Abstract
Background
Dialysis is a rare but serious complication after transcatheter aortic valve replacement. We analyzed the large multicenter TRITAVI (transfusion requirements in transcatheter aortic valve implantation) registry in order to develop and validate a clinical score assessing this risk.
Methods and Results
A total of 10 071 consecutive patients were enrolled in 19 European centers. Patients were randomly assigned (2:1) to a derivation and validation cohort. Two scores were developed, 1 including only preprocedural variables (TRITAVIpre) and 1 also including procedural variables (TRITAVIpost). In the 6714 patients of the derivation cohort (age 82±6 years, 48% men), preprocedural factors independently associated with dialysis and included in the TRITAVIpre score were male sex, diabetes, prior coronary artery bypass graft, anemia, nonfemoral access, and creatinine clearance <30 mL/min per m2. Additional independent predictors among procedural features were volume of contrast, need for transfusion, and major vascular complications. Both scores showed a good discrimination power for identifying risk for dialysis with C‐statistic 0.78 for TRITAVIpre and C‐statistic 0.88 for TRITAVIpost score. Need for dialysis increased from the lowest to the highest of 3 risk score groups (from 0.3% to 3.9% for TRITAVIpre score and from 0.1% to 6.2% for TRITAVIpost score). Analysis of the 3357 patients of the validation cohort (age 82±7 years, 48% men) confirmed the good discrimination power of both scores (C‐statistic 0.80 for TRITAVIpre and 0.81 for TRITAVIpost score). Need for dialysis was associated with a significant increase in 1‐year mortality (from 6.9% to 54.4%; P=0.0001).
Conclusions
A simple preprocedural clinical score can help predict the risk of dialysis after transcatheter aortic valve replacement.
Keywords: acute kidney injury, dialysis, mortality, risk score, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Catheter-Based Coronary and Valvular Interventions, Constraint-Induced Movement Therapy
Nonstandard Abbreviations and Acronyms
- RRT
renal replacement therapy
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
The need for renal replacement therapy post–transcatheter aortic valve replacement is associated with a remarkably high mortality rate of 54% at 1 year.
We found that variables such as the use of nonfemoral vascular access, baseline stage 4 and 5 chronic kidney disease, anemia, male sex, diabetes, and prior coronary artery bypass grafting were significant predictors of the risk of post–transcatheter aortic valve replacement renal replacement therapy and could be used to build a risk score (TRITAVIpre score).
Procedural factors (volume of contrast, need for transfusion, and major vascular complications) only modestly enhance the predictive value of the score (TRITAVIpost score).
What Are the Clinical Implications?
A significant exponential rise in the risk of new‐onset post–transcatheter aortic valve replacement renal replacement therapy is observed in the high‐risk score category compared with the low and intermediate‐risk categories.
This may contribute to identification of patients at risk of needing renal replacement therapy in order to perform all possible preventive measures to lower their risk (such as good hydration before the procedure, limiting contrast use, and avoiding nonfemoral access whenever possible).
Transcatheter aortic valve replacement (TAVR) is an effective treatment for aortic stenosis and it is being proposed to an increasing number of patients, due to its proven benefits in terms of mortality and improvement in quality of life. 1 , 2 However, it is still associated with significant risks despite improved experience and techniques. TAVR is often performed in older patients with multiple comorbidities, including chronic kidney disease (CKD) (with 50%–60% of patients with CKD stage 3 or worse) and it entails a significant risk of worsened kidney function and need for dialysis after the procedure. 3 , 4 , 5
Although relatively rare, newly required renal replacement therapy (RRT) after TAVR is a serious complication, associated with very high mortality (>50% at 1 year). 6 Only a few studies have focused on the risk of RRT after TAVR and, although they have identified possible risk factors for the need for dialysis after TAVR, these data do not allow a clear definition of such risk before the procedure. 6 , 7
Thus, we sought to analyze a large international multicentric registry of patients undergoing TAVR in order to develop and validate a simple risk score that could help to estimate the risk of severe acute kidney injury requiring dialysis of patients undergoing TAVR in contemporary practice.
METHODS
Study Population
The Transfusion Requirements in Transcatheter Aortic Valve Implantation (TRITAVI) is an investigator‐initiated registry designed to collect data on patients with severe aortic stenosis undergoing TAVR that enrolled 11 265 consecutive patients with symptomatic severe aortic stenosis who underwent TAVR at 19 European sites (11 in Italy, 1 in Spain, 1 in Poland, 5 in Finland, and 1 in England) from January 2012 to December 2020. 8 , 9 Local multidisciplinary heart teams evaluated all cases and confirmed eligibility for TAVR. For the present analysis, patients were excluded if they were already on dialysis at the time of the procedure or if they died within 48 hours after the procedure. Centers were contacted in case of missing or inconsistent values and were asked to provide checks or modify results in order to avoid missing data. The study population was randomly divided into a derivation cohort, including two thirds of the population and a validation cohort, including one third of patients. A larger group was selected for the derivation cohort because prediction model development benefits from a larger sample size than its validation. All patients provided written informed consent for the procedure and subsequent data collection. The University of Chieti and Pescara Ethical Committee approved the study. Patient selection, data collection, and data analysis were performed in accordance with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Preprocedural Assessment
Clinical histories, physical examinations, cardiovascular risk factors, pre‐ and postprocedural noninvasive laboratory studies, data such as implanted valve type and size, contrast volume used in the procedure, and procedural complications of the patients were reviewed and recorded. Serum creatinine levels (mg/dL) were measured within 24 hours before the procedure, 24 hours after the procedure, and daily until the patient was discharged. Glomerular filtration rate (GFR) was estimated for each patient using the Cockcroft–Gault formula. 10 CKD was classified as stage 1 with GFR >90 mL/min per m2, stage 2 with GFR 60 to 89 mL/min per m2, stage 3 with GFR 30 to 59 mL/min per m2, stage 4 with GFR 15 to 29 mL/min per m2, and stage 5 with GFR <15 mL/min per m2. 11 Patients already requiring dialysis before TAVR were excluded from the study.
