Abstract
Background
Although tafamidis treatment improves prognosis in patients with wild‐type transthyretin amyloid cardiomyopathy, an optimal surrogate marker monitoring its therapeutic effect remains unclear. This study investigated the association between changes in cardiac biomarkers, high‐sensitivity cardiac troponin T (hs‐cTnT) and B‐type natriuretic peptide (BNP) during the first year after tafamidis treatment and clinical outcomes.
Methods and Results
In 101 patients with wild‐type transthyretin amyloid cardiomyopathy receiving tafamidis at our institution, change in cardiac biomarkers from baseline to 1 year after tafamidis administration and its association with composite outcomes (composite of all‐cause death and hospitalization attributable to heart failure) was assessed. During the follow‐up period (median, 17 months), 16 (16%) patients experienced composite outcomes. The hs‐cTnT level significantly decreased at 1 year after tafamidis treatment, unlike the BNP level. The frequencies of increased hs‐cTnT and BNP levels were significantly higher in those with composite outcomes than in those without (44% versus 15%; P=0.01). Kaplan‐Meier survival analysis showed that patients in whom both hs‐cTnT and BNP levels increased at 1 year after tafamidis had a higher probability of composite outcomes compared with those with decreased hs‐cTnT and BNP levels (log‐rank P<0.01). Cox regression analysis identified increased hs‐cTnT and BNP levels at 1 year after tafamidis administration as an independent predictor of higher cumulative risk of composite outcomes.
Conclusions
Deterioration in cardiac biomarkers during the first year after tafamidis treatment predicted a worse prognosis, suggesting the utility of serial assessment of cardiac biomarkers for monitoring the therapeutic response to tafamidis in patients with wild‐type transthyretin amyloid cardiomyopathy.
Keywords: B‐type natriuretic peptide, high‐sensitivity cardiac troponin T, tafamidis, wild‐type transthyretin amyloid cardiomyopathy
Subject Categories: Cardiomyopathy, Heart Failure
Nonstandard Abbreviations and Acronyms
- ATTR‐CM
transthyretin amyloid cardiomyopathy
- ATTRwt‐CM
wild‐type transthyretin amyloid cardiomyopathy
- ECV
extracellular volume
- hs‐cTnT
high‐sensitivity cardiac troponin T
- LV‐GLS
left ventricular global longitudinal strain
Clinical Perspective.
What Is New?
The association between change in cardiac biomarkers, high‐sensitivity cardiac troponin T, and B‐type natriuretic peptide during the first year after tafamidis treatment in patients with wild‐type transthyretin amyloid cardiomyopathy and clinical outcomes was investigated.
Deterioration in cardiac biomarkers, especially both high‐sensitivity cardiac troponin T and B‐type natriuretic peptide, during the first year after tafamidis treatment predicted worse clinical outcomes.
What Are the Clinical Implications?
Among the disease‐related parameters of wild‐type transthyretin amyloid cardiomyopathy, a combination assessment of both high‐sensitivity cardiac troponin T and B‐type natriuretic peptide levels during tafamidis treatment may enable a higher prediction accuracy of clinical outcomes.
Identifying “nonresponders to tafamidis” may be helpful in selecting more potent antiamyloid treatments.
Transthyretin amyloid cardiomyopathy (ATTR‐CM) is an increasingly recognized cause of progressive heart failure, resulting from amyloid fibril deposition into the myocardium. Tafamidis, the first approved disease‐modifying therapy for wild‐type ATTR‐CM (ATTRwt‐CM), stabilizes the tetrameric form of the transthyretin and inhibits its disintegration into transthyretin monomers and aggregation into amyloid fibrils. In the ATTR‐ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial), long‐term use of tafamidis improved prognosis and reduced cardiovascular‐related hospitalization in patients with ATTRwt‐CM, 1 leading to its approval for treating ATTRwt‐CM in March 2019 in Japan. Although recent studies have validated its therapeutic effect to improve ATTRwt‐CM prognosis, 2 , 3 an optimal surrogate marker for monitoring the therapeutic effect of tafamidis treatment remains unclear.
