Abstract
Zavegepant is a novel gepant administered as a nasal spray approved in the United States at a 10 mg dose for the acute treatment of migraine with or without aura in adults. The cardiovascular safety of zavegepant nasal spray was assessed in both single‐ascending dose (SAD) and multiple‐ascending dose (MAD) studies in healthy participants. The SAD study included 72 participants (54 active/18 placebo) who received 0.1–40 mg zavegepant or placebo. The MAD study included 72 participants (56 active/16 placebo) who received 5–40 mg zavegepant or placebo for 1–14 days. Plasma zavegepant pharmacokinetics and electrocardiographic (ECG) parameters (Fridericia‐corrected QT interval [QTcF], heart rate, PR interval, ventricular depolarization [QRS], T‐wave morphology, and U‐wave presence) were analyzed pre‐ and post‐zavegepant administration. Using pooled data from the SAD and MAD studies, the relationship between time‐matched plasma zavegepant concentrations and QTc interval was assessed using a linear mixed‐effects model to evaluate the potential for QTc interval prolongation. Results showed that single and multiple doses of zavegepant had no significant impact on ECG parameters versus placebo, and there was no concentration‐dependent effect on QTcF interval. The estimated slope of the plasma zavegepant concentration‐QTcF model was −0.053 ms per ng/mL with a 90% confidence interval of −0.0955 to −0.0110 (p = 0.0415), which is not considered clinically meaningful. At doses up to four times the recommended daily dose, zavegepant does not prolong the QT interval to any clinically relevant extent.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Zavegepant (BHV‐3500) is a novel gepant approved in the US as a 10 mg nasal spray for acute migraine treatment with or without aura in adults.
WHAT QUESTION DID THIS STUDY ADDRESS?
Drug‐induced QT prolongation is a significant risk to patients. Therefore, the potential for QTc interval prolongation after administration of intranasal zavegepant was assessed in lieu of a dedicated TQT study.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Single and multiple doses of zavegepant nasal spray had no clinically relevant effect on studied electrocardiogram parameters including QTc interval, HR, PR interval, and QRS duration. Concentration‐QT analysis also showed no clinically meaningful prolongation of the QTc interval at doses up to four times the recommended daily dose of 10 mg zavegepant nasal spray.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
This study supports the cardiovascular safety of 10 mg intranasal zavegepant and showcases the successful application of concentration‐QTc analysis in lieu of a dedicated TQT study.
INTRODUCTION
Migraine, a common neurological disorder, is characterized by unilateral pulsating headache and often associated with symptoms of nausea, photophobia, and/or phonophobia. 1 Migraine afflicts around 15% of the United States population and 14% of the global population. 2 , 3
Calcitonin gene‐related peptide (CGRP), a 37‐amino‐acid peptide produced in the peripheral and central nervous systems that binds to CGRP receptors, has been shown to play a key role in migraine pathophysiology. 1 , 4 Gepants, small‐molecule CGRP receptor antagonists, bind to CGRP receptors and prevent their activation. 5 , 6 Gepants are used for the acute and preventive treatment of migraine, especially in adults who are intolerant or not responsive to triptans. 7 , 8
Zavegepant (Zavzpret™, Pfizer, New York, NY, USA) is a novel gepant approved in the United States as a 10 mg nasal spray for the acute treatment of migraine with or without aura in adults. 9 Zavegepant is the first approved small‐molecule gepant to be administered as a nasal spray. The efficacy and safety of zavegepant nasal spray for the acute treatment of migraine have been demonstrated in two pivotal clinical studies. 10 , 11 Peak plasma concentration after administration of a single 10 mg dose of zavegepant nasal spray is observed at approximately 30 min post‐administration. Zavegepant nasal spray displays slightly less than dose‐proportional pharmacokinetics after single doses up to 40 mg. Zavegepant is primarily metabolized by cytochrome P450 (CYP)3A4 and to a lesser extent by CYP2D6 in vitro; however, in vivo, zavegepant nasal spray undergoes minimal metabolism without producing major metabolites. The primary clearance pathway of zavegepant nasal spray is biliary excretion. 9 , 12 In the human ether‐à‐go‐go related gene (hERG) patch clamp assay, zavegepant showed weak inhibition (9.5% at 10 μM and 22% at 30 μM), which suggests a low potential for QT‐related electrocardiographic (ECG) changes. 13
Drug‐induced QT prolongation is a significant risk to patients and the evaluation of this risk is a critical element of the drug development process. 14 , 15 Therefore, the effects of zavegepant nasal spray on ECG parameters including QTc interval, PR interval, duration of ventricular depolarization (QRS) complex, heart rate (HR), T‐wave morphology, and U‐wave presence were assessed in healthy participants in the zavegepant single‐ascending dose (SAD) and multiple‐ascending dose (MAD) studies. 16 Using pooled data from both the SAD and MAD studies, the relationship between time‐matched plasma zavegepant concentrations and QTc interval was assessed using a linear mixed‐effects model to evaluate the potential for QTc interval prolongation.
METHODS
Study design and participants
The zavegepant SAD and MAD studies were phase I, single‐center, randomized, placebo‐controlled, double‐blind, sequential studies conducted in healthy participants administered zavegepant nasal spray. The SAD study included nine cohorts of eight participants each randomized in a 3:1 ratio to receive either single doses of zavegepant nasal spray (n = 6) at 0.1, 0.3, 1, 3, 5, 10, and 20 mg in one nostril, 20 mg (10 mg in each nostril), and 40 mg (20 mg in each nostril) or matching placebo (n = 2). Participants were dosed sequentially in an ascending pattern with at least 7 days between each dose level. The MAD study included six cohorts. In Cohorts 1–4, 12 participants each were randomized in a 3:1 ratio to receive either zavegepant nasal spray (n = 9) or matching placebo (n = 3). Participants in Cohorts 1–3 received zavegepant nasal spray or placebo once daily at ascending dose levels of 5, 10, or 20 mg for 14 days. Participants in Cohort 4 received two sequential 20 mg sprays separated by 2 h in alternate nostrils for 8 days. The single‐day dosing cohorts (Cohorts 5 and 6) in the MAD study were not included in this analysis as cardiodynamic ECG assessments were not performed. In both studies, participants were followed for 4 days after the last administered dose and all zavegepant nasal spray doses were administered under fasting conditions.
