Abstract
Background
Established cardiovascular disease (CVD) risk prediction functions may not accurately predict CVD risk in people with HIV. We assessed the performance of 3 CVD risk prediction functions in 2 HIV cohorts.
Methods and Results
CVD risk scores were calculated in the Mass General Brigham and Kaiser Permanente Northern California HIV cohorts, using the American College of Cardiology/American Heart Association atherosclerotic CVD function, the FHS (Framingham Heart Study) hard coronary heart disease function and the Framingham Heart Study hard CVD function. Outcomes were myocardial infarction or coronary death for FHS hard coronary heart disease function; and myocardial infarction, stroke, or coronary death for American College of Cardiology/American Heart Association and FHS hard CVD function. We calculated regression coefficients and assessed discrimination and calibration by sex; predicted to observed risk of outcome was also compared. In the combined cohort of 9412, 158 (1.7%) had a coronary heart disease event, and 309 (3.3%) had a CVD event. Among women, CVD risk was generally underestimated by all 3 risk functions. Among men, CVD risk was underestimated by the American College of Cardiology/American Heart Association and FHS hard CVD function, but overestimated by the FHS hard coronary heart disease function. Calibration was poor for women using the FHS hard CVD function and for men using all functions. Discrimination in all functions was good for women (c‐statistics ranging from 0.78 to 0.90) and moderate for men (c‐statistics ranging from 0.71 to 0.72).
Conclusions
Established CVD risk prediction functions generally underestimate risk in people with HIV. Differences in model performance by sex underscore the need for both HIV‐specific and sex‐specific functions. Development of CVD risk prediction models tailored to HIV will enhance care for aging people with HIV.
Keywords: aging, cardiovascular, HIV, risk prediction
Subject Categories: Myocardial Infarction, Risk Factors, Aging, Epidemiology, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- ACC/AHA
American College of Cardiology/American Heart Association
- ATHENA
AIDS Therapy Evaluation in the Netherlands
- FHS
Framingham Heart Study
- FHS CHD
Framingham Heart Study hard coronary heart disease (function)
- FHS CVD
Framingham Heart Study hard cardiovascular disease (function)
- HOPS
HIV Outpatient Study
- KPNC
Kaiser Permanente Northern California
- MGB
Mass General Brigham
- PWH
people with HIV
Clinical Perspective.
What Is New?
Cardiovascular risk prediction scores developed for the general population underestimate risk for people living with HIV, particularly among women.
Underestimation of cardiovascular risk in people living with HIV may result in disparities in preventative care due to inaccurate risk estimation.
What Are the Clinical Implications?
There is an urgent need for tailored strategies to accurately estimate cardiovascular risk in people living with HIV.
Cardiovascular disease (CVD) risk is increased among people with HIV (PWH), with a relative risk that has been shown to be ≈50% to 100% higher compared with that of the general population. 1 , 2 , 3 , 4 The impact of HIV on CVD risk appears to vary by sex, age, and virologic and immunologic status. Traditional CVD risk factors are heightened in HIV 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 yet do not appear to explain the entirety of increased risk, with numerous studies supporting inflammation and immune activation as important factors mediating HIV‐related CVD risk. 12 , 13 , 14 , 15 , 16
To enable optimal management of CVD risk factors and prevention of CVD, accurate risk prediction is essential. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 CVD risk prediction functions that are widely used for the general population have been shown in some studies to be inaccurate in PWH, with underestimation of risk demonstrated. 25 , 26 , 27 , 28 Limitations in the performance of CVD risk prediction functions in PWH have been attributed to the fact that variables included in the functions are limited to traditional CVD risk factors and may not reflect factors unique to HIV and its associated chronic inflammation and immune activation.
In a prior study, we demonstrated that both discrimination and calibration of existing CVD risk prediction functions were poor to moderate for male PWH. 29 In the current study, we conducted a new analysis including both sexes, a longer follow‐up period, and a larger combined HIV group composed of 2 clinically and epidemiologically discrete HIV cohorts. We assessed incidence rates, coefficients, discrimination, and calibration, hypothesizing that CVD risk prediction models would be inaccurate overall and among subgroups in PWH.
Methods
Data are available from the author on reasonable request and pending establishment of an appropriate data‐sharing agreement. The Reporting of Observational Studies in Epidemiology cohort reporting guidelines were used. 30
Study Population
The combined cohort was composed of individuals from the Mass General Brigham (MGB, formerly Partners) HIV cohort, 2 , 29 a longitudinal observational cohort of PWH receiving care through the Mass General Brigham health care system in Boston, Massachusetts, and the Kaiser Permanente Northern California (KPNC) HIV cohort, 31 , 32 a clinic‐based cohort of people enrolled in KPNC, a large integrated health care system in Northern California serving the greater San Francisco Bay Area. A control group without HIV from the same health care system was generated and propensity score matched to individuals in the HIV cohort on the basis of demographic factors and traditional CVD risk factors in up to a 4:1 ratio. Individuals who were followed between January 1, 2000, and January 31, 2020, were eligible for inclusion in the analysis.
The index date was defined as the earliest date lipid values were available for an individual patient after January 1, 2000. The observation period was the index date through the earliest of relevant outcome (coronary heart disease [CHD] or CVD), last encounter, death, or January 31, 2020. Individuals aged 30 to 79 years as of the index date were included, and those with history of CHD or stroke before the index date were excluded. PWH were excluded if the first HIV diagnosis occurred more than a year following the index date given that HIV‐specific factors potentially contributing to risk would not have been present at baseline and during the entirety of follow‐up. Continuity was defined as membership in the Kaiser system or a <2‐year gap in encounters for MGB. The study was approved by the Partners Human Research Committee.
