Abstract
Purpose
The COVID-19 pandemic profoundly affected healthcare systems and patients. There is a need to comprehend the collateral effects of the pandemic on non-communicable diseases. We examined the impact of the pandemic on short-term survival for common solid tumours, including breast, colorectal, head and neck, liver, lung, oesophageal, pancreatic, prostate, and stomach cancer in the UK.
Methods
This was a population-based cohort study of electronic health records from the UK primary care Clinical Practice Research Datalink GOLD database. In sum, 12,259,744 eligible patients aged ≥18 years with ≥1 year’s history identified from January 2000 to December 2022 were included. We estimated age-standardised incidence and short-term (one- and two-year) survival for several common cancers from 2000 to 2019 (in five-year strata) and compared these to 2020–2022 using the Kaplan–Meier method.
Results
Incidence decreased for most cancers in 2020 and recovered to different extents in 2021–2022. Short-term survival improved for most cancers between 2000 and 2019, but then declined, albeit minimally, for those diagnosed in 2020–2022. This was most pronounced for colorectal cancer, with one-year survival falling from 78.8% (95% CI 78%–79.6%) in 2015–2019 to 77% (95% CI 75.6–78.3%) for those diagnosed in 2020–2022.
Conclusion
Short-term survival for many cancers was impacted, albeit minimally, by the pandemic in the UK, with reductions in survivorship from colorectal cancer equivalent to returning to the mortality seen in the first decade of the 2000s. While data on longer-term survival are needed to fully comprehend the impact of COVID-19 on cancer care, our findings illustrate the need for an urgent and substantial commitment from the UK National Health Service to address the existing backlog in cancer screening and diagnostic procedures to improve cancer care and mortality.
Keywords: cancer, COVID-19, colorectal cancer, incidence, pandemic, survival
Introduction
The COVID-19 pandemic left a permanent mark on global public health, with profound implications for healthcare systems and patients. Whilst healthcare systems grappled with the overwhelming demands of treating COVID-19 patients, there emerged a pressing need to comprehend the collateral effects of the pandemic on non-communicable diseases, including cancer. Social restrictions and the prioritisation of urgent over elective healthcare provision imposed by the pandemic impacted screening and diagnostic pathways for many cancers worldwide.1–3 Reduced screening and diagnostic tests inevitably cause delays, leading to more advanced stage at diagnosis and worsened prognosis.
Survival from cancer has generally improved over the last two decades, with combined mortality rates across all cancers and sexes decreasing by around 10%.4 One study estimated that in the UK, the probability of dying from cancer before 80 years of age declined from 0.16 to 0.13 for women and 0.22 to 0.17 for men across 2002–2019.5 The improvement in cancer survival can be attributed to several interconnected factors that have contributed to advancements in cancer prevention: screening, early diagnosis, and treatment.6 These factors have collectively led to better outcomes and increased survival rates for cancer patients. However, the pandemic’s disruptive impact on healthcare systems and cancer care delivery has raised concerns about delayed diagnosis, altered treatment regimens, delays in surgery, and reduced access to essential services, all of which may have adverse consequences for cancer patients’ survival.
The UK’s National Health Service (NHS) was already under pressure prior to the pandemic, suffering from years of reduced funding and substantial challenges, including staff shortages and reduced hospital beds per capita.7 Staffing issues were further compounded because many doctors and nurses were on sick leave or isolating due to COVID-19 exposure. Surgical staff were redeployed to care for COVID-19 patients, and operating theatres and outpatient clinics were closed to free up resources for patients with COVID-19.8 This led to the halting of routine and elective care in the months following the outbreak.9 Despite these efforts, the rapid spread of COVID-19 in the UK impacted health and social care nationally, and the government implemented three national lockdowns.
Literature on the impact of the pandemic on survival from cancers is currently limited, with only a handful of studies addressing this in the UK.10,11 Given the prioritisation of urgent care for COVID-19 during the pandemic on top of a stretched NHS, we hypothesised that delays in the diagnosis and/or lack of cancer screening would lead to increased short-term mortality due to more advanced stages at diagnosis. We speculated that the expected delay in diagnosis and hence potentially reduced short-term survival would be more noticeable for cancers that benefit from early detection and for which we have successful screening programmes (such as breast and colorectal cancer).
The overall aim of this study was to investigate the impact of the COVID-19 pandemic and its management on short-term (one and two years after diagnosis) survival for common solid tumours, including breast, colorectal, head and neck, liver, lung, oesophageal, pancreatic, prostate, and stomach cancer in the UK. Using pseudonymised NHS records, we examined: 1) secular trends in the incidence of several common cancers from 2000–2019 compared to 2020–2022; and 2) short-term survival following diagnosis of the same cancers in 2000–2019 and compared to those diagnosed in 2020–2022.
Methods
Study Design
A population-based cohort study was conducted to examine the incidence and survival for nine cancers using routinely collected primary care data from the Clinical Practice Research Datalink (CPRD) GOLD database December 2022 (version 2022.12.001) in the UK between January 2000 and December 2022.
Study Participants
All patients were required to be aged 18 years or older and have at least one year of history within the database (to characterise patients on key comorbidities) and information on age and sex, excluding individuals diagnosed with the same cancer at any time in the database history. Hence, data from 1999 to 2022 were used. For incidence, the study cohort consisted of individuals present in the database from 1 January 2000 or their first day of eligibility (whichever occurred first). These individuals were followed up to whichever came first: the cancer outcome of interest, date of death, exit from the database, or 31 December 2022 (the end of the study period). For survival analysis, individuals with a newly diagnosed cancer were included. These individuals were followed up from the date of their diagnosis to either date of death, exit from the database, or end of the study period. All outcomes included both sexes, except for breast cancer (females only) and prostate cancer (males only).
