Abstract
Polatuzumab vedotin is a CD79b‐directed antibody–drug conjugate that targets B cells and delivers the cytotoxic payload monomethyl auristatin E (MMAE). The phase III POLARIX study (NCT03274492) evaluated polatuzumab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R‐CHP) as first‐line treatment of diffuse large B‐cell lymphoma (DLBCL). To examine dosing decisions for this regimen, population pharmacokinetic (popPK) analysis, using a previously developed popPK model, and exposure–response (ER) analysis, were performed. The popPK analysis showed no clinically meaningful relationship between cycle 6 (C6) antibody‐conjugated (acMMAE)/unconjugated MMAE area under the concentration–time curve (AUC) or maximum concentration, and weight, sex, ethnicity, region, mild or moderate renal impairment, mild hepatic impairment, or other patient and disease characteristics. In the ER analysis, C6 acMMAE AUC was significantly associated with longer progression‐free and event‐free survival (both p = 0.01). An increase of <50% in acMMAE/unconjugated MMAE exposure did not lead to a clinically meaningful increase in adverse events of special interest. ER data and the benefit–risk profile support the use of polatuzumab vedotin 1.8 mg/kg once every 3 weeks with R‐CHP for six cycles in patients with previously untreated DLBCL.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Prior to this study, no clinically meaningful drug–drug interactions were found between polatuzumab vedotin and rituximab, cyclophosphamide, doxorubicin, and prednisone (R‐CHP) in previously untreated non‐Hodgkin lymphoma (including diffuse large B‐cell lymphoma [DLBCL]). Additionally, no robust population pharmacokinetics or exposure–response analyses are currently available for polatuzumab vedotin in previously untreated DLBCL.
WHAT QUESTION DID THIS STUDY ADDRESS?
This analysis examined whether pharmacokinetic and exposure–response data supported the proposed polatuzumab vedotin dosing regimen (1.8 mg/kg Q3W, for 6 weeks) for the treatment of patients with previously untreated DLBCL.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
This analysis confirms that 1.8 mg/kg Q3W polatuzumab vedotin, in combination with R‐CHP, is appropriate for the wider patient population with previously untreated DLBCL.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
This analysis demonstrates that population pharmacokinetics and exposure–response analyses can provide a comprehensive understanding of the pharmacokinetics and exposure–response relationship of polatuzumab vedotin, and can support dosing guidance for clinicians.
INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is an aggressive form of non‐Hodgkin lymphoma (NHL) and the most commonly diagnosed subtype. 1 Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) has been the standard of care for patients with previously untreated DLBCL; however, 30%–40% of patients relapse or are refractory to treatment. 2
Polatuzumab vedotin is an antibody–drug conjugate consisting of a humanized anti‐CD79b IgG1 monoclonal antibody with activity against dividing B cells. The anti‐mitotic agent monomethyl auristatin E (MMAE) is covalently attached to the antibody via a protease‐cleavable linker. 3 , 4 Polatuzumab vedotin is approved in Europe, Japan, and other countries for use in patients with previously untreated DLBCL based on findings from the phase III POLARIX study. 5 , 6 , 7 , 8 In POLARIX, polatuzumab vedotin administered in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola‐R‐CHP) significantly improved progression‐free survival (PFS) versus R‐CHOP in patients with previously untreated DLBCL (hazard ratio: 0.73; 95% confidence interval: 0.57–0.95; p = 0.02), with a similar safety profile. 8
To evaluate the impact of polatuzumab vedotin dose selection and Pola‐R‐CHP regimen in POLARIX, population pharmacokinetic (popPK) and exposure–response (ER) analyses were performed; both have an important role in supporting dosing decisions and in the approval of oncology drugs. 9 , 10 In a prior study of the PK of polatuzumab vedotin plus R‐CHP in previously untreated NHL (including DLBCL), no clinically meaningful drug–drug interactions were found between polatuzumab vedotin and R‐CHP. 11 Additionally, ER analyses of polatuzumab vedotin plus bendamustine and rituximab (BR) in Phase 1/2 studies supported the use of polatuzumab vedotin (1.8 mg/kg) + BR once every 3 weeks (Q3W) for six cycles in patients with relapsed/refractory (R/R) DLBCL. 12
Here, we report the first popPK, exposure–efficacy, and exposure–safety analyses of the POLARIX study. The current analyses evaluate the impact of intrinsic and extrinsic factors on the PK of polatuzumab vedotin, the relationships between antibody‐conjugated MMAE (acMMAE) exposure and efficacy, and acMMAE and unconjugated MMAE exposure and safety in patients with previously untreated DLBCL.
METHODS
Study design and objectives
Analyses were performed using data from patients with previously untreated DLBCL who received polatuzumab vedotin (1.8 mg/kg Q3W) plus R‐CHP or R‐CHOP in the POLARIX study; full methodology has been described previously. 8 The protocol, which is available, was approved by the institutional review board or ethics committee at each participating institution. 8 PopPK analysis was performed via an external validation approach using a previously developed popPK model (a two‐analyte [acMMAE–MMAE] integrated popPK model, Appendix S1), 13 with the aim of describing the PK of polatuzumab vedotin in POLARIX. The analysis also estimated and summarized individual PK parameters for acMMAE and unconjugated MMAE and estimated individual values of exposure for acMMAE and unconjugated MMAE for further ER analyses.
The exposure–efficacy analysis determined relationships between polatuzumab vedotin exposure (acMMAE cycle [C] 6 area under the concentration–time curve [AUC]) and efficacy endpoints. The exposure–safety analysis investigated relationships between polatuzumab vedotin exposure (acMMAE and unconjugated MMAE C6 AUC and maximum concentration [C max] at C6) and safety endpoints.
