Abstract
Background
Myocardial infarction secondary to spontaneous coronary artery dissection (SCAD) can be traumatic and potentially trigger posttraumatic stress disorder (PTSD). In a large, multicenter, registry‐based cohort, we documented prevalence of lifetime and past‐month SCAD‐induced PTSD, as well as related treatment seeking, and examined a range of health‐relevant correlates of SCAD‐induced PTSD.
Methods and Results
Patients with SCAD were enrolled in the iSCAD (International SCAD) Registry. At baseline, site investigators completed medical report forms, and patients reported demographics, medical/SCAD history, psychosocial factors (including SCAD‐induced PTSD symptoms), health behaviors, and health status via online questionnaires. Of 1156 registry patients, 859 patients (93.9% women; mean age, 52.3 years) completed questionnaires querying SCAD‐induced PTSD. Nearly 35% (n=298) of patients met diagnostic criteria for probable SCAD‐induced PTSD in their lifetime, and 6.4% (n=55) met criteria for probable past‐month PTSD. Of 811 patients ever reporting any SCAD‐induced PTSD symptoms, 34.8% indicated seeking treatment for this distress. However, 46.0% of the 298 patients with lifetime probable SCAD‐induced PTSD diagnoses reported never receiving trauma‐related treatment. Younger age at first SCAD, fewer years since SCAD, being single, unemployed status, more lifetime trauma, and history of anxiety were associated with greater past‐month PTSD symptom severity in multivariable regression models. Greater past‐month SCAD‐induced PTSD symptoms were associated with greater past‐week sleep disturbance and worse past‐month disease‐specific health status when adjusting for various risk factors.
Conclusions
Given the high prevalence of SCAD‐induced PTSD symptoms, efforts to support screening for these symptoms and connecting patients experiencing distress with empirically supported treatments are critical next steps.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04496687.
Keywords: health status, PTSD, SCAD, sleep, trauma, treatment
Subject Categories: Mental Health
Nonstandard Abbreviations and Acronyms
- iSCAD Registry
International SCAD Registry
- SCAD
spontaneous coronary artery dissection
Clinical Perspective.
What Is New?
This is the largest study of posttraumatic stress disorder induced by spontaneous coronary artery dissection conducted to date, with >800 patients from an international, multisite registry.
We demonstrated that >1 in 3 patients met the criteria for probable SCAD‐induced PTSD in their lifetime and highlighted links with adverse health correlates.
What Are the Clinical Implications?
Findings emphasize the importance of screening for posttraumatic stress disorder symptoms after spontaneous coronary artery dissection and connecting those patients experiencing distress postevent with empirically supported treatments.
Although once viewed as a rare cardiovascular condition, spontaneous coronary artery dissection (SCAD) is increasingly acknowledged as an important cause of acute coronary syndrome, particularly among women. 1 Approximately 80% to 90% of patients with SCAD, characterized by the nontraumatic and noniatrogenic separation of the coronary arterial wall by intramural hemorrhage, are women, and SCAD may account for up to 35% of acute coronary syndrome cases in women aged <50 years and up to 43% of acute coronary syndrome cases in pregnant women. 1 , 2 Despite growing awareness of SCAD, its cause, although likely multifactorial, is largely unknown, and optimal evidence‐based management strategies are generally lacking, despite the possibility of recurrence.
To date, several investigations have examined the psychological consequences of SCAD. Depression and anxiety are common after an acute cardiac event, 3 and several cross‐sectional studies with survivors of SCAD have documented elevated symptoms of these mental health conditions. 4 , 5 , 6 Furthermore, some, 7 , 8 but not all, 9 research has found that patients with SCAD report higher depression or anxiety symptom levels compared with cardiac patients without SCAD. The predominance of women in the population of patients with SCAD may explain the high prevalence of post‐SCAD mental health symptoms, because women have approximately twice the lifetime rates of depression and anxiety compared with men. 10 , 11 Additionally, approximately 1 in 3 patients report receiving treatment for SCAD‐related anxiety or depression. 4 , 6 Nevertheless, patients have identified a lack of mental health resources as a leading concern after an initial SCAD event, 12 highlighting the need for greater understanding of the emotional consequences of this cardiac condition and how to best address survivors' mental health needs.
High levels of psychological distress after SCAD may also be due, in part, to the often unexpected and unpredictable onset of SCAD events. Most patients with SCAD lack traditional risk factors associated with cardiovascular disease, which may contribute to the unanticipated nature of the diagnosis. The rare nature of SCAD events and a lack of awareness of SCAD among providers may further exacerbate the emotional impact of these events. These sudden SCAD events can be potentially traumatic experiences that can trigger the development of posttraumatic stress disorder (PTSD) symptoms. There has been a growing awareness of cardiac disease‐induced PTSD, 13 although PTSD after SCAD has received relatively little attention to date compared with other mental health outcomes like depression and anxiety. Nevertheless, there is preliminary evidence of elevated rates of PTSD symptoms in SCAD survivors. 5 , 6
In this observational cohort study, we conducted a comprehensive evaluation of PTSD after SCAD in a large, multicenter, registry‐based patient sample (N=859). Not only did we investigate past‐month symptoms of PTSD induced by patients' worst or most distressing SCAD event, but we queried whether patients ever experienced PTSD symptoms in response to SCAD in their lifetime using validated questionnaires. In addition to documenting the prevalence of traumatic experiences, SCAD‐induced PTSD, and related treatment seeking, we examined a range of health‐relevant correlates of SCAD‐induced PTSD. We hypothesized that patients with SCAD would have high rates of SCAD‐induced PTSD, and that greater PTSD symptom severity would be associated with adverse health behaviors and health status.
Methods
Registry Cohort
The iSCAD (International SCAD) Registry (https://clinicaltrials.gov/ct2/show/NCT04496687) is a prospective, multicenter registry of patients who have had a SCAD event. It is sponsored by the SCAD Alliance, a nonprofit advocacy organization for patients with SCAD. The registry was initiated through collaboration with key stakeholders, including experts in cardiovascular medicine and women's heart health, and survivors of SCAD, and it aims to maintain an independent data repository to advance SCAD research and treatment around the world (for more details, see https://iscadregistry.bidmc.org). At the time of data analysis, there were 22 active sites enrolling patients.
Site investigators identified eligible patients with suspected or diagnosed SCAD. Eligibility criteria included: (1) age >18 years, (2) capable of provided informed consent, and (3) active email address for accessing patient questionnaires. Patients were enrolled in the acute aftermath of a SCAD event or from outpatient cardiovascular medicine clinics based on review of invasive or computed tomography angiographic images. The vast majority of patients were enrolled from outpatient care, with a median of 0.6 years (interquartile range [IQR], 0.2–2.3) since their most recent SCAD event. The iSCAD Registry uses a multimethod approach to data collection. Site investigators completed case report forms about the most recent SCAD event, prior cardiovascular or other medical history, and laboratory and imaging data upon enrollment. At a baseline assessment, patients completed online questionnaires about demographic characteristics, personal and family medical history, information related to circumstances preceding SCAD events, health behaviors, overall disease‐specific health status, and psychosocial factors. Although follow‐up data are collected in the iSCAD Registry, the current study only analyzed data collected at the baseline assessment. All data are coordinated by the PERFUSE academic research organization; PERFUSE investigators also perform core laboratory adjudication of coronary angiograms to confirm the diagnosis of SCAD. Each enrolling site and the PERFUSE coordinating center received institutional review board approval to conduct this research, and participants provided written informed consent. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the PERFUSE Study Group at iscadregistry@bidmc.harvard.edu. This study followed the strengthening the reporting of observational studies in epidemiology cohort reporting guidelines. 14
Measures
Trauma History and PTSD Symptoms
Lifetime exposure to a range of traumatic events was assessed with the Brief Trauma Questionnaire 15 ; patients indicated if they ever experienced 10 types of events. Lifetime cumulative trauma burden was calculated by summing the number of event types reported (range, 0–10). As in prior research, 16 these events were categorized into broad classes of trauma types for analyses: (1) serious accident/disaster, (2) interpersonal or sexual violence, (3) combat, (4) life‐threatening illness/serious injury (other than SCAD), (5) violent death of close other, and (6) witnessed traumatic event.
