Abstract
Baricitinib is approved for the treatment of rheumatoid arthritis (RA) in more than 70 countries, and juvenile idiopathic arthritis (JIA) in the European Union. Population pharmacokinetic (PK) models were developed in a phase 3 trial to characterize PK in pediatric patients with JIA and identify weight‐based dosing regimens. The phase 3, randomized, double‐blind, placebo‐controlled withdrawal, efficacy and safety trial, JUVE‐BASIS, enrolled patients (aged 2 to <18 years) with polyarticular course JIA. During a safety/PK period, baricitinib concentration data from age‐based dose cohorts were compared to concentrations from adult patients receiving 4‐mg QD. PK data were used to develop a population PK model with allometric scaling to determine a weight‐based posology in pediatric patients with JIA that matched the adult 4‐mg exposure. Baricitinib plasma concentrations from 217 pediatric patients were used to characterize PK. Based on the adult model, pediatric PK was best described using a 2‐compartment model with allometric scaling on clearance and volume of distribution and renal function (estimated with glomerular filtration rate [GFR], a known covariate affecting PK of baricitinib) on clearance. The PK modeling suggested the optimal dosing regimen based on weight for pediatric patients as: 2‐mg QD for patients 10 to <30 kg and 4‐mg QD for patients ≥30 kg. The use of a population PK model of baricitinib treatment in adult patients with RA, with the addition of allometric scaling for weight on clearance and volume terms, was useful to predict exposures and identify weight‐based dosing in pediatric patients with JIA.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THIS TOPIC?
Modeling‐based methodology is increasingly used in clinical trial development to predict posology. Allometric scaling of adult pharmacokinetic (PK) parameters is a useful method for determining dosage in pediatric populations.
WHAT QUESTION DID THIS STUDY ADDRESS?
Based on a PK model of baricitinib developed in adult patients, we investigated the use of modeling‐based methodology to characterize baricitinib pharmacokinetics with age‐based dosing in the pediatric population of patients with juvenile idiopathic arthritis (JIA), and predict, a more preferable, weight‐based dosing regimen.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This work represents another example that the adult PK model incorporating allometric scaling with exponent fixed to 0.75 and 1 for clearance‐ and volume‐related parameters can well predict PK in pediatric patients. Model‐based simulations allowed for converting age‐based dosing to weight‐based dosing that was not studied in the clinical trial to optimize dosing in pediatric patients.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
The results from these analyses provide a recommended dosage of baricitinib in a distinct pediatric population, which may influence the posology for other pediatric indications. More broadly, the successful use of PK modeling‐based methodology to determine parameters based on adult models contributes to advances in the development of treatments for vulnerable populations.
INTRODUCTION
Baricitinib is a Janus kinase (JAK) inhibitor demonstrating selectivity for and inhibition of JAK1 and JAK2 with lower potency toward inhibition of JAK3 or TYK2. 1 Juvenile idiopathic arthritis (JIA) is a disease distinct from rheumatoid arthritis (RA) and is defined as a heterogeneous group of rheumatic diseases with onset in patients prior to 16 years of age, persisting for more than 6 weeks, and of unknown etiology. 2 While the etiology of JIA is still poorly understood, its pathogenesis shares several immunological abnormalities as observed in adult patients with RA. 3
Inhibition of JAK‐Signal Transducer and Activator of Transcription (STAT) signaling by baricitinib can target multiple JIA‐associated cytokine pathways and may provide novel therapeutic approaches to disease management. 4 , 5 Baricitinib has demonstrated clinical safety and efficacy in adult patients with RA in 4 completed Phase 3 studies. 6 , 7 , 8 , 9 Throughout the adult RA clinical trial program, baricitinib 4‐mg once daily (QD) was efficacious across domains of efficacy analogous to those in JIA, including signs and symptoms, physical function, reduction of radiographic progression, and other patient‐reported outcomes, such as pain and morning joint stiffness. 10 Furthermore, the 4‐mg QD dose has demonstrated an optimal risk/benefit balance compared to the 2‐mg QD dose. 11 The 4‐mg dose is approved in the EU, Japan, and other geographical regions for the treatment of adult patients with moderate‐to‐severe RA. 12 In addition, baricitinib is an orally ingested product, which may be preferable to injectable biologic agents for both patients and caregivers/legal guardians. 13 In adult patients with RA, baricitinib displays linear PK with minimum accumulation with repeated dosing. 14
In a phase 3 placebo‐controlled withdrawal, efficacy and safety trial, (JUVE‐BASIS; NCT03773978), baricitinib treatment was shown to be efficacious with an acceptable safety profile in the treatment of pediatric patients (aged 2 to <18 years) with polyarticular JIA who have had an inadequate response or intolerance to one or more prior conventional synthetic or biologic disease‐modifying anti‐rheumatic drugs (DMARD). 15 Baricitinib has been approved for the treatment of JIA in the European Union. 16
Modeling‐based methodology is commonly used in clinical trial development to predict dose, dosing frequencies, and performance of investigational products. Optimal dose selection is key to identifying an ideal compromise between efficacy and safety for a distinct patient population. 17 Allometric scaling of adult pharmacokinetic (PK) parameters for predicting the PK in pediatric populations is a useful method for determining dosage in the latter group. 18 , 19
The objective of the current analyses was to leverage the PK model developed for baricitinib in adult patients with RA to identify optimal dosage in pediatric patients with JIA that results in similar exposures to those seen in adult patients with RA receiving 4 mg once daily. Importantly, the results from these analyses aimed to support weight‐based instead of age‐based dosing and to inform prescribing information (i.e., labeling).
METHODS
Patients and study design
This international phase 3, randomized, double‐blind, placebo‐controlled withdrawal, efficacy, and safety trial, enrolled pediatric patients (aged 2 to <18 years) with polyarticular JIA (rheumatoid factor positive [RF+] or negative [RF−]), extended oligoarticular JIA, enthesitis‐related arthritis (ERA), or juvenile psoriatic arthritis (JPsA) who have had an inadequate response or intolerance to one or more prior conventional synthetic or biologic DMARD. 15 The trial consisted of a 2‐week safety/PK period, a 12‐week open‐label lead‐in period (10 weeks for the safety/PK cohort), and an up to 32‐week placebo‐controlled double‐blind withdrawal period.
