Abstract
Background:
The opioid epidemic has seen a drastic increase in the incidence of drug-associated infective endocarditis (IE). No clinical tool exists to predict operative morbidity and mortality in patients undergoing surgery.
Methods:
A multi-institutional database was reviewed between 2011 and 2018. Multivariate logistic regression was fitted in an automated stepwise fashion. The STratification risk analysis in OPerative management of drug-associated IE (STOP) score was constructed. Morbidity was defined as reintubation, prolonged ventilation, pneumonia, renal failure, dialysis, stroke, reoperation for bleeding, and a permanent pacemaker. Cross-validation provided an unbiased estimate of out-of-sample performance.
Results:
A total of 1181 patients underwent surgery for drug-associated IE (median age, 39; interquartile range [IQR], 30–54, 386 women [32.7%], 341 reoperations for prosthetic valve endocarditis [28.9%], 316 patients with multivalve disease [26.8%]). Operative morbidity and mortality were 41.1% and 5.9%, respectively. Predictors of morbidity were dialysis (95% confidence interval [CI], 1.16–2.82), emergent intervention (1.83–4.73), multivalve procedure (1.01–1.98), causative organisms other than Streptococcus (1.09–2.02), and type of valve procedure performed [aortic valve procedure (1.07–2.15), mitral valve replacement (1.03–2.05), tricuspid valve replacement (1.21–2.60)]. Predictors of mortality were dialysis (1.29–5.74), active endocarditis (1.32–83), lung disease (1.25–5.43), emergent intervention (1.69–6.60), prosthetic valve endocarditis (1.24–3.69), aortic valve procedure (1.49–5.92) and multivalve disease (1.00–2.95). Variables maximizing explanatory power were translated into a scoring system. Each point increased odds of morbidity and mortality by 22.0% and 22.4% with an accuracy of 94.0% and 94.1%, respectively.
CONCLUSION:
Drug-related IE is associated with significant morbidity and mortality. An easily-applied risk stratification score may aid in clinical decision-making.
Keywords: cardiac surgery, drug use, endocarditis, valve surgery
1 |. INTRODUCTION
The United States opioid epidemic has implicated a dramatic rise in morbidity and mortality,1 coinciding with a substantial increase in the incidence of infective endocarditis (IE).2 The standard of care for IE entails antibiotic treatment and, if indicated, valve surgery. Early surgical intervention is recommended for patients with complicated IE as defined by heart failure, heart block, risk of embolization, or uncontrolled infection.3 However, mortality rates of up to 20% and morbidity rates of 50% and above4 associated with surgery for active IE,2,5–7 a predominant young patient population fewer traditional cardiovascular comorbidities, as well as inadequate treatment of an underlying substance use disorder,8 make the decision and timing of operative intervention complex. The risk of reinfection and prosthetic valve endocarditis due to ongoing drug use is a particular concern, as repeated surgeries for drug-related endocarditis are associated with higher mortality.2
Data supporting early surgery for IE—regardless of drug use—are not consistent3,9 and the absence of randomization in observational studies makes it difficult to assess the impact of surgery on outcomes and identify predictors of morbidity and mortality for patients undergoing surgery. The objectives of this study are to identify risk factors for short-term morbidity and mortality in patients undergoing surgery for drug-associated IE from the large Multicenter Surgical Endocarditis Collaborative and develop an internally cross-validated clinical risk calculator to aid surgical decision making.
2 |. METHODS
2.1 |. Data collection and study design
The Multicenter Surgical Endocarditis Collaborative was formed among 10 tertiary care centers in the United States. We collected data from each institution’s Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) in a prospective fashion. Patients with a history of “illicit” drug use undergoing surgery for IE between 2011 and 2018 were included. STS data were deidentified from all institutions and kept in a secure folder at the University of Pittsburgh. IRB protocol was approved on 7/9/2019 at the University of Pittsburgh (STUDY19020090).
2.2 |. Statistical analysis
First, data were checked for normality. Descriptive statistics were presented as mean± standard deviation for continuous variables, median (interquartile range) for continuous variables in cases of nonnormality, and percentage (frequency) for categorical variables. Continuous variables were compared using independent sample t-test and Wilcoxon rank-sum test in cases of nonnormality. Chi-square statistics were used to compare categorical variables. All tests were 2-sided with the alpha level set at 0.05 for statistical significance. All statistical analysis and predictive models were coded in R (Version 3.5.3, “Great Truth”). The following R packages were used for analysis: MASS, boot, ROCR, dplyr, caret, sm, and forestplot.
2.3 |. Score generation
The Stratification Risk Analysis in Operative Management of Drug-induced Endocarditis (STOP) score was constructed using the following steps:
Step 1: Identification of candidate variables with plausibility to predict 30-day morbidity and mortality. Outcome measures followed the Society of Thoracic Surgeons (STS) definition of morbidity and mortality after index surgery. Additionally, major operative morbidity was defined as reintubation, prolonged ventilation, pneumonia, renal failure, dialysis, stroke, reoperation for bleeding, and need for a permanent pacemaker. Two separate models were constructed. Stepwise automated forward and backward logistic regression based on Akaike Information Criterion (AIC) as an estimator of out-of-sample prediction error was used to separately identify independent predictors of 30-day morbidity and mortality. All preoperative and intraoperative variables were fed into the model (Table S1). Variables meeting statistical significance for predicting operative morbidity after multivariate regression were: dialysis-dependent renal failure, emergent status, multivalve disease, causative organism other than Streptococcus, and type of procedure performed (aortic valve procedure, mitral valve replacement vs. mitral valve repair, and tricuspid valve replacement vs. tricuspid valve repair. Variables meeting statistical significance for prediciting operative mortality after multivariate regression were dialysis-dependent renal failure, active endocarditis, lung disease at baseline, emergent status, prosthetic valve endocarditis, aortic valve procedure, and multivalve disease.