Cardiovascular Risk Scores
Risk scores assessed included the American College of Cardiology/American Heart Association (ACC/AHA) atherosclerotic CVD function, 23 the FHS (Framingham Heart Study) hard coronary heart disease function (FHS CHD), 18 and the FHS function for global cardiovascular disease (FHS CVD), using an adjustment factor to match the ACC/AHA function outcome. 19 For the ACC/AHA function, coefficients for the specific race–sex group of the individual were employed. 23 Risk prediction functions were selected for inclusion in the study on the basis of frequency of clinical use and availability of risk factor and outcome data specific to the score.
Exposure Variables
Variables in the FHS CHD function include age, prevalence of diabetes, smoking (defined as 3 years before or after index date), systolic and diastolic blood pressure, total cholesterol, and high‐density lipoprotein categories. Variables in the ACC/AHA and FHS CVD functions include age, prevalence of diabetes, smoking (defined as 3 years before or after index date), systolic blood pressure, total cholesterol, high‐density lipoprotein, and antihypertensive treatment. Blood pressure data were extracted from electronic health record coded field data and notes. Smoking status was obtained through application of a natural language processing–based algorithm developed and validated in the MGB HIV cohort to reduce bias 33 and by International Classification of Diseases (ICD‐9 and ICD‐10) codes for KPNC. For vital signs and laboratory values, the closest value was employed within 1 year before or after the index date. For antihypertensive medications, individuals were considered to be on treatment if prescribed 1 year before or after the index date.
Outcomes
Outcomes included myocardial infarction (MI) or coronary death for the FHS CHD, and MI, stroke, or coronary death or death due to stroke for the ACC/AHA and FHS CVD functions. All outcomes in MGB were adjudicated; a validated case ascertainment approach was used for the KPNC cohort. 34 For the MGB cohort, event adjudication was completed for MI and stroke employing teams composed of 3 cardiologists for the MI events and 3 neurologists for the stroke events who were experienced in outcome adjudication. MI definition was based on the Universal Definition of Myocardial Infarction, and stroke definition was based on the American Heart Association/American Stroke Association stroke definition. Cases to be adjudicated were initially identified by ICD code (410 or I21 for MI; and 433, 434, or I63 for stroke). To obtain accurate data on cause of death, mortality data were obtained from the Massachusetts Department of Public Health Office of Data Management and Outcomes Assessment and linked to the data for individuals in the MGB cohort. For KPNC, mortality data were obtained from Kaiser Permanente hospitalization data, quarterly Social Security Administration vital status files, and state death certificate data.
Statistical Analysis
Descriptive statistics (mean, median, quartiles for continuous variables; and frequency/percentage for categorical variables) were calculated overall and for each of the MGB and KPNC cohorts. The Kruskal–Wallis test and χ2 test were used to compare those with and without CVD events. The ability of each risk function (ACC/AHA, FHS CHD, FHS CVD) to predict 10‐year risk was assessed for the combined cohorts (MGB and KPNC) and each cohort individually, separately among women and men using the outcomes predicted by the specific scores. Discrimination of the functions was assessed by the c‐statistic. Calibration was assessed using Demler et al modification of the D'Agostino–Nam χ 2 statistic. 35 , 36 Specifically, calibration was assessed as follows for the risk functions that were investigated: The cohort being studied was divided into tertiles of 10‐year predicted risk. The mean 10‐year predicted risk and the observed Kaplan–Meier 10‐year risk estimate were calculated for each resulting tertile, and the difference between the 2 risks were compared across tertiles using the Demler/D'Agostino–Nam χ 2 statistic. A P value >0.05 (nonsignificant χ 2) suggests good calibration (good agreement of 10‐year predicted risk with observed 10‐year risk). Plots showing the observed and predicted 10‐year risk by tertile of predicted risk are presented (Figure).
Figure 1. Observed and predicted 10‐year risk by tertile of predicted risk.
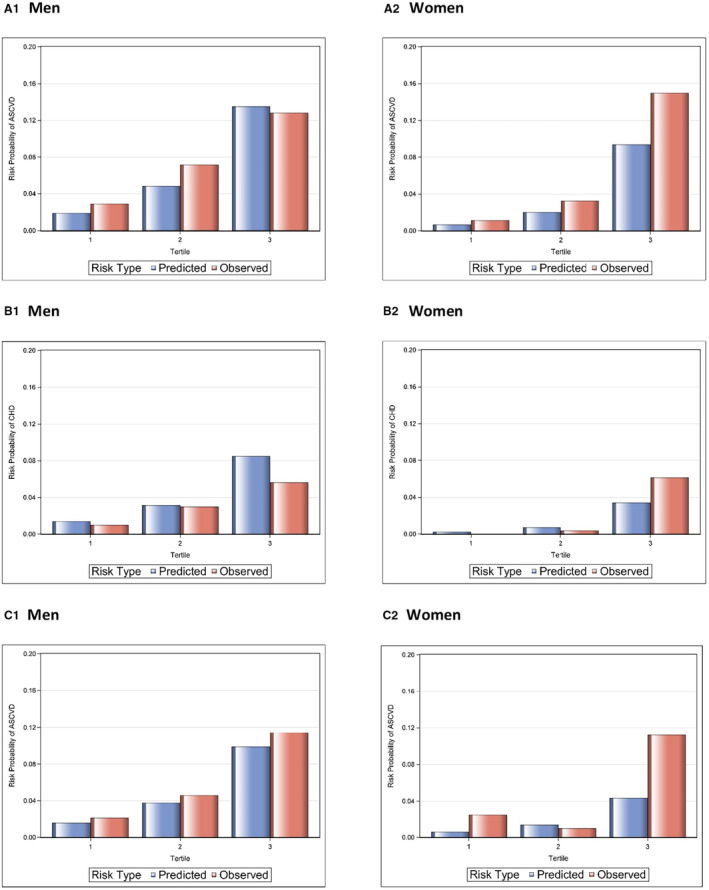
A1 and A2 are for ACC/AHA; B1 and B2 are for FHS CHD; C1 and C2 are for FHS CVD. ACC/AHA indicates American College of Cardiology/American Heart Association; FHS CHD, Framingham Heart Study hard coronary heart disease (function); and FHS CVD, Framingham Heart Study hard cardiovascular disease (function).