Accumulated evidence has suggested the utility of noninvasive disease‐related parameters of ATTRwt‐CM, including native T1 and extracellular volume (ECV), on cardiac magnetic resonance, 4 heart/contralateral ratio on bone scintigraphy, 5 left ventricular global longitudinal strain (LV‐GLS) on echocardiography, 6 and cardiac biomarkers. 7 Among them, cardiac biomarker evaluation can be easily performed with inexpensive cost, irrespective of hospital scales, unlike radiographic parameters. Hence, biomarker measurement may be more suitable for serial and accurate assessment of tafamidis‐mediated therapeutic effect. The elevated cardiac biomarker levels, including high‐sensitivity cardiac troponin T (hs‐cTnT) and B‐type natriuretic peptide (BNP), were identified as established predictors for poor prognosis in patients treated with tafamidis 3 and treatment‐naïve 7 , 8 patients with ATTRwt‐CM. We previously reported that the serum hs‐cTnT level decreased at 1 year after tafamidis administration in patients with ATTRwt‐CM, whereas an increase in hs‐cTnT level was observed in patients with ATTRwt‐CM not receiving tafamidis. 3 However, the association of serial changes in cardiac biomarkers, hs‐cTnT and BNP, during the course of tafamidis treatment with long‐term prognosis in patients with ATTRwt‐CM necessitates further investigation.
This study aimed to investigate the prognostic impact of cardiac biomarker change during the first year after the administration of tafamidis on prognosis and elucidate its utility as a surrogate marker in predicting long‐term prognosis of patients treated with tafamidis with ATTRwt‐CM.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
The study flowchart is shown in Figure 1. In this single‐center retrospective observational study, 116 consecutive patients diagnosed with ATTRwt‐CM and started on tafamidis at Kumamoto University Hospital between March 2019 and October 2022 were enrolled. Of these, 15 patients were excluded for the following reasons: discontinuation of tafamidis during the follow‐up period (n=8), participation in a clinical trial of novel ATTR‐CM treatment other than tafamidis (n=1), and missing data of hs‐cTnT and BNP levels at 1 year follow‐up (n=6; 4 patients died within 1 year after tafamidis commencement). Consequently, the remaining 101 patients with ATTRwt‐CM were finally analyzed. Transthyretin amyloid deposition was confirmed by cardiac biopsy in 86 (85%) patients, gastrointestinal tract biopsy in 8 (8%) patients, skin biopsy in 4 (4%) patients, and removed tenosynovium tissue for carpal tunnel release in 3 (3%) patients. Amyloid deposition was confirmed by Congo‐red staining and apple‐green birefringence examination under cross‐polarized light microscopy. Identification of the amyloid precursor protein was performed using the immunohistochemical staining method. For all patients, the absence of TTR gene mutations was confirmed by genetic test.
Figure 1. Flowchart of this study.
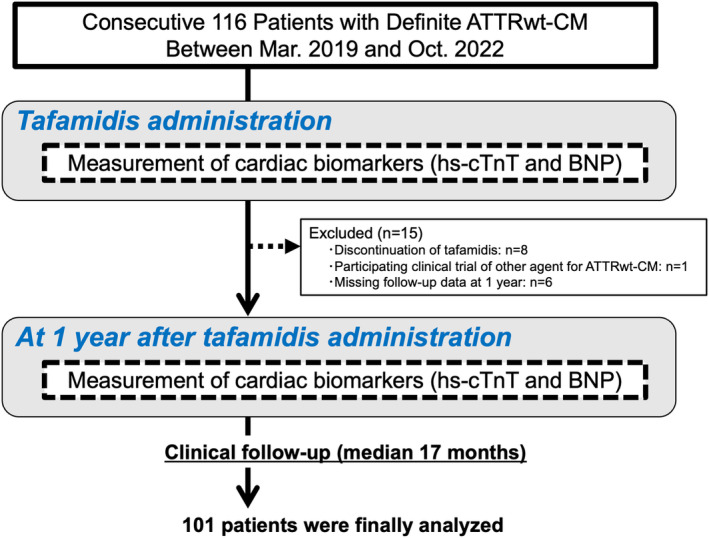
ATTRwt‐CM indicates wild‐type transthyretin amyloid cardiomyopathy; BNP, B‐type natriuretic peptide; and hs‐cTnT, high‐sensitivity cardiac troponin T.
The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and its amendments. The Human Ethics Committee of Kumamoto University approved the study protocol (No. 1590). Informed consent from patients was waived because of the low‐risk nature of our retrospective study and inability to directly obtain consent from all participants. This study protocol was extensively promoted at Kumamoto University Hospital and on our website (available at: http://www.kumadai‐junnai.com) and provided patients the opportunity to withdraw from the study.