Initial doses selected for the safety/PK period, aimed at achieving an exposure of baricitinib in pediatric patients similar to a once‐daily 4‐mg adult‐equivalent dose based on previous PK modeling (data unpublished). Patients received a 4 mg QD in the age groups 12 to <18, and 9 to <12 years, and 2 mg QD for those 6 to <9 and 2 to <6 years. Approximately 5–8 patients were enrolled in each age group for dose confirmation. Enrolment for the safety/PK period was staggered so that the 2‐week assessment period in older groups of patients was completed before younger groups were enrolled. A total of 29 patients were included in the safety/PK period. A Data Monitoring Committee provided reviews of analyses from older groups of patients and confirmed dosage before enrolling younger groups.
Once a dose was confirmed for an age group in the safety/PK period, new patients in that age group were enrolled directly into the 12‐week open‐label lead‐in period. Efficacy was demonstrated by a statistically significant reduction versus placebo in time to flare during the double‐blind withdrawal period as well as a favorable improvement of disease activity (as measured by JIA‐ACR response score) 20 during the study. 15
Sample collection and analysis
The current analyses correspond to blood and plasma samples collected within the safety/PK period and open‐label lead‐in period. Whole blood samples were collected from all patients on days 1, 4, and 14 during the safety/PK period (predose and at 0.25, 0.5, 1, 2, 4, and 6 hours post‐dose) using a microsampling device (Mitra®). One plasma concordance sample was collected from each patient during this period to calculate the blood/plasma (B/P) ratio. The B/P ratio was used to convert the whole blood data to plasma equivalents. Considering the high sampling frequency requirement during the safety/PK period, whole blood (versus plasma) was collected using the Mitra device to reduce the total blood volume requirement, while traditional plasma sample collection was implemented during the open‐label lead‐in. Plasma equivalent concentration‐time data from JIA patients in the safety/PK period were overlayed on model‐predicted 90% CIs (confidence intervals) of adults to confirm exposure matching in pediatric patients with JIA to that observed in adult patients with RA receiving 4‐mg baricitinib QD.
Plasma samples were collected every 2 weeks during the open‐label lead‐in period (pre‐dose and at 0.25, 1, 2 to 4, 4 to 6 h post‐dose). Plasma samples were combined with whole blood data as plasma equivalents from the safety/PK period for the final population PK (PopPK) analysis.
Plasma samples obtained during this study were analyzed for baricitinib using a validated liquid–liquid extraction method at Labcorp Bioanalytical Services, LLC located in Indianapolis, Indiana, USA, and WuXi AppTec (88 FuTe Zhong Road, WaiGaoQiao Free Trade Zone, Shanghai, P.R. China 200131). The LLOQ was 0.200 ng/mL and the ULOQ was 200,000 ng/mL. Dried whole blood samples obtained were analyzed for baricitinib using a validated impact‐assisted extraction method at Altasciences Company Inc. (575 Armand‐Frappier Blvd., Laval, QC, Canada H7V 4B3). The lower limit of quantification (LLOQ) was 0.20 ng/mL and the upper limit of quantification (ULOQ) was 200,00 ng/mL.
Pharmacokinetic model development
Analyses were conducted using non‐linear mixed effects modeling using NONMEM (Version 7.4.2, ICON Development Systems, Gaithersburg, MD, USA) and Perl‐Speaks NONMEM (PsN), (Version 4.8.1, ©2018–2019). 21 Missing values of independent variables (participant characteristic data) were imputed within a participant, using the last observation carried forward (LOCF) method.
A population PK model was previously developed for adult patients with RA. 11 The same model structure was used for the pediatric PK analysis. The base model was a linear 2‐compartment model with zero‐order absorption (including lag time) and a semi‐mechanistic partitioning of apparent total body clearance (CL/F) into apparent renal clearance (CLr/F) dependent on eGFR and apparent non‐renal clearance (CLnr/F); Figure S1. Exponential between‐subject variability (BSV) was included in CLr/F, CLnr/F, central (V1/F), and peripheral (V2/F) volumes of distribution. The absorption duration (D1) parameter included a Box‐Cox‐transformed BSV. Estimates for PK parameters from previous PopPK analyses were adopted as priors for initiating the current PopPK model. An allometric relationship was used for the effect of baseline body weight on clearance‐related parameters (CL/F, CLr/F, and intercompartmental clearance [Q]) with the allometric exponent fixed to 0.75, and for the effect of weight on the central and peripheral volume of distribution (V1/F and V2/F) with the exponent fixed to 1. Fixing the exponents is considered appropriate given the accumulated evidence in the literature. 22 The effect of baseline body weight on CL/F and V/F are shown in Figure 1.
FIGURE 1.
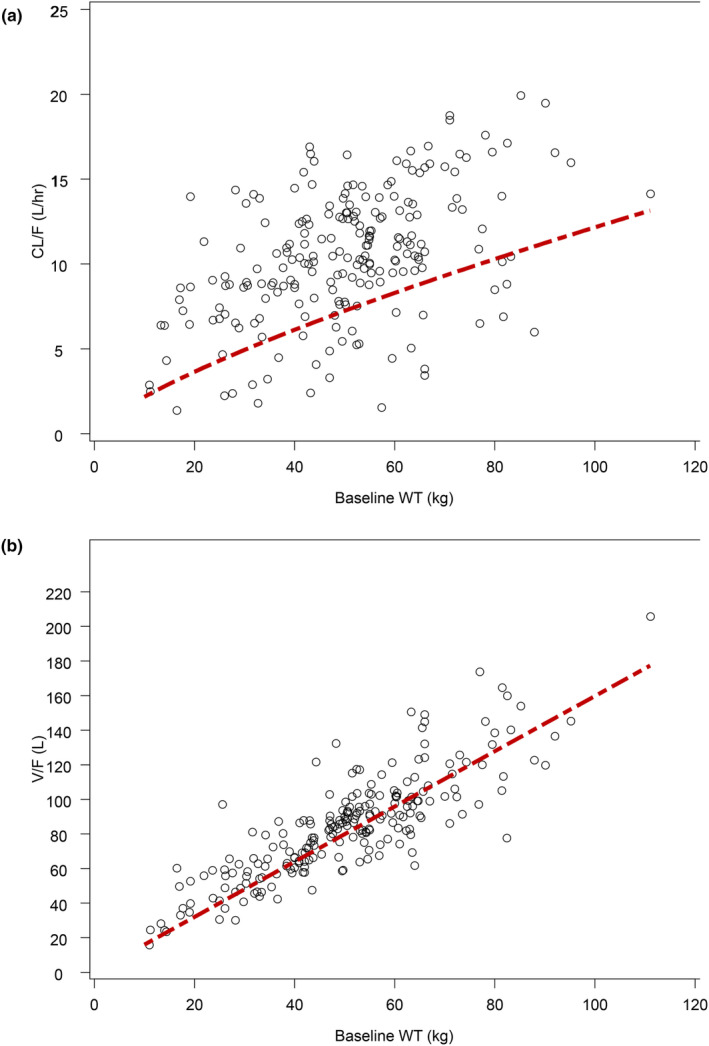
Relationship between weight and individual estimates of (a) apparent clearance and (b) apparent volume of distribution in patients with JIA. CL/F, apparent total body clearance of drug calculated after extravascular administration; JIA, juvenile idiopathic arthritis; V/F, apparent volume of distribution after extravascular administration. The red dashed line represents the model‐estimated relationship between body weight CL/F or V/F.