If the calibration indicated poor fit, recalibration was performed to attempt to improve the fit. For recalibration, the mean values of the risk functions and average incidence rates from the original ACC/AHA, FHS CHD, and FHS CVD functions were substituted with the values derived from the corresponding HIV cohort, 18 and calibration was reassessed.
Results
Baseline Characteristics
Baseline demographic and clinical characteristics are shown in Table 1. There were 9412 individuals overall, with 158 (1.7%) CHD events and 309 (3.3%) CVD events within 10 years from the index date. The overall group had a median age of 45.1 years and was 86.6% men, 17.5% Black individuals, and 16.9% Hispanic individuals. With regard to traditional CVD risk factors, 23.6% had hypertension, 31.7% had hyperlipidemia, 6.1% had diabetes, and 26.1% were smokers. HIV disease characteristics included a median CD4 cell count of 492 cells/mm3, median CD4 cell count nadir of 283 cells/mm3, and median HIV duration of 6.5 years before index date. Overall, 83.2% were on antiretroviral therapy, and 68% were virally suppressed.
Table 1.
Baseline Characteristics
| Overall | CVD event | No CVD event | P value* | |
|---|---|---|---|---|
| (N=9412) | (N=309) | (N=9103) | ||
| Demographics | ||||
| Age, y, median (quartile 1–quartile 3) | 45.1 (39.1–51.4) | 50.9 (45.3–57.1) | 45.0 (38.9–51.2) | <0.0001 |
| Male sex, n (%) | 8148 (86.6) | 272 (88.0) | 7876 (86.5) | 0.4454 |
| Other | 0.0166 | |||
| Black, n (%) | 1649 (17.5) | 62 (20.1) | 1587 (17.4) | |
| Hispanic, n (%) | 1586 (16.9) | 43 (13.9) | 1543 (17.0) | |
| White, n (%) | 4933 (52.4) | 176 (57.0) | 4757 (52.3) | |
| Other, n (%) | 857 (9.1) | 25 (8.1) | 832 (9.1) | |
| Unknown/missing, n (%) | 387 (4.1) | 3 (1.0) | 384 (4.2) | |
| Traditional CVD risk factors | ||||
| Hypertension, n (%) | 2219 (23.6) | 135 (43.7) | 2084 (22.9) | <0.0001 |
| Systolic blood pressure, median (quartile 1–quartile 3) | 122 (112–132) | 128 (116–139) | 122 (112–132) | <0.0001 |
| Diastolic blood pressure, median (quartile 1–quartile 3) | 76 (69–83) | 79 (71–87) | 76 (69–82) | <0.0001 |
| Hypertension treatment, n (%) | 2547 (27.1) | 140 (45.3) | 2407 (26.4) | <0.0001 |
| Hyperlipidemia, n (%) | 2984 (31.7) | 156 (50.5) | 2828 (31.1) | <0.0001 |
| Total cholesterol, median (quartile 1–quartile 3) | 179 (152–207) | 188 (152–220) | 179 (152–207) | 0.0037 |
| HDL cholesterol, median (quartile 1–quartile 3) | 42 (35–51) | 39 (33–48) | 42 (35–51) | <0.0001 |
| Statin treatment, n (%) | 1441 (15.3) | 92 (29.8) | 1349 (14.8) | <0.0001 |
| Diabetes mellitus, n (%) | 573 (6.1) | 42 (13.6) | 531 (5.8) | <0.0001 |
| Smoking, n (%) | 2461 (26.1) | 112 (36.2) | 2349 (25.8) | <0.0001 |
| CHD event, n (%) | 158 (1.7) | 158 (51.1) | 0 (0.0) | <0.0001 |
| HIV‐related factors | ||||
| CD4 count, median (quartile 1–quartile 3) | 492 (316–690) | 451 (268–665) | 495 (318–691) | 0.0146 |
| CD4 count nadir, median (quartile 1–quartile 3) | 283 (132–468) | 190 (64–344) | 286 (135–471) | <0.0001 |
| HIV RNA <400 (copies/mL), n (%) | 6356 (68.0) | 217 (71.1) | 6139 (67.9) | 0.2341 |
| Antiretroviral therapy, n (%) | 7833 (83.2) | 266 (86.1) | 7567 (83.1) | 0.1712 |
| NRTI, n (%)† | 7618 (97.3%) | 257 (96.6%) | 7361 (97.3%) | 0.5166 |
| NNRTI, n (%)† | 3871 (49.4%) | 95 (35.7%) | 3776 (49.9%) | <0.0001 |
| PI, n (%)† | 3281 (41.9) | 160 (60.2) | 3121 (41.2) | <0.0001 |
| INSTI, n (%)† | 1609 (20.5) | 40 (15.0) | 1569 (20.7) | 0.0238 |
| Other, n (%)† | 1450 (18.5) | 42 (15.8) | 1408 (18.6) | 0.2449 |
| Duration HIV, median (Q1, Q3) | 6.5 (1.3–14.1) | 9.7 (3.1–17.2) | 6.4 (1.3–14.0) | <0.0001 |
| Cohort characteristics | ||||
| Year of baseline, median (IQR) | 2009 (2006–2013) | 2008 (2005–2011) | 2009 (2006–2013) | <0.0001 |
| Follow‐up time, median (quartile 1–quartile 3) | 5.2 (2.4–10.0) | 4.3 (2.1–6.9) | 5.3 (2.4–10.0) | <0.0001 |
CHD indicates coronary heart disease; CVD, cardiovascular disease; HDL, high‐density lipoprotein; INSTI, integrase strand transfer inhibitor; IQR, interquartile range, NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; and PI, protease inhibitor.