Dataset and model for popPK analyses
The popPK model used in this analysis, which was based on four clinical studies (DCS4968g, ROMULUS, GO29365, GO29044) of polatuzumab vedotin in patients with B‐cell NHL, was previously described by Lu et al. 13 (Appendix S1). Using an external validation approach, this legacy popPK model was assessed for its ability to describe acMMAE and unconjugated MMAE concentrations following the administration of polatuzumab vedotin (1.8 mg/kg Q3W for six cycles) plus R‐CHP in patients with previously untreated DLBCL. As part of the external validation approach, exposures were assessed by model simulations 14 with empirical Bayes estimates of individual PK parameters without covariate adjustment to reference values, and a hypothetical Q3W 1.8 mg/kg dosing of six cycles. All assessments, either as a PK endpoint or a predictor of safety or efficacy, focused on C6 AUC and/or C6 C max. The exposures achieved after six cycles of treatment were considered most clinically relevant, as maximum acMMAE exposure is expected to be observed in a 6‐cycle dosing regimen, and acMMAE exposure is closest to steady state at this point. 11
The PK of polatuzumab vedotin was characterized by three analytes: total antibody (including conjugated and unconjugated antibody), acMMAE and unconjugated MMAE. Samples were analyzed using validated liquid chromatography methods and tandem mass spectrometric detection with and without immunoaffinity capture for acMMAE and unconjugated MMAE, respectively. 11 The lower limit of quantitation (LLOQ) was 0.3590 ng/mL for acMMAE and 0.0359 ng/mL for unconjugated MMAE. Serum total antibody concentrations were measured using a validated enzyme‐linked immunosorbent assay (ELISA). The indirect sandwich ELISA used anti‐complementarity determining region antibodies to anti‐CD79b monoclonal antibody as the capture reagent, and anti‐human IgG1 framework antibody conjugated to horseradish peroxidase for detection. The LLOQ was 50 ng/mL. Serum concentrations of total antibody were determined in samples taken at C1 day (D) 1 pre‐dose and at the 3‐month post‐treatment follow‐up visit. Serum concentrations of total antibody and plasma concentrations of acMMAE and unconjugated MMAE were determined in samples taken at C1D1 30 min post‐dose, C4D1 pre‐dose and 30 min post‐dose, and at treatment completion/early treatment termination visit.
PopPK analysis/external validation
External validation techniques were used to investigate how well the existing popPK model described PK data from POLARIX. Goodness‐of‐fit and visual predictive check (VPC) plots, normalized prediction distribution errors (NPDE), and conditional predictive checks were generated for further model validation. Individual PK parameters and exposures were generated using empirical Bayes estimates. Individual predictions from the model were used to assess the impact of covariates on acMMAE and unconjugated MMAE C6 exposures (AUC, C max, and trough concentration [C trough]), which were computed using empirical Bayes estimates of individual PK parameters.
Various intrinsic covariates (directly related to the individual patient, such as body weight, sex, age, disease status) and extrinsic covariates (external influences on PK, e.g., geographic region, concomitant medicine), including but not limited to the statistically significant covariates identified in the legacy model, were assessed for their impact on acMMAE and unconjugated MMAE C6 exposures. Continuous covariates were grouped into categories based on specified thresholds, if applicable, and included body weight (<100 kg vs. ≥100 kg), sex (male vs. female), age (≥65 years vs. <65 years), ethnicity (Asian vs. non‐Asian), region (Asia vs. ex‐Asia), hepatic impairment (mild/moderate vs. normal), renal impairment (mild/moderate/severe vs. normal), and Eastern Cooperative Oncology Group performance status (1 vs. 0 and 2 vs. 1). Various disease characteristics were also evaluated including bulky (≥7.5 cm) versus non‐bulky (<7.5 cm) disease, Ann Arbor Stage (3 to 4 vs. 1 to 2), baseline International Prognostic Index score (3 vs. 2 and 4 to 5 vs. 2), cell‐of‐origin (germinal center B‐cell‐like vs. activated B cell), double expressor lymphoma (DEL; i.e., BCL2‐ and MYC‐positive disease by immunohistochemistry) versus non‐DEL, baseline lactate dehydrogenase (LDH) levels (above the upper limit of normal [ULN] vs. ≤ULN), and baseline anti‐drug antibody (ADA) status (positive vs. negative). The body weight cut‐off value used in comparisons (i.e., <100 kg vs. ≥100 kg) was based on the threshold used in popPK studies of brentuximab vedotin. 13 , 15 Further details can be found in the Appendix S1.
Exposure–efficacy analyses
Only C6 AUC for acMMAE was used in the exposure–efficacy analyses as AUC for unconjugated MMAE was considered below the therapeutic range to impact efficacy. This decision is supported by an ER analysis that showed acMMAE, but not unconjugated MMAE, is the main analyte associated with efficacy endpoints for vc‐MMAE antibody‐drug conjugates (such as polatuzumab vedotin), due to their mechanism of action. 16 Efficacy endpoints in the POLARIX study included in this analysis were: investigator‐assessed PFS, investigator‐assessed event‐free survival‐efficacy (EFSeff) and overall survival (OS), and complete response (CR) at the end of treatment by fluorodeoxyglucose‐positron emission tomography, determined by blinded independent central review.
Kaplan–Meier plots and Cox proportional hazards (CPH) modeling were used to evaluate the association between time‐to‐event endpoints (i.e., PFS, EFSeff, OS) and acMMAE C6 AUC. Logistic regression models were used to assess the association between the probability of end‐of‐treatment CR and acMMAE C6 AUC. If a statistically significant effect of exposure (α = 0.05) was observed, a covariate analysis was conducted. Further information can be found in the Appendix S1.
Exposure–safety analyses
The C6 AUC and C6 C max for both acMMAE and unconjugated MMAE were used for exposure–safety analyses. Key adverse events of special interest (AESIs) with Pola‐R‐CHP included grade ≥2 peripheral neuropathy and grade ≥3 neutropenia, febrile neutropenia, infections and infestations, anemia, thrombocytopenia, aspartate aminotransferase increase, alanine aminotransferase increase, bilirubin increase, hepatic toxicity, hyperglycemia, and cardiac arrhythmia.