After confirming the appropriate base model, an assessment of continuous (age) and categorical covariates (gender, JIA sub‐type, ethnicity, race, and Japanese versus non‐Japanese) effects was conducted. Stepwise covariate modeling (SCM) was implemented using Perl‐speaks‐non‐linear effects modeling (NONMEM PsN) to examine these covariates. Key covariate selection criteria for forward inclusion were a p‐value no greater than 0.01 (Δ6.635 minimum objective function for inclusion of 1 parameter), and variance estimate for BSV on the relevant parameter had to decrease by ≥5% for the covariate to be retained in the full model. No covariates met this criterion, therefore backward exclusions were not conducted, hence the base model was the final model.
A visual predictive check (VPC) was performed using PsN to ensure that the model maintained fidelity with the observed data. A non‐parametric bootstrap analysis was performed using PsN to determine the stability and robustness of the final model to produce reliable PK parameter estimates.
RESULTS
Pharmacokinetic sample and demographic summary
The primary population PK dataset contained 1377 plasma concentrations from 217 participants. In total, 116 PK observations (8%) from 94 participants (43%) were excluded from the final analysis dataset (i.e., 106 plasma samples were below the LLOQ, 6 were collected before the dose or within lag time threshold, and 4 led to biologically implausible D1 estimates). Hence, a total of 1261 baricitinib concentrations from 217 participants were used to characterize the PK in these analyses.
The demographic and baseline characteristics of the study population are presented in Table 1. Among the 217 participants, the majority (79%) of patients were between the ages of 12 to <18 years old, 87% weighed ≥30 kg, 69% were female, and 69% were White. The mean weight was 50.2 kg and the mean baseline eGFR was 119 mL/min/1.73 m2.
TABLE 1.
Demographics for patients with JIA used in population pharmacokinetic analyses by age and weight group.
| Parameter | Age (years) | Weight (kg) | All patients | ||||
|---|---|---|---|---|---|---|---|
| 12 to <18 | 9 to <12 | 6 to <9 | 2 to <6 | <30 | ≥30 | ||
| N | 172 | 30 | 9 | 6 | 29 | 188 | 217 |
| Age (years) a | |||||||
| Mean | 14.5 | 10.2 | 6.44 | 3.67 | 8.1 | 14.1 | 13.3 |
| Min, max | 12, 17 | 9, 11 | 6, 8 | 2, 5 | 2, 16 | 9, 17 | 2, 17 |
| Gender (N) | |||||||
| Male | 56 | 7 | 3 | 1 | 8 | 59 | 67 |
| Female | 116 | 23 | 6 | 5 | 21 | 129 | 150 |
| Weight (kg) a | |||||||
| Mean | 55.6 | 35.3 | 19.7 | 14.5 | 22.0 | 54.5 | 50.2 |
| Min, max | 26.1, 111 | 21.9, 63.5 | 13.3, 26.1 | 11.0, 19.2 | 11.0,29.8 | 30.3, 111 | 11.0, 111 |
| Race (N) | |||||||
| White | 114 | 25 | 5 | 5 | 20 | 129 | 149 |
| Black | 4 | 1 | – | – | – | 5 | 5 |
| Asian | 39 | 4 | 4 | 1 | 9 | 39 | 48 |
| Native American | 7 | – | – | – | – | 7 | 7 |
| Native Hawaiian/Other Pacific Islander | – | – | – | – | – | – | – |
| Multiple | 2 | – | – | – | – | 2 | 2 |
| Missing | 6 | – | – | – | – | 6 | 6 |
| Baseline eGFR (mL/min/1.73 m2) a | |||||||
| Mean | 116 | 129 | 141 | 122 | 134 | 119 | |
| Min, max | 66.5, 201 | 82, 179 | 102, 175 | 108, 144 | 82.0, 175 | 66.5, 201 | |
Abbreviations: eGFR, estimated glomerular filtration rate (bedside Schwartz); JIA, juvenile idiopathic arthritis; max, maximum; Min, minimum; N, number of subjects/patients.
At study entry.
Safety/PK period analysis
A total of 29 plasma concordance samples were collected from 29 patients in the safety/PK Period. The whole blood to plasma ratio was determined as the slope of the regression line using time‐matched whole blood and plasma samples. Only 15 matched sample pairs from this study met the inclusion criteria to calculate the B/P ratio (i.e., the concordance samples were drawn within 5 min of each other). The slope was determined to be 1.29 (Figure S2) and was used to convert the whole blood data to plasma equivalents. A total of 179 whole blood samples were collected from 29 patients enrolled in the safety/PK period and converted to plasma equivalents.
