflammation in both the periphery and brain.12,13 Systemic injection of purified LPS is widely used as a model of “sterile inflammation” to recapitulate the known features of neuroinflammation in vivo.14 –16 This suggests that BBB disruption may be essential for the propagation of such blood-born inflammatory processes into the brain, even though the exact sequence of this process has not yet been precisely elucidated. 17 In this context, changes in the endothelium-microglia or endothelium-astrocyte interactions have been shown to play a role.18,19 However, to the best of our knowledge, the impact of increasing doses of LPS on the expression of classical markers of gliosis with respect to concurrent levels of BBB permeability has not been reported.
There are considerable efforts to develop minimally-invasive neuroimaging methods to describe the molecular contexture of neuroinflammation in the living brain. So far studies mainly focused on markers of glial cell density/activation, or endothelial cell activation.8,20,21 In this context, there is a crucial need for quantitative imaging markers to evaluate the status of the BBB during neuroinflammatory processes.22,23 Recently, the fluorinated derivative [18F]2-fluoro-2-deoxy-sorbitol ([18F]FDS) has been repurposed as a quantitative marker of BBB permeability using in vivo positron emission tomography (PET) imaging. 24
In the present study, [18F]FDS PET imaging was performed in a mouse model of endotoxemia to investigate its potential to unveil and quantify BBB permeability after administration of increasing doses of LPS. Changes in BBB permeability, assessed in vivo, were interpreted in the light of the recruitment of glial cells assessed ex vivo in the same individuals.
Methods
Animals
Forty-three male C57BL/6J mice (age 8 weeks, obtained from Janvier, France) were used in this study. All animal of the procedures were used in accordance with the recommendations of the European Community for the care and use of laboratory animals (2010/63/UE) and the French National Committees (French Decret 2013–118). The experimental protocol has been approved by a local ethics committee (Comité d’Ethique en Expérimentation Animale, CEEA n° 44) and the French government (authorization APAFIS#16293-2018072609593031).
Mice were housed in polycarbonate cages (5 individuals per cage) with wood chip bedding and some environmental enrichment. Cages were stored at room temperature (RT, 22°C) and 40% humidity under a regular 12 h dark/light cycle. Mice had access to food and water ad libitum. Caloric and hydration support was added using nutritional hydrogel. All animals were acclimatized for at least 1 week before any experimentation. Animal data are reported in compliance with the ARRIVE guidelines 2.0.
Study design
Given the mortality seen in previous studies using the LPS model of endotoxemia in mice, we aimed for initial group sizes of 9 to 10 individuals. 25 Mice were administered a single intraperitoneal (i.p.) dose of increasing dose of LPS (Escherichia coli O111:B4, Sigma-Aldrich, France) at 3 mg/kg (n = 10), 4 mg/kg (n = 10) or 5 mg/kg (n = 9). In parallel to LPS-treated animals, some mice were injected with sterile saline (vehicle, n = 9 mice in total). Animals were then individually assessed in terms of weight, activity, and general appearance.
Twenty-four hours after LPS injection, surviving mice underwent [18F]FDS PET imaging for determination of BBB permeability. Each imaging session consisted of scanning a maximum of 6 mice from a single radiotracer production, to limit the volume of [18F]FDS to be i.v injected (<150 µL). As a consequence, not all available mice could be scanned 24 h after LPS injection. Animals undergoing PET imaging were randomly selected among surviving individuals. Additional animals (not scanned) were kept alive and monitored for weight and survival analysis. Control mice (vehicle group) were scanned in parallel to LPS-treated mice as part of different imaging sessions to test the repeatability of PET data in mice with intact BBB with respect to the different batches of [18F]FDS. The minimum number of scanned mice per group (n = 4) was set based on the previously reported sensitivity of [18F]FDS PET, and taking the significant mortality observed with the highest doses of LPS into account. 24 After PET acquisition, mice were placed back in their cages.
The day after (to allow for radioactive decay), all surviving animals of the LPS 4 mg/kg and 5 mg/kg groups, 3 animals of the LPS 3 mg/kg group, and 2 control mice were euthanized and their brains removed for ex vivo determination glial cell density, using immunohistochemistry. Five additional mice underwent [18F]FDS PET imaging 48 h after administration of 3 mg/kg of LPS to investigate BBB permeability at the time of immunohistochemistry.
[18F]FDS PET imaging
[18F]FDS was easily synthesized from the chemical reduction of commercially available [18F]2-fluoro-2-deoxy-glucose ([18F]FDG) as previously described.24,26 The radiochemical purity of [18F]FDS observed by radio-thin-layer chromatography analysis was 100 ± 0% (n = 6), confirming the absence of detectable [18F]FDG in the preparation.