Step 2: From the final logistic regression model as selected by AIC, a 19-point score was apportioned for morbidity prediction and 33-point sore was apportioned for mortality prediction using linear prediction:
Step 3: Cross-validation and out-of-sample performance. K-fold cross-validation using the R function “cvglm” in the boot package was used to provide an unbiased estimate of the out-of-sample performance of the presented STOP score stratified by morbidity and mortality. 80% of the data set was used for model training and calibration; the remaining 20% was used for model validation resulting in 5 k-folds. Models were coded with 10,000 iterations and bootstrap replications. Model discriminative power and calibration were assessed using C statistics.
3 |. RESULTS
3.1 |. Cohort statistics at baseline
A total of 1181 patients underwent cardiac surgery for complications related to IE between 2011 and 2018 at 10 centers in the US. Overall operative morbidity and mortality were 41.1% (n = 485) and 5.9% (n = 70), respectively. Patient characteristics and baseline demographics stratified by major operative morbidity and mortality are detailed in Table 1 and Table 2, respectively. Patients suffering major operative morbidity had a higher frequency of hypertension (44.3% vs. 38.5%), diabetes (17.7% vs. 11.5%), hyperlipidemia (30.1% vs. 23.9%), renal failure (GFR: 68.7 ml/h vs. 84.3 ml/h; dialysis: 11.1% vs. 6.2%), peripheral vascular disease (10.3% vs. 6.3%), immunosuppression (8.5% vs. 5.5%), previous MI (15.7% vs. 11.5%), CHF (53.2%, vs. 35.6%), multivalve disease (34.0% vs. 21.6%) and were more likely to presented in shock (12.4% vs. 1.6%). Of note, causative organism other than Streptococcus was associated with higher incidence of morbidity (80.8% vs 74.0%). Similarly, patients sustaining operative mortality had a higher frequency of hypertension (52.9% vs. 40.1%), hyperlipidemia (38.6% vs. 25.7%), renail failure (GFR: 58.4 ml/h vs. 80.8 ml/h; dialysis: 15.7% vs. 7.7%), peripheral vascular disease (15.7% vs. 7.5%), lung disase (15.7% vs. 7.7%), CHF (57.1% vs. 41.9%), prior CABG (12.9% vs. 4.4%), prior valve surgery (48.6% vs. 32.7%), reoperative surgery (48.6% vs. 34.7%), multivalve disease (45.7% vs. 25.5%), shock (18.6% vs. 5.2%), involvement of the native aortic valve (44.3% vs. 33.5%) as well as prosthetic valve endocarditis (42.9% vs. 28.0%).
TABLE 1.
Preoperative and operative patient characteristics stratified by major operative morbidity
| Variable | Major operative morbiditya* |
P value | |
|---|---|---|---|
| Yes (n = 485) | No (n = 696) | ||
| Demographics | |||
| Age | 42 (31‐55) | 38 (29‐52) | .007 |
| Sex | |||
| Female | 152 (31.3) | 234 (33.6) | .411 |
| BMI (kg/m2) | 25.9 (22.3‐31.2) | 24.8 (21.6‐28.4) | <.001 |
| Race | |||
| White | 381 (78.6) | 566 (81.3) | .241 |
| Black | 70 (14.4) | 85 (12.2) | .266 |
| Other | 34 (7.0) | 45 (6.5) | .712 |
| Hypertension | 215 (44.3) | 268 (38.5) | .038 |
| Diabetes | 86 (17.7) | 80 (11.5) | .002 |
| Non‐insulin‐dependent | 51 (10.5) | 47 (6.8) | .021 |
| Insulin‐dependent | 35 (7.2) | 33 (4.7) | .072 |
| Hyperlipidemia | 146 (30.1) | 166 (23.9) | .012 |
| Renal status | |||
| GFR (ml/min) | 68.7 (42.9‐98.1) | 84.3 (64.2‐104.8) | <.001 |
| Dialysis | 54 (11.1) | 43 (6.2) | .002 |
| Peripheral vascular disease | 50 (10.3) | 44 (6.3) | .011 |
| Previous stroke | 130 (26.8) | 177 (25.4) | .597 |
| Lung disease | 47 (9.7) | 49 (7.0) | .074 |
| Immunosuppression | 41 (8.5) | 38 (5.5) | .042 |
| Previous MI | 76 (15.7) | 80 (11.5) | .026 |
| Previous PCI | 22 (4.5) | 20 (2.9) | .129 |
| CHF | 258 (53.2) | 248 (35.6) | <.001 |
| Prior cardiac surgery | |||
| Previous CABG | 27 (5.6) | 27 (3.9) | .171 |
| Previous valve surgery | 169 (34.8) | 228 (32.8) | .450 |
| Incidence | |||
| Re‐operative cardiovascular surgery | 182 (37.5) | 238 (34.2) | .247 |
| Number of valves affected | |||
| 1 | 320 (66.0) | 546 (78.5) | <.001 |
| ≥2 | 165 (34.0) | 150 (21.6) | |
| Presenting in shock | 60 (12.4) | 11 (1.6) | <.001 |
| Urgency of intervention | |||
| Elective | 81 (16.7) | 147 (21.1) | .058 |
| Urgent/emergent | 404 (83.3) | 549 (78.9) | |
| Cultured organism | |||
| Staphylococcus aureus | 173 (35.7) | 212 (30.5) | .060 |
| Streptococcus | 93 (19.2) | 181 (26.0) | .006 |
| Coagulase‐negative staphylococcus | 15 (3.1) | 17 (2.4) | .498 |
| Enterococcus | 63 (13.0) | 86 (12.4) | .747 |
| Fungal | 20 (4.1) | 34 (4.9) | .538 |
| Other | 86 (17.7) | 125 (18.0) | .920 |
| Culture negative | 35 (7.2) | 41 (5.9) | .361 |
| Operative details | |||
| Native valve procedure | |||
| Aortic valve replacement | 161 (33.2) | 242 (34.8) | .705 |
| Mitral valve procedure | |||
| Mitral valve replacement | 107 (22.1) | 131 (18.8) | .034 |
| Mitral valve repair | 45 (9.3) | 89 (12.8) | .140 |
| Tricuspid valve procedure | |||
| Tricuspid valve replacement | 88 (18.4) | 93 (13.4) | .004 |
| Tricuspid valve repair | 38 (7.8) | 72 (10.3) | .275 |
| Bioprosthetic valve replacement | 155 (32.0) | 186 (26.7) | .051 |
Abbreviations: CABG; coronary artery bypass graft, CHF; congestive heart failure, MI; myocardial infarction, PCI; percutaneous coronary intervention.