During the safety/PK period, dosage was performed using age‐based cut‐offs which provided the bases for selecting dosing during the open‐label lead‐in and randomized withdrawal period of JUVE‐BASIS. The observed concentration data from pediatric patients 12 to <18 years and 9 to <12 years receiving baricitinib 4‐mg QD, and pediatric patients 6 to <9 years and 2 to <6 years receiving 2‐mg QD were concordant with the baricitinib exposure in adult patients with RA receiving 4‐mg QD, previously reported; Figure 2. 11 While individual C max values in pediatric patients in the 9 to <12 years (4‐mg) and 2 to <6 years (2‐mg) were slightly higher than the 90th percentile of the adult simulated values, they were within the range of the actual adult concentrations at the 4‐mg dose. In addition, plasma concentrations in pediatric patients decreased quickly over time after reaching C max and remained within the 90% prediction interval of adult concentrations.
FIGURE 2.
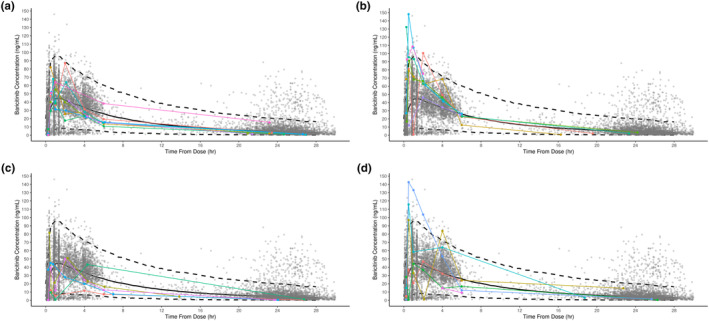
Comparison of observed plasma concentrations of baricitinib in pediatric patients in all age groups with juvenile idiopathic arthritis from Study JUVE‐BASIS and plasma concentrations in adult patients with rheumatoid arthritis receiving baricitinib 4 mg once daily. (a) Pediatric patients aged 12 to <18 years (N = 8) receiving baricitinib 4 mg QD with juvenile idiopathic arthritis. (b) Pediatric patients aged 9 to <12 years (N = 7) receiving baricitinib 4 mg QD with juvenile idiopathic arthritis. (c) Pediatric patients aged 6 to <9 years (N = 5) receiving baricitinib 2 mg QD with juvenile idiopathic arthritis. (d) Pediatric patients aged 2 to <6 years (N = 6) receiving baricitinib 2 mg QD with juvenile idiopathic arthritis. Black solid line and dashed lines are population pharmacokinetic model‐estimated median with a 90% prediction interval of concentrations, and gray circles are observed concentrations at 4 mg once daily (QD) in adult patients with rheumatoid arthritis. Colored lines and symbols are for individual pediatric patients from Study JUVE‐BASIS. Patients that received a higher or lower dose than defined in the protocol were excluded.
Population PK analysis
The adult PK model, with the incorporation of allometric relationship for clearance‐ and volume‐related parameters and a partition of total clearance into renal and non‐renal clearance, well described the PK of baricitinib in pediatric patients with JIA. The goodness of fit plots (Figure S3), and visual predictive check (Figure S4) demonstrated that the model predictions agreed with the observed data reasonably well. Key PK parameter estimates from PopPK modeling are included in Table 2. The bootstrap analysis showed all parameters were estimated with adequate precision. The total clearance estimate (for a weight of 74 kg) is 9.7 L/h (Table 2), similar to that estimated for the adult patients with RA (9.67 L/h). 11 The volume estimates (for a weight of 74 kg) are also similar between the 2 populations (V1/F and V2/F are 92.6 L and 25.6 L, respectively, for pediatric patients [Table 2]; and 95.1 L and 23.3 L, respectively, for adult patients with RA). 23 The relationship between model‐estimated CL/F, V/F, and baseline body weight are shown in Figure 1.
TABLE 2.
Pharmacokinetic and covariate parameters in final population PK model for baricitinib in patients with JIA.
| Model parameter (Unit) | Population mean (%SEE) | BSV a (%SEE) | Mean (95% CI) from bootstrap analysis |
|---|---|---|---|
| D1 (h) | 0.457 (2.76) | 138.4 (13.1) | 0.452 (0.373–0.512) |
| Box‐Cox transformation parameter for D1 | 0.501 (1.40) | – | 0.538 (0.323–0.847) |
| CLnr/F(L/h) b | 3.36 (4.76) | 84.6 (10.3) | 3.32 (3.20–3.46) |
| CLr/F (L/h) b | 6.34 (2.49) | 39.5 (5.30) | 6.41 (6.23–6.52) |
| V1/F (L) c | 92.6 (1.89) | 33.8 (23.2) | 92.6 (90.1–94.5) |
| Q (L/h) d | 2.67 (7.64) | 15.1 (FIX) | 2.76 (2.62–2.88) |
| V2/F (L) e | 25.6 (12.1) | 56.5 (25.2) | 25.1 (21.2–30.1) |
| LAG (h) | 0.147 (2.64) | – | 0.147 (0.144–0.150) |
| Allometric Scaling CL b | 0.75 (FIX) | – | – |
| Allometric Scaling V c , e | 1 (FIX) | – | – |
| Covariate for change in eGFR on CLr/F f | 0.00586 (29.0) | – | 0.00589 (0.00349–0.00852) |
| Covariance for CLnr/F and CLr/F g | – | 0.272 (9.41) | – |
| Covariance for CLr and V1/F g | – | −0.00706 (129) | – |
| Proportional error h | 0.361 (2.07) | – | 0.360 (0.346–0.377) |
Abbreviations: BSV, between‐subject variability; BWT, body weight: CI, confidence interval; CLnr/F, apparent non‐renal clearance; CLr/F, apparent renal clearance; CV, coefficient of variation; D1, absorption duration; ∆eGFR, change in eGFR from baseline; eGFR, estimated glomerular filtration rate; EXP, exponential; FIX, fixed; JIA, juvenile idiopathic arthritis; LAG, absorption lag; PopPK, population pharmacokinetics; Q, intercompartmental clearance; SEE, standard error of estimate; SQRT, square root; V1/F, apparent central volume of distribution; V2/F, apparent peripheral volume of distribution; WTE, weight at entry.
BSV reported as, %CV = (SQRT(EXP(OMEGA(N))‐1))*100.
CL/F = (CLnr/F + CLr/F)*((WTE/74)^0.75).
V1/F = 92.6*((WTE/74)^1.00).
Q = 2.67*((WTE/74)^0.75).