Mice were anesthetized with 2–2.5% isoflurane in O2/air (50/50, v/v). A catheter was then inserted into the tail vein and anesthetized mice were transferred to the µPET scanner. [18F]FDS was administered i.v (6.7 ± 0.6 MBq) using a microinjection pump at 0.3 mL.min−1 during 60 s. All animals were maintained under anesthesia by isoflurane during experiments. A 30 min dynamic whole-body PET acquisition started with radiotracer injection.
Images were acquired on cross-calibrated Inveon microPET/CT or Inveon microPET scanners (Siemens, Knoxville, TN, USA). After each PET scan, a CT scan or a transmission scan was performed for photon attenuation correction. PET images were reconstructed using the 3-dimensional ordered subset expectation maximization with maximum a posteriori algorithm (3 D OSEM/MAP) and corrected for attenuation, random coincidences, and scattering. Data visualization and analysis were performed using PMOD 4.2 (PMOD Technologies LLC, Switzerland). The persons in charge of imaging data analysis were blinded to the group allocation. As a volume of interest (VOI), a spheroid (4 mm length, 1.5 mm width) was positioned in the middle of the brain. A sphere (1.2 mm radius) was positioned on the left ventricle of the heart (blood pool), which was obvious on early time-frames, to generate an image-derived input function (IDIF) (Figure 1). The total volume of the urinary bladder, which was obvious at the latest time frames, was delineated to estimate the urinary clearance (Figure 1). 27 Images were normalized by injected dose and animal weight to be expressed in standardized uptake value (SUV). Time-activity curves (TACs) were then generated. Kinetic modeling of dynamic 30 min PET data was performed using the Logan graphical model to estimate the total volume of distribution of [18F]FDS (VT, mL.cm−3), which describes the brain/blood ratio at equilibrium.24,28 The urinary clearance (mL.min−1) of [18F]FDS was estimated by the total amount of radioactivity in the bladder at the end of PET (kBq), divided by the area under the time-activity curve (AUC, kBq.mL−1.min) of the IDIF.
Figure 1.
Delineation of region of interest in representative [18F]FDS PET images obtained in a control (saline) mouse. These are SUV-normalized PET images. The white arrows indicate the volumes of interest used to delineate the brain (a), the heart blood pool (b), or the urinary bladder (c).
Immunohistochemistry
Resection of the mouse brains for ex vivo immunohistochemistry was performed 24 h after injection of [18F]FDS (i.e. 48 h after injection of LPS) to allow for radioactive decay and avoid radiation exposure during this invasive procedure. LPS-treated mice (n = 3, 4, and 3 for the groups treated with 3, 4, and 5 mg/kg of LPS, respectively), as well as 2 control mice, were euthanized using an overdose of pentobarbital (180 mg/mg; i.p). Mice were then immediately perfused with a large volume of saline (intracardiac perfusion) until complete removal of the circulating blood. Animals were then decapitated and their brains were removed. Resected brains were cryo-frozen using isopentane and liquid nitrogen and stored at −80°C until use. The frozen brains were cut in coronal sections (14 µm thick slices) using a cryostat. For each brain, 3 adjacent slices focused on the hippocampus were investigated. Mouse brain sections were fixed for 15 min at RT in 4% paraformaldehyde (PFA). To block the PFA, the slides were incubated for 5 min RT in PBS containing 50 mM of NH4Cl, and tissue permeabilization was performed with methanol-acetone at a 1:1 volume ratio (−20°C, 5 min) followed by Triton 0.1% in PBS for 5 min at RT. Several washes with PBS were carried out between steps. The non-specific sites were saturated by incubating the slides for 45 minutes at RT in a PBS blocking solution containing 5% BSA (Bovine Albumin Serum) and 0.5% Tween 80. Each slide was incubated for 1 h, in the presence of primary antibodies CD11b (rat anti-mouse Integrin Alpha M, ITGAM 1:500 #ABIN248101, Anticorps-en-ligne, France) and GFAP (chicken anti-rat GFAP IgG 1:500 #AB4674, Abcam) diluted in the blocking solution. After several washes with PBS, to remove excess antibodies, the slides are incubated for 45 minutes at RT with the appropriate secondary antibodies (Alexa Fluor 546 goat anti-rat IgG and Alexa Fluor 647 goat anti-chicken IgG; 1:1000, Invitrogen) in blocking solution. Finally, all the slides are rinsed again, then covered with a mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) for labeling the nuclei (ProLongTM Diamond Antifade Mountant with DAPI, Invitrogen) before sealing with coverslips. The sections were observed using a Zeiss AxioCam fluorescence microscope (Carl Zeiss, Germany). Counting of activated glial cells relative to the total number of nuclei was performed on 8 square areas (0.323 × 0.323 µm) located in the hippocampus of each slide using the ImageJ software. 29 The same fluorescence parameters, including the removal of background noise, were applied for all brain slices. The number of CD11b- and GFAP-positive cells relative to the number of nuclei in the same area is reported as a percentage.