SCAD‐induced PTSD symptoms were assessed with the PTSD checklist for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), 17 a reliable and valid self‐report measure querying the 20 DSM‐5 symptoms of PTSD that can be used for identifying probable PTSD diagnoses. 18 , 19 Patients identified their worst or most distressing SCAD event and answered all questions with respect to that experience. For each symptom, patients indicated whether they ever experienced the symptom in response to their worst/most distressing SCAD event; if so, they then indicated the extent to which they were bothered by the symptom in the past month. As in prior research, 16 responses were aggregated to reflect probable lifetime and past‐month diagnoses of PTSD according to the DSM‐5 symptom criteria (see Data S1 for details). This information was combined to classify patients as having: (1) no lifetime PTSD symptoms, (2) some lifetime PTSD symptoms but no probable diagnosis, (3) probable lifetime (but not past‐month) PTSD, and (4) probable past‐month PTSD (note, these individuals would also meet criteria for lifetime PTSD). In addition, past‐month PTSD symptom severity was calculated by summing the responses to the 20 past‐month symptom questions (range, 0–80). Patients specified the date of their worst/most distressing SCAD event as well, and we calculated the time in years between this event and enrollment in the iSCAD Registry (date of completion of the baseline questionnaires was not recorded).
Treatment Seeking for PTSD Symptoms
Patients indicated whether they ever sought treatment by a professional for SCAD‐induced PTSD symptoms. If they reported seeking treatment, they indicated the type(s) of providers (clergy, primary care physician, psychologist or social worker, psychiatrist, other) and type(s) of treatment received (medication, psychotherapy or talk therapy, other).
Correlates
Patients provided information about demographic characteristics, including age, sex, race, Hispanic or Latino ethnicity, marital status, educational attainment, and current employment status. In regard to SCAD history, patients reported age at first SCAD event, whether they had a history of pregnancy‐related SCAD (among individuals with at least 1 pregnancy), and whether they experienced acute emotional stress or anxiety when first experiencing symptoms of their most recent SCAD event. Site investigators provided information about the event presentation(s) for the most recent SCAD event and treatment(s) performed during hospitalization; investigators could select all options that applied to the most recent event.
Additionally, patients reported their mental health history, specifically whether they had a history of depression or anxiety before their most recent SCAD event. Several health behaviors were also self‐reported, including current smoking and alcohol consumption. Patients described past‐week sleep disturbance using the 6‐item Patient‐Reported Outcomes Measurement Information System Sleep Disturbance Scale, Short Form 6a. 20 , 21 Total raw scores (range, 6–30) were converted to T scores 22 ; higher scores reflect greater sleep disturbance. Patients provided information on overall disease‐specific health status in the past month using the Seattle Angina Questionnaire‐7, a 7‐item disease‐specific patient health status measure assessing symptoms due to chest pain/tightness/angina, functioning, and quality of life. 23 Scores range from 0 to 100; higher scores reflect better health (see Data S1 for details).
Statistical Analysis
Descriptive statistics were calculated for patients who completed the PTSD assessment and compared with the full iSCAD Registry patient cohort. Prevalence of trauma history, SCAD‐induced PTSD, and related treatment seeking were documented, and unadjusted associations between demographic, SCAD history, mental health, health behavior, and disease‐specific health status characteristics with SCAD‐induced PTSD status were tested using χ2 tests for categorical variables and analyses of variance for continuous variables. In addition, multivariable regression was used to identify characteristics associated with past‐month SCAD‐induced PTSD symptom severity; these models included sex, marital status, employment, age at first SCAD, years between the worst/most distressing SCAD event and iSCAD Registry enrollment, lifetime cumulative trauma burden, and history of depression and anxiety before the most recent SCAD event as predictors. Given a high correlation between age at enrollment and age at first SCAD (r=0.93), we only included age at first SCAD in regression analyses. Our approach to selection of the variables included in regression analyses was guided by the broader literature on variables that have been associated with PTSD (including cardiac disease‐induced PTSD) 13 , 24 and by variable associations with SCAD‐induced PTSD status in unadjusted analyses. We considered some SCAD‐related variables (eg, presentation for most recent SCAD event) for potential inclusion as well; however, these variables were not robustly associated with either SCAD‐induced PTSD status or outcomes in the regression analyses, and thus we did not include them in our final models. We also used multivariable linear regression to examine whether past‐month SCAD‐induced PTSD symptom severity was associated with (1) past‐week sleep disturbance and (2) past‐month disease‐specific health status. In separate models, the health‐relevant correlate was the outcome, past‐month SCAD‐induced PTSD symptom severity was the predictor of interest, and we adjusted for several risk factor covariates (sex, marital status, employment, age at first SCAD, years between the worst/most distressing SCAD event and iSCAD Registry enrollment, lifetime cumulative trauma burden, and history of depression and anxiety before the most recent SCAD event). Given that 2 PTSD symptoms pertain to sleep disturbance (ie, nightmares and trouble falling or staying asleep), we conducted a sensitivity analysis excluding those symptoms from the past‐month SCAD‐induced PTSD symptom severity score when examining past‐week sleep disturbance. This sensitivity analysis was conducted to investigate whether PTSD‐specific sleep items were driving any associations with past‐week sleep disturbance. Participants with missing data were excluded from regression analyses; sample size for complete case analysis regression models ranged from n=802 for sleep disturbance to n=805 for past‐month PTSD symptom severity (ie, >90% of participants with SCAD‐induced PTSD data were included in regression analyses). Analyses were conducted in SAS 9.4 (TS1M5; SAS Institute, Cary, NC) and Stata 17.0 (StataCorp, College Station, TX), and P<0.05 indicated statistical significance.
Results
Patient Characteristics
Of the 1156 patients enrolled in the iSCAD Registry through December 27, 2022, 859 individuals completed the PTSD assessment, yielding a 74.3% response rate. Participants completing the PTSD assessment were largely representative of the full iSCAD Registry patient cohort, although they had slightly higher rates of prior depression and anxiety (Table S1). Additionally, participants who did not complete the PTSD assessment had higher rates of missing data on demographics overall compared with completers.
Patient characteristics in the total sample and by SCAD‐induced PTSD status are presented in Table 1. Respondents had a mean age of 52.3 years at enrollment, and the vast majority identified as women (93.9%). The sample was predominantly of White race (89.0%) and non‐Hispanic or Latino ethnicity (95.3%), and most were married or living with a domestic partner (78.8%). Overall, patients were highly educated, with nearly three‐quarters of the sample having at least a college degree (74.5%). Approximately half of the sample was working full‐time at enrollment in the iSCAD Registry.
Table 1.