Logistic regression models were used to assess the relationships between acMMAE/unconjugated MMAE exposure and the probability of key AESIs. Similar logistic regression models addressed relationships between acMMAE/unconjugated MMAE exposure and the probability of dose modification of polatuzumab vedotin due to an AE. Associations between the time to first polatuzumab vedotin dose modification due to any AE and exposure were investigated using Kaplan–Meier plots and CPH models. The association of dose intensities of Pola‐R‐CHP components with acMMAE and unconjugated MMAE C6 AUC and C max was explored graphically by linear regression and summarized by exposure tertiles. Dose intensity was calculated based on the actual doses administered to each patient up to the end‐of‐treatment assessment relative to the planned dose. If a statistically significant effect of exposure (α = 0.05) was observed, a covariate analysis was conducted. See Appendix S1 for further details.
Software
PopPK analyses were conducted via nonlinear mixed‐effects modeling with NONMEM software, Version 7.5.0 (ICON Development Solutions). Exposure–efficacy and exposure–safety analyses, including logistic regression, Kaplan–Meier plots, CPH modeling, and covariate analyses, were performed using R, Version 4.0.2 for Windows (R project, http://www.r‐project.org/).
RESULTS
PopPK analysis
Dataset
Of the 435 safety‐evaluable patients with previously untreated DLBCL who received Pola‐R‐CHP in the POLARIX study, data from 429 patients were included in the current analysis; six patients were excluded due to a lack of quantifiable PK data. Data from the evaluable patients included 1122 acMMAE and 1175 unconjugated MMAE concentration values. Patient covariates are summarized in Table 1.
TABLE 1.
Summary of continuous covariates at baseline and exposure measures for patients included in population pharmacokinetics and exposure–response analyses (N = 429).
| Covariate, units | Mean (SD) | Median (range) |
|---|---|---|
| Weight, kg | 75.8 (20.0) | 74.2 (38.4–228.0) |
| Age, years | 62.9 (11.4) | 65.0 (19.0–80.0) |
| Lactate dehydrogenase, μ/L | 424.0 (421.0) | 297.0 (4.2–4820.0) |
| Albumin, g/L | 36.8 (6.07) | 37.0 (17.1–54.2) |
| Hemoglobin, g/L | 121.0 (19.0) | 123.0 (65.0–170.0) |
| Platelet count, 109/L | 286.0 (121.0) | 259.0 (25.0–881.0) |
| Neutrophil count, 109/L | 6.1 (3.4) | 5.3 (0.46–25.4) |
| B‐cell count, 109/L | 242 (1030) | 90.5 (0–19,100) |
| Log B‐cell count 109/L | 4.4 (1.3) | 4.5 (0–9.9) |
| Neutrophil to lymphocyte ratio | 6.8 (8.1) | 4.3 (0.3–84.7) |
| Tumor size, mm2 | 7420 (12,800) | 4680 (96–227,000) |
| acMMAE C6 AUC, ng*day/mL | 2550 (361) | 2530 (1690–4510) |
| acMMAE C6 C max, ng/mL | 636 (96) | 632 (419–1010) |
| Unconjugated MMAE C6 AUC, ng*day/mL | 15.5 (8.2) | 13.7 (4.1–72.4) |
| Unconjugated MMAE C6 C max, ng/mL | 1.4 (0.6) | 1.3 (0.4–5.4) |
Note: Missing values were replaced by the medians of non‐missing values if missing values were <15%.
Abbreviations: ac, antibody‐conjugated; AUC, area under the concentration–time curve; C, cycle; C max, maximum concentration; MMAE, monomethyl auristatin E; SD, standard deviation.
acMMAE–MMAE integrated model
Estimates of structural fixed‐effects, covariate fixed‐effects, and variance parameters for the final integrated model have been previously published. 13 The previously developed model was run with all parameters fixed using data from the POLARIX study.
The VPC plots for acMMAE and unconjugated MMAE showed acceptable agreement between the simulated and observed data for the 10th percentile and the median, while the model overestimated the 90th percentile of observed data for unconjugated MMAE (Figure S1). The polatuzumab vedotin legacy popPK model was established based on more variable data from studies with intensive PK sampling in more diverse populations, 13 which led to higher than observed variability of predicted unconjugated MMAE concentrations following the first dose in the POLARIX study. As steady state was approached (e.g., C4D1 post‐dose samples, at a nominal time of 1513 h after the first dose), differences between observed and simulated unconjugated MMAE concentrations were much lower than at previous timepoints. Despite this overprediction of variability, the dependencies of the random effects on covariates in POLARIX did not show any strong trends unaccounted for by the legacy popPK model (data not shown). The conditional predictive check confirmed that AUC and C max values based on conditional simulation could be used for the ER analysis and graphical evaluation of covariate effects in the POLARIX study (Figure S2).
The VPC method compares the empirical distribution of observation data with the corresponding model‐based predictions and uses bins to group data. NPDEs are individual comparisons of each observation with the corresponding model‐based prediction, which may serve as a more appropriate approach for model performance assessment. Based on NPDE plots (Figure S3), the model prediction is generally consistent with observations of acMMAE and unconjugated MMAE. Therefore, the legacy popPK model was able to appropriately describe the PK of polatuzumab vedotin in patients with previously untreated DLBCL.
PopPK properties of acMMAE
Based on the external validation results, the previously developed integrated popPK model for acMMAE–MMAE with a parallel linear exponentially declining clearance, a linear time‐dependent non‐specific clearance, and Michaelis–Menten elimination 13 provided a good fit for the acMMAE plasma concentration–time data (definitions shown in Appendix S1). Based on linear clearance at C6, the median C6 terminal half‐life of acMMAE was estimated as 11.8 (range: 5.3–15.2) days.