The preprocedural screening was performed by means of clinical assessment (patient demographics, symptoms, comorbidities, laboratory examinations, and risk evaluation), echocardiography, and multidetector computed tomography.
TAVR Procedure
The operative risk of the patients was evaluated according to the Society of Thoracic Surgeons and the European System for Cardiac Operative Risk Evaluation II (Euro SCORE II) risk scoring methods. TAVR was performed either with balloon‐expandable or self‐expandable at the operator's discretion. Device sizing and the site of femoral access were selected in each case according to clinical judgment based on the results of multidetector computed tomography. For all centers, unfractionated heparin was given during the procedure (70 U/kg) targeting an activated clotting time of 200 to 300 s. Standard postprocedural antiplatelet therapy with clopidogrel 75 mg for 6 months and aspirin 100 mg indefinitely was advised. For patients receiving chronic anticoagulation therapy, treatment was resumed shortly after TAVR. All periprocedural complications, including bleeding and vascular complications, were recorded. Patients' data were entered on a common Excel data sheet, and advancement of data collection and analysis were shared among all study participant centers periodically during the study progress.
Clinical Follow‐Up and End Points
In‐hospital outcomes were collected, and all patients were followed up with a 30‐day clinic visit. The primary end point of the study was the 30‐day occurrence of hemodialysis. The secondary end points were 30‐day all‐cause mortality, nonfatal myocardial infarction, cerebrovascular accident, and stage 2 to 3 acute kidney injury defined according to Valve Academic Research Consortium 3 criteria. 12 Echocardiographic outcomes were evaluated before discharge. Paravalvular aortic regurgitation severity was assessed according to Valve Academic Research Consortium 3 criteria. 12
Statistical Analysis
Categorical variables were summarized as frequencies and percentages. Continuous variables were reported as either mean and SD or median and interquartile range according to their distribution, as assessed by the Shapiro–Wilk's test. Continuous variables were compared using Student t test or Mann–Whitney U tests based on the normality of data. Categorical variables are reported as n (%) and were compared by χ 2 test with Yates' correction for continuity or Fisher exact test as appropriate.
Independent predictors of newly required dialysis after the procedure were derived by multivariable logistic regression analysis. The results of the model were expressed as adjusted hazard ratios and a relative 95% CI. Multivariable models were built using forward stepwise selection, with a P value of <0.05 required for inclusion.
Two separate regression models were developed, one including only preprocedural variables and one also including procedural variables. A weighted risk score was derived for each patient by summing the integer assigned to each variable based on its coefficient B (logOR) in the model (1 point for B 0.5; 2 points for B 0.5–1.0; 4 points for B 1.0–1.5, and 6 points for B >1.5).
The final risk scores were then further categorized into 3 groups based on the distribution of the score: low risk, moderate risk, and high risk. The risk score was tested in the validation data set and model predictive performance was assessed using Harrell's C‐statistic and model calibration by Hosmer‐Lemeshow goodness‐of‐fit test. Receiver operating characteristic curves were generated.
Variables that were significantly associated with 1‐year death at univariate analysis were considered for selection in the multivariable Cox regression model, which was then built using forward stepwise selection, with a P value of <0.05 required for inclusion. Cox regression assumptions were checked by plotting Schoenfeld residuals and by determining log‐minus‐log survival curves. Potential outliers were checked by assessing and plotting DFbeta values (results of this analysis are shown in Figures S1 through S3).
All statistical analyses were performed using SPSS 23. All P values were 2‐tailed and a P value <0.05 was considered indicative of a statistically significant association.
RESULTS
Main Clinical and Procedural Features
Among participants in the multicenter TRITAVI registry, a total of 10 071 TAVR procedures (performed between January 2012 and January 2022) were included in the analysis. One hundred fifty‐nine patients were excluded because of chronic dialysis before TAVR. A total of 6714 patients were randomly assigned to the derivation cohort and 3357 to the validation cohort. Procedural and clinical data of both groups are shown in Table S1. The 2 groups were similar in all clinical and procedural features (including age, renal function, volume of contrast used, and procedural complications). A total of 51 patients in the derivation cohort and 18 patients in the validation cohort needed postprocedural dialysis.
Derivation Cohort
Clinical and procedural features of patients with and without postprocedural dialysis in the derivation cohort are shown in Table 1. Patients with dialysis were younger, had a higher prevalence of diabetes, anemia, peripheral artery disease, previous coronary artery bypass graft, and heart failure as well as worse renal function at baseline, while the severity of aortic stenosis and ejection fraction were not significantly different. Among procedural factors, femoral access was used less frequently among patients who underwent postprocedural dialysis, while the volume of contrast used was significantly larger. Major vascular complications and the need for postprocedural transfusion also occurred more frequently in patients with dialysis.
Table 1.