V2/F = 25.6*((WTE/74)^1.00).
eGFR was estimated using the Bedside Schwartz equation. CLr/F = (apparent renal clearance*((baseline eGFR/93) + covariate for change in eGFR on apparent eGFR‐dependent clearance*(∆eGFR))), where 93 is median eGFR in mL/min/1.73 m2 from the previous popPK analysis.
Covariance between w2.
Standard deviation.
Using the final PK model, the post hoc PK parameter estimates were summarized for patients by dose, and age group; Table 3. The estimated mean CL/F range between age groups was 4.87–10.3 L/h in study participants with JIA, whereas in adult patients with RA the mean CL/F was 9.42 L/h (5th to 95th percentile: 4.44–15.2). The estimated mean half‐life range was 6.39–8.73 h among age groups, shorter than the adult value of 12.5 h.
TABLE 3.
Comparison of post hoc population pharmacokinetic parameter estimates in patients with JIA and rheumatoid arthritis by age and weight.
| Parameters | JIA a | RA b | |||||
|---|---|---|---|---|---|---|---|
| Weight (kg) | <30 | ≥30 | |||||
| Age (years) | 2 to <6 | 6 to <9 | 9 to <12 | 12 to <18 | Adult | ||
| N | 6 | 10 | 29 | 172 | 29 | 188 | 2393 |
| Dose (mg) | 2 | 2 | 4 | 4 | 2 | 4 | 4 |
| CL/F (L/h) c | 4.87 (57) | 6.53 (66) | 8.11 (55) | 10.3 (45) | 6.30 (61) | 10.2 (45) | 9.42 (34) |
| CLr (L/h) | 3.12 (46) | 4.29 (51) | 5.53 (49) | 6.82 (38) | 4.22 (52) | 6.77 (38) | 6.86 (39) |
| CLnr (L/h) | 1.68 (85) | 2.04 (134) | 2.45 (83) | 3.35 (73) | 1.95 (100) | 3.31 (73) | 2.56 (29) |
| V/F (L) c | 27.5 (43) | 41.2 (28) | 62.1 (24) | 88.3 (30) | 41.9 (40) | 86.8 (29) | 108 (19) |
| V1/F (L) | 21.7 (38) | 32.2 (22) | 49.3 (25) | 70.8 (30) | 33.6 (40) | 69.3 (30) | 85.2 (24) |
| V2/F (L) | 4.85 (100) | 7.92 (79) | 12.1 (37) | 16.9 (43) | 7.43 (68) | 16.8 (41) | 23.1 (4) |
| t1/2 (h) | 6.39 (61) | 8.10 (79) | 8.47 (47) | 8.73 (45) | 7.60 (64) | 8.75 (44) | 12.5 (27) |
| AUCτ,ss (ng*h/mL) | 410 (57) | 254 (27) d | 500 (57) e | 386 (45) | 464 (76) | 388 (45) | 483 (40) f |
| C max,ss (ng/mL) | 87.4 (38) | 56.8 (22) d | 79.0 (33) e | 57.7 (28) | 85.7 (40) | 58.1 (28) | 53.3 (22) f |
Note: Values are presented as geometric mean (CV%).
Abbreviations: AUCτ,ss, area under the concentration versus time curve during 1 dosing interval at steady state; CL/F, apparent total body clearance of drug calculated after extravascular administration; CLr/F, apparent renal clearance; CLnr/F, apparent non‐renal clearance; C max,ss, maximum observed drug concentration during a dosing interval at steady state; CV, coefficient of variation; JIA, juvenile idiopathic arthritis; QD, once daily; RA, rheumatoid arthritis; t 1/2, elimination half‐life; V/F, apparent total volume of distribution; apparent central volume of distribution; V2/F, apparent peripheral volume of distribution.
Individual post hoc estimates based on data from the current analysis in patients with JIA.
Individual post hoc estimates based on data combined from the Seven Phase 2 and 3 Studies in patients with RA.
Estimates of CL/F and V/F were based on individual post hoc estimates; CL/F = CLnr/F + CLr/F and V/F = V1/F + V2/F.
N = 8, 2 patients received incorrect dosing, dose‐dependent parameters excluded.
N = 27, 2 patients received incorrect dosing, dose‐dependent parameters excluded.
Values were based on all patients in the respective analysis dataset and a dose normalized to 4 mg QD.
The resulted exposure estimates at the studied doses for the various age groups ranged from 254 to 500 h*ng/mL for AUCτ,ss and 57.7–87.4 ng/mL for C max,ss. The exposure estimates were overall consistent with the exposure at 4‐mg QD in adult patients with RA (AUCτ,ss = 483 ng*h/mL; C max,ss = 53.3 ng/mL).
Weight‐based simulations
The study was implemented by dosing by age groups with 4 mg QD for patients aged 9 to <18 years and 2 mg for patients aged 2 to <9 years. Because weight is a more physiologically relevant factor affecting PK compared to age, simulations using the final model parameters were performed to predict C max,ss and AUCτ,ss at 1, 2, and 4 mg QD to identify an optimal weight cut‐off. Simulations were conducted in the weight range between 10 and 120 kg which contains the range (11.0–111 kg) of weights enrolled in the study. Additionally, 10 kg is the 5th percentile weight for a typical 2‐year‐old. 24 Simulations were conducted by fixing the range of GFR to the study range and varying weight by 10 kg between 10 and 60 kg and then by 20 kg between 60 and 120 kg. The exposures were compared graphically to adult exposures at 4 mg QD; Figure 3. The visual interpretation suggested patients 10 to <30 kg given 2 mg and patients ≥30 kg given 4 mg would provide comparable exposure to adult patients with RA given 4 mg QD. When plotting both the simulated and actual post hoc AUCτ,ss and C max,ss values at 4 mg QD for ≥30 kg and at 2 mg for 10 to <30 kg, both the actual and predicted data are comparable to the exposure at 4 mg QD in adult patients with RA; Figure 4.
FIGURE 3.