In both studies, eligible participants were non‐smoking, healthy male and female individuals aged ≥18 and ≤55 years with a body mass index of >18.5 and <30.0 kg/m2 and a body weight of ≥50 kg for males and ≥45 kg for females. Participants were also required to have a score of 0 on the Sheehan Suicidality Tracking Scale 17 , 18 and a score of >8 on the Brief Smell Identification Test. 19
The key exclusion criteria for both studies included a history of nasal conditions, piercings, or physical findings that may affect the administration or absorption of the nasal product; a sitting systolic blood pressure >140 mmHg or diastolic blood pressure > 90 mmHg; abnormalities on 12‐lead ECG (defined as PR interval ≥ 210 ms, QRS complex ≥120 ms, QT interval ≥ 500 ms, or Fridericia's corrected QT interval [QTcF] ≥450 ms); a history of seizure disorder besides a single childhood febrile seizure; presence of viral hepatitis or a history of liver disease; abnormal hematologic, renal, or liver function laboratory results; positive pregnancy test; history of alcohol or drug abuse; or history of allergic reactions.
The study protocols for the SAD and MAD studies were reviewed and approved by the Advarra Institutional Review Board (Columbia, MD, USA [SAD] and Aurora, ON, Canada [MAD]). Both studies were conducted in compliance with the recommendations from the Declaration of Helsinki; Good Clinical Practices and Good Laboratory Practices as stated in the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines; all applicable regulations, including the Federal Food, Drug and Cosmetic Act, United States applicable Code of Federal Regulations Title 21, Part 50, and Independent Ethics Committee requirements. All participants provided a written informed consent before initiating any study activities. The study activities related to cardiodynamic ECG were performed at ERT Electrocardiogram Central Core Laboratory (Rochester, NY, USA).
Study treatments and procedures
Pharmacokinetic analyses
For the SAD study, blood samples for pharmacokinetic (PK) analyses were collected at the following timepoints: pre‐dose and 0.08, 0.17, 0.33, 0.5, 0.67, 0.83, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 8.0, 12, 24, 48, 72, and 96 h post‐dose. For Cohorts 1–3 in the MAD study, blood samples were collected pre‐dose and at all the same timepoints from 0.08 to 12 h post‐dose on Day 1; pre‐dose on Days 2–13; and at all timepoints pre‐dose through 96 h post‐dose on Day 14. For Cohort 4 in the MAD study, samples were collected pre‐first dose and from 0.17 to 2 h (pre‐second dose) and then 2.17, 2.33, 2.5, 2.83, 3, 3.5, 4, 4.5, 5, 6, 8 and 12 h post‐first dose; pre‐first dose on Days 2–7; and through 96 h post‐first dose on Day 8. PK analyses were performed using Phoenix WinNonlin v.8.0 and inferential statistical analyses were performed using SAS v.9.2.
Plasma concentrations of zavegepant were measured using a validated ultra‐performance liquid chromatography with tandem mass spectrometry. Zavegepant and its internal standard zavegepant‐d8 were extracted from 0.025 mL of human ethylenediaminetetraacetic acid K2 plasma using automated protein precipitation and transferred to a liquid chromatograph with an ACE Excel 2 C18‐PFP, 50 × 3 mm, 2 μm column kept at 40°C. The pump flow was isocratic at a flow rate of 0.500 mL/min. Mobile phase A was Milli‐Q type water/acetonitrile with ammonium acetate and formic acid and mobile phase B was Milli‐Q type water/acetonitrile with ammonium formate and formic acid. An API 5000 mass spectrometer equipped with a turbo‐ion spray source was used for detection in positive ion mode. The mass transitions were 639.4–255.2 m/z for zavegepant and 647.5–255.2 m/z for the internal standard. The lower limit of quantification was 0.4 ng/mL with a validation calibration range of 0.40–400 ng/mL (SAD) and 200 ng/mL (MAD). The between‐run accuracy bias ranged from −5.0% to 2.2% (precision coefficient of variation [CV]: 3.6% to 5.1%) for both studies, and the within‐run accuracy bias was from −8.2% to 1.3% (precision CV: 2.1% to 5.0%) for the SAD study and −6.8% to 0.7% (precision CV: 0.9% to 2.9%) for the MAD study. The data were captured using Analyst software version 1.6.3 (AB Sciex, Toronto, Canada).
Cardiodynamic assessments
In both studies, continuous ECG recordings (Holter) were measured from 1 h pre‐dose to 24 h post‐dose. In the MAD study, continuous ECG recordings were collected on Days 1 and 14 for Cohorts 1–3, and on Days 1 and 8 for Cohort 4. All ECG data were collected using a Global Instrumentation (Manlius, NY, USA) M12R ECG continuous 12‐lead digital recorder. The 12‐lead ECGs for both studies were extracted at a central ECG laboratory at three timepoints (−45, −30, and −15 min) pre‐dose and at timepoints from 0.33 to 24 h post‐dose paired with PK samples.
In both studies, the cardiodynamic ECG end points following the zavegepant or placebo administration included change from baseline in QTcF (ΔQTcF), HR (ΔHR), PR (ΔPR), and QRS (ΔQRS); placebo‐corrected ΔHR (ΔΔHR), ΔQTcF (ΔΔQTcF), ΔPR (ΔΔPR), and ΔQRS (ΔΔQRS); categorical outliers for HR, QTcF, PR, and QRS; and frequency of T‐wave morphology changes and U‐wave presence. Baseline was derived from three pre‐dose timepoints using continuous ECG.