Clinical and Procedural Features in Patients With and Without Postprocedural Dialysis in the Derivation Group
| No dialysis (n=6714) | Postprocedural dialysis (n=51) | P value | |
|---|---|---|---|
| Age, y | 81.7±6.3 | 79.3±7.8 | 0.04 |
| Male sex, % | 47.3 | 66.7 | 0.007 |
| BMI | 26.7±4.8 | 26.8±3.6 | 0.92 |
| Diabetes, % | 29.4 | 45.1 | 0.02 |
| Hypertension, % | 83.0 | 81.6 | 0.82 |
| Smoking, % | 17.3 | 15.7 | 0.91 |
| NYHA III–IV, % | 75.2 | 86.0 | 0.10 |
| Coronary artery disease | 33.9 | 49.0 | 0.03 |
| Prior MI, % | 14.9 | 25.5 | 0.06 |
| Prior PCI, % | 24.9 | 28.6 | 0.62 |
| Prior CABG, % | 12.3 | 26.5 | 0.007 |
| Prior CVA, % | 11.3 | 18.4 | 0.12 |
| PAD, % | 15.9 | 34.7 | 0.001 |
| Active cancer, % | 6.1 | 1.9 | 0.42 |
| Porcelain aorta, % | 4.7 | 8.3 | 0.24 |
| AF, % | 27.2 | 27.5 | 0.92 |
| Anemia, % | 23.9 | 49.0 | <0.0001 |
| Creatinine, mg/dL | 1.2±0.6 | 1.8±1.5 | 0.002 |
| Creatinine clearance | 53±24 | 41±18 | <0.0001 |
| CKD | <0.0001 | ||
| CKD stage 5 (%) | 3.3 | 6.1 | |
| CKD stage 4 (%) | 9.5 | 22.5 | |
| CKD stage 3 (%) | 54.3 | 57.1 | |
| CKD stage 2 (%) | 25.5 | 12.3 | |
| CKD stage 1 (%) | 7.4 | 2.0 | |
| Hemoglobin, g/dL | 12.2±1.7 | 11.2±2.0 | 0.002 |
| STS score | 5.5±4.7 | 9.4±6.9 | 0.001 |
| Euroscore II | 5.8±5.5 | 13.0±15.9 | 0.004 |
| EF, % | 54.4±11.5 | 53.4±13.9 | 0.60 |
| PG mean, mm Hg | 47±15 | 45±15 | 0.31 |
| Aspirin, % | 54.4 | 59.5 | 0.62 |
| P2Y12 inhibitor, % | 21.2 | 21.6 | 0.92 |
| DAPT, % | 14.8 | 19.4 | 0.45 |
| VKA, % | 21.1 | 26.5 | 0.41 |
| Apical access, % | 5.3 | 33.3 | <0.0001 |
| No femoral access, % | 8.2 | 37.3 | <0.0001 |
| Femoral access, % | 91.8 | 62.7 | <0.0001 |
| Corevalve, % | 44.9 | 41.9 | 0.76 |
| Edwards valve, % | 37.2 | 41.2 | 0.56 |
| Valve size | 26.5±2.7 | 26.3±2.5 | 0.65 |
| Valve in valve, % | 2.7 | 5.9 | 0.16 |
| Paravalvular leak | 0.69 | ||
| No paravalvular leak, % | 71.6 | 72.5 | |
| Paravalvular leak I, % | 23.9 | 21.6 | |
| Paravalvular leak II, % | 3.9 | 3.9 | |
| Paravalvular leak III, % | 0.5 | 2.0 | |
| Paravalvular leak, % | 0.1 | 0 | |
| Volume contrast, mL | 194±91 | 262±114 | 0.002 |
| Pacemaker, % | 12.7 | 7.4 | 0.40 |
| Major vascular complications, % | 7.1 | 25.5 | <0.0001 |
| Minor vascular complication, % | 7.9 | 7.8 | 0.98 |
| Transfusion, % | 16.8 | 61.4 | <0.0001 |
| Treatment at discharge | |||
| Single antiplatelet, % | 25.7 | 23.5 | |
| DAPT, % | 28.5 | 21.6 | |
| Oral anticoagulants, % | 25.8 | 29.4 | |
| 30‐d mortality, % | 2.3 | 24.0 | <0.0001 |
| 30‐d stroke/TIA, % | 1.4 | 3.9 | 0.28 |
AF indicates atrial fibrillation; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CVA, cerebrovascular accident; DAPT, dual antiplatelet therapy; EF, ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PG, pressure gradient; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack; and VKA, vitamin K antagonist.
Results of unadjusted analysis evaluating variables associated with need for postprocedural dialysis are shown in Table S2. Significant associations included age, male sex, diabetes, peripheral artery disease, prior coronary artery bypass graft, anemia, no femoral access, creatinine clearance <30 mL/min, the volume of contrast, the occurrence of major vascular complications, and the need for transfusion.
Two separate multivariable analyses (with and without procedural variables) for the prediction of 30‐day postprocedural dialysis were conducted. The results of multivariable analysis on preprocedural features are shown in Table 2. Factors significantly associated with dialysis included male sex, diabetes, prior coronary artery bypass graft, anemia, no femoral access, and creatinine clearance <30 mL/min per m2. A weighted risk score was derived for each patient by summing the integer assigned to each variable based on its coefficient B (logOR) with 1 point for B <0.5; 2 points for B 0.5 to 1.0; 4 points for B 1.0 to 1.5, and 6 points for B >1.5. A TRITAVIpre score was developed from preprocedural data: this score showed good discrimination with C statistics=0.78 (95% CI, 0.71–0.84).
Table 2.