Patient Characteristics Overall and by SCAD‐Induced PTSD Status
| Characteristic | Total (N=859) | SCAD‐induced PTSD status | P value | Valid n | |||
|---|---|---|---|---|---|---|---|
| No lifetime PTSD symptoms (n=48) | Lifetime PTSD symptoms, no diagnosis (n=513) | Lifetime PTSD diagnosis, no past‐month PTSD diagnosis (n=243) | Past‐month PTSD diagnosis (n=55) | ||||
| Demographics | |||||||
| Age at enrollment, y, mean (SD) | 52.3 (10.5) | 58.1 (12.3) | 53.6 (10.0) | 49.9 (10.2) | 45.8 (9.5) | <0.001* | 774 |
| Sex | 0.001† | 858 | |||||
| Women | 806 (93.9%) | 39 (81.3%) | 483 (94.2%) | 230 (95.0%) | 54 (98.2%) | ||
| Men | 52 (6.1%) | 9 (18.8%) | 30 (5.8%) | 12 (5.0%) | 1 (1.8%) | ||
| Race, n (%) | 0.183† | 827 | |||||
| American Indian/Alaska Native | 3 (0.4%) | 0 (0.0%) | 3 (0.6%) | 0 (0.0%) | 0 (0.0%) | ||
| Asian | 15 (1.8%) | 1 (2.2%) | 11 (2.2%) | 1 (0.4%) | 2 (3.8%) | ||
| Black | 60 (7.3%) | 3 (6.5%) | 35 (7.0%) | 14 (6.1%) | 8 (15.1%) | ||
| Native Hawaiian/Pacific Islander | 1 (0.1%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) | 0 (0.0%) | ||
| White | 736 (89.0%) | 42 (91.3%) | 444 (89.3%) | 208 (90.0%) | 42 (79.2%) | ||
| Multiracial | 8 (1.0%) | 0 (0.0%) | 3 (0.6%) | 5 (2.2%) | 0 (0.0%) | ||
| Other | 4 (0.5%) | 0 (0.0%) | 1 (0.2%) | 2 (0.9%) | 1 (1.9%) | ||
| Hispanic or Latino ethnicity, n (%) | 40 (4.7%) | 4 (8.3%) | 19 (3.7%) | 15 (6.2%) | 2 (3.6%) | 0.273† | 859 |
| Marital status, n (%) | 0.019† | 859 | |||||
| Single | 82 (9.5%) | 5 (10.4%) | 36 (7.0%) | 28 (11.5%) | 13 (23.6%) | ||
| Married/domestic partner | 677 (78.8%) | 36 (75.0%) | 420 (81.9%) | 185 (76.1%) | 36 (65.5%) | ||
| Divorced | 76 (8.8%) | 7 (14.6%) | 40 (7.8%) | 23 (9.5%) | 6 (10.9%) | ||
| Widowed | 21 (2.4%) | 0 (0.0%) | 15 (2.9%) | 6 (2.5%) | 0 (0.0%) | ||
| Prefer not to say | 3 (0.3%) | 0 (0.0%) | 2 (0.4%) | 1 (0.4%) | 0 (0.0%) | ||
| Education, n (%) | 0.507† | 859 | |||||
| Some high school | 5 (0.6%) | 1 (2.1%) | 3 (0.6%) | 0 (0.0%) | 1 (1.8%) | ||
| High school graduate | 58 (6.8%) | 4 (8.3%) | 34 (6.6%) | 17 (7.0%) | 3 (5.5%) | ||
| Trade school/apprenticeship | 28 (3.3%) | 0 (0.0%) | 13 (2.5%) | 12 (4.9%) | 3 (5.5%) | ||
| Some college | 125 (14.6%) | 8 (16.7%) | 67 (13.1%) | 39 (16.0%) | 11 (20.0%) | ||
| College graduate | 359 (41.8%) | 21 (43.8%) | 229 (44.6%) | 92 (37.9%) | 17 (30.9%) | ||
| Advanced/professional degree | 281 (32.7%) | 14 (29.2%) | 165 (32.2%) | 82 (33.7%) | 20 (36.4%) | ||
| Not sure or prefer not to say | 3 (0.3%) | 0 (0.0%) | 2 (0.4%) | 1 (0.4%) | 0 (0.0%) | ||
| Current employment status, n (%) | <0.001† | 859 | |||||
| Not employed outside home | 107 (12.5%) | 7 (14.6%) | 59 (11.5%) | 31 (12.8%) | 10 (18.2%) | ||
| Retired | 110 (12.8%) | 9 (18.8%) | 78 (15.2%) | 21 (8.6%) | 2 (3.6%) | ||
| Student | 5 (0.6%) | 0 (0.0%) | 3 (0.6%) | 1 (0.4%) | 1 (1.8%) | ||
| Unemployed | 56 (6.5%) | 2 (4.2%) | 20 (3.9%) | 22 (9.1%) | 12 (21.8%) | ||
| Working part‐time | 118 (13.7%) | 3 (6.3%) | 83 (16.2%) | 25 (10.3%) | 7 (12.7%) | ||
| Working full‐time | 435 (50.6%) | 25 (52.1%) | 258 (50.3%) | 132 (54.3%) | 20 (36.4%) | ||
| Not sure or prefer not to say | 28 (3.3%) | 2 (4.2%) | 12 (2.3%) | 11 (4.5%) | 3 (5.5%) | ||
| SCAD history | |||||||
| Age at first SCAD, y, mean (SD) | 49.7 (10.6) | 55.6 (12.3) | 50.8 (10.0) | 47.3 (10.7) | 44.3 (8.8) | <0.001* | 849 |
| Time between worst SCAD event and registry enrollment, y, median (IQR) | 0.7 (0.2–2.6) | 0.6 (0.1–2.7) | 0.8 (0.2–2.8) | 0.7 (0.2–2.3) | 0.3 (0.2–1.3) | 0.229* | 845 |
| History of pregnancy‐related SCAD for those with at least 1 pregnancy, n (%) | 82/645 (12.7%) | 2/33 (6.1%) | 40/389 (10.3%) | 31/178 (17.4%) | 9/45 (20.0%) | 0.028† | 645 |
| Acute emotional stress/anxiety before SCAD, n (%)‡ | 110 (12.8%) | 3 (6.3%) | 61 (11.9%) | 33 (13.6%) | 13 (23.6%) | 0.043† | 859 |
| Cardiac arrest presentation, n (%)§ | 39 (4.5%) | 0 (0.0%) | 19 (3.7%) | 13 (5.3%) | 7 (12.7%) | 0.007† | 859 |
| Cardiogenic shock presentation, n (%)§ | 3 (0.3%) | 0 (0.0%) | 1 (0.2%) | 2 (0.8%) | 0 (0.0%) | 0.517† | 859 |
| NSTEMI presentation, n (%)§ | 416 (48.4%) | 24 (50.0%) | 251 (48.9%) | 117 (48.1%) | 24 (43.6%) | 0.894† | 859 |
| STEMI presentation, n (%)§ | 218 (25.4%) | 14 (29.2%) | 121 (23.6%) | 65 (26.7%) | 18 (32.7%) | 0.385† | 859 |
| Other presentation, n (%)§ , || | 318 (37.0%) | 22 (45.8%) | 188 (36.6%) | 87 (35.8%) | 21 (38.2%) | 0.611† | 859 |
| CABG performed, n (%)§ | 28 (3.7%) | 0 (0.0%) | 15 (3.3%) | 12 (5.5%) | 1 (2.0%) | 0.231† | 765 |
| PCI performed, n (%)§ | 184 (24.1%) | 10 (21.7%) | 100 (22.4%) | 56 (25.6%) | 18 (35.3%) | 0.202† | 763 |
| Medical therapy only, n (%)§ | 560 (73.6%) | 36 (78.3%) | 337 (75.7%) | 155 (70.8%) | 32 (62.7%) | 0.136† | 761 |
| Prior mental health | |||||||
| History of depression, n (%)‡ | 197 (22.9%) | 8 (16.7%) | 97 (18.9%) | 70 (28.8%) | 22 (40.0%) | <0.001† | 859 |
| History of anxiety, n (%)‡ | 267 (31.1%) | 5 (10.4%) | 138 (26.9%) | 97 (39.9%) | 27 (49.1%) | <0.001† | 859 |
| Trauma‐related information | |||||||
| Lifetime cumulative trauma burden, mean (SD) | 1.5 (1.6) | 1.1 (1.3) | 1.3 (1.4) | 1.8 (1.7) | 2.2 (2.0) | <0.001* | 859 |
| Experienced accident/disaster, n (%) | 251 (29.2%) | 10 (20.8%) | 141 (27.5%) | 82 (33.7%) | 18 (32.7%) | 0.164† | 859 |
| Experienced interpersonal or sexual violence, n (%) | 299 (34.8%) | 13 (27.1%) | 154 (30.0%) | 105 (43.2%) | 27 (49.1%) | <0.001† | 859 |
| Experienced combat, n (%) | 12 (1.4%) | 1 (2.1%) | 3 (0.6%) | 7 (2.9%) | 1 (1.8%) | 0.087† | 859 |
| Experienced illness/injury, n (%) | 239 (27.8%) | 10 (20.8%) | 135 (26.3%) | 76 (31.3%) | 18 (32.7%) | 0.278† | 859 |
| Experienced violent death of close other, n (%) | 143 (16.6%) | 8 (16.7%) | 81 (15.8%) | 40 (16.5%) | 14 (25.5%) | 0.340† | 859 |
| Witnessed traumatic event, n (%) | 130 (15.1%) | 5 (10.4%) | 63 (12.3%) | 45 (18.5%) | 17 (30.9%) | <0.001† | 859 |
| No. of lifetime SCAD‐induced PTSD symptoms ever experienced, mean (SD) | 7.8 (5.1) | 0.0 (0.0) | 5.5 (3.2) | 12.4 (3.1) | 16.3 (2.0) | <0.001* | 859 |
| Past‐month SCAD‐induced PTSD symptom severity, mean (SD) | 10.5 (11.2) | 0.0 (0.0) | 6.0 (6.0) | 15.9 (8.6) | 37.9 (10.9) | <0.001* | 859 |
| Health behaviors | |||||||
| Past‐week sleep disturbance by PROMIS T score, mean (SD) | 51.2 (9.4) | 44.1 (8.8) | 49.4 (8.6) | 54.6 (9.1) | 59.9 (8.7) | <0.001* | 854 |
| Current smoker, n (%) | 20 (2.3%) | 1 (2.1%) | 6 (1.2%) | 8 (3.3%) | 5 (9.1%) | 0.002† | 859 |
| Alcohol consumption, n (%) | 0.055† | 850 | |||||
| Never drinker | 199 (23.4%) | 17 (36.2%) | 122 (24.1%) | 48 (19.9%) | 12 (21.8%) | ||
| <1 drink per wk | 375 (44.1%) | 11 (23.4%) | 215 (42.4%) | 118 (49.0%) | 31 (56.4%) | ||
| 1–7 drinks per wk | 220 (25.9%) | 13 (27.7%) | 134 (26.4%) | 62 (25.7%) | 11 (20.0%) | ||
| >7 drinks per wk | 45 (5.3%) | 4 (8.5%) | 30 (5.9%) | 10 (4.1%) | 1 (1.8%) | ||
| Prefer not to say | 11 (1.3%) | 2 (4.3%) | 6 (1.2%) | 3 (1.2%) | 0 (0.0%) | ||
| Disease‐specific health status | |||||||
| Past‐month health status total score, mean (SD) | 73.1 (16.9) | 83.4 (11.3) | 76.0 (15.6) | 68.4 (17.3) | 58.5 (17.0) | <0.001* | 858 |
CABG indicates coronary artery bypass graft; IQR, interquartile range; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; PROMIS, Patient‐Reported Outcomes Measurement Information System; PTSD, posttraumatic stress disorder; SCAD, spontaneous coronary artery dissection; and STEMI, ST‐segment–elevation myocardial infarction.