Statistical analysis
All results are displayed as mean ± S.D. Statistical analyses and graphs were performed using GraphPad Prism software (version 9.5, La Jolla, CA, USA) or R. 30 The intra-group variability of outcome parameters was estimated by the coefficient of variability (CV, %). Brain VT, blood exposure, and urinary clearance obtained in each group were compared using an ANOVA on Log-transformed data, followed by a Tukey’s post-hoc test. The Log-transformed data were checked for normality of the residuals (Shapiro-Wilk test, p > 0.05) and homoscedasticity (Levene’s test, p > 0.05). The level of significance was set at p < 0.05. Individual brain VT values and the corresponding expression of CD11b and GFAP obtained in each individual were plotted. A nonlinear regression analysis of the data using a one-exponential equation was tested.
Results
No mortality was observed in the vehicle group. However, administration of LPS was associated with significant mortality. The lowest survival was observed in the 5 mg/kg (78% survival on day 1, 34.5% on day 2) whereas survival was similar in the 3 and 4 mg/kg groups (∼90% on day 1, ∼70% on day 2, Supp. Fig. S1A). Weight loss was significant in all groups of LPS-treated mice (p < 0.05) but not in the vehicle group. Maximal weight loss was observed at 48 h after LPS injection, immediately before euthanasia, and was −23.4 ± 3.5% in the 3 mg/kg group, −17.0 ± 2.6% in the 4 mg/kg group, and −17.6 ± 17.22% in the 5 mg/kg group (Supp. Fig. S1B).
Baseline PET images observed in the vehicle group confirmed the negligible BBB penetration of [18F]FDS in vehicle-treated mice. Higher brain PET signal was observed in mice injected with 4 or 5 mg/kg of LPS, but not in animals injected with the lowest dose of LPS (3 mg/kg) (Figures 1 and 2).
Figure 2.
Representative [18F]FDS PET images obtained in the mouse brain 24 h after injection of either saline (vehicle) or lipopolysaccharide (LPS 3, 4, or 5 mg/kg). These are summed (0–30 min) and SUV-normalized PET images.
LPS-induced endotoxemia was associated with a significant increase in [18F]FDS blood exposure for the 5 mg/kg group only (p < 0.05) when compared to vehicle, while no such increase was yet observed at the lower doses (Figure 3). [18F]FDS is predominantly eliminated by renal filtration. 31 A dose-dependent decrease in the urinary clearance was observed and was significant for all tested dose of LPS (Figure 3).
Figure 3.
PET kinetics of [18F]2-fluoro-2-deoxy-sorbitol ([18F]FDS) in mice treated with a dose range of lipopolysaccharide (LPS). The kinetics of radioactivity in the brain, blood pool, and urinary bladder are shown in a, c, and e, respectively. Kinetic modeling was performed to estimate the total brain distribution of (VT, in b), the blood exposure (in d), and the urinary clearance (in f) of [18F]FDS in mice treated with either saline (vehicle, n = 5) or LPS 3 mg/kg (n = 7), LPS 4 mg/kg (n = 4), or LPS 5 mg/kg (n = 4). Data are mean ± S.D. Statistical significance is shown as *p < 0.05, **p < 0.01, ***p < 0.001, and ns: non-significant.
Kinetic modeling was performed using the Logan analysis to estimate the brain VT, which takes any change in the peripheral kinetics of [18F]FDS into account. Representative Logan plots obtained in each group are shown in Supp. Fig. S2. Compared with the vehicle group (n = 5), enhanced BBB permeability was observed after 24 h in LPS-treated animals injected with 4 mg/kg (5.2 ± 2.4-fold increase in VT, n = 4, p < 0.01) and 5 mg/kg (9.0 ± 9.1-fold increase in VT, n = 4, p < 0.01) but not in the 3 mg/kg group, in which the brain VT remained unchanged (n = 7, p > 0.05) (Figure 3). Brain VT of [18F]FDS remained unchanged 48 h after injection of 3 mg/kg of LPS (VT = 0.07 ± 0.01 mL.cm−3, n = 5, p > 0.05). Intra-group variability of brain VT measured 24 h after LPS was CV = 7.4% in the vehicle group, CV = 19.6% in the 3 mg/kg group, CV = 45.6% in the 4 mg/kg group, and CV = 101.3% in the 5 mg/kg group. In the LPS 5 mg/kg group, individual brain VT values ranged from a 1.9 to 10.5-fold increase compared with vehicle, suggesting heterogeneous levels of BBB disruption (Figure 3).