Procedures
People with a diagnosis of a cancer of interest and a denominator cohort were identified from CPRD GOLD to estimate cancer incidence and overall survival. CPRD GOLD contains pseudonymised patient-level information on demographics, lifestyle data, clinical diagnoses, prescriptions, and preventive care contributed by general practitioners (GP) from the UK. It is an established primary care database broadly representative of the UK population.12 This database was mapped to the Observational Medical Outcomes Partnership common data model (CDM).13
Outcomes
We used SNOMED CT diagnostic codes to identify incident cancer events for the nine cancers. Diagnostic codes indicative of either non-malignant cancer or metastasis were excluded, as well as diagnosis code indicative of melanoma and lymphoma occurring in the organs/sites of interest. Metastases were excluded because we intended to examine the impact of the pandemic on patients with incident cancer diagnoses that had not yet entered a clinical care pathway. Hence, the outcomes of interest were local or locally advanced cancers only. The cancer outcome definitions were reviewed with the aid of the CohortDiagnostics R package.14 This package was used to identify additional codes of interest and to remove those highlighted as irrelevant based on feedback from clinicians with oncology expertise through an iterative process. The clinical code lists used to define all cancer outcomes can be found in Supplementary 1. Additionally, a detailed description of all cancer outcomes is provided at https://dpa-pde-oxford.shinyapps.io/EHDENCancerIncPrevCohortDiagShiny. For survival analysis, mortality was defined as all-cause mortality based on date of death records. Mortality data in CPRD GOLD has been previously validated and shown to be over 98% accurate.15
Statistical Analysis
The population characteristics of patients with a diagnosis of each cancer were summarised overall and separately for each calendar year stratum, with median and interquartile range (IQR) used for continuous variables and counts and percentages used for categorical variables. Differences in the counts of comorbidities across time were evaluated by χ2. Characteristics included 16 common chronic diseases, identified by clinical code lists (and reviewed by clinicians).
Annual crude incidence rates (IRs) were calculated for all cancer outcomes from 2000 to 2022. For incidence, the number of events, the observed time at risk, and the IR per 100,000 person years were summarised with 95% CIs. Annual IRs were calculated as the number of incident cancer cases as the numerator and the exact recorded number of person-years in the general population of CPRD GOLD within that year as the denominator.
Using the crude IRs, age-standardised IRs were calculated using the 2013 European Standard Population ESP2013.16 The ESP2013 serves as a standard population with a predefined age distribution where results are adjusted to match this distribution and account for differences in age structures between different populations to ensure fair comparisons. The ESP2013 provides predefined age distribution in five-year age bands; therefore, we collapsed these to obtain distributions for the 10-year age bands used in this study. We used an age distribution of 20–29 years from ESP2013 for age standardisation, as age distributions were not available for the 18- to 29-year age band used in this study.
For survival analysis, we stratified by calendar time of cancer diagnosis (2000–2004, 2005–2009, 2010–2014, 2015–2019, and 2020–2022). To estimate short-term survival at one and two years, we used the Kaplan–Meier (KM) method. Patients whose death and cancer diagnosis occurred on the same date were removed from the survival analysis (0.48%–3.64% of patients depending on cancer). To avoid re-identification, we do not report results with fewer than five cases. All results are presented in an interactive online dashboard at https://dpa-pde-oxford.shinyapps.io/CancerIncSurvCovid.
Code Availability
All codes used for these analyses are publicly available online (https://dpa-pde-oxford.shinyapps.io/CancerIncSurvCovid). Analyses were carried out using R (version 4.2.3).
Patient and Public Involvement
No patients or members of the public were involved in the design, analysis, or interpretation of this study or the reported data, because the study aimed to examine population-level trends and patterns, rather than individual experiences or perspectives.
Ethics Approval
The protocol for this research was approved by the Independent Scientific Advisory Committee for Medicine and Healthcare Products Regulatory Agency database research (protocol 22_001843) and adhered to and complied with local relevant data protection and privacy regulations and the principles of the Declaration of Helsinki.
Results
There were 12,557,753 eligible patients aged 18 years and older with at least one year of history identified from January 2000 to December 2022 from CPRD GOLD, but 5,634,984–11,593,927 finally eligible after applying inclusion/exclusion criteria. The attrition was largely due to not being observed in the database during the time period of interest after applying the age, sex, and history requirements. The attrition table for this study for each cancer can be found in Supplementary 2. A summary of patient characteristics of those with a diagnosis of different cancers is shown in Table 1.
Table 1.