Kruskal‐Wallis P value.
χ2 P value.
For most recent SCAD event.
Investigators could select as many options as applied to the most recent SCAD event; thus, percentages total >100%.
Other presentations included asymptomatic, atypical chest pain, stable angina, and unstable angina.
Average age at first SCAD was 49.7 years, and 12.7% of patients with at least 1 pregnancy experienced pregnancy‐related SCAD. For the most recent SCAD event, most patients presented as non–ST‐segment–elevation myocardial infarction (48.4%), and 12.8% of patients reported experiencing acute emotional stress/anxiety before the event. Most cases were treated with medical therapy only during hospitalization for the most recent SCAD event (73.6%).
Trauma History and Prevalence of SCAD‐Induced PTSD
The majority of patients (65.0%, n=558) reported experiencing at least 1 traumatic event during their lifetime other than their SCAD event, and the mean cumulative lifetime trauma burden was 1.5 (SD, 1.6). As shown in the Figure, the most frequently reported traumatic event type was interpersonal/sexual violence, with over one‐third of patients experiencing this type of trauma in their lifetime, followed by accidents/disasters and illness/injury (other than SCAD).
Figure . Lifetime prevalence of traumatic event types among iSCAD Registry patients.
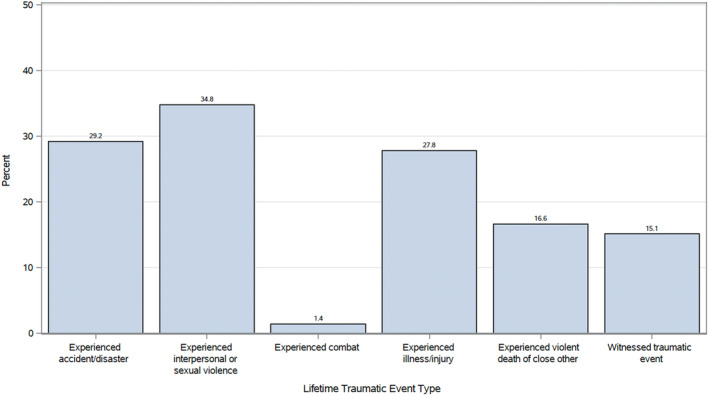
iSCAD Registry indicates International Spontaneous Coronary Artery Dissection Registry.
The median time in years between the worst/most distressing SCAD event and enrollment in the iSCAD Registry was 0.7 years (IQR, 0.2–2.6). Nearly 35% of patients (n=298) met criteria for probable SCAD‐induced PTSD in their lifetime (Table 1). Of those with probable lifetime SCAD‐induced PTSD, 18.5% (n = 55; 6.4% of the total sample) met the criteria for probable past‐month PTSD. Furthermore, a sizeable proportion of respondents (59.7%) reported ever experiencing some PTSD symptoms in response to their worst/most distressing SCAD event in their lifetime, although they did not reach the level of a probable diagnosis. In the total sample, the mean number of lifetime SCAD‐induced PTSD symptoms ever experienced was 7.8 (SD, 5.1; range, 0–20); the number of lifetime symptoms endorsed was larger for greater and more enduring manifestations of SCAD‐induced PTSD symptoms (Table 1). Consistent with probable diagnostic status, mean past‐month PTSD symptom severity was greatest among patients with probable past‐month PTSD.
Treatment for SCAD‐Induced PTSD
Of the 811 patients who reported at least some lifetime SCAD‐induced PTSD symptoms, 34.8% indicated seeking treatment for this distress, with treatment‐seeking rates highest among those with probable past‐month PTSD (65.5%) and lifetime (but not past‐month) probable PTSD (51.4%; Table 2). However, nearly half (46.0%, n=137) of patients with probable SCAD‐induced PTSD diagnoses in their lifetime reported never receiving trauma‐related treatment. Additionally, nearly a quarter of patients with some lifetime SCAD‐induced PTSD symptoms that did not reach the level of a probable diagnosis nevertheless sought treatment by a professional. Overall, psychologists or social workers were the most commonly seen provider (20.1%), followed by primary care physicians (15.3%) and psychiatrists (10.0%). Rates of engagement with these professionals were highest for individuals with probable past‐month PTSD, ranging from 21.8% for psychiatrists to 34.5% for psychologists/social workers to 36.4% for primary care physicians. Furthermore, psychotherapy or talk therapy was the most frequent type of treatment received overall (26.8%). However, 49.1% and 45.5% of individuals with probable past‐month PTSD reported receiving psychotherapy or talk therapy and medication, respectively.
Table 2.
Prevalence of Treatment Seeking and Receipt for SCAD‐Induced PTSD Symptoms
| Treatment seeking and receipt | Total (N=811) | SCAD‐induced PTSD status | |||
|---|---|---|---|---|---|
| Lifetime PTSD symptoms, no diagnosis (n=513) | Lifetime PTSD diagnosis, no past‐month PTSD diagnosis (n=243) | Past‐month PTSD diagnosis (n=55) | P value | ||
| Sought treatment, n (%) | 282 (34.8%) | 121 (23.6%) | 125 (51.4%) | 36 (65.5%) | <0.001 |
| Type of professional,* n (%) | |||||
| Clergy | 21 (2.6%) | 6 (1.2%) | 13 (5.3%) | 2 (3.6%) | 0.003 |
| Primary care physician | 124 (15.3%) | 53 (10.3%) | 51 (21.0%) | 20 (36.4%) | <0.001 |
| Psychologist or social worker | 163 (20.1%) | 64 (12.5%) | 80 (32.9%) | 19 (34.5%) | <0.001 |
| Psychiatrist | 81 (10.0%) | 29 (5.7%) | 40 (16.5%) | 12 (21.8%) | <0.001 |
| Other | 28 (3.5%) | 15 (2.9%) | 9 (3.7%) | 4 (7.3%) | 0.236 |
| Type of treatment,* n (%) | |||||
| Medication | 145 (17.9%) | 56 (10.9%) | 64 (26.3%) | 25 (45.5%) | <0.001 |
| Psychotherapy or talk therapy | 217 (26.8%) | 86 (16.8%) | 104 (42.8%) | 27 (49.1%) | <0.001 |
| Other | 19 (2.3%) | 12 (2.3%) | 7 (2.9%) | 0 (0.0%) | 0.443 |
P values reflect χ2 comparisons across levels of SCAD‐induced PTSD status for a given professional or treatment type. PTSD indicates posttraumatic stress disorder; and SCAD, spontaneous coronary artery dissection.
Patients could select 1 or more of these options.
Correlates of SCAD‐Induced PTSD Status
Demographics
SCAD‐induced PTSD status was significantly associated with several demographic characteristics. Specifically, younger age at enrollment and being a woman, single, or unemployed were associated with greater and more enduring manifestations of SCAD‐induced PTSD symptoms (Table 1).
SCAD History
Several SCAD‐related characteristics were associated with SCAD‐induced PTSD status (Table 1). Younger age at first SCAD was associated with greater and more enduring manifestations of SCAD‐induced PTSD. Among patients with at least 1 pregnancy, history of pregnancy‐related SCAD was most prevalent among patients with probable past‐month PTSD (20.0%). Patients with probable past‐month PTSD were also most likely to report that they experienced acute emotional stress/anxiety before their most recent SCAD event (23.6%). Of the various event presentations for the most recent SCAD event, only presenting with cardiac arrest was significantly associated with SCAD‐induced PTSD status. The percentage of participants with this presentation was highest among those with probable past‐month PTSD (12.7%); in contrast, rates of other presentations were generally comparable across groups. Type of in‐hospital treatment for the most recent SCAD event was not significantly associated with SCAD‐induced PTSD status.