Kruskal–Wallis test was used to generate P values for continuous variables, and χ2 test was used to generate P values for categorical variables. P values are for the comparison of individuals with a CVD event vs those with no CVD event.
Percent of those on antiretroviral therapy.
Compared with those who did not experience a CVD event, individuals who experienced an event were older (median age, 50.9 versus 45.0), were more likely to be men (88.0% versus 86.5%), were less likely to be Hispanic individuals (13.9% versus 17.0%), and had shorter median follow‐up time (4.3 versus 5.3 years). Individuals with CVD events were more likely to have hypertension (43.7% versus 22.9%), hyperlipidemia (50.5% versus 31.1%), and diabetes (13.6% versus 5.8%); were more likely to be smokers (36.2% versus 25.8%); were more likely to be on antihypertensive treatment (45.3% versus 26.4%) and statins (29.8% versus 14.8%); and had higher median systolic blood pressure (128 versus 122), diastolic blood pressure (79 versus 76), and total cholesterol (188 versus 179) and lower high‐density lipoprotein cholesterol (39 versus 42). In terms of HIV characteristics, individuals with CVD events had a longer median HIV duration (9.7 versus 6.4 years), higher rates of antiretroviral therapy use (86.1% versus 83.1%) and viral suppression (71.1% versus 67.9%), lower median CD4 cell count (451 versus 495 cells/mm3) and lower median CD4 nadir (190 versus 286 cells/mm3).
When baseline characteristics were assessed by cohort, there were 7260 individuals in the KPNC cohort and 2152 in the MGB cohort (Table S1). Individuals from KPNC were more likely to be Hispanic individuals (18.1% versus 12.6%), and individuals from MGB were more likely to be women (24.3% versus 10.2%) and Black individuals (26.5% versus 14.9%) and have longer median follow‐up time (7.0 versus 4.9 years). From a cardiovascular risk perspective, individuals from KPNC had higher rates of hyperlipidemia (33.4% versus 26.0%) and statin use (16.8% versus 10.3%), similar rates of hypertension (23.2% versus 24.7%), and lower rates of diabetes (5.5% versus 8.1%) and smoking (21.0% versus 43.6%). Individuals from KPNC had higher median CD4 cell counts (509 versus 438 cells/mm3) and median nadir CD4 cell counts (310 versus 181 cells/mm3) and a higher rate of HIV viral suppression (73.9% versus 48.3%).
Cardiovascular Risk Scores
The median follow‐up time, events, and CVD risk scores are shown in Table 2. For the ACC/AHA risk score, median predicted 10‐year event risk was 1.9% for women and 4.8% for men. Predicted risk for FHS CHD was 0.7% for women and 3.1% for men and for FHS CVD was 1.3% for women and 3.7% for men. CVD risk scores by cohort are shown in Table S2.
Table 2.
CVD Risk Scores: Median Follow‐Up, Events, Discrimination, and Calibration
| N | Median follow‐up, y (quartile 1–quartile 3) | Events | Median risk score (quartile 1–quartile 3) | Discrimination c‐statistic (95% CI) | Calibration χ 2 | Calibration P value | |
|---|---|---|---|---|---|---|---|
| ACC/AHA | |||||||
| Women | 810 | 6.40 (2.96–10.0) | 31 | 0.019 (0.009–0.046) | 0.780 (0.694–0.866) | 4.22 | 0.1210 |
| Men | 5926 | 5.39 (2.48–10.0) | 248 | 0.048 (0.025–0.087) | 0.706 (0.672–0.7404) | 10.55 | 0.005 |
| FHS CHD | |||||||
| Women | 1261 | 6.32 (2.85–10.0) | 15 | 0.007 (0.003–0.014) | 0.901 (0.846–0.956) | 3.43 | 0.1800 |
| Men | 8133 | 5.16 (2.34–10.0) | 143 | 0.031 (0.018–0.054) | 0.718 (0.673–0.763) | 24.12 | <0.0001 |
| FHS CVD | |||||||
| Women | 1261 | 6.24 (2.81–10.0) | 36 | 0.013 (0.008–0.026) | 0.784 (0.712–0.857) | 13.52 | 0.0012 |
| Men | 8133 | 5.09 (2.30–10.0) | 267 | 0.037 (0.021–0.065) | 0.723 (0.690–0.756) | 6.37 | 0.0413 |
ACC/AHA indicates American College of Cardiology/American Heart Association; CVD, cardiovascular disease; FHS CHD, Framingham Heart Study hard coronary heart disease (function); and FHS CVD, Framingham Heart Study hard caddiovascular disease (function).