Baseline characteristics of patients at the time of cancer diagnosis for this study from 2000–2022
| Breast | Colorectal | Liver | Stomach | Prostate | Pancreatic | Head and Neck | Lung | Oesophageal | |
|---|---|---|---|---|---|---|---|---|---|
| n | 88,196 | 55,317 | 4198 | 7444 | 66,903 | 10,391 | 12,831 | 46,922 | 15,621 |
| Sex (male), n (%) | 0 | 30,640 (55%) | 3005 (72%) | 4668 (63%) | 66,903 (100.0%) | 5169 (50%) | 8859 (69%) | 25,231 (54%) | 10,669 (68%) |
| Age (years), median (IQR) | 63 (52–73) | 72 (63–80) | 71 (63–78) | 75 (67–82) | 72 (65–78) | 73 (64–80) | 64 (56–73) | 72 (65–79) | 72 (63–79) |
| Age group, n (%) | |||||||||
| 18–29 | 285 | 12 | 14 | 20 | 6 | 6 | 96 (1%) | 24 | 12 |
| 30–39 | 2950 (3%) | 545 (1.0%) | 28 (1%) | 70 (1%) | 11 | 35 | 265 (2%) | 129 | 65 |
| 40–49 | 11,540 (13%) | 2080 (4%) | 143 (3%) | 233 (3%) | 458 (1%) | 317 (3%) | 1093 (9%) | 1041 (2%) | 480 (3%) |
| 50–59 | 22,421 (25%) | 6580 (12%) | 585 (14%) | 614 (8%) | 6023 (9%) | 1170 (11%) | 3075 (24%) | 4781 (10%) | 2017 (13%) |
| 60–69 | 23,043 (26%) | 13,830 (25%) | 1122 (27%) | 1387 (19%) | 20,773 (31%) | 2615 (25%) | 3844 (30%) | 12,602 (27%) | 4144 (27%) |
| 70–79 | 15,599 (18%) | 17,737 (32%) | 1410 (34%) | 2626 (35%) | 25,843 (39%) | 3412 (33%) | 2897 (23%) | 17,294 (37%) | 5013 (32%) |
| 80–89 | 9872 (11%) | 12,446 (22%) | 825 (20%) | 2123 (29%) | 12,222 (18%) | 2402 (23%) | 1337 (10%) | 9894 (21%) | 3287 (21%) |
| 90+ | 2486 (2.9%) | 1971 (4%) | 71 (2%) | 368 (5%) | 1567 (2%) | 434 (4%) | 224 (2%) | 1227 (3%) | 603 (4%) |
| History, median (IQR) days | 3190 (1609–5037) | 3540 (1915–5335) | 4046 (2166–5766) | 3366 (1824–5109) | 3682 (1964–5457) | 3809 (2127–5572) | 3399 (1828–5180) | 3706 (2035–5453) | 3602 (1957–5408) |
| Number of GP visits, mean (SD) | 21.89 (16.86) | 28.95 (18.58) | 39.27 (21.82) | 32.79 (19.08) | 28.68 (16.97) | 35.57 (19.55) | 27.07 (18.72) | 35.17 (20.30) | 30.48 (19.12) |
| Atrial fibrillation | 2803 (3%) | 3804 (7%) | 321 (8%) | 568 (8%) | 4498 (7%) | 660 (6%) | 569 (4%) | 3319 (7%) | 1049 (7%) |
| Heart failure | 1457 (2%) | 1958 (4%) | 191 (5%) | 356 (5%) | 2142 (3%) | 351 (3%) | 290 (2%) | 2250 (5%) | 610 (4%) |
| Ischaemic heart disease | 3920 (4%) | 5544 (10%) | 547 (13%) | 1010 (14%) | 7845 (12%) | 1139 (11%) | 1042 (8%) | 6424 (14%) | 1737 (11%) |
| Cerebrovascular disease | 2698 (3%) | 3305 (6%) | 278 (7%) | 571 (8%) | 3914 (6%) | 667 (6%) | 724 (6%) | 3964 (8%) | 1009 (6%) |
| Hyperlipidaemia | 5924 (7%) | 5123 (9%) | 323 (8%) | 647 (9%) | 6830 (10%) | 1038 (10%) | 1012 (8%) | 4726 (10%) | 1429 (9%) |
| Hypertensive disorder | 18,077 (20%) | 15,228 (28%) | 1270 (30%) | 2053 (28%) | 19,612 (29%) | 2957 (28%) | 3017 (24%) | 12,865 (27%) | 4354 (28%) |
| Pulmonary embolism | 575 (1%) | 708 (1%) | 58 (1%) | 108 (1%) | 644 (1%) | 181 (2%) | 79 (1%) | 823 (2%) | 226 (1%) |
| Venous thrombosis | 3130 (4%) | 2680 (5%) | 197 (5%) | 388 (5%) | 2602 (4%) | 690 (7%) | 392 (3%) | 2551 (5%) | 792 (5%) |
| Type 2 diabetes | 5206 (6%) | 6061 (11%) | 1156 (28%) | 891 (12%) | 6364 (10%) | 2149 (21%) | 1017 (8%) | 5089 (11%) | 1708 (11%) |
| Chronic liver disease | 223 | 202 | 900 (21%) | 24 | 138 | 44 | 179 (1%) | 257 (1%) | 96 (1%) |
| Renal impairment | 6606 (7%) | 6906 (12%) | 682 (16%) | 1063 (14%) | 7757 (12%) | 1439 (14%) | 1034 (8%) | 6374 (14%) | 1997 (13%) |
| COPD | 2773 (3%) | 3394 (6%) | 385 (9%) | 670 (9%) | 4114 (6%) | 769 (7%) | 1213 (9%) | 11,547 (25%) | 1373 (9%) |
| Gastrointestinal haemorrhage | 4386 (5%) | 10,359 (19%) | 474 (11%) | 735 (10%) | 4792 (7%) | 795 (8%) | 783 (6%) | 3208 (7%) | 1194 (8%) |
| Osteoarthritis | 15,004 (17%) | 10,753 (19%) | 955 (23%) | 1662 (22%) | 13,393 (20%) | 2416 (23%) | 1946 (15%) | 10,151 (22%) | 3109 (20%) |
| Depressive disorder | 13,100 (15%) | 5478 (10%) | 555 (13%) | 785 (11%) | 5029 (8%) | 1267 (12%) | 1671 (13%) | 6613 (14%) | 1611 (10%) |
| Dementia | 1067 (1%) | 752 (1%) | 63 (2%) | 136 (2%) | 632 (1%) | 167 (2%) | 163 (1%) | 783 (2%) | 220 (1%) |
| COVID-19 (2020–2022) | 1202 (18.73%) | 832 (17.25%) | 82 (14.72%) | 59 (13.79%) | 872 (14.77%) | 154 (15.16%) | 202 (17.67%) | 774 (17.25%) | 226 (16.36%) |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
As detailed in Table 1, breast cancer was the most common cancer, followed by prostate, colorectal, and lung. Males were more likely to have a cancer diagnosis apart from breast cancer and for pancreatic cancer where there were no sex differences in numbers diagnosed. Cancer diagnoses were more common with increasing age, peaking in those aged 70–79 years for all cancers, apart from those diagnosed with breast or head and neck cancer, where more diagnoses were in those aged 60–69 years. For breast, prostate, and head and neck cancers, these patients had the lowest percentages of comorbidities, whereas those with a diagnosis of liver, stomach, and lung cancers had the highest percentages of comorbidities.