Note: Data presented as median (interquartile range), n (%).
Defined as reintubation, prolonged ventilation, pneumonia, renal failure, dialysis, stroke, reoperation for bleeding, and pacemaker.
TABLE 2.
Preoperative and operative patient characteristics stratified by operative mortality
| Variable | Operative mortality |
P value | |
|---|---|---|---|
| Yes (n =70) | No (n = 1111) | ||
| Demographics | |||
| Age | 39 (30–53) | 43 (32–56) | .085 |
| Sex | |||
| Women | 25 (35.7) | 361 (32.5) | .577 |
| BMI (kg/m2) | 27.7 (22.6–32.3) | 25.1 (21.8–29.4) | .017 |
| Race | |||
| White | 55 (78.6) | 892 (80.3) | .727 |
| Black | 10 (14.3) | 145 (13.1) | .767 |
| Other | 5 (7.1) | 74 (6.7) | .876 |
| Hypertension | 37 (52.9) | 446 (40.1) | .038 |
| Diabetes | 13 (18.1) | 158 (14.0) | .339 |
| Non‐insulin‐dependent | 6 (8.6) | 92 (8.3) | .932 |
| Insulin‐dependent | 6 (8.6) | 62 (5.6) | .297 |
| Hyperlipidemia | 27 (38.6) | 285 (25.7) | .019 |
| Renal status | |||
| GFR (ml/min) | 58.4 (33.5–87.4) | 80.8 (56.2–104.1) | <.001 |
| Dialysis | 11 (15.7) | 86 (7.7) | .019 |
| Peripheral vascular disease | 11 (15.7) | 83 (7.5) | .013 |
| Previous stroke | 22 (31.4) | 285 (25.7) | .285 |
| Lung disease | 11 (15.7) | 85 (7.7) | .014 |
| Immunosuppression | 6 (8.6) | 73 (6.6) | .500 |
| Previous MI | 14 (20.0) | 142 (12.8) | .072 |
| Previous PCI | 3 (4.3) | 39 (3.5) | .736 |
| CHF | 40 (57.1) | 466 (41.9) | .018 |
| Prior cardiac surgery | |||
| Previous CABG | 9 (12.9) | 45 (4.1) | .001 |
| Previous valve surgery | 34 (48.6) | 363 (32.7) | .007 |
| Incidence | |||
| Re‐operative cardiovascular surgery | 34 (48.6) | 386 (34.7) | .019 |
| Number of valves affected | |||
| 1 | 38 (54.3) | 828 (74.5) | <.001 |
| ≥2 | 32 (45.7) | 283 (25.5) | |
| Presenting in shock | 13 (18.6) | 58 (5.2) | <.001 |
| Urgency of intervention | |||
| Elective | 9 (12.9) | 219 (19.7) | .159 |
| Urgent/emergent | 61 (87.1) | 892 (80.3) | |
| Cultured organism | |||
| Staphylococcus aureus | 24 (34.3) | 361 (32.5) | .756 |
| Streptococcus | 11 (15.7) | 263 (23.7) | .126 |
| Coagulase‐negative staphylococcus | 3 (4.3) | 29 (2.6) | .402 |
| Enterococcus | 11 (15.7) | 138 (12.4) | .421 |
| Fungal | 3 (4.3) | 51 (4.6) | .906 |
| Other | 12 (17.1) | 199 (17.9) | .871 |
| Culture negative | 6 (8.6) | 70 (6.3) | .453 |
| Operative details | |||
| Native valve procedure | |||
| Aortic valve replacement | 31 (44.3) | 372 (33.5) | <.001 |
| Mitral valve procedure | |||
| Mitral valve replacement | 10 (14.3) | 228 (20.5) | .632 |
| Mitral valve repair | 7 (10.0) | 127 (11.4) | .784 |
| Tricuspid valve procedure | 7 (10.0) | 174 (15.7) | .523 |
| Tricuspid valve replacement | 5 (7.1) | 105 (9.5) | .909 |
| Tricuspid valve repair | |||
| Bioprosthetic valve replacement | 30 (42.9) | 311 (28.0) | .008 |
Abbreviations: CABG, coronary artery bypass graft; CHF, congestive heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Note: Data presented as median (interquartile range), n (%).
3.2 |. Operative details
Isolated aortic valve replacement was the most common procedure performed for IE (32.4%), followed by isolated mitral valve procedures (21.2%) and isolated tricuspid valve procedures. Multivalve procedures were performed in 26.8% of patients. The mitral valve was repaired in 32.1% of cases, the tricuspid valve was repaired in 38.6% of cases. 28.9% were reoperations for prosthetic valve endocarditis.
3.3 |. STOP score, identification of candidate variables, and score generation
An automated stepwise approach was chosen to select independent predictors of both operative morbidity and mortality. Using β-coefficients generated from multivariable logistic regression, weights were assigned to independent variables to create the Stratification Risk Analysis in Operative Management of Drug-induced Endocarditis (STOP). Two models were created to assess independent predictors for major operative morbidity and operative mortality. Both final models consisted of seven variables maximizing explanatory power.