Mental Health Factors
Mean cumulative lifetime trauma burden increased monotonically with greater and more enduring manifestations of SCAD‐induced PTSD symptoms (Table 1). Although approximately one‐quarter to one‐third of the total sample reported a history of depression or anxiety before the most recent SCAD event, prevalence of these mental health conditions was highest among patients with greater and more enduring manifestations of SCAD‐induced PTSD symptoms.
Health Behaviors
SCAD‐induced PTSD status was linked to several health behaviors (Table 1). The greatest past‐week sleep disturbance was reported by individuals with probable past‐month PTSD (mean T score, 59.9); this group also had the highest rate of current smoking, at nearly 1 in 10 patients. In contrast, alcohol consumption was not significantly associated with SCAD‐induced PTSD status.
Disease‐Specific Health Status
Mean past‐month disease‐specific health status decreased monotonically with greater and more enduring manifestations of SCAD‐induced PTSD symptoms (Table 1).
Adjusted Analyses of SCAD‐Induced PTSD Symptom Severity
Results of the multivariable regression examining past‐month SCAD‐induced PTSD symptom severity are presented in Table 3. Being single (ß=0.10, P=0.003), unemployed status (ß=0.13, P<0.001), not being employed outside the home (ß=0.09, P=0.007), younger age at first SCAD (ß=−0.21, P<0.001), fewer years since the worst/most distressing SCAD event and registry enrollment (ß=−0.15, P<0.001), greater lifetime cumulative trauma burden (ß=0.18, P<0.001), and a history of anxiety before the most recent SCAD event (ß=0.20, P<0.001) were significantly associated with higher past‐month SCAD‐induced PTSD symptom levels. Age and time‐related factors, along with trauma and anxiety‐related variables, had some of the strongest associations with past‐month SCAD‐induced PTSD symptom severity.
Table 3.
Parameters From Adjusted Regression Model Examining Patient Characteristics Associated With Past‐Month SCAD‐Induced PTSD Symptom Severity
| Variable in regression model | b (95% CI) | β | P value |
|---|---|---|---|
| Sex (women=reference) | −2.47 (−5.34 to 0.39) | −0.05 | 0.090 |
| Marital status | |||
| Divorced | −0.16 (−2.65 to 2.33) | −0.00 | 0.900 |
| Single | 3.65 (1.26 to 6.03) | 0.10 | 0.003* |
| Widowed | −0.34 (−4.78 to 4.10) | −0.00 | 0.881 |
| Married/domestic partner (reference) | … | … | … |
| Employment status | |||
| Employed part‐time | 0.61 (−1.45 to 2.67) | 0.02 | 0.563 |
| Not employed outside home | 3.02 (0.84 to 5.20) | 0.09 | 0.007* |
| Retired | 0.47 (−1.96 to 2.90) | 0.01 | 0.704 |
| Student | 2.23 (−6.44 to 10.90) | 0.02 | 0.614 |
| Unemployed | 5.50 (2.68 to 8.32) | 0.13 | <0.001* |
| Employed full‐time (reference) | … | … | … |
| Age at first SCAD | −0.22 (−0.30 to −0.14) | −0.21 | <0.001* |
| Time between worst SCAD event and registry enrollment, y | −0.44 (−0.63 to −0.25) | −0.15 | <0.001* |
| Lifetime cumulative trauma burden | 1.26 (0.81 to 1.71) | 0.18 | <0.001* |
| Reported depression before most recent SCAD event | 1.59 (−0.23 to 3.41) | 0.06 | 0.087 |
| Reported anxiety before most recent SCAD event | 4.64 (3.01 to 6.27) | 0.20 | <0.001* |
Sample size for regression models based on complete case analysis=805. Model R 2=22.13%. Both unstandardized (b) and standardized (β) regression coefficients are presented for completeness. PTSD indicates posttraumatic stress disorder; and SCAD, spontaneous coronary artery dissection.
Significant at P<0.05.
Adjusted Associations of SCAD‐Induced PTSD Symptom Severity With Sleep Disturbance and Disease‐Specific Health Status
In regression models adjusting for demographic, SCAD‐related, and mental health variables, past‐month SCAD‐induced PTSD symptom levels were significantly positively associated with past‐week sleep disturbance (ß=0.42, P<0.001) and significantly negatively associated with past‐month disease‐specific health status (ß=−0.37, P<0.001; Table 4). In both sets of models, PTSD symptoms emerged as the significant predictor with the largest effect size, with only sex (ß=−0.08, P=0.016), unemployed status (ß=0.06, P=0.049), and trauma burden (ß=0.08, P=0.018) also significantly associated with sleep disturbance, and only unemployed status (ß=−0.11, P=0.001) and years between the worst/most distressing SCAD event and registry enrollment (ß=0.14, P<0.001) also significantly associated with disease‐specific health status. Furthermore, when excluding the sleep‐related symptoms of PTSD from the total symptom severity score in a sensitivity analysis, past‐month SCAD‐induced PTSD symptoms remained significantly positively associated with sleep disturbance, albeit with a slightly attenuated effect size (β=0.34, P<0.001).
Table 4.
Parameters From Adjusted Regression Models Examining the Associations of Past‐Month SCAD‐Induced PTSD Symptom Severity With Past‐Week Sleep Disturbance and Past‐Month Disease‐Specific Health Status
| Past‐week sleep disturbance | Past‐month disease‐specific health status | |||||
|---|---|---|---|---|---|---|
| Variable in regression model | b (95% CI) | β | P value | b (95% CI) | β | P value |
| Sex (women=reference) | −2.94 (−5.31 to −0.56) | −0.08 | 0.016* | 2.11 (−2.26 to 6.48) | 0.03 | 0.344 |
| Marital status | ||||||
| Divorced | 1.24 (−0.84 to 3.31) | 0.04 | 0.241 | 1.68 (−2.12 to 5.48) | 0.03 | 0.387 |
| Single | −0.66 (−2.65 to 1.33) | −0.02 | 0.514 | 0.40 (−3.26 to 4.05) | 0.01 | 0.831 |
| Widowed | −2.18 (−5.86 to 1.51) | −0.04 | 0.247 | −5.59 (−12.54 to 1.36) | −0.05 | 0.115 |
| Married/domestic partner (reference) | … | … | … | … | … | … |
| Employment status | ||||||
| Employed part‐time | −0.64 (−2.34 to 1.07) | −0.02 | 0.465 | −1.05 (−4.19 to 2.09) | −0.02 | 0.513 |
| Not employed outside home | −0.05 (−1.88 to 1.78) | −0.00 | 0.959 | −0.67 (−4.01 to 2.67) | −0.01 | 0.695 |
| Retired | 1.01 (−1.01 to 3.03) | 0.04 | 0.326 | −1.86 (−5.58 to 1.85) | −0.04 | 0.325 |
| Student | −0.88 (−8.07 to 6.31) | −0.01 | 0.810 | −5.47 (−18.69 to 7.76) | −0.03 | 0.417 |
| Unemployed | 2.37 (0.01 to 4.73) | 0.06 | 0.049* | −7.28 (−11.62 to −2.94) | −0.11 | 0.001* |
| Employed full‐time (reference) | … | … | … | … | … | … |
| Age at first SCAD | 0.02 (−0.05 to 0.08) | 0.02 | 0.634 | 0.07 (−0.05 to 0.18) | 0.04 | 0.267 |
| Time between worst SCAD event and registry enrollment, y | −0.04 (−0.20 to 0.12) | −0.02 | 0.608 | 0.62 (0.33 to 0.92) | 0.14 | <0.001* |
| Lifetime cumulative trauma burden | 0.47 (0.08 to 0.85) | 0.08 | 0.018* | −0.14 (−0.85 to 0.56) | −0.01 | 0.687 |
| Reported depression before most recent SCAD event | 1.33 (−0.18 to 2.85) | 0.06 | 0.085 | −0.48 (−3.27 to 2.30) | −0.01 | 0.734 |
| Reported anxiety before most recent SCAD event | 1.03 (−0.35 to 2.41) | 0.05 | 0.145 | −1.32 (−3.86 to 1.21) | −0.04 | 0.306 |
| Past‐month PTSD symptoms | 0.35 (0.30 to 0.41) | 0.42 | <0.001* | −0.55 (−0.66 to −0.45) | −0.37 | <0.001* |
Sample size for regression models based on complete case analysis=802 for sleep disturbance and 804 for disease‐specific health status. Both unstandardized (b) and standardized (β) regression coefficients are presented for completeness. Model R 2=26.08% for sleep disturbance and 21.52% for disease‐specific health status. PTSD indicates posttraumatic stress disorder; and SCAD, spontaneous coronary artery dissection.
Significant at P<0.05.