Comparison of patient characteristics of those diagnosed in 2020–2022 with the other calendar-year groups (2000–2004, 2005–2009, 2010–2014, and 2015–2019) showed similar socio-demographics with very similar age and sex distributions, regardless of the year of diagnosis. Regarding comorbidities, whilst there were significant differences across all time points for various conditions across cancers, the most notable differences between 2020 to 2022 compared to previous years were greater frequencies of: 1) atrial fibrillation in colorectal, liver, lung, oesophageal, prostate, and stomach cancers; 2) cerebrovascular disease in colorectal, lung, and liver cancers; 3) venous thrombosis in colorectal, lung, oesophageal, and prostate cancers; 4) COPD in colorectal, head and neck, and liver cancers; 5) type 2 diabetes in colorectal, head and neck, liver, lung, pancreatic, prostate, and stomach cancers; 6) hypertensive disorder in head and neck and liver cancers; 7) chronic liver disease in liver and prostate cancers; 8) osteoarthritis in liver, lung, pancreatic, and prostate cancers; 9) depressive disorder in liver and prostate cancers; 10) pulmonary embolism in lung and oesophageal cancers; and 11) gastrointestinal haemorrhage in oesophageal cancers. Detailed patient characteristics stratified by calendar year of diagnosis in five-year strata can be found in Supplementarys 3.1–3.9.
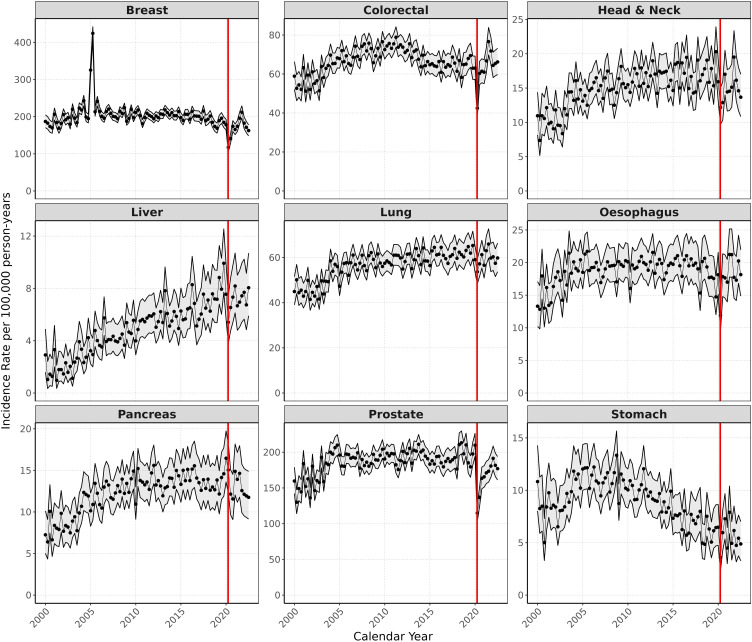
Regarding secular trends in the incidence of cancer, annual age-standardised IRs increased from 2000 to 2019 for all cancers except breast, colorectal, oesophageal, and stomach cancer (Figure 1). Apart from pancreatic cancer, IRs decreased for all cancers in 2020 and recovered to different extents in 2021–2022. For breast, colorectal, oesophageal, and pancreatic cancers, IRs in 2021 were higher than 2019, whereas for head and neck, liver, lung, prostate, and stomach cancers, IRs were still lower in 2021 than before the pandemic in 2019. Results showing the crude annualised IRs can be found in Supplementary 4.
Figure 1.
Age-standardised annual incidence (per 100,000 person years) for nine cancers from 2000 to 2022 (red line indicating start of COVID pandemic in 2020). Grey bands indicate 95% CIs.
Positive trends in the survival for most cancers were observed in the years 2000–2019. Improvements in one- and two-year survival were more obvious for liver, lung, and prostate cancer (one-year survival increased from 35% to 46.5% for liver cancer, 33.1% to 45.4% for lung cancer, and 90.8% to 94.9% for prostate cancer from 2000 to 2019) and minimal or non-significant for head and neck and pancreatic cancers (one-year survival increased from 80.7% to 81.2% for head and neck cancer and from 22.3% to 28.4% for pancreatic cancer from 2000–2019) (Table 2).
Table 2.