3.4 |. Prediction of operative morbidity
Cumulative operative morbidity was 41.1% and consisted of reintubation (9.1%), prolonged ventilation (27.5%), pneumonia (5.2%), renal failure (8.8%), dialysis (5.9%), stroke (2.0%), reoperation for bleeding (4.9%) and need for permanent pacemaker (15.2%). Variables meeting statistical significance in multivariate regression were (Figure 1A): Dialysis-dependent renal failure (odds ratio [OR], 1.80; 95% confidence interval [CI], 1.16–2.82; p = .009), need for emergent surgical intervention (OR, 2.91; CI, 1.83–4.73), multivalve disease (OR, 1.41; 95%CI, 1.01–1.98), causative organism other than Streptococcus (OR, 1.48; 95%CI 1.09–2.02), aortic valve replacement (OR, 1.51; 95%CI, 1.07–2.15), mitral valve replacement (OR, 1.45; 95%CI, 1.03–2.05) and tricuspid valve replacement (OR, 1.77; 95%CI, 1.21–2.60).
FIGURE 1.
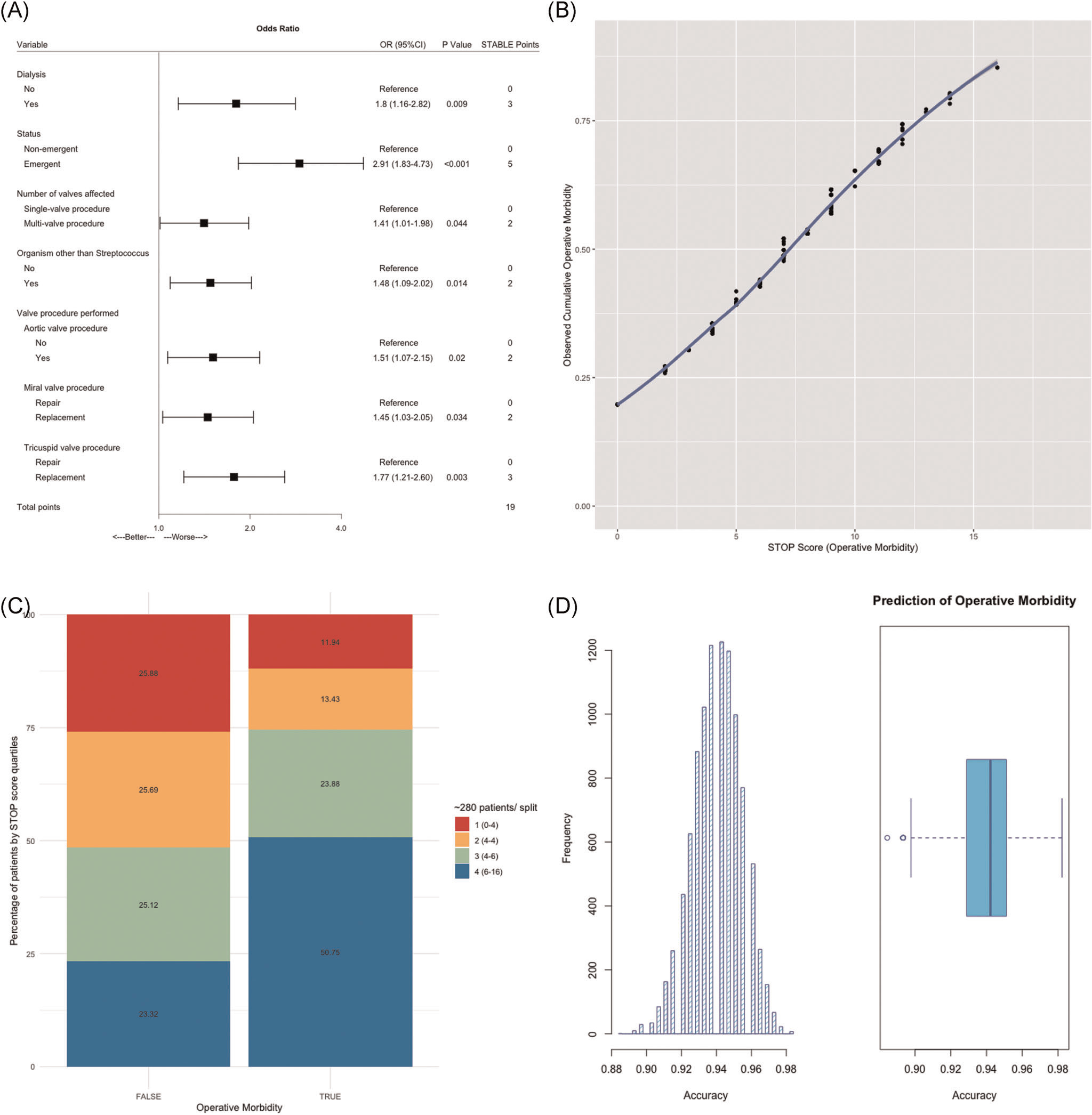
Performance of the STOP score in predicting operative morbidity. (A) Final multivariate logistig regression. The model was selected based on AIC. STOP risk points for each selected independent variable via approximation of the magnitude of β-coefficients. (B) STOP score is presented as a function of predicted operative morbidity. Each point increased odds of operative morbidity by 22% (OR, 1.22; 95% CI, 1.16–1.28; p < .001). (C) STOP score by quartile split. The majority of patients with operative morbidity (50.8%) had STOP scores in the highest quartile (6–16 points). (D) Out-of-sample performance. K-fold cross-validation with 10,000 bootstrap replications provided an unbiased estimate of out-of-sample performance and yielded a receiver operating characteristic area under the curve (ROC AUC) of 94.0%. AIC, Akaike Information Criterion; CI, confidence interval; OR, odds ratio; STOP, STratification risk analysis in OPerative management of drug-associated IE