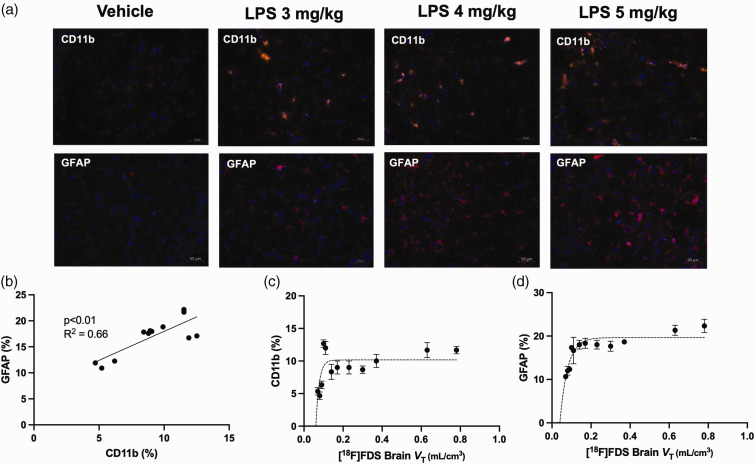
Immunohistochemistry performed in animals from each group confirmed the increased abundance of glial cells associated with endotoxemia (Figure 4). CD11b and GFAP labeling respectively indicate strong microglial as well as astrocytic density in response to LPS treatment. Immunolabelling in vehicle-treated animals showed only minor CD11b-staning and microglia with the typical surveilling/ramified phenotype, whereas LPS treatment showed stronger CD11b staining of activated microglia. Similar results were found for GFAP-labelled astrocytes and a significant correlation was demonstrated between the expression of CD11b and GFAP in the brains of tested mice (R2 = 0.66, p < 0.01, Figure 4(b)). Higher expression of CD11b and GFAP was observed in mice injected with 3 mg/kg of LPS, while integrity of the BBB remained intact, as investigated with [18F]FDS. Higher doses of LPS did not further increase the expression of CD11b and GFAP but significantly increased BBB permeability (i.e brain VT of [18F]FDS), leading to a non-linear correlation between BBB permeability and expression of CD11b and GFAP, respectively (Figure 4(c) and (d)).
Figure 4.
Expression of glial cell markers relative to blood-brain-barrier (BBB) permeability, assessed using [18F]2-fluoro-2-deoxy-sorbitol ([18F]FDS) PET imaging in mice treated with increasing doses of lipopolysaccharide (LPS). The percentage of GFAP and CD11b-positive cells was measured using immunohistochemistry in the brain of 12 mice that received different doses of lipopolysaccharide saline (vehicle, n = 2) or LPS 3 mg/kg (n = 3), LPS 4 mg/kg (n = 4), or LPS 5 mg/kg (n = 3). Representative immunohistochemistry slices obtained in each condition are shown in a. Correlation between expression of CD11b and GFAP, expressed as a percentage of total nuclei from resected brain slices as markers of microglia or astrocytes, respectively, are shown in b. Correlation between the total brain distribution of [18F]FDS (VT), used for quantitative estimation of BBB permeability and corresponding expression CD11b and GFAP obtained in the same individuals are shown in c and d, respectively.
Discussion
In this study, the impact of a dose range of LPS on BBB permeability and the recruitment of glial cells was compared in mice. To this end, [18F]FDS PET imaging was performed as an in vivo marker of BBB permeability, followed by ex vivo immunochemistry targeting microglia (CD11b) and astrocytes (GFAP), as key regulators of inflammatory responses in the CNS. These complementary assessments were performed in the same individuals, thus making the best with the minimally invasive nature of [18F]FDS PET imaging.