Survival probabilities (and 95% CIs) for all cancer outcomes at one and two years after diagnosis stratified by calendar year group
| Cancer | Calendar years | One year survival, % (95% CI) | Two-year survival, % (95% CI) |
|---|---|---|---|
| Breast | 2000–2004 | 93.7 (93.3–94.1) | 88.3 (87.8–88.8) |
| 2005–2009 | 95.2 (94.0–95.5) | 91.0 (90.7–91.4) | |
| 2010–2014 | 95.6 (95.3–95.9) | 91.3 (90.9–91.6) | |
| 2015–2019 | 95.9 (95.5–96.2) | 92.1 (91.7–92.6) | |
| 2020–2022 | 95.7 (95.1–96.2) | 91.9 (91.0–92.8) | |
| Colorectal | 2000–2004 | 77.1 (76.2–78.0) | 65.9 (64.9–66.9) |
| 2005–2009 | 77.8 (77.2–78.5) | 66.5 (65.8–67.3) | |
| 2010–2014 | 79.5 (78.9–80.1) | 69.1 (68.3–69.8) | |
| 2015–2019 | 78.8 (78.0–79.6) | 68.5 (67.6–69.5) | |
| 2020–2022 | 77.0 (75.6–78.3) | 68.2 (66.4–70.0) | |
| Head and neck | 2000–2004 | 80.7 (78.8–82.6) | 70.8 (68.7–73.0) |
| 2005–2009 | 79.8 (78.4–81.2) | 69.3 (67.7–70.8) | |
| 2010–2014 | 82.4 (81.2–83.7) | 72.0 (70.5–73.5) | |
| 2015–2019 | 81.2 (79.7–82.7) | 71.7 (70.0–73.5) | |
| 2020–2022 | 77.6 (74.9–80.4) | 68.7 (65.2–72.4) | |
| Liver | 2000–2004 | 35.0 (30.0–40.9) | 21.0 (16.8–26.3) |
| 2005–2009 | 35.8 (32.8–39.1) | 21.2 (18.6–24.1) | |
| 2010–2014 | 43.1 (40.4–46.0) | 28.5 (26.0–31.3) | |
| 2015–2019 | 46.5 (43.5–49.7) | 30.7 (27.9–33.8) | |
| 2020–2022 | 46.6 (42.0–51.6) | 30.8 (25.9–36.7) | |
| Lung | 2000–2004 | 33.1 (31.9–34.3) | 18.4 (17.4–19.4) |
| 2005–2009 | 35.4 (34.5–36.2) | 20.1 (19.3–20.8) | |
| 2010–2014 | 39.9 (39.0–40.7) | 24.7 (23.9–25.5) | |
| 2015–2019 | 45.4 (44.4–46.4) | 30.6 (29.6–31.6) | |
| 2020–2022 | 45.2 (43.5–46.9) | 31.4 (29.7–33.3) | |
| Oesophagus | 2000–2004 | 40.7 (38.8–42.8) | 23.0 (21.3–24.8) |
| 2005–2009 | 42.3 (40.8–43.8) | 24.0 (22.7–25.3) | |
| 2010–2014 | 46.3 (44.8–47.8) | 28.1 (26.7–29.6) | |
| 2015–2019 | 45.3 (43.5–47.1) | 26.3 (24.6–28.0) | |
| 2020–2022 | 42.4 (39.4–45.6) | 24.8 (21.7–28.5) | |
| Pancreas | 2000–2004 | 22.3 (20.0–24.8) | 11.4 (9.7–13.4) |
| 2005–2009 | 23.3 (21.7–25.0) | 12.1 (10.9–13.5) | |
| 2010–2014 | 26.1 (24.5–27.7) | 12.6 (11.4–14.0) | |
| 2015–2019 | 28.4 (26.5–30.5) | 14.9 (13.3–16.6) | |
| 2020–2022 | 25.9 (22.9–29.2) | 13.9 (11.2–17.2) | |
| Prostate | 2000–2004 | 90.8 (90.2–91.4) | 82.4 (81.7–83.2) |
| 2005–2009 | 92.6 (92.2–93.0) | 85.6 (85.1–86.1) | |
| 2010–2014 | 94.4 (94.1–94.8) | 88.6 (88.1–89.0) | |
| 2015–2019 | 94.9 (94.5–95.2) | 89.1 (88.5–89.6) | |
| 2020–2022 | 94.5 (93.8–95.2) | 88.9 (87.8–90.1) | |
| Stomach | 2000–2004 | 41.7 (38.9–44.6) | 27.0 (24.5–29.6) |
| 2005–2009 | 40.8 (38.9–42.9) | 24.9 (23.2–26.8) | |
| 2010–2014 | 44.0 (41.9–46.3) | 28.1 (26.1–30.1) | |
| 2015–2019 | 46.9 (43.9–50.1) | 32.1 (29.3–35.2) | |
| 2020–2022 | 45.1 (40.0–50.9) | 33.1 (27.6–39.7) |
In contrast, the first three years of the pandemic (2020–2022) saw a numerical reduction in short-term (one-year) survival for most cancers (Table 2). More specifically, one-year survival significantly dropped after a colorectal cancer diagnosis, from 78.8% (95% CI 78%–79.6.1%) in 2015–2019 to 77% (95% CI 75.6%–78.3%) in 2020–2022. A numerical decrease in one-year survival was also observed for three other cancers: from 81.2% (95% CI 79.7%–82.7%) to 77.6% (95% CI 74.9%–80.4%) for head and neck cancer; from 45.3% (95% CI 43.5%–47.1%) to 42.4% (95% CI 39.4%–45.6%) for oesophageal cancer; from 28.4% (95% CI 26.5%–30.5%) to 25.9% (95% CI 22.9–29.2%) for pancreatic cancer; and from 46.9% (95% CI 43.9%–50.1%) to 45.1% (95% CI 40%–50.9%) for stomach cancer. Liver, lung, breast, and prostate cancers were an exception, with no observable decline in one-year survival in 2020–2022.
Survival after two years since diagnosis also indicated that those diagnosed during 2020–2022 had lower survival for some cancers (Table 2), but samples were small. We observed slight numerical decreases in two-year survival for those diagnosed in 2015–2019 compared to those diagnosed in 2020–2022 for three cancers: from 92.1% (95% CI 91.7%–92.6%) to 91.9% (95% CI 91%–92.8%) for breast cancer; from 71.7% (95% CI 70%–73.5%) to 68.7% (95% CI 65.2%–72.4%) for head and neck cancer; and from 26.3% (95% CI 24.6%–28%) to 24.8% (95% CI 21.7%–28.5%) for oesophageal cancer, though it should be noted that CIs between these calendar-year survival trends were overlapping. Two-year survival for colorectal, lung, liver, pancreatic, prostate, and stomach cancers remained unchanged when comparing those diagnosed in 2015–2019 and those diagnosed in 2020–2022.