3.5 |. STOP score distribution, performance, and cross-validation
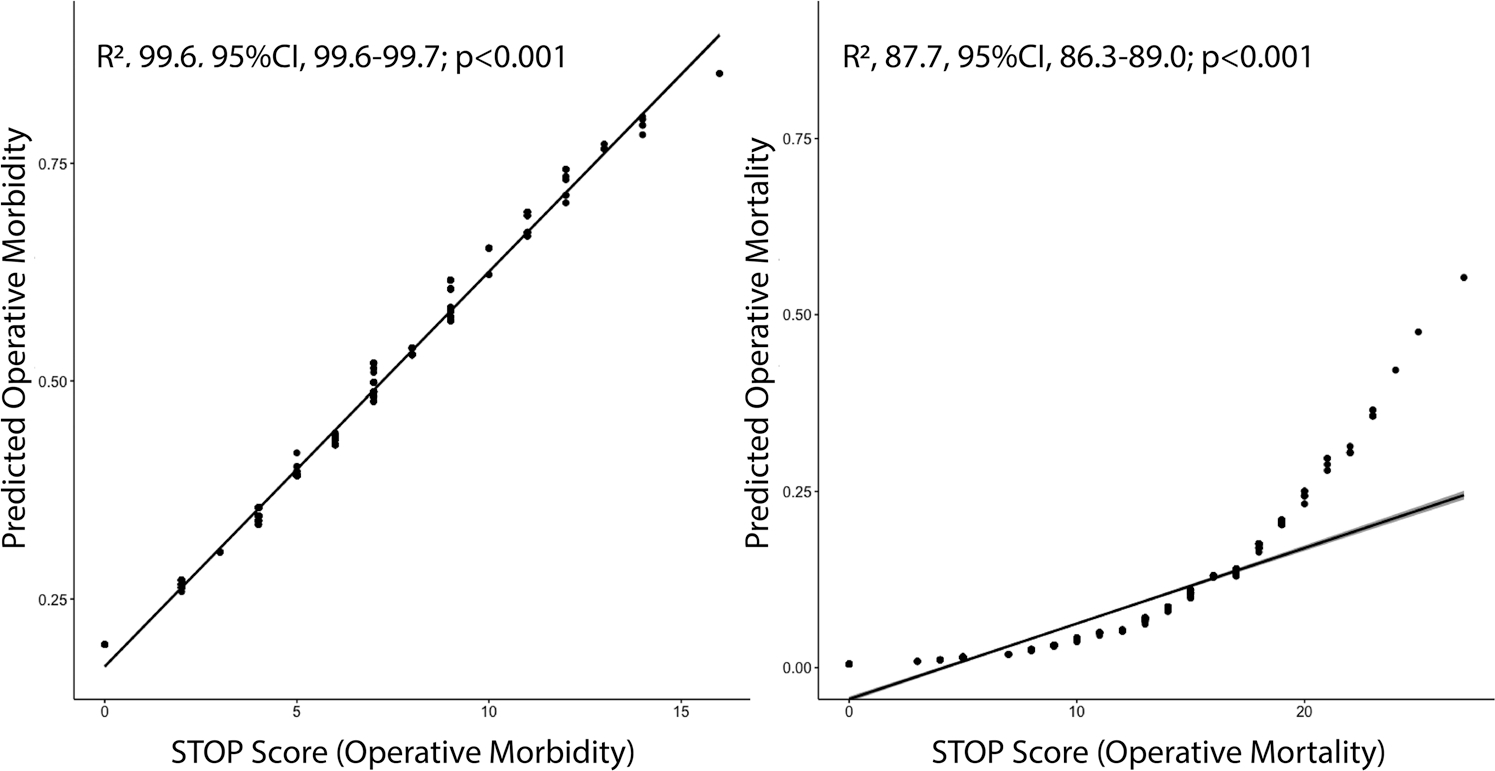
STOP scores in the derivation data set predicting operative morbidity ranged from 0 points to 16 points, with a possible total of 19 points. Predicted operative morbidity ranged from 19.7% (0 STOP points) to 84.9% (16 STOP points, Figure 1B). No patient in the derivation data set reached the maximal score of 19 points which translated to predicted morbidity of 91.0%. Both mean (6.8 vs. 4.9, p < .001) and median (6 vs. 4, p < .001) STOP scores were significantly higher for patients suffering operative morbidity. The STOP score led to the excellent characterization of operative morbidity status and highly correlated with the observed outcome (R2, 99.6, 95% CI, 99.6–99.7; p < .001, (Figure 3). As a continuous variable the STOP score was highly associated with operative morbidity (OR, 1.22; 95%CI, 1.16–1.28; p < .001). Each point increased the odds of morbidity by 22% (Figure 1B). When splitting the STOP score into quartiles, the majority of patients with operative morbidity (50.8%) had STOP scores in the highest quartile (6–16 points). Observed morbidity by quartile split of the STOP score is detailed in Figure 1C. Plots with Kernel smoothing stratified by operative morbidity and empirical cumulative distribution plots (Supplemental Figure 1) were generated. Again, the STOP score was higher among patients suffering operative morbidity (p < .001). Out-of-sample performance as assessed by cross-validation with 10,000 bootstrap replications yielded an unbiased receiver operating characteristic (ROC) area under the curve (AUC) accuracy of 94.0%, reflecting excellent model performance (Figure 1D).
FIGURE 3.