In non-clinical in vitro and ex vivo research, low molecular weight (MW, g/mol) hydrophilic molecules such as sucrose (MW = 342 Da) or mannitol (MW = 182) are preferred to estimate the “paracellular” (i.e between-cell pathway) permeability of the BBB with enhanced sensitivity to detect subtle change.32 –34 Translational imaging techniques such as contrast-enhanced MRI using gadolinium (Gd)-based contrast agents such as gadoterate (MW = 558) or Gd-DTPA (Gd-diethylene triamine pentaacetic acid, MW = 546),35,36 and brain SPECT using [99mTc]Tc-DTPA (MW = 487) 37 can be used to detect BBB disruption. However, as compared with MRI or SPECT, PET benefits from absolute quantitative performances so that estimation of BBB permeability using kinetic modeling is possible. 38 So far, the predominant use of MRI and SPECT instead of PET to investigate BBB integrity in animals or humans is likely due to the wide availability of corresponding imaging probes. 39 Consistently, PET imaging using radiolabeled low- or high-MW makers of BBB integrity such as [18F]1-fluoro-1-deoxy-D-mannitol (MW = 183) 40 or [11C]inulin (MW = 6179), 41 which production requires a cyclotron and radiochemistry facilities, did not reach mainstream application in neuroscience research. PET imaging using [18F]FDS (MW = 183 Da) was previously suggested as a quantitative marker of permeability following BBB disruption induced by focused ultrasound. 24 In a translational perspective, [18F]FDS has virtually no uptake in the healthy human brain. Furthermore, data on biodistribution and radiation dosimetry in healthy volunteers indicate that [18F]FDS is safe, well tolerated, and suitable for use in humans. 42 This may justify further clinical evaluation of [18F]FDS for in vivo determination of BBB permeability in the diseased brain.
In the present study, higher brain uptake of [18F]FDS was observed in mice injected with either 4 or 5 mg/kg of LPS, suggesting increased BBB permeability. Moreover, high variability in brain kinetics of [18F]FDS was observed in the group injected with LPS 5 mg/kg compared with other groups. We first hypothesized that enhanced brain PET signal, as well as variability, may be associated with changes in the peripheral kinetics of [18F]FDS. Indeed, injection of LPS is often used as a model of sepsis and induces acute kidney injury and renal failure. 43 [18F]FDS was initially developed as a renal filtration agent and its peripheral kinetics is very sensitive to changes in renal function. 27 ,31 Consistently, our results show that LPS induces a significant decrease in the urinary clearance of [18F]FDS, which translates into changes in blood kinetics.
Given important changes in the IDIF between groups, and between individuals of the same group, kinetic modeling is essential for the correct estimation of the BBB permeation of [18F]FDS, as described by the brain VT. 44 Arterial blood sampling, which is the state-of-the-art method for determination of the arterial input function (AIF) for PET kinetic modeling, is particularly challenging in mice. Given the high intra-group variability, an IDIF method using the heart blood pool as a region was used for kinetic modeling. To our knowledge, the validity of this IDIF method using concomitant arterial blood sampling and PET scanning has not been validated in mice. Although uptake of [18F]FDS by the heart tissue is extremely low, it cannot be excluded that the IDIF may not accurately reflect the AIF, due to partial volume effect or motion artifact in mice. As a consequence, absolute brain VT values obtained using the AIF may be different than those obtained using the corresponding IDIF. However, it can be reasonably assumed that the use of IDIF is sufficient to take any changes in the peripheral kinetics of [18F]FDS into account for the correct estimation of BBB permeability.
The Logan graphical analysis was used to estimate VT as an outcome parameter to describe the BBB permeability of [18F]FDS. This model does not allow for estimation of the vascular volume of the brain, which may account for differences in the brain PET signal between groups. The 1-tissue compartment model, which enables estimation of the vascular volume, did not correctly fit all brain PET data of the study (data not shown). Baseline brain VT values obtained in vehicle-treated mice, were 0.074 ± 0.005 mL.cm−3, which is consistent with the vascular volume of the mouse brain estimated using invasive in situ brain perfusion procedures (0.06–0.13 mL.g−1). 45 This suggests negligible BBB penetration of [18F]FDS in mice with intact BBB. Low variability VT values in this vehicle group (CV = 7.4%) suggests low variability of [18F]FDS PET imaging as a method to estimate BBB permeability in vivo. As a consequence, the high variability observed after the injection of LPS 5 mg/kg group (CV = 101.3%) probably reflects different levels of BBB permeability in this group.