In addition, the SAD and MAD data were pooled and a linear mixed‐effects model with a treatment effect‐specific intercept was fitted for zavegepant plasma concentrations to assess the effect of zavegepant on ΔΔQTcF.
A positive control was not included in the SAD or MAD studies. Because the zavegepant doses reached up to 40 mg, fourfold the therapeutic dose of 10 mg, the studied exposures were deemed sufficient to provide conclusive evidence of any potential clinically relevant effects of zavegepant on QTc per International Conference on Harmonisation (ICH) E14 guidelines. 20 , 21
Statistical analyses
Two sets of statistical analyses were conducted to evaluate the effect of zavegepant on cardiodynamic assessments. These included a separate evaluation of post‐dose timepoints for each study (QT/QTc analysis) and a pooled concentration‐QT analysis (PK/QTc analysis).
The QT/QTc analysis was conducted using a linear mixed‐effects model with ΔQTcF as the dependent variable; time, study drug, and time by treatment as fixed effects; and baseline QTcF as a covariate. For each timepoint, the least squares (LS) mean estimates with two‐sided 90% confidence interval (CI) were calculated to compare zavegepant versus placebo. Similar analyses, using a similar model, were performed for HR, PR, and QRS.
In both studies, the QT/QTc analysis population consisted of all participants who received at least one dose of zavegepant or placebo, who had measurements at baseline as well as on‐treatment, and who had at least one after dose timepoint with a valid change in ΔQTcF.
The PK/QTc analysis involved modeling of the relationship between plasma zavegepant concentrations and ΔQTcF by using a linear mixed‐effects model and pooled data from the SAD and MAD studies. 22 Prior to performing the pooled concentration‐QTc analysis, homogeneity was assessed by Levene's test to determine whether group variances were equal. The model used ΔQTcF as the dependent variable and estimated treatment, time, and centered baseline effects on the model intercept. Zavegepant quantitation values below the limit of quantitation were replaced with one‐half the lower limit of quantitation for the calculation of log maximum observed plasma concentration (C max).
The geometric mean of the individual C max values for participants on each dose of zavegepant was used to derive the predicted effect and two‐sided 90% CI for ΔΔQTcF. In the MAD study, the predictions were based on C max values observed on Day 14 for Cohorts 1–3 and Day 8 for Cohort 4. If the upper bounds of the 2‐sided 90% CI for predicted ΔΔQTcF were <10 ms, then zavegepant would most likely not cause clinically concerning QTc prolongation.
The concentration‐QTc analysis was performed using the PK/QTc analysis population. The assumptions of the pre‐specified model were assessed by conducting exploratory analyses, including (i) lack of drug effect on HR assessed using time course of HR stratified by dose (Figure S1); (ii) QTc independent from HR assessed using linear regression between QTc and RR interval (Figure S2); (iii) lack of hysteresis assessed using time course of mean concentrations, ΔQTcF and ΔΔQTcF (Figures S3 and S4); (iv) linear relationship between concentration and ΔQTcF assessed using a linear regression line with a locally weighted scatter plot smoothing regression line across all concentrations (Figures S5, S6). The observed PK profiles are provided in Figure S7.
In the SAD study, it was determined a sample size of 56 participants (42 participants dosed with zavegepant and 14 with placebo) was needed to achieve 1064 PK‐QTc pairs, and thus, provide at least 94% power with two‐sided 90% CI to exclude the possibility that zavegepant caused more than a 10‐ms effect on QTc at clinically relevant plasma levels, including C max, based on the concentration‐QTc analysis. 22 This assumed zavegepant's true effect was an increase of 3 ms for ΔQTcF and a standard deviation (SD) of 7 ms. In the MAD study, the goal was to enroll 12 participants per cohort, which was not based on power calculations.
All statistical analyses were performed using SAS v9.4 or higher (SAS Institute, Cary, NC, USA).
RESULTS
Participant characteristics and demographics
In the SAD study, the QT/QTc analysis population comprised 72 participants. The PK/QTc analysis population consisted of 59 participants, due to undetectable PK plasma concentrations in all 12 participants from Cohort 1 (0.1 mg dose); two participants from the 0.3 mg dose group and one participant from the 1 mg dose group in Cohort 3. The concentration‐QTc analysis in the SAD study included 1218 PK‐ΔQTcF data pairs. In the MAD study, the QT/QTc and PK/QTc analysis populations consisted of 48 participants from Cohorts 1 to 4 and no participants from Cohorts 5 to 6. The concentration‐QTc analysis in the MAD study involved 1600 PK‐ΔQTcF data pairs. The baseline demographics of the study participants are summarized in Table 1.
TABLE 1.
Summary of demographic characteristics (safety population).
| SAD study (N = 72) | MAD study (N = 72) | |
|---|---|---|
| Age, years, mean (SD) | 41.2 (9.7) | 40.8 (10.4) |
| Male, n (%) | 40 (55.6) | 59 (81.9) |
| White, n (%) | 66 (91.7) | 61 (84.7) |
| Hispanic or Latino, n (%) | 70 (97.2) | 21 (29.2) |
| Not Hispanic or Latino, n (%) | 2 (2.8) | 51 (70.8) |
| Weight, kg, mean (SD) | 73.4 (9.4) | 76.0 (9.6) |
| BMI, kg/m2, mean (SD) | 26.4 (2.7) | 25.4 (2.4) |
Note: PK/QTc population was n = 59 for the SAD study and n = 48 for the MAD study.
Abbreviations: BMI, body mass index; MAD, multiple‐ascending dose; SAD, single‐ascending dose; SD, standard deviation.