Simulation of PK exposures following polatuzumab vedotin 1.8 mg/kg Q3W dosing showed the mean (standard deviation) AUC and C trough for acMMAE increased steadily from C1 to C6, from 1730 (345) ng*day/mL to 2480 (351) ng*day/mL for AUC, and from 10.5 (3.68) ng/mL to 22.7 (6.75) ng/mL for C trough. This increase was likely due to a decrease in drug clearance over time. The acMMAE exposure was higher for C3 than C1, with a 31.0% increase in AUC and a 78.1% increase in C trough (i.e., C4D1 pre‐dose) versus C2D1 pre‐dose. For C6 versus C1, AUC was 43.4% higher and C trough was 116.2% higher. By C3, acMMAE exposures approached C6 exposures, where maximum acMMAE exposures were achieved for the fixed duration of six cycles of treatment. For acMMAE, C3 values as a percentage of C6 values were 92% for AUC, 99% for C max, and 82% for C trough (i.e., C4D1 pre‐dose), and respective C6 values as a percentage of model‐predicted steady state values (i.e., with hypothetical continued dosing past C6) were 90%, 99%, and 80%.
PopPK properties of unconjugated MMAE
Based on the external validation results, the integrated popPK model for acMMAE–MMAE described the PK of unconjugated MMAE well. 13 Unconjugated MMAE demonstrated formation rate‐limited kinetics, and C max and AUC decreased with repeated dosing of polatuzumab vedotin during C1 to C6. For C3 versus C1, unconjugated MMAE decreased by 34.0% for AUC and 45.2% for C max, and for C6 versus C1, the respective decreases were 36.2% and 48.5%. C trough values for unconjugated MMAE exposures remained low throughout C1 to C6 (range: 0.113–0.154 ng/mL).
Covariate evaluation
Based on C6 exposures, weight, sex, ethnicity (Figure 1a–d), region, mild or moderate renal impairment, mild hepatic impairment based on National Cancer Institute classification, and other patient and disease characteristics (data not shown) were not associated with notable differences in acMMAE or unconjugated MMAE exposures. The POLARIX popPK analysis dataset included data from 429 patients with a median body weight of 74.4 kg (range: 38.4 to 228.0 kg). Comparisons of exposure by body weight are shown in Table S1. The acMMAE C6 AUC was 14% higher and C6 C max was 18% higher in patients weighing ≥100 kg than in those weighing <100 kg, and unconjugated MMAE C6 AUC was 54% higher and C6 C max was 48% higher for the same body weight comparison.
FIGURE 1.
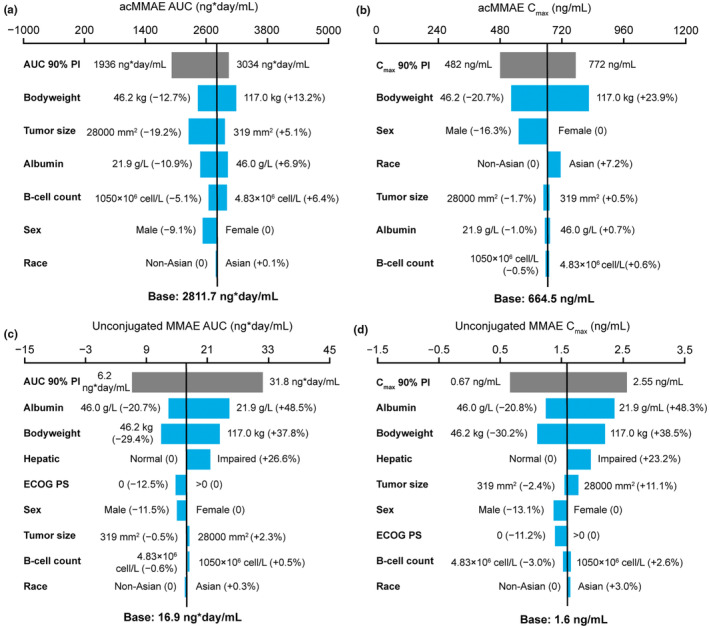
Impact of covariates on exposure at cycle 6 following polatuzumab vedotin 1.8 mg/kg Q3W dosing. (a) acMMAE AUC. (b) acMMAE C max. (c) Unconjugated MMAE AUC. (d) Unconjugated MMAE C max. Base, as represented by the black vertical line, refers to the predicted cycle 6 exposure (C trough) of unconjugated MMAE in a typical patient. The gray bar shows the minimum and maximum exposure range across the entire population based on individual predictions. Each blue bar represents the influence of a single covariate on the cycle 6 exposure after repeated polatuzumab vedotin treatment at a dose of 1.8 mg/kg Q3W for six cycles. The label at the left end of the bar represents the covariate being evaluated. For each covariate, if continuous, two subjects were generated with extreme covariate values (2.5th and 97.5th percentile); if categorical, one subject from each category was created, with other covariates fixed at reference value (continuous) or reference category (categorical). The length of each bar describes the potential impact of that particular covariate on unconjugated MMAE exposure at cycle 6, with the percentage value in the parentheses at each end representing the percent change of exposure from the base. The most influential covariate is at the top of the plot for each exposure parameter. The typical patient is a white male patient with DLBCL receiving 1L Pola‐R‐CHP with a baseline body weight of 75 kg, baseline albumin of 35 g/L, baseline tumor size of 5000 mm2, normal hepatic function, baseline ECOG performance status score of 1, and baseline B‐cell count as 90 × 106 cell/L. 1L, first‐line; ac, antibody‐conjugated; AUC, area under the concentration–time curve; C max, maximum concentration; C trough, trough concentration; DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; MMAE, monomethyl auristatin E; Pola‐R‐CHP, polatuzumab vedotin plus rituximab and cyclophosphamide, doxorubicin, and prednisone; Q3W, every 3 weeks.
Similar acMMAE exposures were observed in patients with moderate hepatic impairment compared with those with normal function (within 1.5% for both AUC and C max; Table S2). Patients with moderate hepatic impairment had moderately higher unconjugated MMAE C6 exposures than those with normal hepatic function (46% for AUC and 35% for C max), which is in line with model‐predicted covariate relationships.