The causal relationship between the peripheral inflammation and BBB integrity involves different mediators whose response to LPS may considerably vary between individuals.46,47 Further experiments are therefore needed to correlate the level of inflammatory cytokines with BBB permeability. 48
Data obtained in mice treated with 3 mg/kg LPS showed recruitment of microglial cells and astrocytes after 48 h while brain distribution of [18F]FDS remains unchanged at 24 and 48 h. In a previous study, Banks and colleagues reported increased levels of cytokines and glial activation and BBB disruption 24 h after i.p. injection of 3 mg/kg of LPS (from Salmonella typhimurium) using [14C]sucrose as an investigational probe. 12 Assuming similar purity, this suggests that LPS from Salmonella typhimurium is more potent than LPS from Escherichia coli used in our study at inducing BBB disruption, a property already described depending on bacterial species, strain, and environmental conditions. 49 Furthermore, by using complementary in vivo/in vitro methods, they concluded that astrocytes and microglia play little role in LPS-induced BBB disruption. 12 In turn, the present study shows that LPS-induced recruitment of glial cells can be observed in mice without disruptive changes in BBB integrity. This advocates that BBB disruption is not a prerequisite for induction of neuroinflammation triggered by peripheral endotoxemia, but that other mechanisms may come into play e.g. the suggested dual role of microglia in maintaining or disrupting BBB integrity. 19 This is consistent with the negligible brain penetration of LPS, 50 suggesting that peripheral inflammation may propagate to the brain to trigger neuroinflammation before disruptive changes of the BBB. Our results ultimately illustrate the extent to which the BBB, in this mouse model, can maintain its integrity in the presence of endotoxemia.
Interestingly, increasing doses of LPS intensified BBB permeability but did not increase the expression of CD11b and GFAP, as markers of glial density. This non-linear relationship illustrates differences in the magnitude and dynamics of the regulation of BBB permeability and the recruitment of glial cells during endotoxemia. Conversely, expression of CD11b and GFAP were significantly correlated, suggesting joined microglial and astrocytic regulation by LPS.
Sepsis is associated with multiple organ failure which is probably linked with damage to biological membranes. 51 The use of whole-body PET imaging with [18F]FDS enabled comparison of loss in BBB integrity with renal function and animal physiology. Twenty-four hours after the injection of 3 mg/kg of LPS, there was a significant weight loss and a strong decrease in renal function, while BBB integrity remained intact. This suggests that the BBB is less vulnerable to LPS-induced damage compared to peripheral organs such as the kidneys.
Sorbitol is a metabolic substrate that is utilized by Enterobacteriaceae, which use a metabolic pathway that is absent in mammalian cells. 52 [18F]FDS PET is therefore receiving much attention as a means to localize infection induced by these bacteria, including in the CNS. 53 Our data, obtained in a mouse model of “sterile” endotoxemia induced by the LPS of Escherichia coli, suggests that BBB disruption is required for effective brain delivery of [18F]FDS, as evidenced from early time frames (0–30 min) after injection, even in the absence of living bacteria. 52 However, dynamic PET data in mouse models have convincingly shown that late SUV values (120–135 min) predominantly reflect the presence of bacteria rather than non-specific uptake by the brain tissue across the leaky BBB. 52
Unlike [18F]FDG, [18F]FDS is not recognized by specific membrane transporters in mammals. 26 Moreover, [18F]FDS is a hydrophilic molecule that poorly penetrates plasma membranes. As a consequence, [18F]FDS exhibits very poor diffusion into cells and low intracellular retention. 26 In the brain, sorbitol can be endogenously produced in cells from the conversion of glucose, as the first step of the polyol pathway. 54 Sorbitol is then rapidly converted into fructose by the sorbitol dehydrogenase. 54 However, the 2-hydroxyl group of sorbitol is a critical functional group for its recognition by sorbitol dehydrogenase. [18F]FDS, with substitution by fluorine at the 2-position, completely abrogates this recognition. 26 Without any known transport and/or trapping mechanism found for cell retention, it may be hypothesized that the brain uptake of [18F]FDS across the disrupted BBB is non-specific, and is predominantly driven by the concentration gradient that exists between the plasma and the interstitial system of the brain. Nevertheless, as for other markers of BBB integrity, 55 the mechanisms underlying the tissue accumulation of [18F]FDS and its slow clearance from the brain remain to be investigated.
Conclusion
This mouse study shows that almost maximum recruitment of microglia or astrocytes induced by peripheral endotoxemia can be achieved while BBB integrity remains unchanged. Higher amounts of LPS dose-dependently enhanced BBB permeability but did not further increase the abundance of microglia or astrocytes. This suggests a biphasic effect of increasing doses of LPS that first induces glial activation, followed by BBB disruption with higher LPS doses.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X241236755 for Imaging quantitative changes in blood-brain barrier permeability using [18F]2-fluoro-2-deoxy-sorbitol ([18F]FDS) PET in relation to glial cell recruitment in a mouse model of endotoxemia by Sarah Leterrier, Sébastien Goutal, Gaëlle Hugon, Maud Goislard, Wadad Saba, Benoit Hosten, Simon Specklin, Alexandra Winkeler and Nicolas Tournier in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We thank the staff of the SHFJ platform for technical assistance.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was performed on a platform member of France Life Imaging network (grant ANR-11-INBS-0006).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: NT and AW contributed to the conception and design of the work; SL, WS, GH, MG, and SS performed the experiments. SL and SG analyzed the data. SL, AW, and NT interpreted the data. SL, NT, and AW have drafted the work. BH substantively revised the manuscript. All co-authors read and approved the submitted version.