Multivariable Analysis in the Derivation Group (Preprocedural Factors) with TRITAVIpre Score
| B | SE | OR (95% CI) | P value | Points | |
|---|---|---|---|---|---|
| Male sex | 0.72 | 0.27 | 2.06 (1.20–3.55) | 0.01 | 2 |
| Diabetes | 0.51 | 0.26 | 1.66 (1.01–2.76) | 0.04 | 2 |
| Anemia | 0.91 | 0.26 | 2.48 (1.49–4.18) | <0.001 | 2 |
| Prior CABG | 0.68 | 0.29 | 1.96 (1.10–3.53) | 0.02 | 2 |
| No femoral access | 1.45 | 0.27 | 4.26 (2.39–7.61) | <0.0001 | 4 |
| GFR <30 mL/min per m2 | 1.03 | 0.29 | 2.80 (1.45–4.58) | <0.0001 | 4 |
CABG indicates coronary artery bypass graft; GFR, glomerular filtration rate; OR, odds ratio; and TRITAVI, transfusion requirements in transcatheter aortic valve implantation.
A second multivariable analysis including also procedural features added as predictors the volume of contrast, need for transfusion, and occurrence of major vascular complications. In this model, factors associated with postprocedural dialysis included male sex, diabetes, prior coronary artery bypass graft, no femoral access, creatinine clearance <30 mL/min, volume of contrast, occurrence of major vascular complications, and need for transfusion (Table 3). Anemia was no longer significant in this analysis including postprocedural factors. Interestingly, other procedural factors, including the presence of paravalvular leaks and the type of valve used, were not associated with the risk of dialysis.
Table 3.
Multivariable Analysis in the Derivation Group (Including Procedural Factors) With TRITAVIpost Score
| B | SE | OR (95% CI) | P value | Points | |
|---|---|---|---|---|---|
| Male sex | 0.83 | 0.28 | 2.29 (1.21–4.35) | 0.01 | 2 |
| Diabetes | 0.63 | 0.27 | 1.87 (1.03–3.40) | 0.04 | 2 |
| Prior CABG | 0.74 | 0.30 | 2.09 (1.04–4.21) | 0.04 | 2 |
| No femoral access | 1.61 | 0.32 | 5.05 (2.52–10.09) | 0.001 | 6 |
| Contrast per 100 mL | 0.32 | 0.09 | 1.38 (1.12–1.70) | 0.002 | 1 |
| Creatinine clearance <30 mL/min per m2 | 0.92 | 0.30 | 2.52 (1.36–4.66) | 0.01 | 2 |
| Transfusion | 1.74 | 0.29 | 5.67 (2.92–11.0) | <0.0001 | 6 |
| Major vascular complication | 0.94 | 0.33 | 2.56 (1.21–5.41) | 0.01 | 2 |
CABG indicates coronary artery bypass graft; OR, odds ratio; and TRITAVI, transfusion requirements in transcatheter aortic valve implantation.
A TRITAVIpost score was developed according to this analysis with a good discrimination power for identifying risk for dialysis with a C statistic=0.88 (95% CI, 0.81–0.92).
Both scores showed significant increments in risk with rising risk scores (Figure 1A for TRITAVIpre and Figure 1B for TRITAVIpost score).
Figure 1. Predicted risk of dialysis in the derivation cohort according to risk score values from Model 1 (A) and Model 2 (B).
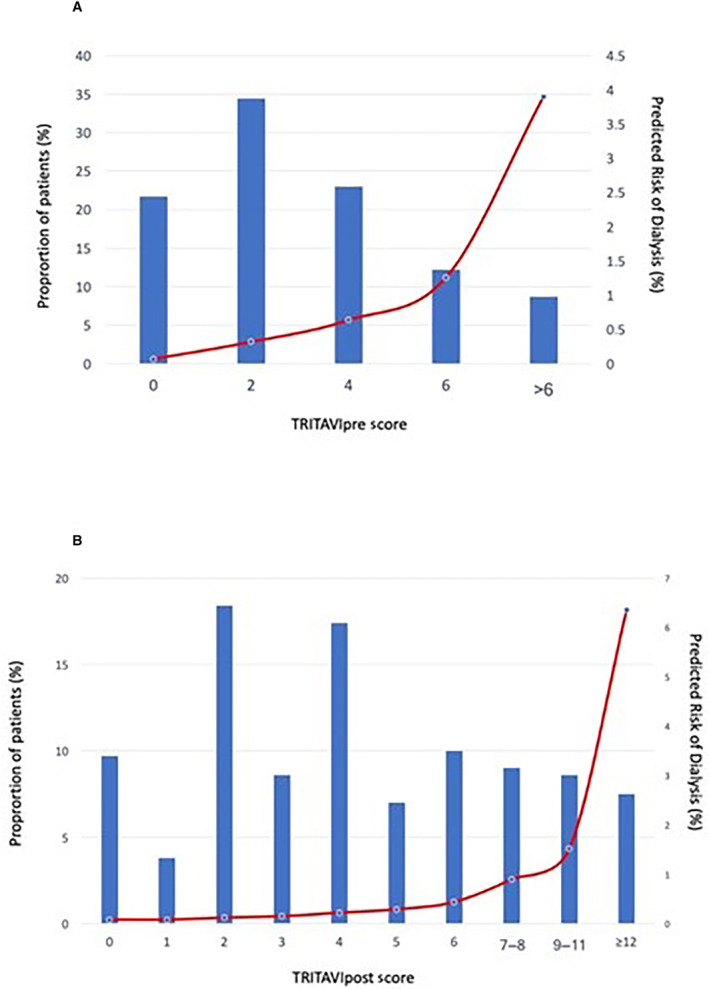
Bars show the proportion of patients (left axes) and lines show the predicted risk of dialysis (right axes). TRITAVI indicates transfusion requirements in transcatheter aortic valve implantation.