Discrimination
Discrimination was assessed using the c‐statistic (Table 2). For women, discrimination was good for all 3 risk scores, with c‐statistics of 0.78 for ACC/AHA, 0.90 for FHS CHD, and 0.78 for FHS CVD. The c‐statistic for the FHS CHD risk score may have been impacted by the low number of events. For men, discrimination was moderate, with c‐statistics of 0.71 for ACC/AHA, 0.72 for FHS CHD, and 0.72 for FHS CVD. When evaluated within each cohort, discrimination remained good for women for both KPNC and MGB and moderate for men for both sites (Table S2).
Calibration
Calibration, or goodness of fit for the models, was assessed by the Demler/D'Agostino–Nam χ 2 statistic and P values, using tertiles of predicted risk (Table 2). For women, calibration was poor for the FHS CVD risk score, with a χ 2 P value of 0.001. For men, calibration was poor for all 3 functions, with P values of 0.005 for ACC/AHA, <0.001 for FHS CHD, and 0.04 for FHS CVD. Calibration was similarly poor for all groups able to be assessed when evaluated separately by cohort (Table S2).
We recalibrated all of the functions to try to improve model fit by using baseline survival and mean risk factor values from the combined HIV cohort instead of the original ACC/AHA, FHS CHD, or FHS CVD cohorts' values. Recalibration did not significantly improve model fit, with the exception of the FHS CHD risk score in men (Demler/D'Agostino–Nam P=0.224 for recalibrated score, increased from P<0.0001 before recalibration) and the ACC/AHA risk score in women (P=0.892 for recalibrated score, increased from P=0.121 before recalibration). All other recalibration P values were <0.01.
Observed Versus Predicted Risk
When observed and predicted 10‐year risk of event for each risk factor model were compared, there was a general pattern of underestimating risk, although differences between observed and predicted risk varied depending on the tertile of baseline risk, specific risk function, and sex (Figure). Among women, CVD risk was underestimated by all 3 risk functions for most tertiles of predicted risk. Among men, CVD risk was underestimated by the ACC/AHA function among the lower and middle predicted risk tertiles and underestimated for all tertiles for the FHS CVD function. For the FHS CHD function, risk was overestimated, with predicted risk exceeding observed risk. Predicted risk, observed risk, and risk ratios for each tertile by function are shown in Table 3; observed versus predicted plots are shown in the Figure.
Table 3.
Observed and Predicted Risk by Tertile
| ACC/AHA | FHS CHD | FHS CVD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |
| Men* | |||||||||
| Predicted risk | 0.019 | 0.049 | 0.135 | 0.014 | 0.031 | 0.085 | 0.016 | 0.038 | 0.099 |
| Observed risk | 0.029 | 0.072 | 0.128 | 0.010 | 0.030 | 0.056 | 0.022 | 0.046 | 0.115 |
| Predicted‐to‐observed risk ratio | 0.66 | 0.68 | 1.05 | 1.41 | 1.03 | 1.52 | 0.73 | 0.83 | 0.86 |
| Women† | |||||||||
| Predicted risk | 0.007 | 0.020 | 0.094 | 0.002 | 0.007 | 0.034 | 0.006 | 0.014 | 0.043 |
| Observed risk | 0.011 | 0.033 | 0.150 | NA | 0.004 | 0.061 | 0.025 | 0.010 | 0.113 |
| Predicted‐to‐observed risk ratio | 0.64 | 0.61 | 0.63 | NA | 1.75 | 0.56 | 0.24 | 1.40 | 0.38 |
ACC/AHA indicates American College of Cardiology/American Heart Association; FHS CHD, Framingham Heart Study hard coronary heart disease (function); and FHS CVD, Framingham Heart Study hard caddiovascular disease (function).
Demler/D'Agostino–Nam χ 2 (P‐value) for men: χ 2=10.55 (P=0.005) for ACC/AHA; χ 2=24.12 (P<0.0001) for FHS CHD; χ 2=6.37 (P=0.0413) for FHS CVD.
Demler/D'Agostino–Nam χ 2 (P‐value) for women: χ 2=4.22 (P=0.1210) for ACC/AHA; χ 2=3.43 (P=0.1800) for FHS CHD; χ 2=13.52 (P=0.0012) for FHS CVD.
Comparison of Coefficients
For each risk score, regression coefficients were compared qualitatively between the original function and those generated for the same risk factors in the HIV data set (both cohorts combined) among women and men (Table S3). For the majority of coefficients, the magnitude and direction of the association with the outcome of the specific function were similar for the original function and those generated from the HIV data set.
Assessment of CVD Risk Scores in Individuals Without HIV
We assessed model fit in a matched control cohort of individuals without HIV (Table S1). Calibration was poor for women and men for all 3 risk scores, with significant χ 2 P values, with the exception of the FHS CVD score for women in the control group. In the control group, recalibration improved model fit for women and men for the FHS CHD score. Observed and predicted 10‐year risk of event for each risk score were assessed in the control group. There was a general pattern of overestimating risk, with predicted events exceeding observed events in the upper tertile of risk across all 3 functions for women, with the exception of FHS CVD risk score (Table S4).