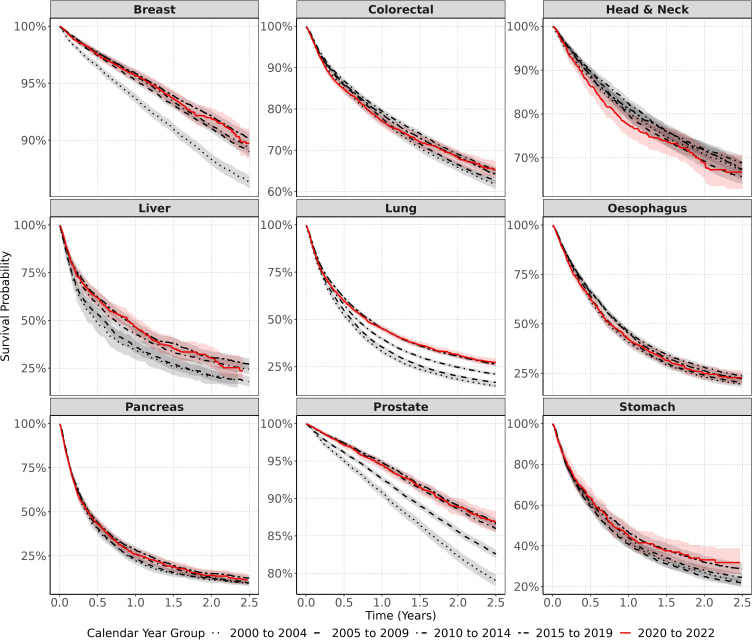
The KM survival curves for all nine cancers from the date of diagnosis to two-year follow-up are shown in Figure 2. Individual plots for each cancer can be found in Supplementarys 5.1–5.9. The numbers at risk, events, and censoring at each of the time points in the KM plots for each cancer shown in Figure 2 are included in Supplementary 6. Cancer survival curves depicted in Figure 2 are consistent with the one- and two-year survival probabilities shown in Table 2. Survival trends tended to improve in the years 2000–2019, but then declined for breast, head and neck, oesophageal, and pancreatic cancers after their diagnosis in 2020–2022.
Figure 2.
Kaplan–Meier survival curves for all nine cancers stratified by calendar time of cancer diagnosis. Shaded bands indicate 95% CIs.
Discussion
Statement of Principal Findings
The incidence of cancer diagnoses in the UK decreased in the first year of the pandemic (2020), recovering to different extents in 2021 for most cancers. This was more obvious for breast, colorectal, lung, and oesophageal cancer, with rates of new diagnosis in 2021 and 2022 higher than those recorded immediately before the pandemic in 2019. One- and two-year survival after a diagnosis of cancer increased in the years 2000–2019 for most cancers and more clearly for cancers that benefit from novel treatments including antivirals (eg, liver cancer) and immunotherapies (eg, lung cancer). Cancers included in national screening programmes (eg, breast, colorectal) also improved prognosis in these same years, likely due to earlier diagnosis.
In contrast with observable improvements over the previous 20 years, the first two years of the pandemic led to a decline, albeit minimal, in short-term survival for some cancers (breast, head and neck, colorectal, oesophageal, and pancreatic cancer) in the UK. This decline was most pronounced for colorectal cancers, with reductions in survivorship equivalent to the return to mortality rates seen in the first decade of the 2000s. Although only a difference of 1.4%, this represents a substantial number of patients for whom survival could have been improved through early detection and treatment, which were halted during the pandemic.3,17 We found no evidence that this decrease in survivorship could be due to differences in socio-demographics: patients diagnosed with cancer in 2020–2022 were of similar age and sex and had similar comorbidities compared to those diagnosed in previous years. However, it should be noted that there was a greater frequency of some comorbid conditions in patients diagnosed during the pandemic compared to previous years, and this may account for decreases in survival. In line with this, there may be competing causes of death other than cancer, particularly due to COVID-19 infection during this time. Whilst it is pertinent to account for the impact of COVID-19 infection on mortality in the cancer population, cause of death is not captured by the CPRD dataset. As such, it was not possible to quantify this potential competing cause of death from COVID-19. Future research should account for this so that it is possible to delineate the impact of the pandemic on cancer survival whilst ruling out COVID-19 infection itself as a contributing cause.
Strengths of the Study
The large sample and over 20 years of follow-up provided by CPRD GOLD make this a unique dataset for longitudinal analyses. Cancer incidence before the pandemic reported here is in line with national data from Cancer Research UK statistics for all cancers, providing confidence in the validity of our estimates.18 The high validity and completeness of mortality data, with over 98% accuracy when compared to national mortality records,15 allowed us to demonstrate the impact of the COVID-19 pandemic on short-term survival, one of the key outcomes in cancer care.
Weaknesses of the Study
Our study also had limitations. First, we used primary care data without linkage to cancer registry, potentially leading to misclassification and delayed recording. However, previous validation studies have shown high accuracy and completeness of cancer diagnoses in primary care records.19 Second, our use of primary care records precluded us from studying tumour histology, staging, or cancer therapies, which can all impact survival. Third, the composition of the CPRD GOLD database has changed over time. With the advent of the CPRD AURUM database, some English practices were transferred out of GOLD into AURUM from 2021. Therefore, the data from 2021 onwards are more representative of Scotland and Wales and less representative of England and Northern Ireland than earlier datacuts.