Validation Cohort
The results of multivariable analysis in the validation cohort were similar to the derivation cohort, although with larger confidence intervals. Analysis of the validation cohort confirmed the good discrimination power of both scores, with C=0.80 (95% CI, 0.72–0.88) for TRITAVIpre score and C=0.81 (95% CI, 0.72–0.91) for TRITAVIpost score. Receiver operating characteristic curves for the TRITAVIpre and TRITAVIpost scores for both derivation and validation cohorts are shown in Figure 2.
Figure 2. Assessment of risk discrimination for the derivation and the validation cohorts.
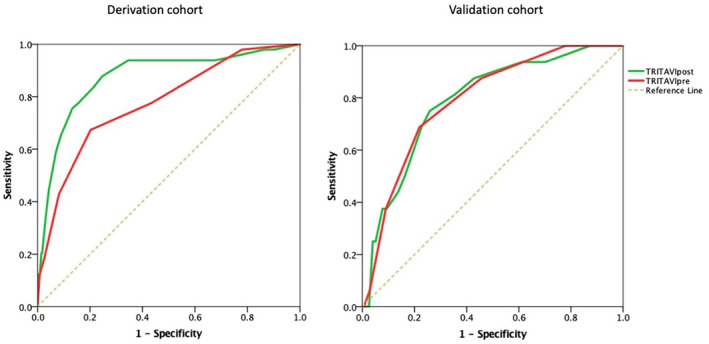
The C‐statistic was 0.78 for Model 1 (TRITAVIpre) and 0.87 for Model 2 (TRITAVIpost) in the derivation cohort (left panel), and 0.80 for Model 1 (TRITAVIpre) and 0.83 for Model 2 (TRITAVIpost) in the validation cohort (right panel). TRITAVI indicates transfusion requirements in transcatheter aortic valve implantation.
Assessment of Goodness of Fit
Patients in the derivation and validation cohorts were stratified into 3 groups according to the TRITAVIpre risk scores: low risk (score 0–2, 56.2% of the derivation cohort and 53.8% of the validation cohort), moderate risk (score 3–6, 35.3% of the derivation cohort and 37.2% of the validation cohort), and high risk (score >6, 8.5% of the derivation cohort and 9.0% of the validation cohort). The observed and predicted rates of postprocedural dialysis for TRITAVIpre risk score in the derivation and validation cohorts (Figures 3A and 3B, left panels) showed a clear increase in risk across the 3 groups (Hosmer‐Lemeshow goodness of fit: P=0.31 in the derivation and P=0.25 in the validation group).
Figure 3. Assessment of goodness of fit in the 3 risk groups for the derivation cohort (A) and the validation cohort (B).
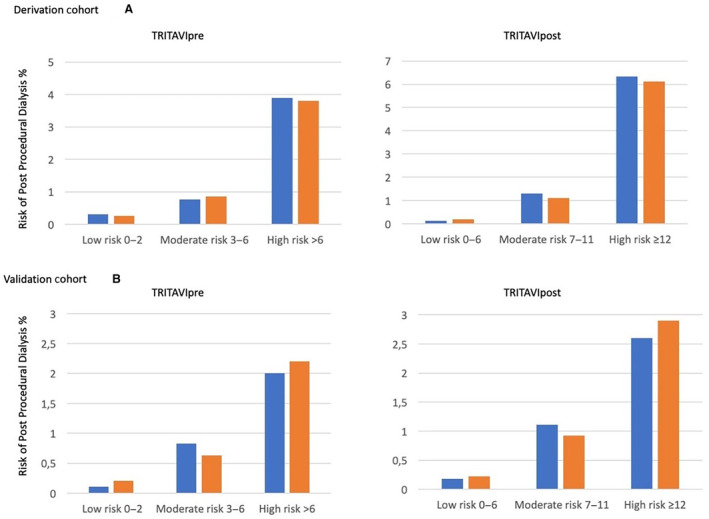
The observed and predicted rates of postprocedural dialysis for TRITAVIpre risk score in the derivation and validation cohorts showed a Hosmer‐Lemeshow goodness of fit: P=0.31 in the derivation (A) and P=0.25 in the validation group (B). Need for dialysis increased gradually in particular in the highest of 3 risk score groups (from 0.3% to 3.9%). The observed and predicted rates of postprocedural dialysis for TRITAVIpost score in the derivation and validation cohorts showed a Hosmer‐Lemeshow goodness of fit: P=0.58 for the derivation (A) and P=0.84 for the validation group (B). Need for dialysis increased in particular in the highest of the 3 score groups (from 0.1% to 6.2%). The corresponding risk score thresholds for each risk group were ≤2, 3 to 6, and >6 in TRITAVIpre, and ≤6, 7 to 11, and ≥12 in TRITAVIpost. Blue bars indicate observed values and orange bars indicate predicted values. TRITAVI indicates transfusion requirements in transcatheter aortic valve implantation.
A similar analysis for the TRITAVIpost score allowed stratification into 3 groups: low risk (score 0–6, 74.9% of the derivation cohort and 73.9% of the validation cohort), moderate risk (score 7–11, 17.6% of the derivation cohort and 18.2% of the validation cohort), and high risk (score ≥12, 7.5% of the derivation cohort and 7.9% of the validation cohort). The observed and predicted rates of postprocedural dialysis for TRITAVIpost score in the derivation and validation cohorts (Figures 3A and 3B, right panels) showed a sharp increase of risk in particular for the high‐risk group (Hosmer‐Lemeshow goodness of fit: P=058 for derivation and P=0.84 for the validation group).