Discussion
In the largest study of SCAD‐induced PTSD conducted to date, with >800 patients from a multisite registry, we demonstrated that developing PTSD symptoms in response to a SCAD event is common, with nearly 35% of patients meeting criteria for probable SCAD‐induced PTSD in their lifetime and 6.4% of the total sample meeting criteria for probable past‐month PTSD. Furthermore, nearly half of patients who developed probable SCAD‐induced PTSD reported never receiving trauma‐related treatment, highlighting a notable treatment gap.
Probable SCAD‐induced PTSD prevalence in this sample (34.7% for lifetime and 6.4% for past‐month) was substantially higher than rates observed in the general population. Rates of lifetime PTSD in the United States are estimated at 6.1% to 8.3% overall among trauma‐exposed individuals, 25 , 26 although women are approximately twice as likely to develop PTSD in their lifetime than men. 26 , 27 In addition, a large recent study of middle‐aged and older women estimated lifetime and past‐month prevalence of probable PTSD among trauma‐exposed individuals, measured in the same way as we did here, at 10.5% and 1.5%, respectively. 16 Furthermore, the PTSD prevalence rates we observed in this sample of patients with SCAD are high even compared with PTSD induced by other cardiovascular events. For example, clinically significant symptoms of PTSD have been observed in ≈12% of patients after acute coronary syndrome, 28 25% of patients after stroke or transient ischemic attack, 29 and 32% of patients after cardiac arrest. 30 However, much of the literature on PTSD induced by cardiovascular events has examined rates of elevated PTSD symptoms rather than applying the DSM‐5 PTSD diagnostic criteria, as we did here. Additionally, our sample was predominantly women, which contrasts with much cardiovascular research in which women have traditionally been underrepresented. 31 Moreover, research examining lifetime, in addition to past‐month, symptoms of PTSD triggered by cardiovascular events has been lacking. Research on SCAD‐induced PTSD to date has only examined current PTSD symptoms, with 1 large study estimating probable PTSD based on an elevated symptom score cutoff in 7.8% of the sample. 6 Together, our findings highlight that symptoms of PTSD after SCAD that align with the diagnostic criteria for the disorder may occur in >1 in 3 patients at some point after their SCAD event, pointing to the need for greater awareness of this particular mental health consequence of SCAD among treatment providers.
Rates of lifetime trauma exposure (beyond the SCAD event that patients experienced) were also high, with 65% reporting at least 1 other traumatic event. Although this finding is not unique, with the vast majority of individuals (50%–89%) experiencing a traumatic event during their lifetimes, 25 , 26 , 27 , 32 the rates of exposure to certain trauma types were notable. In particular, over one‐third of patients had experienced some form of interpersonal or sexual violence in their lifetime. Although high rates of trauma exposure in patients presenting with cardiovascular events have been noted, 33 these findings in patients with SCAD suggest that an appreciation of prior traumatic experiences may serve patients in their interactions with health care providers. Further research is needed to determine whether incorporating elements of trauma‐informed care into cardiology clinical settings may be beneficial for patients. 34
Over a third of patients who experienced some symptoms of PTSD in response to their SCAD event indicated seeking treatment for this distress, with rates highest among those with probable past‐month PTSD (65.5%) and lifetime (but not past‐month) probable PTSD (51.4%). Patients receiving treatment most often interacted with psychologists or social workers, followed by their primary care physician, and psychotherapy was generally received more often than pharmacotherapy. The extent to which patients consulted with their cardiovascular care providers on SCAD‐induced PTSD symptoms was not assessed specifically, although only 3.5% of patients who reported treatment seeking specified interacting with a professional other than clergy, primary care physicians, psychologists or social workers, or psychiatrists. Because the patient–cardiovascular provider relationship can be a particularly salient one for patients with SCAD, exploring psychological treatment seeking and receipt in the context of this relationship is an important topic for future research with high clinical relevance. We also found that nearly half of patients with probable SCAD‐induced PTSD in their lifetime reported never receiving trauma‐related treatment. Lack of access to evidence‐based mental health care is well established, 35 and our results mirror other research demonstrating that a sizeable proportion of individuals with PTSD do not get treatment, ranging from approximately one‐third of middle‐aged and older women with PTSD in the United States to half of individuals with PTSD in high‐income countries. 16 , 25 Thus, it is of interest to examine whether efforts to connect patients who are struggling with PTSD symptoms after SCAD with mental health professionals can help to close this treatment gap. Because patients receive medical care by a cardiologist, and many of these patients are ultimately seen in specialized clinics with expertise in SCAD, offering mental health care through these treatment settings may be a promising avenue for engaging patients. Another notable finding is that nearly a quarter of patients whose SCAD‐induced PTSD symptoms did not reach the level of a probable diagnosis reported seeking trauma‐related treatment. This result suggests that even subthreshold symptoms of PTSD after SCAD may be associated with clinically meaningful distress or impairment, a finding that is echoed in the broader PTSD literature, 36 , 37 and may benefit from being addressed in treatment.
Several demographic and psychosocial correlates of SCAD‐induced PTSD were identified in this large registry sample, including younger age (along with younger age at first SCAD), being a woman, single, or unemployed, fewer years between the worst/most distressing SCAD event and registry enrollment, greater cumulative lifetime trauma burden, and a history of anxiety or depression. Several of these factors (eg, younger age, being a woman, unemployment) have been linked to PTSD either in patients with SCAD 6 or in the broader PTSD literature. 24 In contrast, relatively few SCAD‐related medical factors (eg, treatment strategy) were robustly related to manifestations of SCAD‐induced PTSD, consistent with prior research in patients with SCAD. 6 Some, 4 , 38 but not all, 6 research in patients with cardiac events has found that undergoing percutaneous coronary intervention is associated with lower rates of psychological distress, including PTSD, but we did not observe differential associations between various treatments for the most recent SCAD event and PTSD. Longitudinal research that tracks SCAD‐related psychological distress before and after receiving various treatments is needed to better understand how interventions may relate to emotional responses to SCAD. Additionally, of the various event presentations for the most recent SCAD event, only presenting with cardiac arrest was significantly associated with SCAD‐induced PTSD. The percentage of participants with this presentation was highest among those with probable past‐month SCAD‐induced PTSD, which parallels findings of particularly high rates of PTSD symptoms in patients with cardiac arrest defined more broadly. 30 Together, these results suggest that cardiac arrest may be an important presentation with respect to PTSD risk.
We also observed links between SCAD‐induced PTSD and relevant health behaviors. Probable past‐month SCAD‐induced PTSD was associated with greater rates of current smoking and sleep disturbance, though not alcohol use. The mean sleep disturbance score for this group of patients was nearly 1 SD above the mean of the US general population. In adjusted models, past‐month SCAD‐induced PTSD symptom severity was positively associated with past‐week sleep disturbance, and it had the largest effect size by far of the various potential risk factors. Sleep disturbance is a hallmark symptom of PTSD that also has relevance for cardiovascular health, 39 and it is possible that SCAD‐induced PTSD symptoms may contribute to sleep problems in this patient population. However, results were similar in a sensitivity analysis in which the PTSD‐specific sleep items were excluded from the total symptom severity score, suggesting that broad manifestations of PTSD, and not just sleep‐related symptoms, may have relevance for sleep patterns in patients with SCAD. Given the cross‐sectional nature of the study, it is not possible to determine the directionality of this association and whether there is a causal link; longitudinal research is needed to address these relations.
SCAD‐induced PTSD symptoms were also associated with worse disease‐specific health status in the past month, with mean disease‐specific health status reflecting symptoms of chest pain, chest tightness, and angina, and related impairment decreasing monotonically with greater and more enduring manifestations of SCAD‐induced PTSD symptoms. In models accounting for a range of risk factors, past‐month PTSD symptom severity was negatively associated with past‐month disease‐specific health status. Furthermore, only 2 other characteristics were significantly associated with disease‐specific health status (unemployed status and years between the worst and most‐distressing SCAD event and registry enrollment), and SCAD‐induced PTSD symptom severity had a substantially larger effect size. Longitudinal research is needed to assess whether SCAD‐induced PTSD is an aspect of mental health in patients with SCAD that is particularly relevant for functional status and outcomes.