Discussion
Our findings expand upon a growing body of literature demonstrating that CVD risk prediction algorithms designed for the general population underperform in PWH, systematically underestimating risk in this population. Assessing 3 widely used CVD risk prediction functions in a multisite HIV cohort representing discrete regions and care settings, we found that established risk functions fit poorly and underestimate risk, particularly among women, and that this finding persists regardless of baseline risk. To our knowledge, our study was the first to evaluate the performance of FHS CVD risk prediction functions in PWH using sex‐specific analyses. The findings suggest that current models to predict CVD risk in PWH are likely to result in disparities in CVD preventative care due to underestimation of risk, underscoring the urgent need for tailored CVD risk prediction strategies for PWH.
The rationale for suboptimal performance of CVD risk prediction models in HIV is based on the increasingly well‐delineated mechanism of HIV‐associated CVD. 37 , 38 , 39 , 40 While traditional CVD risk factors are present at heightened rates in PWH and likely contribute to the observed increased risk of CVD events, 2 , 6 , 7 there is still excess CVD risk that is unexplained by established risk factors alone. 12 , 15 , 41 Inflammation and immune dysregulation in PWH have been associated with subclinical markers of coronary atherosclerosis and with coronary inflammation. 42 , 43 , 44 Moreover, clinical correlates that reflect inflammation and immune activation (CD4 cell count and HIV RNA) have been linked to increased MI and stroke event rates. 3 , 45 , 46 The metabolic effects of antiretroviral therapy are being increasingly delineated and may contribute to CVD risk both through potentiation of traditional risk factors and through separate mechanisms. 47 , 48 While factors specific to HIV infection and its associated inflammatory state contribute to CVD risk, none of these factors are reflected in established risk prediction models, which were designed in populations that differ demographically and clinically from HIV groups. 18 , 23 The predicted risk generated from existing models consequently reflects only a subset of risk factors and incompletely represents the multifactorial mechanism of CVD in HIV.
Previous studies have demonstrated consistent underestimation of risk when traditional risk prediction functions have been evaluated in PWH. A large study from the Center for AIDS Research Network of Integrated Clinical Systems found that the pooled cohort equations (ACC/AHA) underestimated risk, especially among women and Black individuals and those of low or intermediate predicted risk. 26 Another US‐based study from the HOPS (HIV Outpatient Study) cohort found that the pooled cohort equations and Data Collection on Adverse Effects of Anti‐HIV Drugs Study (D:A:D) equations underestimated risk by nearly 20%. 27 Most recently, a study from the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort found that while all models discriminated well, the pooled cohort equations, Systematic COronary Risk Evaluation (SCORE), and D:A:D models underestimated risk in low/intermediate‐risk individuals. 28 Previous data from our cohort, evaluating men with a follow‐up time of 5 years, demonstrated poor fit for 3 established models including the ACC/AHA and 2 FHS models, with observed CVD rates exceeding predicted rates among the majority of risk groups. 29 While prior investigations have been largely consistent in their underestimation of risk, gaps in knowledge remain, and the present study adds new and clinically relevant knowledge to this growing body of literature. Notably, the largest US‐based study to date evaluating CVD risk functions in HIV was not able to include stroke events in the composite CVD end point. Our study evaluates 2 risk scores that include stroke in the composite outcome and has rigorous outcome ascertainment for stroke events in addition to MI events. Other prior studies to date have not evaluated CVD risk scores separately in women and men. Our study significantly expands on prior work from this cohort as well as previous studies from other cohorts by providing the first sex‐specific analyses of FHS risk prediction functions in PWH. In the current study, we expand upon previous findings with data encompassing 2 cohorts that separately evaluates women and has a longer follow‐up period to approximate that used to develop the original risk prediction models. Our results reinforce the finding that the performance of established risk prediction models is suboptimal in an HIV population. Calibration, as assessed by Demler/D'Agostino–Nam χ 2 test, was poor for men with HIV among all functions evaluated and for women for the FHS CVD risk score, indicating that these models do not fit well in an HIV population and underestimate risk for the majority of groups. Discrimination, or the model's capacity to order risk, was assessed using the c‐statistic and was demonstrated to be moderate overall for men and good for women.
Findings from sex‐specific analyses of the performance of CVD risk prediction functions highlight the importance of tailored risk estimation strategies in HIV‐associated CVD among women. Results from analyses assessing calibration demonstrate poor model fit for all 3 functions among men and better fit among women for the ACC/AHA and FHS CHD functions. However, when observed versus predicted events were compared, poor fit among men was explained by overestimation of risk for many groups. In contrast, among women, risk was more consistently underestimated, with observed events exceeding predicted events in most risk groups across baseline risk and function. These findings can be interpreted in the context of differences in HIV‐associated CVD among women. Studies have shown that while absolute risk of CVD events remains lower for women compared with men living with HIV, relative risk is increased among women, 2 , 49 with earlier data from the MGB HIV cohort showing a relative risk of MI comparing HIV to non‐HIV of 2.98 for women versus 1.40 for men. 2 Biomarkers of inflammation have been shown to be increased in women living with HIV compared with men, 50 , 51 and inflammatory and immune biomarkers have been shown to be associated with coronary plaque and disease in women with HIV, 51 , 52 with some biomarkers showing stronger associations with CVD outcomes in women compared with men. 50 If underestimation of CVD risk in PWH is explained by key missing risk factors in risk scores, including inflammation, then the fact that women have higher levels of inflammation that relate to CVD outcomes is consistent with greater underestimation of risk compared with men with HIV. Our findings underscore the importance of both HIV‐ and sex‐specific CVD risk estimation strategies.