Correlation of STOP score with predicted operative morbidity and mortality. The STOP score led to the excellent characterization of operative morbidity status and highly correlated with the observed outcome (R2, 99.6; 95% CI, 99.6–99.7; p < .001). It also highly correlated with the observed outcome (R2, 87.7; 95% CI, 86.3–89.0; p < .001). CI, confidence interval; OR, odds ratio; STOP, STratification risk analysis in OPerative management of drug-associated IE
3.6 |. Prediction of operative mortality
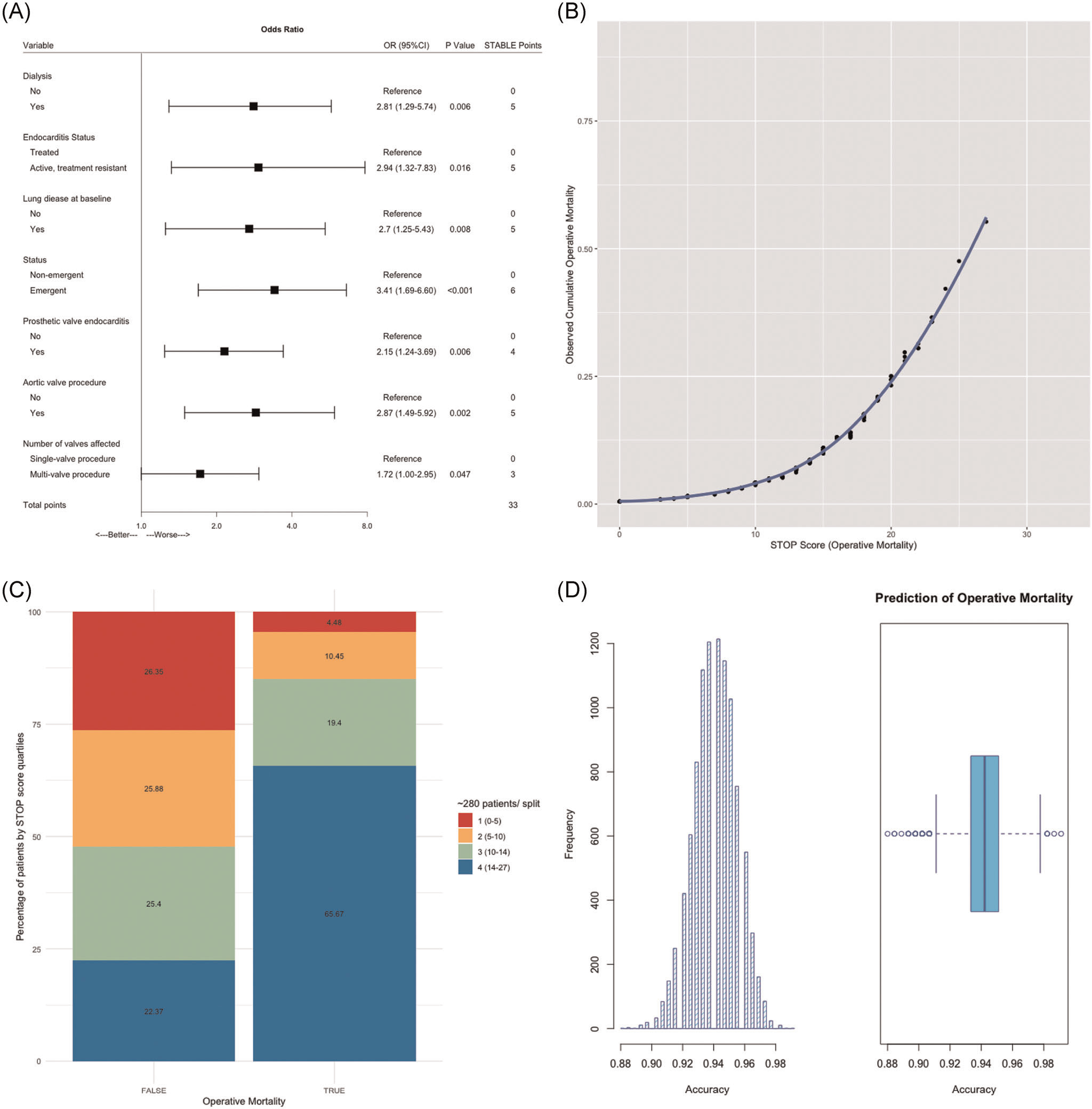
Operative mortality was 5.9%. Variables maximizing explanatory power (Figure 2A) were dialysis-dependent renal failure (OR, 281; 95% CI, 1.29–5.74), active endocarditis at time of surgery (OR, 2.94; 95% CI, 1.32–7.83), lung disease (OR, 2.70; 95% CI, 1.25–5.43), emergent surgical intervention (OR, 3.41; 95% CI, 1.69–6.60), prosthetic valve endocarditis (OR, 2.15; 95% CI, 1.24–3.69), aortic valve replacement (OR, 2.87; 95% CI, 1.49–5.92), and multivalve disease (OR, 1.72; 95% CI, 1.00–2.95).
FIGURE 2.
Performance of the STOP score in predicting operative mortality. (A) Final multivariate logistig regression. The model was selected based on AIC. STOP risk points for each selected independent variable via approximation of the magnitude of β-coefficients. (B) STOP score is presented as a function of predicted operative mortality. Each point increased odds of operative mortality by 22.4% (OR, 1.22; 95% CI, 1.16–1.29; p < .001). (C) STOP score by quartile split. The majority of patients with operative mortality (65.7%) had STOP scores in the highest quartile (14–27 STOP points), conversely, the majority of patients alive during the same time period (26.4%) had STOP scores in the lowest quartile (0–5 STOP points). (D) Out-of-sample performance. K-fold cross-validation with 10,000 bootstrap replications provided an unbiased estimate of out-of-sample performance and yielded a Receiver Operating Characteristic Area Under the Curve (ROC AUC) of 94.1%. AIC, Akaike Information Criterion; CI, confidence interval; OR, odds ratio; STOP, STratification risk analysis in OPerative management of drug-associated IE
3.7 |. STOP score distribution, performance, and cross-validation
STOP scores in the derivation data set predicting operative mortality ranged from 0 to 27 STOP points correlating with a predicted mortality of 0.5%–56.2% (Figure 2B). No patient in the derivation data set achieved the maximal score of 33 points. Higher STOP score of 28–33 points correlated with an operative mortality of 61.1%–81.2%. Both mean (14.7 vs. 9.4, p < .001) and median (15.0 vs. 10.0, p < .001) STOP scores were significantly higher for patients with operative mortality.