Patient and disease characteristics were not associated with differences in acMMAE or unconjugated MMAE C6 exposures. ADAs were only detected in 1.4% (6/429) of PK‐evaluable patients. For patients who were ADA‐positive versus those who were ADA‐negative, acMMAE exposures were similar, whereas unconjugated MMAE exposures were 30%–31% lower (Table S3). Comparisons of C6 exposures with other covariates are shown in Tables S3 to S25.
Exposure–efficacy analyses
Exposure–efficacy analyses were conducted in 429 patients from the Pola‐R‐CHP arm and 439 patients from the R‐CHOP arm of POLARIX. The six patients in the Pola‐R‐CHP arm who were excluded from the popPK analyses were also excluded from the exposure–efficacy analyses. A summary of the exposure–efficacy results is shown in Table 2. CPH modeling demonstrated a significant relationship between acMMAE AUC C6 and PFS, and acMMAE AUC C6 and EFSeff (both p = 0.01). Baseline bulky disease was identified as a significant covariate in both models; the exposure–efficacy relationship remained significant with baseline bulky disease included in the models. Also, baseline peripheral B‐cell count was found to be a significant covariate in the PFS model, although it did not remain in the final model at α = 0.001 during the backward elimination process. Increased acMMAE C6 exposure was associated with improved PFS (p = 0.011); however, no statistically significant correlation was observed between acMMAE AUC and OS, and the probability of end‐of‐treatment CR was not associated with AUC C6 acMMAE exposure (Figure 2). Kaplan–Meier analysis (stratified by acMMAE AUC C6 tertiles) also indicated longer PFS and EFSeff with higher acMMAE C6 exposure, with no clear trends for OS (Figure S4).
TABLE 2.
Summary of the exposure–efficacy analysis (N = 429): base models.
| Endpoint | Analysis type | Number of patients with an event, n (%) | p value |
|---|---|---|---|
| PFS | CPH model | 105 (25.4) | 0.011 |
| EFSeff | 110 (25.6) | 0.010 | |
| OS | 51 (11.9) | 0.117 | |
| CR at EOT | Logistic regression | 339 (79.0) | 0.149 |
Abbreviations: CPH, Cox proportional hazards; CR, complete response; EFSeff, event‐free survival‐efficacy; EOT, end of treatment; OS, overall survival; PFS, progression‐free survival.
FIGURE 2.
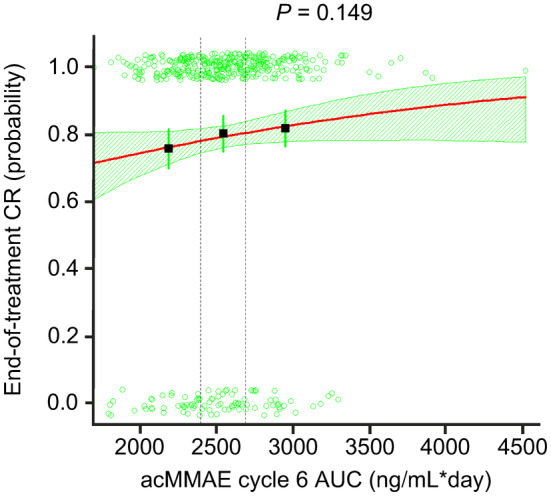
Exposure–efficacy analysis. Logistic regression for CR at EOT versus acMMAE AUC. Dashed vertical lines show bounds of exposure groups. ac, antibody‐conjugated; AUC, area under the concentration–time curve; CR, complete response; EOT, end of treatment; MMAE, monomethyl auristatin E.
Exposure–safety analyses
Exposure–safety analyses were performed in 429 patients with previously untreated DLBCL who were treated with polatuzumab vedotin 1.8 mg/kg Q3W in POLARIX. The six patients in the Pola‐R‐CHP arm who were excluded from the popPK analyses were also excluded from the exposure–safety analyses. Table 3 shows a summary of the results from the exposure–safety analysis.
TABLE 3.
Summary of the exposure–safety analysis (N = 429): base models.
| Analysis type | Factor analyzed | Number of patients with event, n (%) | p value | |||
|---|---|---|---|---|---|---|
| acMMAE | Unconjugated MMAE | |||||
| C6 AUC | C6 C max | C6 AUC | C6 C max | |||
| Logistic regression (AEs) | Grade ≥3 neutropenia | 179 (41.7) | 0.450 | 0.636 | 0.001 | 0.004 |
| Grade ≥3 febrile neutropenia | 58 (13.5) | 0.188 | 0.504 | <0.0005 | 0.001 | |
| Grade ≥2 neuropathy | 60 (14.0) | 0.042 | 0.003 | 0.525 | 0.827 | |
| Grade ≥3 infections or infestations | 65 (15.2) | 0.099 | 0.089 | <0.0005 | <0.0005 | |
| Grade ≥3 anemia | 52 (12.1) | 0.028 | 0.060 | <0.0005 | <0.0005 | |
| Grade ≥3 thrombocytopenia | 22 (5.1) | 0.011 | 0.020 | <0.0005 | <0.0005 | |
| Dose modification due to AE | 34 (7.9) | 0.199 | 0.394 | 0.866 | 0.757 | |
| CPH model | Time to first dose modification | 34 (7.9) | 0.164 | 0.341 | 0.797 | 0.689 |
| Linear regression (dose intensities) | Polatuzumab vedotin | – | 0.166 | 0.050 | <0.0005 | <0.0005 |
| Rituximab | – | 0.007 | 0.001 | <0.0005 | <0.0005 | |
| Doxorubicin | – | 0.035 | 0.006 | 0.001 | 0.001 | |
| Cyclophosphamide | – | 0.029 | 0.003 | <0.0005 | <0.0005 | |
| Prednisone | – | 0.563 | 0.623 | 0.390 | 0.341 | |
Abbreviations: ac, antibody‐conjugated; AE, adverse event; AUC, area under the concentration–time curve; C, cycle; C max, maximum concentration; CPH, Cox proportional hazards; MMAE, monomethyl auristatin E.