Research in Context
In line with our results, UK cancer statistics demonstrate the increasing trend in cancer incidence over recent decades.18,20 Reasons for these increases are multifaceted and vary per cancer type, but generally include diagnostic testing (eg, PSA for prostate cancer), public awareness of symptomology (eg, for breast cancer), and an ageing population.20 A notable trend observed is a significant increase in breast cancer incidence during 2004–2005. One potential explanation for this could be the implementation of the Quality and Outcomes Framework in 2004. This evaluates the performance of general practices across various disease areas, including cancer, and offers financial incentives for meeting specific quality targets. Consequently, there may have been increased focus on screening, diagnostics, and reporting of cancer cases during this period. Moreover, from 2001 to 2004, many screening units in the UK expanded their screening programs to include women aged 65–70 years.21 This expansion could have contributed to the observed surge in cancer diagnoses during this time frame.
Unsurprisingly, the COVID-19 pandemic halted screening programmes and diagnostic appointments, and may have altered patients’ health-seeking behaviour in an attempt to contain the virus.3 These alterations led to reduced cancer diagnoses, as evidenced by our results (with the exception of pancreatic cancer), as well as other European data.11,22,23 However, there may be other reasons for the reduction in cancer diagnoses during the pandemic. For example, reduced exposure to certain cancer risk factors due to relatively healthier lifestyles during lockdown (such as decreased smoking, decreased alcohol consumption, and healthier dietary and sleep patterns24,25) may have halted tumour development. An alternative explanation is that undiagnosed cancer patients may have died from COVID-19 infection. Finally, delays or inaccuracies in cancer data collection and reporting may affect the apparent incidence. Regardless of the explanation, our results contrast with data from the United States showing an increase in diagnoses of lung, colorectal, pancreatic, breast, and prostate cancers in 2020 compared to 2019,26 and illustrate the selective channelling of healthcare resources in the UK during the first 2 years of the pandemic. Not surprisingly, 2021–2022 saw a relative increase in incidence of these cancers compared to 2020, which suggests attempts to compensate for those diagnoses missed during the pandemic. Our data for the first time show that cancer incidence have largely recovered since 2020, yet rates of diagnosis need to continue to exceed expected rates in the forthcoming years to account for the backlog in diagnoses accumulated in 2020.
Possible reasons that the incidence of pancreatic cancer did not decrease during the pandemic compared to other cancers include the fact that pancreatic cancer is usually diagnosed at an advanced stage, as there are not good predictors for screening and early diagnosis. Cancer arising at the pancreatic head is usually diagnosed when jaundice appears due to compression of the bile duct. Patients with such symptoms as jaundice attended hospital during the pandemic, and the diagnosis was generally determined without a relevant delay. Cancers arising at the body or tail of the pancreas present non-specific symptoms and are usually diagnosed at a very advanced stage, when the patient has very severe symptoms of weight loss and debility. In such conditions, CT scans for diagnosis during the pandemic were probably prioritised and diagnoses not remarkably delayed. In fact, it is possible that they were even scheduled sooner than usual, because CT scans for other non-urgent indications were delayed and there was greater availability of the scanner.
One major implication of reductions in cancer incidence is that diagnoses and treatments have been delayed, affecting patient survival. This may be particularly prominent for cancers included in screening programmes, such as colorectal cancers. Whilst the literature on survival estimates post-pandemic is sparse, some global estimates align with our UK-based estimates demonstrating the impact of diagnostic delays and reduction in care/availability of treatments on cancer survival, with an increase in total deaths from most cancers during 2019–2021.26 That said, during 2020–2022 the one-year survival rate of liver, lung, breast, and prostate cancers, as well as the two-year survival rate of colorectal, lung, liver, pancreatic, prostate, and stomach cancers, did not decrease. There are many possible explanations as to why this may be. For example, this may be because these particular cancers benefited from increased remote monitoring or adaptation of treatment protocols during and immediately following the pandemic. Alternatively, patients may have been more motivated during the COVID-19 pandemic to adhere more strictly to treatment plans, adopt healthier lifestyles, and prioritize self-care measures to optimize their health and recovery.
Conclusion
Taken together with previous research, our results show evidence that short-term survival from cancer was impacted by the management of the COVID-19 pandemic in the UK. The combination of a decline in diagnoses followed by an increase in short-term mortality for many cancers and more clearly for colorectal malignancies provide evidence of delays in diagnosis during the first two years of the pandemic. However, other factors, such as reduced care/unavailability of certain cancer treatments, and COVID-19 infection, may have contributed to reduced survival of those diagnosed with cancer during the pandemic. Our findings illustrate the need for an immediate and intense investment from the NHS to resolve the current backlog in cancer screening and diagnostic procedures to improve cancer survival. Datasets with longer follow-up are now needed to fully comprehend the impact of COVID-19 on long-term cancer care and survival.
Acknowledgments
This study was funded by the European Health Data & Evidence Network (EHDEN) and the Optimal treatment for Patients with Solid Tumours in Europe through Artificial Intelligence (OPTIMA) initiative, which has received funding from the Innovative Medicines Initiative 2 (IMI2) Joint Undertaking under grant agreements 806968 and 101034347, respectively. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. The views communicated within are those of OPTIMA and EHDEN. Neither the IMI nor the European Union, EFPIA, or any associated partners are responsible for any use that may be made of the information contained herein. AWR would like to thank Professor Ismail Gögenur and Dr Mikail Gögenur for discussions regarding development of the colorectal cancer phenotyping used in this study. The study funders had no role in the conceptualisation, design, data collection, analysis, decision to publish, or preparation of the manuscript. This paper is also available on medRxiv as a preprint, which can be found here: https://doi.org/10.1101/2023.09.14.23295563
Disclosure
NB reports personal fees from Sleep Universal, Roche and Theramex, outside the submitted work. DPA receives funding from the UK National Institute for Health and Care Research (NIHR) in the form of a senior research fellowship. DPA’s group received partial support from the Oxford NIHR Biomedical Research Centre. DPA’s department has received grant/s from Amgen, Chiesi-Taylor, Lilly, Janssen, Novartis, and UCB Biopharma. His research group has received consultancy fees from AstraZeneca and UCB Biopharma. Amgen, Astellas, Janssen, Synapse Management Partners, and UCB Biopharma have funded or supported training programmes organised by DPA’s department. ÀRS reports honoraria for presentations, lectures, speakers, support for attending meetings and travel from Janssen, Astellas, and Bayer and support for attending meetings and travel from Ipsen outside the submitted work. FX-A-J is funded by Fundación Cientifica AECC (LABAE18025AVIL) and Plan Estatal de I+D+I of the Instituto de Salud Carlos III (FIS PI15/02047 and FIS PI18/0844). DN reports grants from OPTIMA and EHDEN. All other authors declare no conflicts of interest in this work.