Several limitations of the current investigation merit acknowledgement. First, all data were collected as part of the iSCAD Registry baseline assessment and thus cannot address the causal or directional nature of associations. We look forward to harnessing the follow‐up assessments administered in the iSCAD Registry to explore changes in SCAD‐induced PTSD symptoms over time and in relation to treatment seeking and health outcomes. A longer follow‐up window will also provide an opportunity to reassess rates of treatment seeking for SCAD‐induced PTSD symptoms. Second, because our sample comprised patients participating in a multisite registry, our study is characterized by limitations such as selection bias, recall bias, and issues with misclassification and representativeness. Our sample was predominantly White, non‐Hispanic or non‐Latina women. Additional research in more diverse patient samples, as demonstrated in recent community‐based cohorts, 40 , 41 is needed. Third, data were missing for some variables, and over a quarter of patients in the iSCAD Registry did not complete the baseline questionnaires that included the assessment of SCAD‐induced PTSD. We also lacked information for patients who were approached for the iSCAD Registry but did not elect to participate. Nevertheless, patients who completed the PTSD questionnaire were similar to those in the full registry cohort, and our 74.3% response rate is higher than in other registry samples. 6 Fourth, we used a self‐reported questionnaire to assess SCAD‐induced PTSD symptoms and assign probable diagnostic status based on DSM‐5 symptom criteria, as in prior research. 16 Although this questionnaire is well validated, can be used to assign provisional diagnoses of PTSD, and is more feasible for use in clinical settings, 18 , 19 research with gold‐standard clinical interviews to determine full diagnostic criteria is needed. By focusing on assessing symptoms of SCAD‐induced PTSD, we also were unable to determine whether patients had a history of PTSD symptoms that developed in response to other types of traumatic experiences as well. Despite these limitations, this largest study analyzing SCAD‐induced PTSD conducted to date has several unique strengths, including (1) integrating patients from multiple clinics, (2) incorporating information from patients and provider site investigators, (3) assessing lifetime and past‐month probable diagnoses of SCAD‐induced PTSD, and (4) investigating associations between SCAD‐induced PTSD and a range of health‐relevant correlates.
Conclusions
Our investigation of >800 patients with SCAD suggests that SCAD‐induced PTSD symptoms are a common mental health consequence for these patients. Furthermore, these symptoms are linked to clinical characteristics that are relevant to the course of disease, including adverse health behaviors and disease‐specific health status. Moreover, nearly half of patients who developed probable SCAD‐induced PTSD reported never receiving trauma‐related treatment. The high prevalence of SCAD‐induced PTSD symptoms and this treatment gap are particularly notable given that a lack of mental health resources after SCAD is a leading concern for patients. 12 Efforts to support screening for PTSD symptoms after SCAD and connecting those patients experiencing distress postevent with empirically supported treatments are critical next steps.
Patient Perspective
As a survivor who has journeyed through the challenges of SCAD, I am thankful for the ongoing research dedicated to understanding the intricate connection between SCAD and PTSD. I think it is incredibly important that those engaged in post‐SCAD care recognize that the emotional aftermath of SCAD can be just as, if not more, stressful than the event itself. SCAD survivors require not only medical support but also emotional support to truly heal after facing SCAD.
As a patient diagnosed with a rare disease, it is important to acknowledge the additional emotional aspect that is required in the healing process. The diagnosis of a rare condition, where even medical professionals have limited information, increased my anxiety and uncertainty surrounding its cause and likelihood of recurrence. Although the initial heart attack and hospitalization were emotionally challenging for me, the period following my discharge from the hospital proved even more challenging. The uncertainty took a toll on my emotional healing and perspective for my future health.
During my time in the hospital, although I experienced moments of anxiety, it was when I was discharged that my anxiety seemed to intensify. Losing the comforting presence of medical professionals and the reassuring presence of the machines that monitored my heart's every beat left me feeling vulnerable and uncertain about the path ahead.
I vividly recall the realization that, with SCAD, life could slip away in the blink of an eye, with no warning, no chance to seek help. Faced with this unsettling uncertainty and the gravity of SCAD's potential impact, adapting to a sense of “normalcy” was a challenge. With time, I found strength through cardiac rehabilitation, where I learned to trust my healing heart once more and the fearful moments gradually gave way to a newfound sense of confidence. The journey after my discharge was undeniably stressful, but it also carried the precious gift of a second chance at life.
It was not until the 1‐year mark of my SCAD journey that I found myself grappling with PTSD. The moment arrived unexpectedly, as my spouse and I were leaving a restaurant. I glanced at my watch and realized it was the exact time we had dialed 911 a year earlier. Just then, an ambulance rushed past us, heading in the direction of my home, the same path it had taken that day. At that moment, I was overwhelmed with anxiety. Later that evening, as I lay in bed, the memories flooded back as I recalled the smell of the hospital sheets, the hospital machines beeping, and the profound fear that gripped me during my hospital stay. These recollections weighed heavily on my already anxious heart, and it became apparent that I needed help. I made an appointment with my cardiologist, as well as my physician, and was diagnosed with PTSD.
Twelve years have passed since my diagnosis of PTSD, and although my life has returned to normal, there are moments when my PTSD resurfaces. Whenever my heart flutters or the mere thought of something resembling the feeling of my SCAD experiences, anxiety instantaneously takes over me. External triggers, like appointments with physicians, the wailing sirens of ambulances, or the hospital environment, cause me to have anxiety. Even my annual cardiology appointment fills me with anxiety. The fear of discovering something awry and facing hospitalization looms over me. This anxiety extends to my visits to the primary doctor and dentist as well. These settings take me back to those moments of crippling fear and uncertainty.
PTSD is another aspect of SCAD that SCAD survivors are faced with. There is an urgent need for health care providers to acknowledge its significance, diagnose it, and provide the necessary care. Survivors bear the weight of this emotional battle alongside their physical struggles, and it is important that it is addressed with compassion and understanding.
I am so thankful for the compassion and empathy displayed by my medical team. They understood that my journey was not just about the physical toll on my body; it encompassed the emotional turbulence I was navigating as well. Their holistic approach, addressing not only my physical needs but also my emotional and mental well‐being, played a crucial role in my journey to heal from SCAD.
Sources of Funding
This work was supported by the SCAD Alliance and funding from the National Heart, Lung, and Blood Institute (R01HL139614, R01HL160850 to J.A.S.).
Disclosures
Dr Kim is the Chair of the Scientific Advisory Board of SCAD Alliance, a funder of this work. This is an unpaid position. She has no relationships with industry. Dr Wood is a member of the Scientific Advisory Board of SCAD Alliance; this is an unpaid position. She has no relationships with industry. Dr Chi receives research grant support paid to the Beth Israel Deaconess Medical Center, Harvard Medical School from Bayer, Janssen Scientific Affairs, and CSL Behring. Dr Kadian‐Dodov receives research support from Philips Healthcare, paid to the Cardiovascular Institute at Mount Sinai. None of this work is relevant to the research in this article. Dr Gibson receives research support from SCAD Alliance. K. Leon is cofounder and executive director of the SCAD Alliance; this is an unpaid position. Dr Naderi is a member of the Scientific Advisory Board of the SCAD Alliance; this is an unpaid position. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1
Acknowledgments
The iSCAD Registry was developed in partnership with patients with SCAD who recognized the need for a collaborative international research model accessible to patients and investigators. Patients simultaneously raised funds to support the registry, answered surveys to develop the study questions, and generously shared their medical and personal information as active enrolled participants in the registry. The authors would like to acknowledge these patients; this work would not have been possible without their time, effort, generosity, and dedication. The authors would also like to acknowledge the many coinvestigators and research staff at the enrolling sites who volunteered their time to complete this work.
This article was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032819
For Sources of Funding and Disclosures, see page 14.