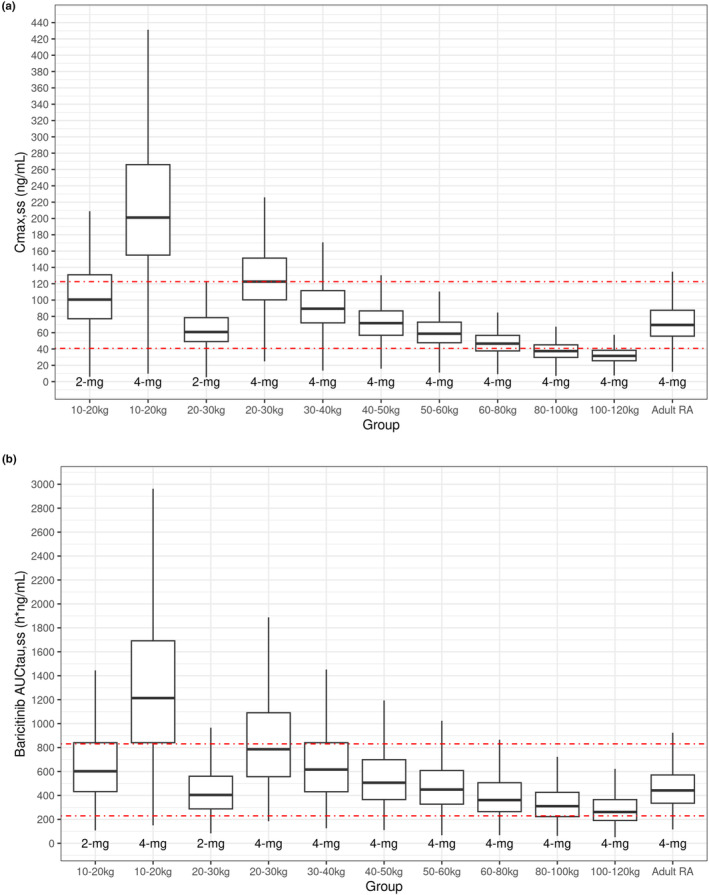
Boxplots of simulated (a) C max,ss and (b) AUCtau,ss by weight ranges in patients with JIA compared to adult patients with RA. AUCtau,ss, area under the concentration versus time curve during a dosing interval at steady state; C max,ss, maximum observed drug concentration during a dosing interval at steady state; JIA, juvenile idiopathic arthritis; QD, once daily; RA, rheumatoid arthritis. The dashed red lines indicate 5th and 95th percentiles for adult patients with RA. Box for adult RA are based on PK modeling with data combined from 7 Phase 2 and Phase 3 studies in patients with adult RA.
FIGURE 4.
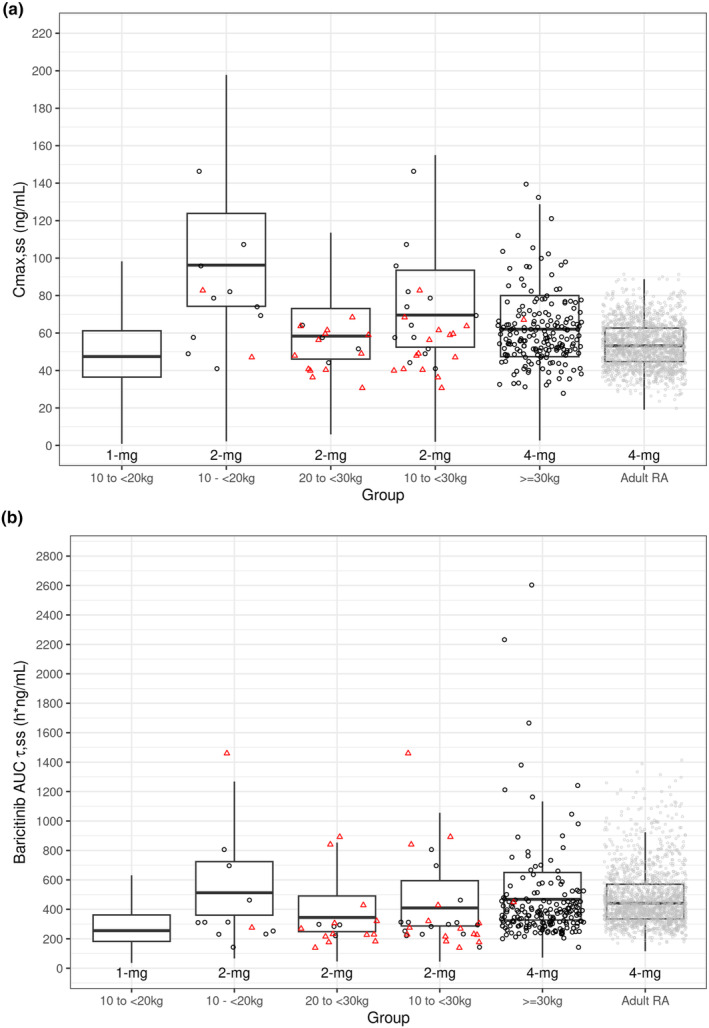
Boxplots of simulated post hoc (a) C max,ss and (b) AUCτ,ss at 2 mg and 4 mg QD for pediatric patients with JIA, compared to adult patients with RA, with JUVE‐BASIS and adult RA individual post hoc data overlaid. AUCτ,ss, area under the concentration versus time curve during a dosing interval at steady state; JIA, juvenile idiopathic arthritis; PK, pharmacokinetics; QD, once daily; RA, rheumatoid arthritis. Boxes were based on simulations without including the residual error. The box for adult RA was based on PK modeling with data combined from seven Phase 2 and Phase 3 Studies in patients with adult RA. Gray circles = adult RA at 4 mg individual post hoc estimates. Black and red symbols were individual post hoc estimates for pediatric JIA patients with a dose normalized to 2 or 4 mg based on the proposed dose for the weight group: Black circles = patient received dose for weight group; Red triangles = patient did not receive dose for weight group and data dose‐normalized.
The post hoc PK parameter estimates for patients in the JIA Phase 3 study by weight group with a weight cut‐off of 30 kg are summarized in Table 3 and compared with those in adult patients with RA. The resulted exposure estimates (AUC and C max) at 4 mg QD for patients ≥30 kg and at 2 mg QD for patients <30 kg, compared with dosing by age groups, are more consistent with the exposures at 4 mg QD in adult patients with RA.