The characteristics of patients on the basis of CVD event status suggest that both traditional and nontraditional risk factors contribute to HIV‐associated CVD. As anticipated, individuals with CVD events had more traditional risk factors: older age; higher rates of hypertension, hyperlipidemia, diabetes, and smoking; higher blood pressure and total cholesterol and lower high‐density lipoprotein cholesterol. Yet the characteristics of individuals who experienced an event also reflect the HIV‐specific mechanistic factors that drive excess CVD risk: a longer duration of HIV infection likely reflecting chronic inflammation and lower CD4 and nadir CD4 cell counts reflecting more pronounced immune dysregulation. Interestingly, while higher HIV viral load has been associated with CVD events, 3 , 45 , 53 those with an outcome event in our study had higher rates of viral suppression. The measure of viral suppression used in the analysis was made at baseline, not at the time of the event; thus, changes in viral suppression over time could partially explain this finding.
Overall, our study indicates that current CVD risk prediction scores could be improved to estimate risk more accurately in PWH. While the models evaluated performed modestly when ordering risk, they were poor for most groups when matching predicted to observed risk, indicating that existing models are not reliable for accurate clinical risk estimation. Importantly, the majority of individuals in the study were in lower or moderate predicted risk categories, as indicated by median calculated risk score, in which there is clinical uncertainty about indications for CVD risk factor interventions and in which accurate risk estimation is thus critical. Moreover, while CVD risk score model fit was also poor when evaluated in a control group, the patterns of over‐ and underestimation differed from those in the HIV group, with more overestimation of risk in the control group. These results suggest that established risk scores are not well calibrated for many cohorts of patients including PWH.
These findings further underscore the need for tailored CVD risk scores for PWH, which could be developed through integration of HIV‐specific CVD risk factors (eg, HIV RNA, CD4 cell count nadir, antiretroviral medications linked to cardiometabolic risk) with traditional CVD risk factors in new functions.
Our study design reflects many strengths, including a study population drawn from 2 large, well‐established HIV cohorts that represent clinically and geographically distinct populations and highly accurate outcome ascertainment. Limitations of our study include low event numbers among women, limiting conclusions for the FHS CHD risk prediction model in particular. Additionally, outcome ascertainment may have been limited by decreased capture of out‐of‐system events at MGB, introducing potential bias, although capture of more observed events would further strengthen the finding of underestimation of risk. KPNC is a closed system, thus ensuring near complete ascertainment.
In conclusion, we found established CVD risk prediction functions to systematically underestimate risk in PWH. In our study encompassing 2 established HIV cohorts, risk estimation differed across subgroups and risk functions, but model fit was overall poor, with greater underestimation of risk among women. Our findings underscore the importance of developing CVD risk prediction functions tailored to PWH, in which CVD risk factor profiles do not match those of the general population and further tailored to women with HIV. Application of current risk prediction paradigms for PWH is likely to underestimate risk and thus impact clinical decision making, resulting in potential missed opportunities for risk modification and counseling. Adapting CVD risk prediction models to better reflect the risk factor profiles of PWH will help to ensure equity in the delivery of CVD preventative care.
Sources of Funding
This work was supported by the National Institutes of Health (R01HL132786 to Drs Triant and D'Agostino).
Disclosures
Dr Lo has served as a consultant for Viiv Healthcare. Dr Grinspoon has received grants to Institution from Gilead, KOWA, Viiv, none related to this project; and has consulted for Theratechnologies unrelated to this project. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Acknowledgments
The authors are grateful to the Partners HealthCare Research Patient Data Registry group for facilitating use of their database. Additional Information: Coauthor Joseph M. Massaro, PhD, died October 19, 2022.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029228
For Sources of Funding and Disclosures, see page 9.
References
- 1. Currier JS. Cardiovascular risk associated with HIV therapy. J Acquir Immune Defic Syndr. 2002;31:S16–S23. doi: 10.1097/00126334-200209011-00004 [DOI] [PubMed] [Google Scholar]
- 2. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation. 2018;138:1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179 [DOI] [PubMed] [Google Scholar]
- 6. Friis‐Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d'Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. [DOI] [PubMed] [Google Scholar]
- 7. Grinspoon S, Carr A. Cardiovascular risk and body‐fat abnormalities in HIV‐infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811 [DOI] [PubMed] [Google Scholar]
- 8. Hadigan C, Meigs JB, Corcoran C, Rietschel P, Piecuch S, Basgoz N, Davis B, Sax P, Stanley T, Wilson PW, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541 [DOI] [PubMed] [Google Scholar]
- 9. Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978 [DOI] [PubMed] [Google Scholar]
- 10. Seaberg EC, Munoz A, Lu M, Detels R, Margolick JB, Riddler SA, Williams CM, Phair JP. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8 [DOI] [PubMed] [Google Scholar]
- 11. Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV‐1‐infected individuals: a nationwide, population‐based cohort study. Clin Infect Dis. 2013;56:727–734. doi: 10.1093/cid/cis933 [DOI] [PubMed] [Google Scholar]
- 12. Strategies for Management of Antiretroviral Therapy Study G , El‐Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, et al. CD4+ count‐guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 13. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV‐infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, Williams I, Drummond F, Duprez D, Belloso WH, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV‐1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215 [DOI] [PubMed] [Google Scholar]