References
- 1. Hayes SN, Kim ES, Saw J, Adlam D, Arslanian‐Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. doi: 10.1161/CIR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tweet MS, Kok SN, Hayes SN. Spontaneous coronary artery dissection in women: what is known and what is yet to be understood. Clin Cardiol. 2018;41:203–210. doi: 10.1002/clc.22909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy B, Le Grande M, Alvarenga M, Worcester M, Jackson A. Anxiety and depression after a cardiac event: prevalence and predictors. Front Psychol. 2020;10:3010. doi: 10.3389/fpsyg.2019.03010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang JJ, Tweet MS, Hayes SE, Gulati R, Hayes SN. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. doi: 10.1097/HCR.0000000000000030 [DOI] [PubMed] [Google Scholar]
- 5. Edwards KS, Vaca KC, Naderi S, Tremmel JA. Patient‐reported psychological distress after spontaneous coronary artery dissection: evidence for post‐traumatic stress. J Cardiopulm Rehabil Prev. 2019;39:E20–E23. doi: 10.1097/HCR.0000000000000460 [DOI] [PubMed] [Google Scholar]
- 6. Johnson AK, Hayes SN, Sawchuk C, Johnson MP, Best PJ, Gulati R, Tweet MS. Analysis of posttraumatic stress disorder, depression, anxiety, and resiliency within the unique population of spontaneous coronary artery dissection survivors. J Am Heart Assoc. 2020;9:e014372. doi: 10.1161/JAHA.119.014372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saw JW, Starovoytov A, Birnie T, Prakash R, Heydari‐Kamjani M, Isserow S, Taylor C, Chan S, Ignaszewski A. Comparison of psychosocial questionnaires between spontaneous coronary artery dissection (SCAD) and non‐SCAD populations undergoing cardiac rehabilitation program after myocardial infarction. J Am Coll Cardiol. 2016;67:1936. doi: 10.1016/S0735-1097(16)31937-4 [DOI] [Google Scholar]
- 8. Tulloch H, Bouchard K, Brownrigg J, Coutinho T. Depression and anxiety before and after cardiac rehabilitation: comparing patients with and without spontaneous coronary artery dissection. Can J Cardiol. 2023;39:350–352. doi: 10.1016/j.cjca.2023.01.009 [DOI] [PubMed] [Google Scholar]
- 9. Krittanawong C, Kumar A, Johnson KW, Luo Y, Yue B, Wang Z, Bhatt DL. Conditions and factors associated with spontaneous coronary artery dissection (from a national population‐based cohort study). Am J Cardiol. 2019;123:249–253. doi: 10.1016/j.amjcard.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 10. Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/S0165-0327(02)00426-3 [DOI] [PubMed] [Google Scholar]
- 11. McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baechler CJ, Witt DR, Lohese O, Benson G. Spontaneous coronary artery dissection and evidence‐based medicine. Am J Cardiol. 2022;171:65–68. doi: 10.1016/j.amjcard.2022.01.046 [DOI] [PubMed] [Google Scholar]
- 13. Vilchinsky N, Ginzburg K, Fait K, Foa EB. Cardiac‐disease‐induced PTSD (CDI‐PTSD): a systematic review. Clin Psychol Rev. 2017;55:92–106. doi: 10.1016/j.cpr.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 15. Schnurr P, Vielhauer M, Weathers F, Findler M. The brief trauma questionnaire. 1999. Scale Available from the National Center for PTSD at www.ptsd.va.gov.
- 16. Sampson L, Jha SC, Roberts AL, Lawn RB, Nishimi KM, Ratanatharathorn A, Sumner JA, Kang JH, Kubzansky LD, Rimm EB, et al. Trauma, post‐traumatic stress disorder, and treatment among middle‐aged and older women in the Nurses' health study II. Am J Geriatr Psychiatry. 2022;30:588–602. doi: 10.1016/j.jagp.2021.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM‐5 (PCL‐5). 2013. Scale Available from the National Center for PTSD at www.ptsd.va.gov.
- 18. Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL‐5) in veterans. Psychol Assess. 2016;28:1379–1391. doi: 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- 19. Wortmann JH, Jordan AH, Weathers FW, Resick PA, Dondanville KA, Hall‐Clark B, Foa EB, Young‐McCaughan S, Yarvis JS, Hembree EA, et al. Psychometric analysis of the PTSD Checklist‐5 (PCL‐5) among treatment‐seeking military service members. Psychol Assess. 2016;28:1392–1403. doi: 10.1037/pas0000260 [DOI] [PubMed] [Google Scholar]
- 20. Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, Johnston KL, Pilkonis PA. Development of short forms from the PROMIS™ sleep disturbance and sleep‐related impairment item banks. Behav Sleep Med. 2012;10:6–24. doi: 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky‐Cade MA, Pilkonis PA. Development and validation of patient‐reported outcome measures for sleep disturbance and sleep‐related impairments. Sleep. 2010;33:781–792. doi: 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. PROMIS Sleep Disturbance Scoring Manual (2022). Manual available at https://staging.healthmeasures.net/images/PROMIS/manuals/PROMIS_Sleep_Disturbance_Scoring_Manual.pdf
- 23. Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7:640–647. doi: 10.1161/CIRCOUTCOMES.114.000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tortella‐Feliu M, Fullana MA, Pérez‐Vigil A, Torres X, Chamorro J, Littarelli SA, Solanes A, Ramella‐Vravaro V, Vilar A, González‐Parra JA, et al. Risk factors for posttraumatic stress disorder: an umbrella review of systematic reviews and meta‐analyses. Neurosci Biobehav Rev. 2019;107:154–165. doi: 10.1016/j.neubiorev.2019.09.013 [DOI] [PubMed] [Google Scholar]
- 25. Koenen K, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, Karam EG, Meron Ruscio A, Benjet C, Scott K, et al. Posttraumatic stress disorder in the world mental health surveys. Psychol Med. 2017;47:2260–2274. doi: 10.1017/S0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldstein RB, Smith SM, Chou SP, Saha TD, Jung J, Zhang H, Pickering RP, Ruan WJ, Huang B, Grant BF. The epidemiology of DSM‐5 posttraumatic stress disorder in the united Staets: results from the National Epidemiologic Survey on alcohol and related conditions‐III. Soc Psychiatry Psychiatr Epidemiol. 2016;51:1137–1148. doi: 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- 28. Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta‐analytic review. PLoS One. 2012;7:e38915. doi: 10.1371/journal.pone.0038915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ, Kronish IM. Prevalence of PTSD in survivors of stroke and transient ischemic attack: a meta‐analytic review. PLoS One. 2013;8:e66435. doi: 10.1371/journal.pone.0066435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal S, Presciutti A, Cornelius T, Birk J, Roh DJ, Park S, Claassen J, Elkind MWV, Edmondson D. Cardiac arrest and the subsequent hospitalization induced posttraumatic stress is associated with 1‐year risk of major adverse cardiovascular events and all‐cause mortality. Crit Care Med. 2019;47:e502–e505. doi: 10.1097/CCM.0000000000003713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tobb K, Kocher M, Bullock‐Palmer RP. Underrepresentation of women in cardiovascular trials‐it is time to shatter this glass ceiling. Am Heart J Plus: Cardiol Res Pract. 2022;13:100109. doi: 10.1016/j.ahjo.2022.100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J Trauma Stress. 2013;26:537–547. doi: 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Young J, Schweber A, Sumner JA, Chang BP, Cornelius T, Kronish IM. Impact of prior trauma exposure on the development of PTSD symptoms after suspected acute coronary syndrome. Gen Hosp Psychiatry. 2021;68:7–11. doi: 10.1016/j.genhosppsych.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hargrave AS, Sumner JA, Ebrahimi R, Cohen BE. Posttraumatic stress disorder (PTSD) as a risk factor for cardiovascular disease: implications for future research and clinical care. Curr Cardiol Rep. 2022;24:2067–2079. doi: 10.1007/s11886-022-01809-y [DOI] [PubMed] [Google Scholar]
- 35. Patel V, Maj M, Flisher AJ, De Silva MJ, Koschorke M, Prince M, Zonal WPA; Member Society Representatives . Reducing the treatment gap for mental disorders: a WPA survey. World Psychiatry. 2010;9:169–176. doi: 10.1002/j.2051-5545.2010.tb00305.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: findings from a community survey. Am J Psychiatry. 1997;154:1114–1119. doi: 10.1176/ajp.154.8.1114 [DOI] [PubMed] [Google Scholar]
- 37. Grubaugh AL, Magruder KM, Waldrop AE, Elhai JD, Knapp RG, Frueh BC. Subthreshold PTSD in primary care: prevalence, psychiatric disorders, healthcare use, and functional status. J Nerv Ment Dis. 2005;193:658–664. doi: 10.1097/01.nmd.0000180740.02644.ab [DOI] [PubMed] [Google Scholar]
- 38. Edmondson D, Birk JL, Ho VT, Meli L, Abdalla M, Kronish IM. A challenge for psychocardiology: addressing the causes and consequences of patients' perceptions of enduring somatic threat. Am Psychol. 2018;73:1160–1171. doi: 10.1037/amp0000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meinhausen C, Prather AA, Sumner JA. Posttraumatic stress disorder (PTSD), sleep, and cardiovascular disease risk: a mechanism‐focused narrative review. Health Psychol. 2022;41:663–673. doi: 10.1037/hea0001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen S, Merchant M, Mahrer KN, Lundstrom RJ, Naderi S, Goh AC. Spontaneous coronary artery dissection: clinical characteristics, management, and outcomes in a racially and ethnically diverse community‐based cohort. Perm J. 2019;23:18.278. doi: 10.7812/TPP/18.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen S, Merchant M, Mahrer KN, Ambrosy AP, Lundstrom RJ, Sahar N. Pregnancy‐associated spontaneous coronary artery dissection: clinical characteristics, outcomes, and risk during subsequent pregnancy. J Invasive Cardiol. 2021;33:E457–E466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