SAD and MAD study cardiodynamic assessments
Effect on QTcF in the SAD and MAD studies
In both studies, the LS mean ΔQTcF in different zavegepant cohorts followed the pattern observed with placebo. In the SAD study, the LS mean ΔΔQTcF ranged from −5.5 ms (8 h in the 0.1 mg cohort) to 6.7 ms (12 h in the 10 mg cohort) with no indication of dose dependency (Figure 1a). The QTcF exceeded 450 ms only in one participant receiving placebo, whose QTcF readings were between 450 and 480 ms at two timepoints. In the MAD study, the LS mean ΔΔQTcF ranged from −6.2 to 8.0 ms on Day 1 with no indication of dose dependency (Figure 1b,c). On Days 8 and 14, the LS mean ΔΔQTcF in the higher 10 mg, 20 mg, and 2 × 20 mg dose groups ranged from −4.9 to 7.5 ms (Figure 1c,d). On Day 14, in the 5 mg dose group, the LS mean ΔΔQTcF ranged from 4.5 to 13.7 ms (6 h post‐dose), with no indication of dose dependency. There were no participants with QTcF >450 ms. There were no treatment emergent T‐wave morphology changes or U‐waves on active treatment in either the SAD or MAD studies.
FIGURE 1.
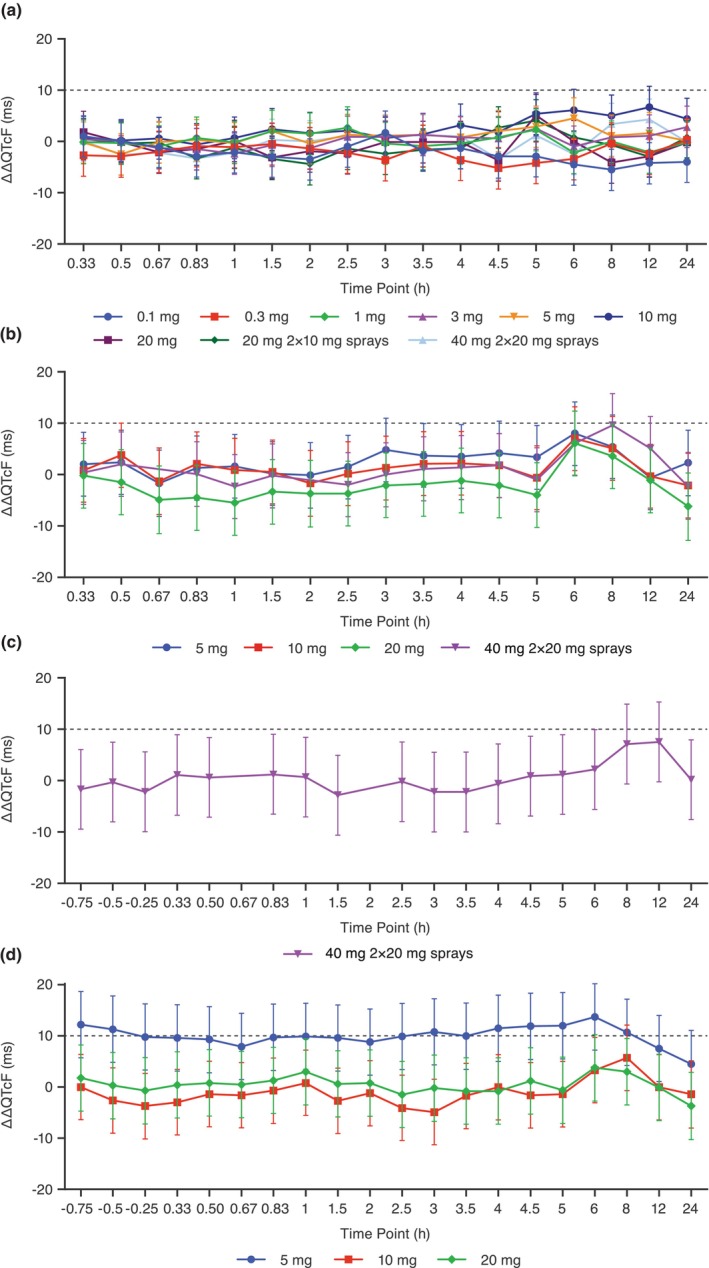
LS mean placebo‐corrected change from baseline QTcF (ΔΔQTcF) across timepoints for the QT/QTc analysis population in the (a) SAD study and on (b) Day 1, (c) Day 8, and (d) Day 14 in the MAD study. Points and error bars represent LS mean and 90% CI based on a linear mixed‐effects model. The dashed gray line represents a 10 ms increase in QTcF. CI, confidence interval; LS, least squares; MAD, multiple‐ascending dose; SAD, single‐ascending dose; ΔΔQTcF, placebo‐corrected change from baseline QT interval corrected using Fridericia's formula.
Effects on heart rate, and PR and QRS intervals in the SAD and MAD studies
In both studies, the LS mean ΔHR in different zavegepant cohorts followed the pattern observed with placebo (Figure S1). In the SAD study, the LS mean ΔΔHR across all dose cohorts and after dosing timepoints ranged from −5.0 bpm (1 h after dosing in the 40 mg cohort) to 6.2 bpm (20 min after dosing in the 20 mg [2 × 10 mg] cohort) (Figure 2a). In the MAD study, the LS mean ΔΔHR ranged from −5.0 bpm (12 h after dosing on Day 14 in the 20 mg cohort) to 4.4 bpm (0.33 h after dosing on Day 1 in the 20 mg cohort), except for an LS mean ΔΔHR of 14.7 bpm that occurred at 24 h after dosing on Day 1 with zavegepant 20 mg (Figure 2b–d). There were no outliers in HR change for either the SAD or MAD studies.
FIGURE 2.