- 16. Triant VA, Grinspoon SK. Immune dysregulation and vascular risk in HIV‐infected patients: implications for clinical care. J Infect Dis. 2011;203:439–441. doi: 10.1093/infdis/jiq084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.CIR.83.1.356 [DOI] [PubMed] [Google Scholar]
- 18. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; Group CHDRP . Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180 [DOI] [PubMed] [Google Scholar]
- 19. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 20. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 21. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 22. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.STR.22.3.312 [DOI] [PubMed] [Google Scholar]
- 23. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task Force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 24. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task Force on practice guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 25. Bibbins‐Domingo K; Force USPST . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task Force recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 26. Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, Budoff MJ, Mathews WC, Kitahata MM, Saag MS, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the centers for AIDS research network of integrated clinical systems. JAMA Cardiol. 2017;2:155–162. doi: 10.1001/jamacardio.2016.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thompson‐Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr, Skarbinski J, Chmiel JS, Hart R, Wei SC, Loustalot F, Brooks JT, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis. 2016;63:1508–1516. doi: 10.1093/cid/ciw615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Zoest RA, Law M, Sabin CA, Vaartjes I, van der Valk M, Arends JE, Reiss P, Wit FW. Predictive performance of cardiovascular disease risk prediction algorithms in people living with HIV. J Acquir Immune Defic Syndr. 2019;81:562–571. doi: 10.1097/QAI.0000000000002069 [DOI] [PubMed] [Google Scholar]
- 29. Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, D'Agostino RB Sr. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137:2203–2214. doi: 10.1161/CIRCULATIONAHA.117.028975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 31. Silverberg MJ, Leyden W, Hurley L, Go AS, Quesenberry CP Jr, Klein D, Horberg MA. Response to newly prescribed lipid‐lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150:301–313. doi: 10.7326/0003-4819-150-5-200903030-00006 [DOI] [PubMed] [Google Scholar]
- 32. Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, Hurley LB, Marcus JL, Quesenberry CP Jr, Silverberg MJ. Declining relative risk for myocardial infarction among HIV‐positive compared with HIV‐negative individuals with access to care. Clin Infect Dis. 2015;60:1278–1280. doi: 10.1093/cid/civ014 [DOI] [PubMed] [Google Scholar]
- 33. Regan S, Meigs JB, Grinspoon SK, Triant VA. Determinants of smoking and quitting in HIV‐infected individuals. PLoS One. 2016;11:e0153103. doi: 10.1371/journal.pone.0153103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610 [DOI] [PubMed] [Google Scholar]
- 35. D'Agostino RB, Nam BH. Evaluation of the Performance of Survival Analysis Models: Discrimination and Calibration Measures. Vol 23. Elsevier Science B.V; 2004. [Google Scholar]
- 36. Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness‐of‐fit in the survival setting. Stat Med. 2015;34:1659–1680. doi: 10.1002/sim.6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feinstein MJ. HIV and cardiovascular disease: from insights to interventions. Top Antivir Med. 2021;29:407–411. [PMC free article] [PubMed] [Google Scholar]
- 38. So‐Armah K, Benjamin LA, Bloomfield GS, Feinstein MJ, Hsue P, Njuguna B, Freiberg MS. HIV and cardiovascular disease. Lancet HIV. 2020;7:e279–e293. doi: 10.1016/S2352-3018(20)30036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. So‐Armah K, Freiberg MS. HIV and cardiovascular disease: update on clinical events, special populations, and novel biomarkers. Curr HIV/AIDS Rep. 2018;15:233–244. doi: 10.1007/s11904-018-0400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019;140:e98–e124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. doi: 10.1097/QAD.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, Feldpausch MN, Martin A, Melbourne K, Triant VA, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment‐naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016;1:474–480. doi: 10.1001/jamacardio.2016.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein‐Grobusch K. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One. 2017;12:e0169986. doi: 10.1371/journal.pone.0169986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS. 2016;11:216–225. doi: 10.1097/COH.0000000000000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV‐infected and non‐HIV‐infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–358. doi: 10.1097/QAI.0b013e31825c7f24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Friis‐Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El‐Sadr W, Thiebaut R, De Wit S, Kirk O, Fontas E, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744 [DOI] [PubMed] [Google Scholar]
- 48. Elion RA, Althoff KN, Zhang J, Moore RD, Gange SJ, Kitahata MM, Crane HM, Drozd DR, Stein JH, Klein MB, et al. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr. 2018;78:62–72. doi: 10.1097/QAI.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. doi: 10.1097/QAD.0b013e328339192f [DOI] [PubMed] [Google Scholar]
- 50. Schnittman S, Beck‐Engeser G, Shigenaga J, Ahn H, Nance R, York V, Delaney J, Heckbert S, Tirschwell D, Chow F, et al. Sex modifies the association between inflammation and vascular events in treated HIV. Paper Presented at: Conference on Retroviruses and Opportunistic Infection. 2021. Virtual.
- 51. Zanni MV, Foldyna B, McCallum S, Burdo TH, Looby SE, Fitch KV, Fulda ES, Autissier P, Bloomfield GS, Malvestutto CD, et al. Sex differences in subclinical atherosclerosis and systemic immune activation/inflammation among people with human immunodeficiency virus in the United States. Clin Infect Dis. 2023;76:323–334. doi: 10.1093/cid/ciac767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in HIV‐infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lang S, Mary‐Krause M, Simon A, Partisani M, Gilquin J, Cotte L, Boccara F, Costagliola D. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV‐infected individuals. Clin Infect Dis. 2012;55:600–607. doi: 10.1093/cid/cis489 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