ORCID iDs: Alexandra Winkeler https://orcid.org/0000-0003-3397-2570
Nicolas Tournier https://orcid.org/0000-0002-0755-2030
Supplementary material
Supplemental material for this article is available online.
References
- 1.Banks WA. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016; 15: 275–292. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simard M, Arcuino G, Takano T, et al. Signaling at the gliovascular interface. J Neurosci 2003; 23: 9254–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kugler EC, Greenwood J, MacDonald RB. The “Neuro-Glial-Vascular” unit: the role of glia in neurovascular unit formation and dysfunction. Front Cell Dev Biol 2021; 9: 732820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo EH, Rosenberg GA. The neurovascular unit in health and disease. Stroke 2009; 40: S2–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takata F, Nakagawa S, Matsumoto J, et al. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci 2021; 15: 661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masdeu JC, Pascual B, Fujita M. Imaging neuroinflammation in neurodegenerative disorders. J Nucl Med 2022; 63: 45S–52S. [DOI] [PubMed] [Google Scholar]
- 8.Gauberti M, Fournier AP, Docagne F, et al. Molecular magnetic resonance imaging of endothelial activation in the central nervous system. Theranostics 2018; 8: 1195–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004; 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 2012; 19: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banks WA, Gray AM, Erickson MA, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflammation 2015; 12: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandiego CM, Gallezot J-D, Pittman B, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A 2015; 112: 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrzypczak-Wiercioch A, Sałat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules 2022; 27: 5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batista CRA, Gomes GF, Candelario-Jalil E, et al. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int J Mol Sci 2019; 20: 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vizuete AFK, Fróes F, Seady M, et al. Early effects of LPS-induced neuroinflammation on the rat hippocampal glycolytic pathway. J Neuroinflammation 2022; 19: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun 2017; 60: 1–12. [DOI] [PubMed] [Google Scholar]
- 18.Ronaldson PT, Davis TP. Regulation of blood-brain barrier integrity by microglia in health and disease: a therapeutic opportunity. J Cereb Blood Flow Metab 2020; 40: S6–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun 2019; 10: 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Camp N, Lavisse S, Roost P, et al. TSPO imaging in animal models of brain diseases. Eur J Nucl Med Mol Imaging 2021; 49: 77–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain P, Chaney A, Carlson ML, et al. Neuroinflammation PET imaging: current opinion and future directions. J Nucl Med 2020; 61: 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris WJ, Asselin M-C, Hinz R, et al. In vivo methods for imaging blood-brain barrier function and dysfunction. Eur J Nucl Med Mol Imaging 2022; 50: 1051–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassidim Y, Veksler R, Lublinsky S, et al. Quantitative imaging assessment of blood-brain barrier permeability in humans. Fluids Barriers Cns 2013; 10: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugon G, Goutal S, Dauba A, et al. [18F]2-fluoro-2-deoxy-sorbitol PET imaging for quantitative monitoring of enhanced blood-brain barrier permeability induced by focused ultrasound. Pharmaceutics 2021; 13: 1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starr ME, Ueda J, Takahashi H, et al. Age-dependent vulnerability to endotoxemia is associated with reduction of anticoagulant factors activated protein C and thrombomodulin. Blood 2010; 115: 4886–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z-B, Wu Z, Cao Q, et al. The synthesis of 18F-FDS and its potential application in molecular imaging. Mol Imaging Biol 2008; 10: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner RA, Wakabayashi H, Chen X, et al. Functional renal imaging with 2-deoxy-2-18F-fluorosorbitol PET in rat models of renal disorders. J Nucl Med 2018; 59: 828–832. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747. [DOI] [PubMed] [Google Scholar]
- 29.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: a language and environment for statistical computing, www.R-project.org/ (2014, accessed 27 February 2024).