Postprocedural Dialysis and Survival
Kaplan–Meier curves showing the unadjusted relationships between postprocedural dialysis and 1‐year total mortality are shown in Figure 4 with an unadjusted 1‐year mortality of 54.2% in patients with dialysis and 6.9% in patients without dialysis, P <0.0001 (after excluding patients dying in the first 48 hours after the procedure). Survival curves clearly diverged in the first weeks after the procedure. Postprocedural dialysis remained the strongest predictor of 1‐year mortality after adjustment for other factors associated with mortality (including diabetes, baseline heart failure with New York Heart Association functional class III to IV, presence of coronary disease, peripheral artery disease, ejection fraction, anemia, no femoral access, major vascular complications) with hazard ratio 4.72 (95% CI, 3.19–6.98). Factors significantly associated with 1‐year mortality at multivariable analysis are shown in Table 4.
Figure 4. Cumulative incidence of 1‐year death in patients with (red line) and without (green line) need for dialysis after TAVR, with 95% CI.
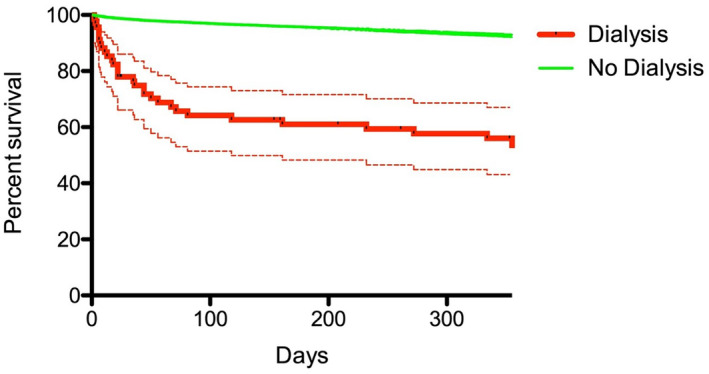
TAVR indicates transcatheter aortic valve replacement.
Table 4.
Factors Associated With 1‐Year Mortality in Multivariable Analysis
| HR (95% CI) | P | |
|---|---|---|
| Postprocedural dialysis | 4.72 (3.19–6.98) | <0.0001 |
| Major vascular complications | 1.42 (1.11–1.82) | 0.006 |
| Heart failure (NYHA III–IV) | 1.52 (1.23–1.89) | <0.0001 |
| Anemia | 1.35 (1.13–1.60) | 0.001 |
| No femoral access | 1.47 (1.17–1.85) | 0.001 |
HR indicates hazard ratio; and NYHA, New York Heart Association.
DISCUSSION
In this study, we assessed the risk of postprocedural RRT in a large population of patients undergoing TAVR, and we developed and validated a simple risk model to predict this risk.
Our simple risk score, including the use of alternative (nonfemoral) vascular access, baseline renal function (stage 4 and 5 CKD), anemia, male sex, diabetes, and prior coronary artery bypass graft, was able to predict the risk of postprocedural RRT. The inclusion of procedural features only marginally improves the predictive value of the score. Postprocedural dialysis is associated with a poor outcome after TAVR, with high mortality at 30 days and 1 year.
In the last 10 years, TAVR has been one of the fastest‐growing procedures in interventional cardiology and it is now offered to an ever‐expanding range of patients. Although the risk of complications during TAVR has been declining due to improved devices and better operator experience, TAVR is still associated with significant mortality occurring after the procedure. The reduction of periprocedural risks underscores the importance of identifying factors associated with mortality in the first weeks after the procedure.
Several large studies have shown that the most important risk factors associated with mortality after TAVR are CKD 13 and, even more, postprocedural acute kidney injury. 14 , 15 , 16 , 17 Many studies have focused on acute kidney injury after cardiovascular procedures and specifically after TAVR, but data dealing with dialysis (notably the most dreadful outcome) have been more limited. 6 , 7
Large registries have consistently shown that the need for postprocedural RRT after TAVR is associated with an ominous outcome, with >50% mortality at 1 year (mostly occurring in the first 6 months). 6 , 7 Also, because long‐term RRT may be considered an intolerable outcome for some patients, our score might provide an easy and valuable tool for shared decision‐making in the setting of the Heart Team meeting. 18 The TRITAVIpre score can be used to identify patients at higher risk of RRT in order to use all possible preventive measures to lower their risk (such as good hydration before the procedure, limiting contrast use, and avoiding nonfemoral access whenever possible). Inclusion of procedural features can only marginally improve on the predictive value of the preprocedural score.
Earlier studies have reported a high incidence of dialysis after TAVR (up to 21%), 19 , 20 but such incidence has decreased over time: 2.3% in the UK‐TAVI registry (for years 2013–2014) 6 and 1.9% in the large Society of Thoracic Surgery/American College of Cardiology (STS/ACC) registry in the United States (in patients undergoing TAVR between 2011 and 2015). 7 Our large multicenter European registry found an even lower incidence of dialysis after TAVR, with an incidence of only 0.7% (years 2011–2021), probably reflecting contemporary practice and possibly a higher threshold for the use of dialysis in continental European practice compared with the United States. Exclusion of all patients already in dialysis before TAVR from our database was also important. Still, this complication maintains its strong association with mortality after TAVR. Indeed, in our database, dialysis was associated with >50% mortality at 1 year.