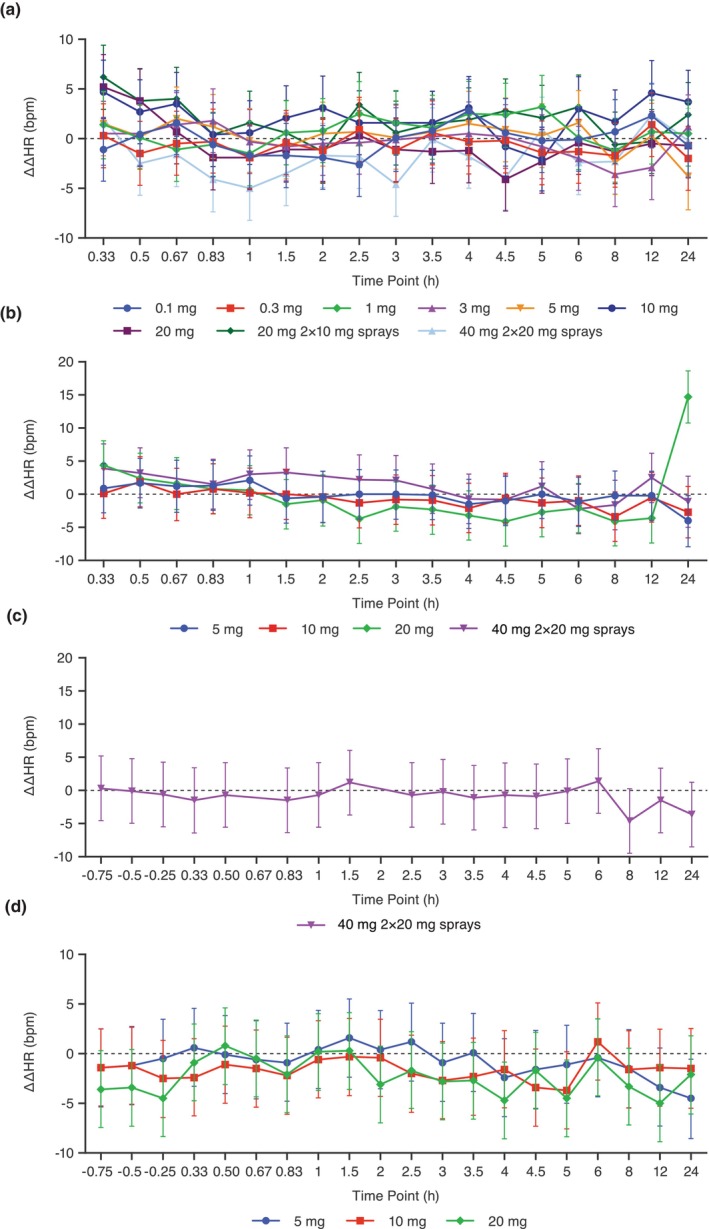
LS mean placebo‐corrected change from baseline heart rate (ΔΔHR) across timepoints for the QT/QTc analysis population in the SAD study (a), and on Day 1 (b), Day 8 (c), and Day 14 (d) in the MAD study. Points and error bars represent LS mean and 90% CI based on a linear mixed‐effects model. The dashed gray line represents a 10 bpm increase in HR. CI, confidence interval; HR, heart rate; LS, least squares; MAD, multiple‐ascending dose; SAD, single‐ascending dose; ΔΔHR, placebo‐corrected change from baseline heart rate.
In both the SAD and MAD studies, zavegepant did not have clinically relevant effects on cardiac conduction at the doses studied. In the SAD study, the LS mean ΔPR varied with no indication of dose dependency, and the LS mean ΔΔPR ranged across dose cohorts from −7.8 ms (3 h after dosing in the 10 mg cohort) to 6.5 ms (30 min after dosing in the 3 mg cohort). In the MAD study, the LS mean ΔΔPR in the MAD study ranged from −6.8 ms (0.33 h after dosing on Day 14 in the 20 mg cohort) to 5.4 ms (6 h after dosing on Day 1 in the 20 mg cohort), except for an LS mean ΔΔPR of −13.7 ms with zavegepant 20 mg at 24 h after dosing on Day 1.
In both the SAD and MAD studies, the LS mean ΔQRS was small, and the ΔΔQRS ranged across all dose cohorts and after dosing timepoints from −1.9 to 1.1 ms in the SAD study and − 1.3 to 0.8 ms on Day 1 and −2.6 to 0.8 ms on Days 8 and 14 in the MAD study. There were no PR or QRS outliers in either study.
Pooled SAD and MAD studies concentration‐QTc assessment
While the results of Levene's test for assessing homogeneity were significant at the 0.05 level, plots of standardized residuals across fitted values of ΔQTcF and different studies (Figures S8–S14) did not highlight any inadequacies resulting from using pooled SAD and MAD data. Exploratory analysis of other model assumptions, including linearity of the concentration‐QTc relationship, also showed no signs of the pre‐specified model being inadequate for concentration‐QTc assessment. The goodness‐of‐fit plot showed that the predicted ΔΔQTcF values are close to the ΔΔQTcF across all but 2 of the lower deciles of zavegepant plasma concentrations (Figure S15). The CI width of the model‐predicted ΔΔQTcF is likely due to minimal data at high zavegepant concentrations from the SAD study. Therefore, the proposed model provides a reasonable representation of the ΔΔQTcF and zavegepant relationship.
For pooled data from the SAD and MAD studies, the pre‐specified linear mixed‐effects model provided an acceptable fit for zavegepant plasma concentrations and ΔΔQTcF (Figure 3a). The estimated slope of the linear relationship between zavegepant concentration and ΔΔQTcF was negative and statistically significant (−0.053 ms per ng/mL [90% CI, −0.0955 to −0.0110 ms per ng/mL; p = 0.0415]) with a small, not statistically significant intercept of 0.99 ms at the 10% level. A full description of parameter estimates for the model is provided in Table S1.
FIGURE 3.