Higher acMMAE C6 exposures were associated with increased incidence of grade ≥2 peripheral neuropathy (p = 0.042 and p = 0.003 for AUC and C max, respectively), grade ≥3 anemia (p = 0.028 for AUC only), and grade ≥3 thrombocytopenia (p = 0.011 and p = 0.020 for AUC and C max, respectively; Figure 3a–c; Table S26). The covariate analyses were performed for acMMAE AUC C6 only; baseline hemoglobin and baseline LDH were identified as significant covariates for grade ≥3 anemia at the α = 0.01 level. Higher unconjugated MMAE exposures were associated with increased incidence of grade ≥3 neutropenia (p = 0.001 and p = 0.004 for AUC and C max, respectively), febrile neutropenia (p < 0.0005 and p = 0.001 for AUC and C max, respectively), infections/infestations (p < 0.0005 for both AUC and C max), anemia (p < 0.0005 for both AUC and C max), and thrombocytopenia (p < 0.0005 for both AUC and C max; Figure 3d–h). The covariate analysis for unconjugated MMAE exposure (also performed for AUC only) identified hemoglobin as a significant covariate for grade ≥3 anemia, and Asian ethnicity for grade ≥3 neutropenia at the α = 0.01 level. The acMMAE and unconjugated MMAE exposures were not associated with the probability of dose modification due to AEs (Figure S5a–d) or time to first dose modification due to AEs (Figure S6a–d).
FIGURE 3.
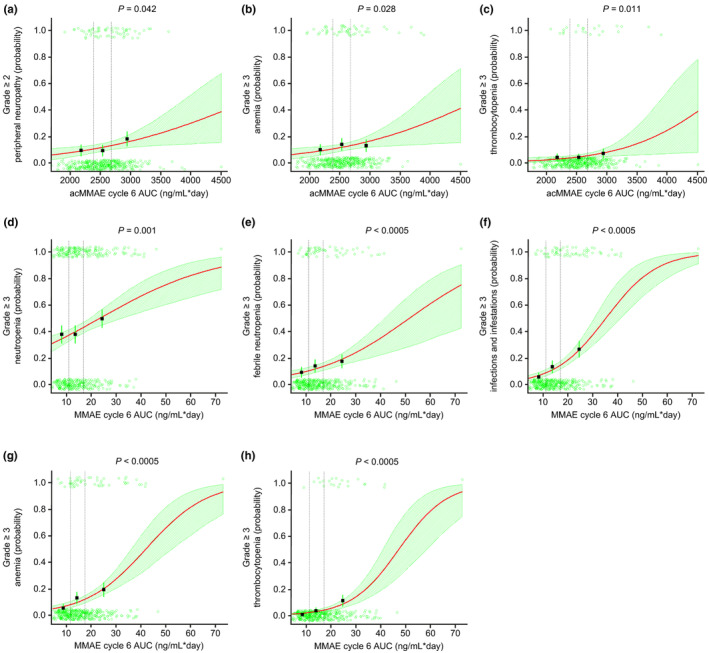
Exposure–safety analyses. Logistic regression for (a) grade ≥2 peripheral neuropathy, (b) grade ≥3 anemia, (c) grade ≥3 thrombocytopenia, versus acMMAE AUC, and (d) grade ≥3 neutropenia, (e) grade ≥3 febrile neutropenia, (f) grade ≥3 infections/infestations, (g) grade ≥3 anemia, and (h) grade ≥3 thrombocytopenia, versus unconjugated MMAE AUC. Dashed vertical lines show bounds of exposure groups. ac, antibody‐conjugated; AUC, area under the concentration–time curve; MMAE, monomethyl auristatin E.
Statistically significant relationships between increased acMMAE and unconjugated MMAE C6 exposures and decreased dose intensity of polatuzumab vedotin were observed (α = 0.05, acMMAE: p = 0.050 for C max; MMAE: p < 0.0005 for both AUC and C max). Statistically significant relationships were also observed between increased acMMAE and unconjugated MMAE C6 exposures and decreased dose intensity of rituximab, doxorubicin, and cyclophosphamide (Figure S7a–d). The correlations were not considered clinically relevant due to the high overall dose intensity across exposure tertiles.
DISCUSSION
Patients with R/R DLBCL tend to have poor survival outcomes 2 ; therefore, it is crucial that effective treatments with manageable safety profiles are developed for use in the first‐line setting, to avoid the risk of relapse or lack of response to treatment. In the POLARIX study, PFS was higher with Pola‐R‐CHP versus R‐CHOP, and safety profiles were comparable. 8 This analysis assessed the dose and regimen of polatuzumab vedotin 1.8 mg/kg Q3W for six cycles plus R‐CHP in patients with DLBCL in POLARIX, using a previously established integrated popPK model 13 and ER analyses.
The previously developed popPK model provided a good description of acMMAE and unconjugated MMAE concentrations following the administration of intravenous polatuzumab vedotin in patients with previously untreated DLBCL in POLARIX. Unconjugated MMAE AUC and C max decreased over C1 to 6, possibly due to higher nonlinear unconjugated MMAE clearance rate at lower concentrations and a lower relative fraction of formation of unconjugated MMAE from acMMAE over time. Therefore, repeated dosing of polatuzumab vedotin did not result in the accumulation of acMMAE.