DISCUSSION
Study JUVE‐BASIS is the first study conducted in pediatric patients with JIA receiving baricitinib. A PK model was previously applied to inform initial dose selection for pediatric patients based on PK data in adult patients with RA using the exposure matching approach. 25 A 2‐week safety/PK period was incorporated in JUVE‐BASIS and confirmed exposures at the initial doses selected for pediatric patients in the various age groups. The doses confirmed during the safety/PK period were implemented during the remainder of the phase 3 trial (ie, the open‐label lead‐in and double‐blind withdrawal period), and baricitinib was shown to be efficacious with an acceptable safety profile for the treatment of polyarticular course JIA. 15 The population PK analysis using data from all periods of the study well characterized the PK in this pediatric population and allowed for optimization of pediatric dosing with the appropriate weight cut‐off values.
A PK model for baricitinib was previously developed for adult patients with RA. 11 The adult PK model is a two‐compartment model with zero‐order absorption and partitioning of total CL/F into CLr/F and CLnr/F. The CLr/F term is correlated with the eGFR estimated with modification of diet in renal disease (MDRD) and represents mainly renal filtration and secretion. The CLnr/F term largely represented hepatic metabolism and may also include some renal secretion. The model structure for CL/F was set up in this manner based on the knowledge that renal excretion represents the primary elimination route for baricitinib. It is known that renal function and drug‐metabolizing enzymes largely mature around the age of 2 years, 26 and Study JUVE‐BASIS enrolled patients 2–18 years old, therefore further maturation is not expected to affect the relationship between CLr/F and CLnr/F. Moreover, since the CLr/F term was estimated as a function of individual eGFR, the model structure provides an accurate estimate for the CLr/F regardless of the age of a given patient.
Given the elimination mechanism of baricitinib as a small molecule and that only patients older than 2 years were enrolled in JUVE‐BASIS, weight was the main factor that might contribute to the difference in the PK between adult and pediatric patients. It was assumed a priori that body weight would have a significant effect on the clearance and volume‐related PK parameters and these relationships were assumed to follow the typical allometric scaling. 22 The adult PK model in combination with the allometric relationship sufficiently described the PK data in this trial. No additional covariates other than weight and renal function, both incorporated into the model structure, were identified. Specifically, after the effect of weight was accounted for, age was not a significant covariate on the clearance or volume terms. VPCs suggest concordance between model‐predicted and observed data over time, across body weight and age groups with all parameters being adequately estimated.
In the allometric function, the exponent was fixed to 0.75 for CL‐related parameters (CLr/F, CLnr/F, and Q) and to 1 for volume‐related parameters (V1/F and V2/F). For comparison, additional modeling was performed allowing the allometric exponents to be derived. The analysis estimated the exponent of 0.48 and 0.70, respectively for CL‐ and V‐related parameters. Fixing the exponents is considered appropriate given accumulated evidence in literature 22 and relatively sparse data available in the current study, especially for the younger age group. In addition, dose recommendation for pediatric patients with various weight groups will not be different if based on the approach of estimating the exponents.
The JIA phase 3 study was conducted using a dose assignment based on age. The age‐based dosing was converted to weight‐based dosing, using modeling and simulations, given that weight is a more physiologically relevant patient factor affecting PK and is a covariate on clearance and volume of distribution in the PopPK model. There was a high correlation between age and weight (Figure S5). The effect of weight on the C max and AUC was further evaluated to identify an optimal cut‐off weight value for dosing. In Figures 3 and 4, the boxes for the AUC and C max estimates were generated using simulations for 5000 patients with JIA in the various weight groups and adult patients with RA using the respective final pediatric and adult PK model, to account for possible correlations among patient factors such as renal function and weight. Individual post hoc AUC estimates from pediatric JIA and adult patients with RA were also overlaid for comparison. No patients received 1‐mg QD in JUVE‐BASIS, hence no individual estimates were shown. As shown by the 3 boxes on the right in Figure 4b, the AUC data as summarized by the box plot are completely overlapping between pediatric patients with JIA (2‐mg for 10 to <30 kg and 4 mg for ≥30 kg) and adult patients with RA (4‐mg). Notably, the median AUC for 2 mg in the 10 to <20 kg weight group (second box from left) is also comparable to that of the adult. When the dose is reduced to 1 mg QD for the 10 to <20 kg weight group, the resulting AUC is 42% lower than that for 4 mg in adult patients with RA at the median levels. The C max data (Figure 4a) are largely overlapping between pediatric patients with JIA (2 mg for 20 to <30 kg and 4‐mg for ≥30 kg) and adult patients with RA (4‐mg). The C max at a dose of 2 mg QD for the weight group of 10 to <20 kg is estimated to be higher than the adult (approximately 46% higher at the mean level). Although C max at 1 mg QD would appear to better match the adult C max at 4 mg QD, the corresponding AUC is 42% lower, which would be likely to compromise efficacy.
Based on the established exposure‐efficacy and exposure‐safety relationship from the large dataset from adults, C max is not expected to have a relevant effect on efficacy and safety. Careful evaluation of the safety data from JUVE‐BASIS did not identify an increased risk of adverse events in patients with JIA weighing less than 30 kg. Matching AUC is considered as the most important acceptance criterion as it is most relevant to the efficacy and safety of baricitinib based on the exposure‐response relationship established in adults. 11 In the situation where a given dose produced comparable AUC but 46% higher C max, it is considered that the currently recommended dose is more appropriate from a benefit–risk point of view, than a lower dose that provides comparable C max but lower AUC. A weight cut‐off of 30 kg was therefore selected based on the simulated relationship between baricitinib exposure and weight for pediatric patients weighing 10 kg and above. This weight cut‐off is also approximately the median weight of pediatric patients at an age of 9 years used as the age cut‐off for dose assignment in the trial.
In the safety/PK period, we demonstrated the effectiveness of microsampling using the Mitra® VAMS® blood collection device in a small number of pediatric patients. 27 The advantage of microsampling over traditional phlebotomy techniques will allow greater access to PK data in the pediatric population. 27
We show the application of the PK model developed for adult patients with RA with the addition of allometric scaling for weight on clearance and volume terms for predicting exposures in pediatric populations. Furthermore, the JIA pediatric PK model allowed the simulation by weight ranges to select the appropriate weight grouping for dose optimization. The final accepted doses are 2 mg QD for patients 10 to <30 kg and 4 mg QD for patients ≥30 kg.