- 31.Werner RA, Ordonez AA, Sanchez-Bautista J, et al. Novel functional renal PET imaging with 18F-FDS in human subjects. Clin Nucl Med 2019; 44: 410–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders NR, Dziegielewska KM, Møllgård K, et al. Markers for blood-brain barrier integrity: how appropriate is evans blue in the twenty-first century and what are the alternatives? Front Neurosci 2015; 9: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noorani B, Chowdhury EA, Alqahtani F, et al. LC–MS/MS-based in vitro and in vivo investigation of blood–brain barrier integrity by simultaneous quantitation of mannitol and sucrose. Fluids Barriers CNS 2020; 17: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernal J, Valdés-Hernández M. d C, Escudero J, et al. A four-dimensional computational model of dynamic contrast-enhanced magnetic resonance imaging measurement of subtle blood-brain barrier leakage. NeuroImage 2021; 230: 117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elschot EP, Backes WH, Postma AA, et al. A comprehensive view on MRI techniques for imaging blood-brain barrier integrity. Invest Radiol 2021; 56: 10–19. [DOI] [PubMed] [Google Scholar]
- 37.Yang F-Y, Wang H-E, Lin G-L, et al. Micro-SPECT/CT-based pharmacokinetic analysis of 99mTc-diethylenetriaminepentaacetic acid in rats with blood-brain barrier disruption induced by focused ultrasound. J Nucl Med 2011; 52: 478–484. [DOI] [PubMed] [Google Scholar]
- 38.Arif WM, Elsinga PH, Gasca-Salas C, et al. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J Control Release 2020; 324: 303–316. [DOI] [PubMed] [Google Scholar]
- 39.Tournier N, Comtat C, Lebon V, et al. Challenges and perspectives of the hybridization of PET with functional MRI or ultrasound for neuroimaging. Neuroscience 2021; 474: 80–93. [DOI] [PubMed] [Google Scholar]
- 40.Elmaleh D, Shoup T, Bonab A, et al. Evaluation of 1-deoxy-1-[18F]fluoro-D-mannitol as a brain imaging tracer for measuring osmotic disruption following cancer therapy. J Nucl Med 2014; 55: 1123–1123. [Google Scholar]
- 41.Hara T, Iio M, Tsukiyama T, et al. Measurement of human blood brain barrier integrity using 11C-inulin and positron emission tomography. Eur J Nucl Med 1988; 14: 173–176. [DOI] [PubMed] [Google Scholar]
- 42.Zhu W, Yao S, Xing H, et al. Biodistribution and radiation dosimetry of the Enterobacteriaceae-specific imaging probe [(18)F]fluorodeoxysorbitol determined by PET/CT in healthy human volunteers. Mol Imaging Biol 2016; 18: 782–787. [DOI] [PubMed] [Google Scholar]
- 43.Yoo J-Y, Cha DR, Kim B, et al. LPS-Induced acute kidney injury is mediated by Nox4-SH3YL1. Cell Rep 2020; 33: 108245. [DOI] [PubMed] [Google Scholar]
- 44.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 45.Dagenais C, Rousselle C, Pollack GM, et al. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J Cereb Blood Flow Metab 2000; 20: 381–386. [DOI] [PubMed] [Google Scholar]
- 46.Galea I. The blood–brain barrier in systemic infection and inflammation. Cell Mol Immunol 2021; 18: 2489–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copeland S, Warren HS, Lowry SF, et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 2005; 12: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Ran M, Li H, et al. New insight into neurological degeneration: inflammatory cytokines and blood–brain barrier. Front Mol Neurosci 2022; 15: 1013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation 2019; 16: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun 2010; 24: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kozlov AV, Grillari J. Pathogenesis of multiple organ failure: the impact of systemic damage to plasma membranes. Front Med (Lausanne) 2022; 9: 806462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weinstein EA, Ordonez AA, DeMarco VP, et al. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci Transl Med 2014; 6: 259ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mota F, De Jesus P, Jain SK. Kit-based synthesis of 2-deoxy-2-[18F]-fluoro-D-sorbitol for bacterial imaging. Nat Protoc 2021; 16: 5274–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang JJ, Jiang L, Hamza M, et al. The human brain produces fructose from glucose. JCI Insight 2: e90508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasschaert M, Weller RO, Schroeder JA, et al. Retention of gadolinium in brain parenchyma: pathways for speciation, access, and distribution. A critical review. J Magn Reson Imaging 2020; 52: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X241236755 for Imaging quantitative changes in blood-brain barrier permeability using [18F]2-fluoro-2-deoxy-sorbitol ([18F]FDS) PET in relation to glial cell recruitment in a mouse model of endotoxemia by Sarah Leterrier, Sébastien Goutal, Gaëlle Hugon, Maud Goislard, Wadad Saba, Benoit Hosten, Simon Specklin, Alexandra Winkeler and Nicolas Tournier in Journal of Cerebral Blood Flow & Metabolism