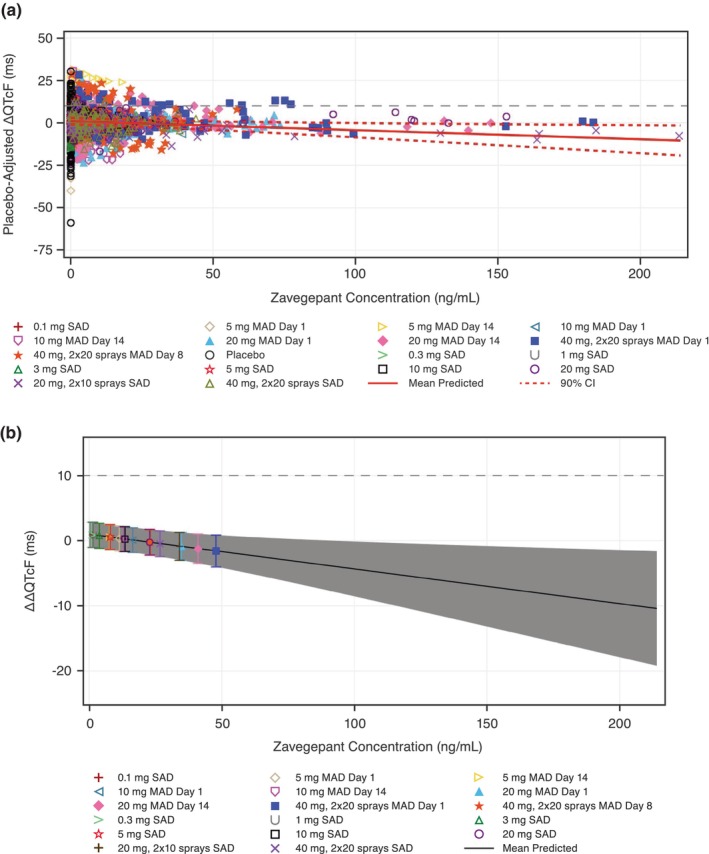
Scatter plot of (a) observed plasma zavegepant plasma concentrations and placebo‐corrected change from baseline QTcF (ΔΔQTcF) and (b) model‐predicted ΔΔQTcF across deciles of plasma zavegepant concentrations using pooled data from SAD and MAD studies. The dashed gray lines represent a 10 ms increase in ΔΔQTcF. (a) The solid red line with dashed red lines denotes the model‐predicted mean ΔΔQTcF with 90% CI, calculated from the equation ΔΔQTcF (ms) = 0.99 (ms) – 0.053 (ms/ng/mL) × plasma zavegepant concentrations (ng/mL). The plotted points denote the pairs of observed plasma drug concentrations and estimated ΔΔQTcF by participants for each active dose group and placebo group. The individually estimated placebo‐adjusted ΔQTcF i,k (ΔΔQTcF i,k ) equals the individual ΔQTcF i,k for participant i administered zavegepant or placebo at timepoint k minus the estimation of the time effect at timepoint k. (b) The solid black line with gray shaded area denotes the model‐predicted mean ΔΔQTcF with 90% CI, calculated from the equation ΔΔQTcF (ms) = 0.99 (ms) – 0.053 (ms/ng/mL) × zavegepant plasma concentrations (ng/mL). The non‐gray shaded areas denote the estimated mean (90% CI) ΔΔQTcF with plotted points at the geometric mean C max of zavegepant. BLQ values for the calculation of log C max are replaced with one‐half lower limit of quantitation. BLQ, below limit of quantitation; CI, confidence interval; C max, maximum observed concentration; MAD, multiple‐ascending dose; SAD, single‐ascending dose; ΔΔQTcF, placebo‐corrected change from baseline in QT interval corrected using Fridericia's formula.
The predicted ΔΔQTcF interval at the geometric mean peak zavegepant concentration for doses in the SAD and MAD studies is shown in Table 2. The predicted ΔΔQTcF effect on the geometric mean C max on Day 14 after 20 mg zavegepant (40.9 ng/mL) was −1.19 ms (90% CI, −3.45–1.08 ms) and was −0.23 ms (90% CI, −2.19–1.73 ms) at the geometric mean C max on Day 8 after zavegepant 40 mg (2 × 20 mg sprays) (23.0 ng/mL) (Figure 3b). Therefore, an effect on ΔΔQTcF exceeding 10 ms could be excluded for plasma zavegepant geometric mean concentrations up to ~47.7 ng/mL (Figure 3b).
TABLE 2.
Predicted ΔΔQTcF interval at geometric mean peak zavegepant concentration (SAD and MAD studies pooled) (PK/QTc analysis population).
| Zavegepant treatment | Zavegepant geometric mean C max (ng/mL) | ΔΔQTcF estimates (90% CI) |
|---|---|---|
| SAD study | ||
| 0.1 mg | 1.2 | 0.93 (−1.02, 2.87) |
| 0.3 mg | 1.1 | 0.94 (−1.01, 2.88) |
| 1 mg | 1.3 | 0.92 (−1.02, 2.86) |
| 3 mg | 3.7 | 0.80 (−1.13, 2.72) |
| 5 mg | 7.8 | 0.58 (−1.33, 2.48) |
| 10 mg | 13.4 | 0.28 (−1.62, 2.18) |
| 20 mg | 22.6 | −0.21 (−2.17, 1.75) |
| 20 mg, 2 × 10 mg sprays | 33.9 | −0.81 (−2.94, 1.31) |
| 40 mg, 2 × 20 mg sprays | 26.7 | −0.43 (−2.43, 1.58) |
| MAD study | ||
| 5 mg Day 1 | 11.4 | 0.39 (−1.51, 2.29) |
| 5 mg Day 14 | 7.6 | 0.59 (−1.32, 2.50) |
| 10 mg Day 1 | 16.3 | 0.12 (−1.79, 2.04) |
| 10 mg Day 14 | 13.0 | 0.30 (−1.60, 2.20) |
| 20 mg Day 1 | 34.7 | −0.85 (−2.99, 1.28) |
| 20 mg Day 14 | 40.9 | −1.19 (−3.45, 1.08) |
| 40 mg, 2 × 20 mg sprays Day 1 | 47.7 | −1.55 (−3.98, 0.89) |
| 40 mg, 2 × 20 mg sprays Day 8 | 23.0 | −0.23 (−2.19, 1.73) |
Note: Based on a linear mixed‐effects model with ΔQTcF as the dependent variable, time‐matched zavegepant plasma concentration as an explanatory variate, centered baseline QTcF as an additional covariate, treatment (active = 1 or placebo = 0) and time as fixed effects, and a random intercept and slope per subject. Participants who had only BLQ values for the calculation of log C max are replaced with ½ lower limit of quantitation.