AUTHOR CONTRIBUTIONS
R.L.D., C.S.E.II, R.W., S.Y.K., and X.Z. wrote the manuscript; R.L.D., C.S.E.II, S.Y.K., and X.Z. designed the research; R.L.D. and S.Y.K. performed the research; R.L.D., C.S.E.II, D.B.R., R.W., J.A., S.Y.K., and X.Z. analyzed the data.
FUNDING INFORMATION
Funded by Eli Lilly and Company under license from Incyte.
CONFLICT OF INTEREST STATEMENT
R.L.D. was an employee and shareholder of Eli Lilly and Company at the time of this study. C.S.E.II was an employee and shareholder of Eli Lilly and Company at the time of this study and is currently employed with Metrum Research Group. D.B.R., R.W., J.A., S.Y.K., and X. Z are employees and shareholders of Eli Lilly and Company.
RESEARCH INVOLVING HUMAN PARTICIPANTS
Informed consent, or assent when appropriate, was provided for participation in the study and prior to any study‐specific procedures. The protocol was approved by institutional review boards or ethics committees from all participating sites according to local requirements and was conducted according to the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki.
Supporting information
Figure S1.
ACKNOWLEDGMENTS
The authors thank the participants, caregivers, and investigators for their participation in this study. Eric A. Rodriguez, Ph.D., of Eli Lilly and Company, provided writing and editorial assistance.
Decker RL, Steven Ernest C II, Radtke DB, et al. A population pharmacokinetic model using allometric scaling for baricitinib in patients with juvenile idiopathic arthritis. CPT Pharmacometrics Syst Pharmacol. 2024;13:970‐981. doi: 10.1002/psp4.13131
C.S. Ernest II was an employee of Eli Lilly and Company at the time of this study.
REFERENCES
- 1. Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184:5298‐5307. [DOI] [PubMed] [Google Scholar]
- 2. Martini A, Lovell DJ, Albani S, et al. Juvenile idiopathic arthritis. Nat Rev Dis Primers. 2022;8:5. [DOI] [PubMed] [Google Scholar]
- 3. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767‐778. [DOI] [PubMed] [Google Scholar]
- 4. van den Ham HJ, de Jager W, Bijlsma JW, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology (Oxford). 2009;48:899‐905. [DOI] [PubMed] [Google Scholar]
- 5. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138‐2149. [DOI] [PubMed] [Google Scholar]
- 6. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376:652‐662. [DOI] [PubMed] [Google Scholar]
- 7. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374:1243‐1252. [DOI] [PubMed] [Google Scholar]
- 8. Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA‐BUILD study. Ann Rheum Dis. 2017;76:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease‐modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69:506‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al‐Salama ZT, Scott LJ. Baricitinib: a review in rheumatoid arthritis. Drugs. 2018;78:761‐772. [DOI] [PubMed] [Google Scholar]
- 11. Zhang X, Chua L, Ernest C 2nd, et al. Dose/exposure‐response modeling to support dosing recommendation for phase III development of Baricitinib in patients with rheumatoid arthritis. CPT Pharmacometrics Syst Pharmacol. 2017;6:804‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor PC, Laedermann C, Alten R, et al. A JAK inhibitor for treatment of rheumatoid arthritis: the Baricitinib experience. J Clin Med. 2023;12:4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winnick S, Lucas DO, Hartman AL, Toll D. How do you improve compliance? Pediatrics. 2005;115:e718‐e724. [DOI] [PubMed] [Google Scholar]
- 14. Shi JG, Chen X, Lee F, et al. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J Clin Pharmacol. 2014;54:1354‐1361. [DOI] [PubMed] [Google Scholar]
- 15. Ramanan AV, Quartier P, Okamoto N, et al. Baricitinib in juvenile idiopathic arthritis: an international, phase 3, randomised, double‐blind, placebo‐controlled, withdrawal, efficacy, and safety trial. Lancet. 2023;402(10401):555‐570. [DOI] [PubMed] [Google Scholar]
- 16. European Commission Public Health – Union of Register of medicinal products . https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant. Accessed September 25, 2023.
- 17. Love SB, Brown S, Weir CJ, et al. Embracing model‐based designs for dose‐finding trials. Br J Cancer. 2017;117:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu Q, Peters SA. A retrospective evaluation of allometry, population pharmacokinetics, and physiologically‐based pharmacokinetics for pediatric dosing using clearance as a surrogate. CPT Pharmacometrics Syst Pharmacol. 2019;8:220‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahmood I, Tegenge MA. A comparative study between allometric scaling and physiologically based pharmacokinetic modeling for the prediction of drug clearance from neonates to adolescents. J Clin Pharmacol. 2019;59:189‐197. [DOI] [PubMed] [Google Scholar]
- 20. Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202‐1209. [DOI] [PubMed] [Google Scholar]
- 21. Lindbom L, Ribbing J, Jonsson EN. Perl‐speaks‐NONMEM (PsN)–a Perl module for NONMEM related programming. Comput Methods Prog Biomed. 2004;75:85‐94. [DOI] [PubMed] [Google Scholar]
- 22. Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941‐2952. [DOI] [PubMed] [Google Scholar]
- 23. FDA . Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) . Retrieved January 24, 2024, from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000ClinPharmR.pdf
- 24. Centers for Disease Control and Prevention . Data Table of Weight‐for‐age Charts. https://www.cdc.gov/growthcharts/html_charts/wtage.htm#males. Accessed October 24, 2023.
- 25. Purohit VS, Ports WC, Wang C, Riley S. Systemic tofacitinib concentrations in adult patients with atopic dermatitis treated with 2% tofacitinib ointment and application to pediatric study planning. J Clin Pharmacol. 2019;59:811‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson TN, Rostami‐Hodjegan A, Tucker GT. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin Pharmacokinet. 2006;45:931‐956. [DOI] [PubMed] [Google Scholar]
- 27. Wickremsinhe ER, Decker RL, Lee LB, et al. Microsampling in pediatric studies: pharmacokinetic sampling for baricitinib (Olumiant™) in global pediatric studies. Bioanalysis. 2023;15:621‐636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.