The UK TAVI registry found that the need for dialysis after TAVR was associated with several factors, including baseline creatinine clearance, diabetes, and ejection fraction <30%, 6 use of nonfemoral approach, use of self‐expanding valve, and presence of significant aortic regurgitation after TAVR. 6 The US STS/ACC TVT registry found only an association between the need for RRT and preprocedural creatinine clearance (<60 mL/min per m2 and even more significantly when <30 mL/min per m2) while other factors were not taken into account. 7 Our large registry confirms and expands such previous findings: a strong association between the need for RRT and baseline renal function (creatinine clearance <30 mL/min per m2) and the use of a nonfemoral approach was identified. However, a moderate but significant association with male sex, diabetes, presence of anemia, previous coronary artery bypass graft, the volume of contrast used, and procedural vascular complications was documented. Baseline ejection fraction, type of valve used, and presence of significant paravalvular leak were not associated with the need for RRT. In our study, reflecting contemporary practice, a significant paravalvular leak was relatively uncommon (3–4 paravalvular leaks only 0.6% in our study, while 7.9% of patients in the UK TAVI registry had a moderate/severe aortic regurgitation). 6 Also, we did not find any difference between different types of valves: it is possible that the reported association between balloon‐expanding valve and RRT was due to the exclusive use of the Edwards valve in nonfemoral (in particular apical) TAVRs. Also, ejection fraction was not significantly associated with RRT in our data. Although all studies have found a significant association between baseline kidney function and risk of RRT and complications after TAVR, in our database less than one‐third of cases of RRT occurred in patients with severe CKD (stage 4 or 5).
Previous analyses have shown the lack of discriminatory power of classic scores used for coronary angiography for identifying the risk of renal failure after TAVR. 21 Indeed, patients undergoing TAVR seem to have a lower risk of acute kidney injury compared with patients undergoing coronary angiography, despite a much worse risk profile. 22 In the present study, for the first time, we found that a simple score, based on preprocedural features, may help predict risk of RRT after TAVR. Adding procedural features (in particular volume of contrast and major vascular complications) only moderately improves the discriminatory power of the score. This is similar to what was reported in previous large studies on scores for predicting kidney damage during coronary angiography. 23 It is possible that operators tried to limit the use of contrast in patients with baseline renal failure, thus limiting its impact on renal failure. On the other hand, the association between TAVR and worsening renal failure leading to dialysis is likely related to many factors other than contrast use, including procedural hypotension, peripheral embolization from calcific aortic atheroma, systemic inflammation, and transfusions. Because TAVR is often performed in elderly and frail patients, multiple comorbidities are also likely to play a much larger role than in younger and fitter patients in whom coronary procedures with contrast medium are performed.
Limitations
RRT after TAVR has become infrequent in contemporary practice; thus, even our large international registry includes only a relatively limited number of patients with dialysis after TAVR. For this reason, it is not possible to include in the analysis subgroups with higher score values and higher risk of RRT, because inclusion of these smaller subgroups would impair the statistical significance of the analysis.
However, it should be emphasized that postprocedural dialysis, although being rare in current practice, is by far the single most relevant risk factor for 1‐year mortality (Table 4). Thus, even identifying a complication occurring in a small number of patients has an important clinical value.
We have tested our score both in derivation and validation groups, to confirm its clinical value. However, further studies should test the predictive value of the score in other populations (in particular in a non‐European population). It should also be considered that TAVR is still a fast‐evolving procedure. Thus further technical improvements may change its risk profile; conversely, coronary angiography and angioplasty are now quite mature procedures, where risk scores are expected to maintain their validity.
CONCLUSIONS
Our study was able to determine factors associated with the need for RRT in a large contemporary population of patients undergoing TAVR. A simple preprocedural clinical score can help assess the risk of this severe complication, which is associated with high mortality.
Sources of Funding
The present study was funded by Institutional Funds of ‘G. D'Annunzio’ University of Chieti‐Pescara and of ‘La Sapienza’ University, Rome, Italy.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S3
This manuscript was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032955
For Sources of Funding and Disclosures, see page 11.
References
- 1. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana G, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232 [DOI] [PubMed] [Google Scholar]
- 2. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 3. Barbash IM, Segev A. The plan was to replace the valve not the kidneys. JACC Cardiovasc Interv. 2017;10:2076–2077. doi: 10.1016/j.jcin.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 4. Gupta T, Goel K, Kolte D, Khera S, Villablanca PA, Aronow WS, Bortnick AE, Slovut DP, Taub CC, Kizer JR, et al. Association of chronic kidney disease with in‐hospital outcomes of transcatheter aortic valve replacement. JACC Cadiovasc Interv. 2017;10:2050–2060. doi: 10.1016/j.jcin.2017.07.044 [DOI] [PubMed] [Google Scholar]