Abbreviations: BLQ, below limit of quantitation; CI, confidence interval; MAD, multiple‐ascending dose; PK, pharmacokinetics; SAD, single‐ascending dose; ΔQTcF, change from baseline in QTcF; ΔΔQTcF, placebo‐corrected change from baseline QT interval corrected using Fridericia's formula.
DISCUSSION
The zavegepant SAD and MAD studies included an evaluation of the effects of zavegepant nasal spray on ECG parameters in healthy participants. Single and multiple doses of zavegepant nasal spray had no clinically relevant effect on other ECG parameters, including HR, PR interval, QRS duration, T‐wave morphology, or U‐wave presence. In both studies, mean ∆QTcF and ∆HR in zavegepant‐treated participants generally followed the pattern observed with placebo including doses with a higher geometric mean C max than the therapeutic dose of 10 mg. Per ICH E14 guidelines, 20 the use of robust concentration‐QTc data from these studies at zavegepant doses up to 40 mg and concentrations up to fourfold the geometric mean Cmax of the therapeutic dose of 10 mg, eliminates the need for inclusion of a positive control (i.e., moxifloxacin), and replaces a dedicated thorough QT (TQT) study. 21
The results of the concentration‐QTc analysis from the individual studies (not shown) and the pooled analysis revealed no clinically meaningful prolongation of the QTc interval with a margin of more than fourfold the geometric mean C max relative to the therapeutic 10 mg nasal spray dose. Based on the pooled concentration analysis, a ΔΔQTcF effect >10 ms can be excluded for zavegepant geometric mean plasma concentrations up to ~47.7 ng/mL.
The highest clinical exposures observed after a single 10 mg zavegepant nasal spray dose were in subjects with moderate hepatic impairment with a geometric mean C max of 23.1 ng/mL. 23 The 40 mg zavegepant nasal spray dose in the MAD study, with a geometric mean C max of 47.7 ng/mL, approximately fourfold higher than that of the 10 mg zavegepant nasal spray clinical dose, is considered sufficiently supratherapeutic to ensure coverage for intrinsic and extrinsic factors that might increase zavegepant exposures. Specifically, the C max after the 40 mg supratherapeutic dose is approximately twofold higher than that observed or anticipated following administration of 10 mg zavegepant nasal spray in the context of the largest intrinsic factor (moderate hepatic impairment) that increases zavegepant exposure. In addition, concomitant administration of zavegepant with itraconazole, a strong CYP3A4 inhibitor, at steady state did not have a clinically relevant effect on zavegepant exposure. 9 Therefore, based on the concentration‐QT analysis at doses as high as 40 mg, zavegepant nasal spray at concentrations well above those produced from the clinical dose of 10 mg, is not expected to prolong the QTc interval.
The study has several strengths, including the use of a concentration‐QTc analysis, which provides a robust approach to assessing the effects of an investigational agent on the QT interval in phase I studies that replaces a thorough QT study. 24 In addition, the analysis used pooled data from two studies and covered a wide range of doses.
In summary, single and multiple doses of zavegepant nasal spray had no clinically relevant effect on studied ECG parameters including QTc interval, HR, PR interval, and QRS duration. Concentration‐QT analysis also showed no clinically meaningful prolongation of the QTc interval at doses up to four times the recommended daily dose of 10 mg zavegepant nasal spray.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. R. Bertz, R. Bhardwaj, M.K.D., M.S.A., B.A.M., and R.S.C. designed the research. M.D., J.M., B.A.M., and R.C. performed the research. R.Bertz, R.Bhardwaj, and J.L. analyzed the data.
FUNDING INFORMATION
This study was supported by Biohaven Pharmaceuticals, which was acquired by Pfizer in October 2022.
CONFLICT OF INTEREST STATEMENT
J.H.H. and J.L. are employed by and hold stock/options in Pfizer. R.Bertz, M.D., and B.M. are employees of Biohaven Pharmaceuticals and own Biohaven stock and/or stock options. R.S.C. was an employee of Biohaven Pharmaceuticals, owns stock in Biohaven Ltd, was an employee of Pfizer, has received research payments from Pfizer, and provides services to Collima LLC which has had consulting agreements with Pfizer, Aptose Biosciences Inc., Manistee Therapeutics, and Vida Ventures Management Co., LLC. J.M. was an employee of Biohaven Pharmaceuticals at the time of this study and owns Biohaven stock. R.B. and M.S.A. are employees of Certara Strategic Consulting and were paid consultants to Biohaven, which was acquired by Pfizer in October 2022, for this study.
Supporting information
Data S1:
ACKNOWLEDGMENTS
The medical writing support was provided by Janna Afanasjeva, PharmD, Sai Tanikella, MPharm, and Amy C. Porter, PhD, ISMPP CMPP™ of Certara Synchrogenix and was funded by Biohaven Pharmaceuticals, which was acquired by Pfizer in October 2022.
Hughes JH, Bertz R, Bhardwaj R, et al. Concentration‐QTc and cardiac safety analysis of single and multiple zavegepant nasal spray doses in healthy participants to support approval. CPT Pharmacometrics Syst Pharmacol. 2024;13:1044‐1054. doi: 10.1002/psp4.13140
DATA AVAILABILITY STATEMENT
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/data‐and‐results for more information.