The AUC and C max based on nominal dosing were used in the ER analysis, to avoid bias caused by a correlation in time between response and lower exposures due to dose modifications, both of which are more likely the longer a patient is on study. Moreover, AUC and C max based on nominal dosing isolated the impact of assigned target polatuzumab vedotin dose and associated steady‐state exposure on safety and efficacy and was not subject to confounding by complex interactions between time and treatment‐related or disease‐related changes to polatuzumab vedotin dosing. Importantly, the choice of nominal versus actual polatuzumab vedotin dose as the exposure metric is less likely to confound the interpretation of ER relationships with high dose intensity of polatuzumab vedotin in POLARIX. In the Pola‐R‐CHP arm, 92% of patients received six cycles of polatuzumab vedotin, and 94% completed 6 cycles of Pola‐R‐CHP. ER simulations further confirm that average concentration up to an event time may result in causal confounding, particularly in scenarios that prompt the use of this metric (such as dosing patterns). 14
Changes in unconjugated MMAE exposures did not lead to a clinically meaningful increase in the frequency of AESIs. This observation is consistent with previous ER analyses in patients with R/R DLBCL. 12 Based on logistic regression of unconjugated MMAE exposures with the probability of AESIs, a 50% increase in unconjugated MMAE AUC from 13.8 to 20.7 ng*day/mL and unconjugated MMAE C max from 1.3 to 2.0 ng/mL is not expected to result in a clinically meaningful increase in the incidence of AESIs. Similarly, a 50% increase in acMMAE AUC from 2530 to 3790 ng*day/mL and acMMAE C max of 629 to 943 ng/mL is not expected to result in a clinically meaningful increase in the incidence of AESIs (no more than ~22% increase in absolute risk). Despite increased AEs with increased exposure, the benefit–risk profile was still considered favorable.
As expected, patients who weighed ≥100 kg had higher exposure than those who weighed <100 kg due to body weight‐based dosing. These differences in exposure due to weight are not expected to have a clinically relevant impact on safety based on the exposure‐safety analysis. The clinical safety data did not show strong evidence that the safety profile of Pola‐R‐CHP was worse in patients weighing ≥100 kg than in those <100 kg; however, only 43 patients in the Pola‐R‐CHP arm weighed ≥100 kg. The incidence of fatal AEs, serious AEs, and grade 3 to 5 AEs were similar in patients who weighed ≥100 kg compared with those who weighed <100 kg. Overall, these data suggest that weight‐adjusted dosing and dose capping are not required.
Patients with moderate hepatic impairment had moderately higher unconjugated MMAE exposures, which may cause safety concerns due to an increased risk of peripheral neuropathy and neutropenia, and other AESIs. 17 , 18 However, the logistic regression of unconjugated MMAE exposures with the probability of AESIs implies that this will not result in a clinically meaningful increase in the incidence of AESIs. Also, the presence of ADAs in serum did not appear to have a clinically meaningful impact on the PK or exposure of acMMAE or unconjugated MMAE. Of note, given the small number of patients with ADA‐positive status (n = 6; 1.4%), this may require further investigation.
At a polatuzumab vedotin dose of 1.8 mg/kg Q3W, the ER analysis suggested that lower exposure to polatuzumab vedotin may be associated with lower toxicity; however, lower exposure was also associated with lower efficacy. Higher polatuzumab vedotin exposures were associated with decreased dose intensities of polatuzumab vedotin, rituximab, doxorubicin, cyclophosphamide, and prednisone, although this was not considered clinically relevant as the overall dose intensity for each agent was high. The analysis also suggested that increased acMMAE exposure was associated with prolonged PFS and EFSeff. C trough was calculated but not included in the analysis, as it was considered less relevant to safety and efficacy outcomes than acMMAE/MMAE AUC and C max. Given the aggressive nature of DLBCL and the importance of achieving a cure in the first‐line setting, the benefit–risk profile was still considered favorable for polatuzumab vedotin 1.8 mg/kg Q3W.
Limitations of the analyses include small sample sizes for some covariates, including ADA status, and data were limited to single dosing of polatuzumab vedotin at 1.8 mg/kg Q3W. This approach can cause confounding of ER results and limits the exposure range included in the analyses. 17 It is known that the ER relationships for biologics with only one dose level may be confounded due to the potential for the extent of disease to impact the PK of a therapeutic antibody. Given that extent of disease may also be related to both safety and efficacy, it is not possible to distinguish between an effect of PK on disease and an effect of disease on PK. 18 , 19 , 20 , 21
In conclusion, the PK profile of polatuzumab vedotin in the POLARIX study was similar to that previously described in patients with NHL. 12 The starting dose of polatuzumab vedotin 1.8 mg/kg Q3W is appropriate for patients with DLBCL based on the covariates investigated, which are representative characteristics of the wider DLBCL patient population. The ER data and benefit–risk profile support the use of polatuzumab vedotin 1.8 mg/kg Q3W with R‐CHP for six cycles in patients with previously untreated DLBCL.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript; R. Deng, L.G., T.L., C.F., L.S., M.Z.L., R. Dere, C. Lee, G.M., and J.H. designed the research; R. Deng, M.Z.L., D.M., and C. Li performed the research; R. Deng, Q.L., and P.A. analyzed the data.
FUNDING INFORMATION
This study was funded by F. Hoffmann‐La Roche Ltd.
CONFLICT OF INTEREST STATEMENT
T.L., Q.L., P.A., M.Z.L., R. Dere, G.M., J.H., C. Li, and D.M. are employees of Genentech, Inc., and receive F. Hoffmann‐La Roche Ltd. stocks/stock options. R. Deng and L.G. are paid consultants for Genentech, Inc. C. Lee was an employee of Genentech, Inc. at the time of the analysis. C.R.F. reports consultancy for AbbVie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech, Inc./F. Hoffmann‐La Roche Ltd, Genmab, Gilead, Karyopharm, N‐Power Medicine, Pharmacyclics/Janssen, Seagen, Spectrum; holds stock options in Foresight Diagnostics, N‐Power Medicine; and receives research funding from 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, BostonGene, Celgene, Cellectis EMD, Gilead, Genentech, Inc./F. Hoffmann‐La Roche Ltd, Guardant, Iovance, Janssen, Kite, MorphoSys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research. LHS reports consultancy for AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, MorphoSys, F. Hoffmann‐La Roche Ltd, Genentech, Inc., Sandoz, Seagen, Servier, Teva, Takeda, TG Therapeutics, Verastem; research funding from F. Hoffmann‐La Roche Ltd, Genentech, Inc., Teva; and honoraria from AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, MorphoSys, F. Hoffmann‐La Roche Ltd, Genentech, Inc., Sandoz, Seagen, Servier, Teva, Takeda, TG Therapeutics, and Verastem.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank the participating patients and their families, the investigators, research nurses, study coordinators, and operations staff, as well as the Lymphoma Academic Research Organisation, and the Data and Safety Monitoring Committee. Third‐party medical writing assistance, under the direction of the authors, was provided by Carla Smith, MSc, of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann‐La Roche Ltd.