- 5. Crimi G, De Marzo V, De Marco F, Conrotto F, Oreglia J, D'Ascenzo F, Testa L, Gorla R, Esposito G, Sorrentino S, et al. Acute kidney injury after transcatheter aortic valve replacement mediates the effect of chronic kidney disease. J Am Heart Assoc. 2022;11:e024589. doi: 10.1161/JAHA.121.024589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferro CJ, Law JP, Doshi SN, de Belder M, Moat N, Mamas M, Hildick‐Smith D, Ludman P, Townend JN. UK TAVI steering group and the National Institute for Cardiovascular Outcomes Research. Dialysis following transcatheter aortic valve replacement: risk factors and outcomes: an analysis from the UK TAVI (transcatheter aortic valve implantation) registry. JACC Cadiovasc Interv. 2017;10:2040–2047. doi: 10.1016/j.jcin.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 7. Hansen JW, Foy A, Yadav P, Gilchrist IC, Kozak M, Stebbins A, Matsouaka R, Vemulapalli S, Wang A, Wang DD, et al. Death and dialysis after transcatheter aortic valve replacement: an analysis of the STS/ACC TVT registry. J Am Coll Cardiol Intv. 2017;10:2064–2075. doi: 10.1016/j.jcin.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 8. Zimarino M, Barbanti M, Dangas GD, Testa L, Capodanno D, Stefanini GG, Radico F, Marchioni M, Amat‐Santos I, Piva T, et al. Early adverse impact of transfusion after transcatheter aortic valve replacement: a propensity‐matched comparison from the TRITAVI registry. Circ Cardiovasc Interv. 2020;13:e009026. doi: 10.1161/CIRCINTERVENTIONS.120.009026 [DOI] [PubMed] [Google Scholar]
- 9. Rodriguez‐Gabella T, Zimarino M, Barbanti M, Testa L, Capodanno D, Stefanini GG, Radico F, Fabbiocchi F, Piva T, Saia F, et al. Sex based analysis of the impact of red blood cell transfusion and vascular or bleeding complications related to TAVI—the TRITAVI‐women study. Int J Cardiol. 2021;333:69–76. doi: 10.1016/j.ijcard.2021.02.066 [DOI] [PubMed] [Google Scholar]
- 10. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415 [DOI] [PubMed] [Google Scholar]
- 11. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):S1–S150. [DOI] [PubMed] [Google Scholar]
- 12. VARC‐3 WRITING COMMITTEE , Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, Bax JJ, Leipsic JA, Blanke P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. 2021;77(21):2717–2746. doi: 10.1016/j.jacc.2021.02.038 [DOI] [PubMed] [Google Scholar]
- 13. Chiarito M, Mehran R. Transcatheter aortic valve replacement in patients with end‐stage renal disease: is “better than nothing” good enough? Catheter Cardiovasc Interv. 2020;96:1110–1112. doi: 10.1002/ccd.29341 [DOI] [PubMed] [Google Scholar]
- 14. Barbash IM, Ben‐Dor I, Dvir D, Maluenda G, Xue Z, Torguson R, Satler LF, Pichard AD, Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. doi: 10.1016/j.ahj.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 15. Koifman E, Segev A, Fefer P, Barbash I, Sabbag A, Medvedovsky D, Spiegelstein D, Hamdan A, Hay I, Raanani E, et al. Comparison of acute kidney injury classifications in patients undergoing transcatheter aortic valve implantation: predictors and long‐term outcomes. Catheter Cardiovasc Interv. 2016;87:523–531. doi: 10.1002/ccd.26138 [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto M, Hayashida K, Mouillet G, Hovasse T, Chevalier B, Oguri A, Watanabe Y, Dubois‐Randé JL, Morice MC, Lefèvre T, et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol. 2013;62:869–877. doi: 10.1016/j.jacc.2013.04.057 [DOI] [PubMed] [Google Scholar]
- 17. Allende R, Webb JG, Munoz‐Garcia AJ, de Jaegere P, Tamburino C, Dager AE, Cheema A, Serra V, Amat‐Santos I, Velianou JL, et al. Advanced chronic kidney disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes and prognostic markers from a large cohort of patients. Eur Heart J. 2014;35:2685–2696. doi: 10.1093/eurheartj/ehu175 [DOI] [PubMed] [Google Scholar]
- 18. Coylewright M, O'Neill E, Sherman A, Gerling M, Adam K, Xu K, Grande SW, Dauerman HL, Dodge SE, Sobti NK, et al. The learning curve for shared decision‐making in symptomatic aortic stenosis. JAMA Cardiol. 2020;5:442–448. doi: 10.1001/jamacardio.2019.5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferro CJ, Chue CD, de Belder MA, Moat N, Wendler O, Trivedi U, Ludman P, Townend JN; UK TAVI Steering Group , National Institute for Cardiovascular Outcomes Research . Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): an analysis of the UK TAVI registry. Heart. 2015;101:546–552. doi: 10.1136/heartjnl-2014-307041 [DOI] [PubMed] [Google Scholar]
- 20. Gargiulo G, Sannino A, Capodanno D, Perrino C, Capranzano P, Barbanti M, Stabile E, Trimarco B, Tamburino C, Esposito G. Impact of postoperative acute kidney injury on clinical outcomes after transcatheter aortic valve implantation: a meta‐analysis of 5971 patients. Catheter Cardiovasc Interv. 2015;86:518–527. doi: 10.1002/ccd.25867 [DOI] [PubMed] [Google Scholar]
- 21. Rosa VEE, Campos CM, Bacelar A, Abizaid AAC, Mangione JA, Lemos PA, Esteves V, Caramori P, Sampaio RO, Tarasoutchi F, et al. Performance of prediction models for contrast‐induced acute kidney injury after transcutaneous aortic valve replacement. Cardiorenal Med. 2021;11:166–173. doi: 10.1159/000517058 [DOI] [PubMed] [Google Scholar]
- 22. Venturi G, Pighi M, Pesarini G, Ferrero V, Lunardi M, Castaldi G, Setti M, Benini A, Scarsini R, Ribichini FL. Contrast‐induced acute kidney injury in patients undergoing TAVI compared with coronary interventions. J Am Heart Assoc. 2020;9:e017194. doi: 10.1161/JAHA.120.017194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehran R, Owen R, Chiarito M, Baber U, Sartori S, Cao D, Nicolas J, Pivato CA, Nardin M, Krishnan P, et al. A contemporary simple risk score for prediction of contrast‐associated acute kidney injury after percutaneous coronary intervention: derivation and validation from an observational registry. Lancet. 2021;398:1974–1983. doi: 10.1016/S0140-6736(21)02326-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