REFERENCES
- 1. International Headache Society . Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 2. Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: updated age, sex, and socioeconomic‐specific estimates from government health surveys. Headache. 2021;61:60‐68. doi: 10.1111/head.14024 [DOI] [PubMed] [Google Scholar]
- 3. Stovner LJ, Hagen K, Linde M, Steiner TJ. The global prevalence of headache: an update, with analysis of the influences of methodological factors on prevalence estimates. J Headache Pain. 2022;23:34. doi: 10.1186/s10194-022-01402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters GL. Migraine overview and summary of current and emerging treatment options. Am J Manag Care. 2019;25:S23‐s34. doi: 10.1111/j.1526-4610.2012.02185.x [DOI] [PubMed] [Google Scholar]
- 5. Altamura C, Brunelli N, Marcosano M, Fofi L, Vernieri F. Gepants – a long way to cure: a narrative review. Neurol Sci. 2022;43:5697‐5708. doi: 10.1007/s10072-022-06184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Puledda F, Silva EM, Suwanlaong K, Goadsby PJ. Migraine: from pathophysiology to treatment. J Neurol. 2023;270:3654‐3666. doi: 10.1007/s00415-023-11706-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ailani J, Burch RC, Robbins MS. The American headache society consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61:1021‐1039. doi: 10.1111/head.14153 [DOI] [PubMed] [Google Scholar]
- 8. Burch RC, Ailani J, Robbins MS. The American headache society consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2022;62:111‐112. doi: 10.1111/head.14245 [DOI] [PubMed] [Google Scholar]
- 9. FDA . Zavegepant prescribing information. 2023. Accessed July 18, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216386s000lbl.pdf
- 10. Croop R, Madonia J, Stock DA, et al. Zavegepant nasal spray for the acute treatment of migraine: a phase 2/3 double‐blind, randomized, placebo‐controlled, dose‐ranging trial. Headache. 2022;62:1153‐1163. doi: 10.1111/head.14389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipton RB, Croop R, Stock DA, et al. Safety, tolerability, and efficacy of zavegepant 10 mg nasal spray for the acute treatment of migraine in the USA: a phase 3, double‐blind, randomised, placebo‐controlled multicentre trial. Lancet Neurol. 2023;22:209‐217. doi: 10.1016/s1474-4422(22)00517-8 [DOI] [PubMed] [Google Scholar]
- 12. Bhardwaj R, Donohue MK, Stringfellow J, et al. Absorption, distribution, metabolism, and elimination of 5 mg [14C]‐zavegepant in healthy male subjects after a single intravenous infusion dose. Headache. 2022;62:101‐102. [Google Scholar]
- 13. Chaturvedula PV, Mercer SE, Pin SS, Thalody G, Xu C, Conway CM, Keavy D, Signor L, Cantor GH, Mathias N, Moench P, Denton R, Macci R, Schartman R, Whiterock V, Davis C, Macor JE, Dubowchik GM Discovery of (R)‐N‐3‐(7‐methyl‐1H‐indazol‐5‐yl)‐1‐(4‐(1‐methylpiperidin‐4‐yl)‐1‐oxopropan‐2‐yl)‐4‐(2‐oxo‐1,2‐dihydroquinolin‐3‐yl)piperidine‐1‐carboxamide (BMS‐742413): a potent human CGRP antagonist with superior safety profile for the treatment of migraine through intranasal delivery. Bioorg Med Chem Lett 2013;23:3157–3161. 10.1016/j.bmcl.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 14. Valentin JP, Hoffmann P, Ortemann‐Renon C, et al. The challenges of predicting drug‐induced QTc prolongation in humans. Toxicol Sci. 2022;187:3‐24. doi: 10.1093/toxsci/kfac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grenier J, Paglialunga S, Morimoto BH, Lester RM. Evaluating cardiac risk: exposure response analysis in early clinical drug development. Drug Healthc Patient Saf. 2018;10:27‐36. doi: 10.2147/dhps.S133286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertz R, Donohue MK, Madonia J, et al. Safety, Tolerability, and Pharmacokinetics of Single and Multiple Ascending Doses of Intranasal Zavegepant in Healthy Adults. AAN 2022 Annual Meeting Abstract. 2022. Accessed August 5, 2023. https://index.mirasmart.com/aan2022/PDFfiles/AAN2022‐001003.html
- 17. Sheehan DV, Giddens JM, Sheehan IS. Status update on the Sheehan‐suicidality tracking scale (S‐STS) 2014. Innov Clin Neurosci. 2014;11:93‐140. doi: 10.1136/bmj.g3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan suicidality tracking scale (Sheehan‐STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6:26‐31. [PMC free article] [PubMed] [Google Scholar]
- 19. El Rassi E, Mace JC, Steele TO , et al. Sensitivity analysis and diagnostic accuracy of the brief smell identification test in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:287‐292. doi: 10.1002/alr.21670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. International Conference on Harmonisation E14 Clinical Evaluation of QT. Accessed January 30, 2024. https://www.ich.org/page/efficacy‐guidelines
- 21. Darpo B, Borin M, Ferber G, et al. ECG evaluation as part of the clinical pharmacology strategy in the development of new drugs: a review of current practices and opportunities based on five case studies. J Clin Pharmacol. 2022;62:1480‐1500. doi: 10.1002/jcph.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garnett C, Bonate PL, Dang Q, et al. Scientific white paper on concentration‐QTc modeling. J Pharmacokinet Pharmacodyn. 2018;45:383‐397. doi: 10.1007/s10928-017-9558-5 [DOI] [PubMed] [Google Scholar]
- 23. Bhardwaj R, Donohue MK, Madonia J, et al. Pharmacokinetics of Zavegepant nasal spray 10 mg in subjects with moderate hepatic impairment. Headache. 2023;63:152. doi: 10.1111/head.14523 [DOI] [Google Scholar]
- 24. International Conference on Harmonisation . Guidance on E14 clinical evaluation of QT/QTc interval prolongation and Proarrhythmic potential for non‐antiarrhythmic drugs; availability. Notice. Fed Regist. 2005;70:61134‐61135. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1:
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. See https://www.pfizer.com/science/clinical‐trials/data‐and‐results for more information.