Similar to the prediction of morbidity, the STOP score led to the excellent characterization of operative mortality status and highly correlated with the observed outcome (R2, 87.7; 95%CI, 86.3–89.0; p < .001; Figure 3). As a continuous variable, the STOP score was highly associated with operative mortality (OR, 1.22; 95% CI, 1.16–1.29; p < .001). Each point increased the odds of operative mortality by 22.4% (Figure 2B). When splitting the STOP score into quartiles (Figure 2C), the majority of patients with operative mortality (65.7%) had STOP scores in the highest quartile (14–27 STOP points). When looking into patients alive during the same time period, the majority of patients (26.4%) had STOP scores in the lowest quartile (0–5 STOP points). Observed mortality by quartile split of the STOP score is detailed in Figure 2C. Again, plots with Kernel smoothing stratified by operative mortality and empirical cumulative distribution plots were generated (Supplemental Figure 2).
Out-of-sample performance as assessed by cross-validation with 10,000 bootstrap replications yielded an unbiased receiver operating characteristic (ROC) area under the curve (AUC) accuracy of 94.1%, reflecting excellent model performance (Figure 2D).
4 |. DISCUSSION
Infective endocarditis (IE) is a complex disease with wide variations in both clinical course and prognosis.10 While established and validated criteria exist for the diagnosis of IE (Duke Criteria),11,12 the decision to perform surgery in complicated IE is primarily based on observational data and expert opinions.13 Further, little is known about patient-level prognostic factors for morbidity and mortality. Our goal was to (1) use data from the large Multicenter Surgical Endocarditis Collaborative including 10 high-volume centers in the USA, (2) apply automated statistical algorithms to derive a simplified tool using readily available clinical variables at the time of IE diagnosis for predicting operative morbidity and operative mortality, (3) apportion risk points for each selected independent variable via approximation of the magnitude of β-coefficients and (4) validate out-of-sample performance in an unbiased fashion using cross-validation algorithms. The resulting tool, the STOP score, compiles 7 patient-level variables to separately characterize and predict short-term morbidity and mortality. STOP score points are additive in nature with a maximum of 19 possible points for characterization of morbidity and 33 points for characterization of mortality and are highly correlated with operative outcomes for IE (morbidity: R2, 99.6; 95% CI, 99.6–99.7; p < .001; mortality: R2, 87.7; 95% CI, 86.3–89.0; p < .001). This strong association translates into a 22.0% relative increase in operative morbidity and 22.4% relative increase in operative mortality for each added STOP point from baseline.
In our multi-institutional collaboration of 1181 IE patients2 with low median age and few comorbidities2 undergoing surgery for IE overall operative morbidity and mortality was rather low with 41.1% and 6%, respectively. Major morbidity was defined as reintubation, prolonged ventilation, pneumonia, renal failure, dialysis, stroke, reoperation for bleeding, and need for a permanent pacemaker. We have previously reported our multi-institutional investigation of risk of IE-related reoperative valve surgery2 and now characterize independent predictors of operative morbidity and mortality in form of a risk scoring system including more centers and a larger patient cohort.
Previous studies have evaluated clinical characteristics associated with short-term morbidity and mortality in IE.4,6,14 Chu et al.6 identified diabetes (OR, 2.48), staphylococcus aureus as causative organism (OR, 2.06), APACHE II score (OR, 1.07), and embolic events (OR, 2.79) as independent predictors for in-hospital mortality in 267 IE patients as defined by modified Duke criteria. In the subsequent large International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) on 2781 patients admitted to 58 hospitals in 25 countries, prosthetic valve involvement (OR, 1.47), age (OR, 1.30), pulmonary edema (OR, 1.79), staphylococcus aureus infection (OR, 1.54), coagulase-negative staphylococcal infection (OR 1.50), mitral valve vegetation (OR 1.34), and paravalvular complications (OR, 2.25) were associated with an increased risk of in-hospital death.15 Even though both studies6,15 aimed at identifying predictors of short-term mortality, the discrepancy in risk factor profiles compared to our study might be explained by the fact that both studies included patient cohorts treated by surgical and medical approaches. Further, identified variables were not used to derive a clinically applicable risk calculator.
Similar to our approach, previous reports attempted to derive and validate prognostic risk score models for IE patients from multivariate models for both operative morbidity and mortality.4,13,14 Gaca et al.4 reported operative morbidity of 53% and operative mortality of 8.2% for 19,543 patients undergoing surgery for IE in the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) between 2002 and 2008 and used logistic regression to derive a simplified risk scoring model. Overall, identified predictors for the combined endpoint morbidity or mortality were comparable to our study with a reported model predictive accuracy of 75.8%4: emergency or salvage status, multiple valve procedure, prior valve surgery, active endocarditis, preoperative hemodialysis, and renal failure. In addition, women, BSA > 1.9, age >60 years, prior CABG, preoperative balloon pump or inotropic support, diabetes NYHA class IV, and arrythmia were described as independent predictors. Despite the large patient collective, the STS ACSD did not use a validation cohort to assess model calibration and performance.4 Our presented study is unique in the sense that predictors were separately characterized for morbidity and mortality and internal cross-validation was applied to obtain an unbiased estimate of out-of-sample performance and model predictive accuracy of ≥94.0% after 10,000 bootstrap replications far exceed the power of other platforms.4
Longer-term studies have tried to identify risk factors for mortality beyond 30 days.13,14 The ICE-PCS investigators identified host factors (age, dialysis), IE characteristics (prosthetic or nosocomial IE, causative organism, left-sided valve vegetation), and IE complications (severe heart failure, stroke, paravalvular complication, and persistent bacteremia) as risk factors for 6-month mortality in 4049 patients.14 The authors created a simplified risk model by weight adjustment of these variables.14 Hasbun et al.13 used five baseline features (comorbidity [p = .03], abnormal mental status [p = .02], moderate to severe congestive heart failure [p = .01], bacterial etiology [p < .001], and medical therapy without valve surgery [p = .002]) to classify patients with left-sided IE into four prognostic groups.