Clinical Follow‐Up Protocol and Prognosis
Baseline clinical demographics, including medical history, laboratory data, echocardiography, and medication use for treating heart failure at the time of tafamidis commencement, were obtained. Clinical follow‐up at 1 year after tafamidis commencement included the collection of hs‐cTnT and BNP levels, echocardiographic parameters, and information on medication use for treating heart failure. According to the change in the hs‐cTnT and BNP levels at 1 year after tafamidis administration, the participants were assigned to subgroups using 2 methods. First, they were assigned to the hs‐cTnT and BNP increase or decrease groups independent of the degree of change. Second, they were divided into the increase (percentage change in the hs‐cTnT or BNP level >10%), stable (percentage change in the hs‐cTnT or BNP level between −10% and 10%), and decrease (percentage change in the hs‐cTnT or BNP <−10%) groups.
Clinical events during the follow‐up period, since the measurement of hs‐cTnT and BNP levels at 1 year after tafamidis commencement, were collected according to medical records and telephone interview with the patient or family member. The incidence of adverse events, including all‐cause death and hospitalization attributable to heart failure, was assessed. Composite outcomes were defined as a combination of all‐cause death and hospitalization attributable to heart failure. Hospitalization attributable to heart failure was defined as an unplanned or emergent hospitalization attributable to heart failure, for which enrichment of heart failure treatment, including intravenous administration of diuretics, was required. According to this definition, whether a hospitalization was attributable to heart failure or other causes was determined by each cardiologist, who was not blinded to the patient's clinical data to ensure appropriate management for the patient. The association between serial change in cardiac biomarkers before and 1 year after tafamidis treatment and the occurrence of clinical events during the follow‐up period was assessed. Enrichment of heart failure treatment was defined as a dose increase or addition of diuretics (loop and thiazide diuretics and tolvaptan), or mineralocorticoid receptor antagonists at 1 year after tafamidis treatment from the baseline. The dose of loop diuretics was described by the furosemide dose. In cases receiving azosemide, 30 mg azosemide was considered to be equivalent to 20 mg furosemide. 9
Measurement of Cardiac Biomarkers and Radiographic Parameters
Plasma BNP concentrations were measured using the MI02 Shionogi BNP kit from Abbott Japan (Matsudo, Japan). Serum hs‐cTnT levels were measured using the Elecsys 2010 Troponin T HS kit purchased by Roche Diagnostics (Indianapolis, IN). The patients were stratified into the high‐ or low‐risk group according to the biomarker staging system we previously reported in patients with ATTRwt‐CM using hs‐cTnT, BNP, and estimated glomerular filtration rate. 8 Echocardiography was performed using Epiq 7G (Philips, Bothell, WA). LV‐GLS was assessed using TomTec (TomTec Imaging Systems, Munich, Germany), a postprocessing software. Left ventricular chamber size, wall thickness, left atrial diameter, and left ventricular ejection fraction (LVEF) were measured using transthoracic echocardiography. LVEF was calculated using the modified Simpson method.
Cardiac magnetic resonance studies were performed using a 3.0‐T magnetic resonance imaging scanner (Ingenia CX, R5.4; Philips Healthcare, Best, the Netherlands). T1 mapping in a midventricular short‐axis section of the left ventricle was performed using the modified Look‐Locker inversion recovery sequence to analyze the native T1 value (normal value in our institution, 1200–1260 ms). ECV value was calculated using ECV maps produced from analyzing precontrast and postcontrast images with a postprocessing workstation (Ziostation2; Ziosoft, Tokyo, Japan), as previously described. 10 99mTc‐labeled pyrophosphate scintigraphy was performed using a GE Discovery 670 dual‐headed single‐photon emission computed tomography camera with low‐energy, high‐resolution collimators (GE Healthcare, Waukesha, WI). Three hours after radiotracer administration, anterior and lateral planar heart views were obtained. Cardiac uptake of 99mTc‐labeled pyrophosphate was evaluated by both planar and single‐photon emission computed tomography images. The heart/contralateral ratio was quantitatively calculated via the total counts in a region of interest over the heart to background counts in a similarly sized region of interest over the contralateral chest.