Deng R, Gibiansky L, Lu T, et al. Population pharmacokinetics and exposure–response analyses of polatuzumab vedotin in patients with previously untreated DLBCL from the POLARIX study. CPT Pharmacometrics Syst Pharmacol. 2024;13:1055‐1066. doi: 10.1002/psp4.13141
DATA AVAILABILITY STATEMENT
For eligible studies, qualified researchers may request access to individual patient‐level clinical data through the clinical study data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). For up‐to‐date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, available at: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked owing to a potential increase in risk of patient reidentification.
REFERENCES
- 1. Robertson J, Barr R, Shulman L, Forte G, Magrini N. Essential medicines for cancer: WHO recommendations and national priorities. Bull World Health Organ. 2016;94:735‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sehn L, Salles G. Diffuse large B‐cell lymphoma. N Engl J Med. 2021;384:842‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti‐CD79b antibody‐drug conjugate, anti‐CD79b‐vc‐MMAE, for the treatment of non‐Hodgkin lymphoma. Blood. 2009;114:2721‐2729. [DOI] [PubMed] [Google Scholar]
- 4. Pfeifer M, Zheng B, Erdmann T, et al. Anti‐CD22 and anti‐CD79B antibody drug conjugates are active in different molecular diffuse large B‐cell lymphoma subtypes. Leukemia. 2015;29:1578‐1586. [DOI] [PubMed] [Google Scholar]
- 5. Chugai Pharmaceutical Co. Ltd . Chugai Obtains Regulatory Approval for POLIVY for Additional Indication of Previously Untreated Diffuse Large B‐cell Lymphoma. https://www.chugai‐pharm.co.jp/english/news/detail/20220824150000_944.html. Accessed February 26, 2024.
- 6. ClinicalTrials.gov . A Phase III, multicenter, randomized, double‐blind, placebo‐controlled trial comparing the efficacy and safety of polatuzumab vedotin in combination with rituximab and CHP (R‐CHP) versus rituximab and CHOP (R‐CHOP) in previously untreated patients with diffuse large B‐cell lymphoma. https://clinicaltrials.gov/ct2/show/NCT03274492. Accessed February 26, 2024.
- 7. POLIVY® Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product‐information/polivy‐epar‐product‐information_en.pdf. Accessed February 29, 2024.
- 8. Tilly H, Morschhauser M, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B‐cell lymphoma. N Engl J Med. 2022;386:351‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minchella K, Xu H, Al‐Huniti N. Exposure‐response methods and dose approval of new oncology drugs by FDA from 2005 to 2015. J Clin Oncol. 2016;34:2530. [Google Scholar]
- 10. Overgaard R, Ingwersen S, Tornøe C. Establishing good practices for exposure‐response analysis of clinical endpoints in drug development. CPT Pharmacometrics Syst Pharmacol. 2015;4:565‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shemesh C, Agarwal P, Lu T, et al. Pharmacokinetics of polatuzumab vedotin in combination with R/G‐CHP in patients with B‐cell non‐Hodgkin lymphoma. Cancer Chemother Pharmacol. 2020;85:831‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu T, Gibiansky L, Li X, et al. Exposure‐safety and exposure‐efficacy analyses of polatuzumab vedotin in patients with relapsed or refractory diffuse large B‐cell lymphoma. Leuk Lymphoma. 2020;61:2905‐2914. [DOI] [PubMed] [Google Scholar]
- 13. Lu D, Lu T, Gibiansky L, et al. Integrated two‐analyte population pharmacokinetic model of polatuzumab vedotin in patients with non‐Hodgkin lymphoma. CPT Pharmacometrics Syst Pharmacol. 2020;9:48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiens MR, French JL, Rogers JA. Confounded exposure metrics. CPT Pharmacometrics Syst Pharmacol. 2024;13:187‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suri A, Mould D, Song G, Kinley J, Venkatakrishnan K. Population pharmacokinetics of brentuximab vedotin in adult and pediatric patients with relapsed/refractory hematologic malignancies: model‐informed hypothesis generation for pediatric dosing regimens. J Clin Pharmacol. 2020;60:1585‐1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li C, Zhang C, Li Z, et al. Clinical pharmacology of vc‐MMAE antibody–drug conjugates in cancer patients: learning from eight first‐in‐human phase 1 studies. MAbs. 2019;12:e1699768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody‐drug conjugates. MAbs. 2016;8:659‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawakatsu S, Bruno R, Kågedal M, et al. Confounding factors in exposure‐response analyses and mitigation strategies for monoclonal antibodies in oncology. Br J Clin Pharmacol. 2021;87:2493‐2501. [DOI] [PubMed] [Google Scholar]
- 19. Dai HI, Vugmeyster Y, Mangal N. Characterizing exposure response relationship for therapeutic monoclonal antibodies in immuno oncology and beyond: challenges, perspectives and prospects. Clin Pharmacol Ther. 2020;108:1156‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Booth B, Rahman A, Kim G, Huang SM, Zineh I. Toward greater insights on pharmacokinetics and exposure response relationships for therapeutic biologics in oncology drug development. Clin Pharmacol Ther. 2017;101:582‐584. [DOI] [PubMed] [Google Scholar]
- 21. Turner DC, Kondic AG, Anderson KM, et al. Pembrolizumab exposure‐response assessments challenged by association of cancer cachexia and catabolic clearances. Clin Cancer Res. 2018;24:5841‐5849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient‐level clinical data through the clinical study data request platform. At the time of writing this request, the platform is Vivli (https://vivli.org/ourmember/roche/). For up‐to‐date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, available at: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked owing to a potential increase in risk of patient reidentification.