In accordance with previously published work, we noticed large variations in prognosis after risk stratification into prognostic groups.13 To provide clinical examples, a patient presenting for first-time surgery for active IE with singular valve involvement, maintained renal function, however, baseline lung disease would accumulate 11 points on the STOP score scale with a predicted 30-day mortality of 1.5%. On the other end of the spectrum, a patient presenting for reoperative surgery for active IE due to drug use with multivalve involvement and impaired renal function at baseline requiring emergent intervention would accumulate 24 STOP points with a drastically worse short-term prognosis and predicted 30-day mortality of 17.8%.
Although our study has several methodological advantages due to cohort size and statistical algorithms applied, we must recognize several limitations. The presented cohort of patients represents a surgical subset of patients with injection drug-induced IE and, therefore, we were unable to compare outcomes to those who were treated with medical therapy alone or include the medical cohort into our statistical modeling. Further, time from clinical diagnosis to surgery was not recorded and patients failing initial medical management with a secondary indication for surgery versus patients meeting initial criteria for surgery were not accounted for in the model, introducing further potential bias.
In conclusion, the surgical patient with IE remains a significant challenge. We provide a novel and internally cross-validated clinical tool to predict operative morbidity and operative mortality with high predictive accuracy. This simplified risk calculator may permit risk stratification and is intended to aid surgical decision-making. Future studies should consider evaluating this tool in populations of patients managed both medically and surgically to understand the relative benefits and risks of surgery in IE management.
4.1 |. Study limitations
Lastly, several limitations are inherent to the study design. Even though data were collected prospectively, this study presents a retrospective cohort study. The current STS risk calculator does not capture multivalve procedures, therefore STS predicted risk of mortality (PROM) scores were not available in all patients, and comparisons relating to predictive power and risk stratification between STS PROM score and STOP score were not feasible. Data were collected from large tertiary centers well experienced in the management of endocarditis patients and results might differ in smaller centers. Twenty patients had missing data and were excluded from this study. Further bias might be introduced by not including more patient-specific baseline variables such as detailed echocardiographic data as well as operative details such as bypass and cross-clamp times.
Supplementary Material
ABBREVIATIONS:
- IE
infective endocarditis
- STOP
STratification risk analysis in OPerative management of drug-associated IE
- STS
society of thoracic surgery
Footnotes
CONFLICT OF INTEREST
TG serves on the advisory board for Abbott. AK serves on the young advisory board for Medtronic. TK receives personal fees from Edwards, Medtronic, Abbott and Baylis. IS receives institutional research funding from Medtronic and Atricure. None of these are related to this manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Cook CC, Rankin JS, Roberts HG, et al. The opioid epidemic and intravenous drug-associated endocarditis: a path forward. J Thorac Cardiovasc Surg. 2019;159:1273–1278. [DOI] [PubMed] [Google Scholar]
- 2.Mori M, Bin Mahmood SU, Schranz AJ, et al. Risk of reoperative valve surgery for endocarditis associated with drug use. J Thorac Cardiovasc Surg. 2020;159:1262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang DH. Timing of surgery in infective endocarditis. Heart. 2015; 101(22):1786–1791. [DOI] [PubMed] [Google Scholar]
- 4.Gaca JG, Sheng S, Daneshmand MA, et al. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg. Jan 2011;141(1):98–106. [DOI] [PubMed] [Google Scholar]
- 5.Delahaye F, Alla F, Béguinot I, et al. In-hospital mortality of infective endocarditis: prognostic factors and evolution over an 8-year period. Scand J Infect Dis. 2007;39(10):849–857. [DOI] [PubMed] [Google Scholar]
- 6.Chu VH, Cabell CH, Benjamin DK, et al. Early predictors of in-hospital death in infective endocarditis. Circulation. 2004;109(14):1745–1749. [DOI] [PubMed] [Google Scholar]
- 7.Kemp CD, Arnaoutakis GJ, George TJ, et al. Valve surgery for infective endocarditis is associated with high hospital charges. J Heart Valve Dis. 2013;22(1):110–117. [PubMed] [Google Scholar]
- 8.Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481–485. [DOI] [PubMed] [Google Scholar]
- 9.Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466–2473. [DOI] [PubMed] [Google Scholar]
- 10.Sy RW, Chawantanpipat C, Richmond DR, Kritharides L. Development and validation of a time-dependent risk model for predicting mortality in infective endocarditis. Eur Heart J. 2011;32(16):2016–2026. [DOI] [PubMed] [Google Scholar]
- 11.Sekeres MA, Abrutyn E, Berlin JA, et al. An assessment of the usefulness of the Duke criteria for diagnosing active infective endocarditis. Clin Infect Dis. 1997;24(6):1185–1190. [DOI] [PubMed] [Google Scholar]
- 12.Hoen B, Selton-Suty C, Danchin N, et al. Evaluation of the Duke criteria versus the Beth Israel criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 1995;21(4):905–909. [DOI] [PubMed] [Google Scholar]
- 13.Hasbun R, Vikram HR, Barakat LA, Buenconsejo J, Quagliarello VJ. Complicated left-sided native valve endocarditis in adults: risk classification for mortality. JAMA. 2003;289(15):1933–1940. [DOI] [PubMed] [Google Scholar]
- 14.Park LP, Chu VH, Peterson G, et al. Validated Risk Score for Predicting 6-Month Mortality in Infective Endocarditis. J Am Heart Assoc. 2016;5(4):e003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murdoch DR. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