References
- 1.Machii R, Takahashi H. Japanese cancer screening programs during the COVID-19 pandemic: changes in participation between 2017-2020. Cancer Epidemiol. 2023;82:102313. doi: 10.1016/j.canep.2022.102313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkatout I, Biebl M, Momenimovahed Z, et al. Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol. 2021;11:675038. doi: 10.3389/fonc.2021.675038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay NL, Pineda-Moncusí M, Jödicke AM, et al. The impact of the UK COVID-19 lockdown on the screening, diagnostics and incidence of breast, colorectal, lung and prostate cancer in the UK: a population-based cohort study. MedRxiv. 2023. doi: 10.1101/2023.07.21.23292937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Research UK. Cancer mortality statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/mortality#heading-Zero. Accessed September 28, 2023.
- 5.Rashid T, Bennett JE, Muller DC, et al. Mortality from leading cancers in districts of England from 2002 to 2019: a population-based, spatiotemporal study. Lancet Oncol. 2023;25:86–98. doi: 10.1016/S1470-2045(23)00530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–1505. doi: 10.1016/S1470-2045(19)30456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alderwick H. Is the NHS overwhelmed? BMJ. 2022;376:051. doi: 10.1136/bmj.o51 [DOI] [PubMed] [Google Scholar]
- 8.Carr A, Smith JA, Camaradou J, Prieto-Alhambra D. Growing backlog of planned surgery due to covid-19. BMJ. 2021;2021:372. [DOI] [PubMed] [Google Scholar]
- 9.McLellan A, Abbasi K. The NHS is not living with covid, it’s dying from it. BMJ. 2022;2022:378. [DOI] [PubMed] [Google Scholar]
- 10.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 12.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. 2015;22(3):553–564. doi: 10.1093/jamia/ocu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert J, Rao G, Schuemie M, Ryan P, Weaver J. CohortDiagnostics: diagnostics for OHDSI Cohorts. Available from: https://ohdsi.github.io/CohortDiagnostics. Accessed 2, August 2023.
- 15.Gallagher AM, Dedman D, Padmanabhan S, Leufkens HG, de Vries F. The accuracy of date of death recording in the clinical practice research Datalink GOLD database in England compared with the office for national statistics death registrations. Pharmacoepidemiol Drug Saf. 2019;28(5):563–569. doi: 10.1002/pds.4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eurostat Taskforce. Revision of the European Standard Population - Report of Eurostat’s task force. Available from: https://ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/ks-ra-13-028. Accessed August 2, 2023.
- 17.Mazidimoradi A, Hadavandsiri F, Momenimovahed Z, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer diagnosis and treatment: a systematic review. J Gastrointest Can. 2021;2021:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Research UK. Cancer incidence for all cancers combined. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/all-cancers-combined. Accessed August 9, 2023.
- 19.Strongman H, Williams R, Bhaskaran K. What are the implications of using individual and combined sources of routinely collected data to identify and characterise incident site-specific cancers? A concordance and validation study using linked English electronic health records data. BMJ open. 2020;10(8):e037719. doi: 10.1136/bmjopen-2020-037719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddams J, Utley M, Moller H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br J Cancer. 2012;107(7):1195–1202. doi: 10.1038/bjc.2012.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett R, Blanks R, Moss S. Evaluation of extension of breast screening to women aged 65–70 in England using screening performance measures. Br J Cancer. 2009;100(7):1043–1047. doi: 10.1038/sj.bjc.6604981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mora N, Guiriguet C, Cantenys R, et al. Cancer diagnosis in primary care after second pandemic year in Catalonia: a time-series analysis of primary care electronic health records covering about 5 million people. Family Practice. 2023;40(1):183–187. doi: 10.1093/fampra/cmac083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skovlund CW, Friis S, Dehlendorff C, Nilbert MC, Mørch LS. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta oncologica. 2021;60(1):20–23. doi: 10.1080/0284186X.2020.1858235 [DOI] [PubMed] [Google Scholar]
- 24.Nindenshuti PM, Caire-Juvera G. Changes in diet, physical activity, alcohol consumption, and tobacco use in adults during the COVID-19 pandemic: a systematic review. Inquiry. 2023;60:00469580231175780. doi: 10.1177/00469580231175780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey V, Mohan R, Kumar A, Gangadevi P, Kurien N. The impact of the COVID-19 outbreak on lifestyle-related behavior among the general population. Cureus. 2023;15(9):e45756. doi: 10.7759/cureus.45756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Concepcion J, Yeager M, Alfaro S, et al. Trends of cancer screenings, diagnoses, and mortalities during the COVID-19 pandemic: implications and future recommendations. Am Surg. 2022;14:31348221091948. doi: 10.1177/00031348221091948 [DOI] [PMC free article] [PubMed] [Google Scholar]