Statistical Analysis
Continuous variables were expressed as mean±SD and compared using the t‐test when data exhibited a normal distribution. Nonnormally distributed continuous data were presented as median (interquartile range) and compared using the Wilcoxon signed‐rank test. Categorical data were expressed as frequencies and percentages, and compared using the χ2 test. Clinical data at baseline and at 1 year after tafamidis administration were compared by linear mixed‐effect model if the variables were normally distributed and by Wilcoxon signed‐rank test if the variables were nonnormally distributed. Because the data for hs‐cTnT and BNP levels were nonnormally distributed, they were logarithmically transformed. Paired cartegorical data were compared using the McNemar test. Kaplan‐Meier survival analysis was conducted to compare the composite outcome‐free survival rate during the follow‐up period between the divided groups depending on the serial biomarker change from baseline to 1 year after tafamidis administration. Furthermore, Cox regression analysis was conducted to compute the hazard ratio (HR) and 95% CI as estimates of the incidence of composite outcomes and evaluate the association between the biomarker change and appearance of composite outcomes. Proportional hazards assumption was confirmed by log‐minus‐log analysis using SPSS, version 26 (IBM Corp, Armonk, NY). We selected covariates previously reported to be associated with prognosis of ATTR‐CM: hs‐cTnT, 11 BNP, 8 LVEF, 6 LV‐GLS, 6 native T1, 4 and ECV. 4 Because continuous variables' effects in the Cox regression analysis for composite outcomes were represented by nonlinear functions, we conducted multivariable models using restricted cubic splines analysis to estimate the nonlinear covariates' effects. Restricted cubic splines analysis was performed by R, version 4.0.5 (R Foundation for Statistical Computing). Other statistical analyses were performed using JMP, version 17 (SAS Institute, Cary, NC). Statistical significance was set at P<0.05.
Results
Baseline Demographics and Serial Changes in Cardiac Biomarkers After Tafamidis Treatment
During the median follow‐up period of 17 months (interquartile range, 10–25 months) since the 1 year follow‐up after tafamidis administration, 16 (16%) patients had composite outcomes. Baseline demographics of all patients in this study and comparison of the baseline demographics among the patients with and without composite outcomes are shown in Table 1. The mean±SD age of all participants was 75.2±5.3 years, and 86% of patients were men. One‐third of patients had a history of prior hospitalization attributable to heart failure. The median values of the hs‐cTnT and BNP level at baseline were 0.049 ng/mL and 167 pg/mL, respectively. The one‐third of patients were categorized as the high‐risk group based on the biomarker staging system. A total of 70 (69%) patients received diuretics. Compared with patients without composite outcomes, those with composite outcomes had significantly lower body mass index and estimated glomerular filtration rate; had a higher prevalence of New York Heart Association class III and history of heart failure hospitalization; and had higher hs‐cTnT, BNP, and ECV levels.
Table 1.
Baseline Demographics of All Participants
| Variable | Overall (n=101) | Composite outcomes (+) (n=16) | Composite outcomes (−) (n=85) | P value |
|---|---|---|---|---|
| Age, y | 75.2±5.3 | 76.8±6.3 | 75.0±5.0 | 0.22 |
| Male sex | 87 (86) | 13 (81) | 74 (87) | 0.55 |
| Body mass index, kg/m2 | 23.3±3.2 | 21.8±2.0 | 23.6±3.3 | 0.04 |
| NYHA≧III | 30 (30) | 9 (56) | 21 (25) | 0.01 |
| Prior heart failure hospitalization | 30 (30) | 9 (56) | 21 (25) | 0.01 |
| Hypertension | 50 (50) | 6 (38) | 44 (52) | 0.30 |
| Diabetes | 26 (26) | 7 (44) | 19 (22) | 0.07 |
| Atrial fibrillation | 56 (55) | 10 (63) | 46 (54) | 0.54 |
| hs‐cTnT, ng/mL | 0.049 (0.036–0.072) | 0.083 (0.056–0.100) | 0.044 (0.035–0.060) | <0.01 |
| BNP, pg/mL | 167 (102–267) | 248 (146–521) | 166 (93–249) | 0.02 |
| eGFR, mL/min per 1.73 m2) | 52.7±13.8 | 46.9±15.3 | 53.8±13.3 | 0.06 |
| Biomarker staging* | ||||
| Score 0–1 (low risk) | 68 (67) | 3 (19) | 65 (76) | <0.01 |
| Score 2–3 (high risk) | 33 (33) | 13 (81) | 20 (24) | |
| IVSd, mm | 15.6±2.3 | 15.1±1.9 | 15.6±2.4 | 0.44 |
| LVEF, % | 50.2±10.3 | 46.5±10.3 | 50.9±10.2 | 0.12 |
| QRS duration, ms | 112±27 | 117±29 | 111±27 | 0.46 |
| H/CL ratio† | 1.93±0.35 | 1.98±0.25 | 1.92±0.36 | 0.61 |
| Native T1, ms‡ | 1427±54 | 1456±52 | 1423±54 | 0.08 |
| ECV, %§ | 57.1±13.8 | 69.3±10.2 | 55.2±13.3 | <0.01 |
| ACEI/ARB | 47 (47) | 6 (38) | 41 (48) | 0.43 |
| ARNI | 4 (4) | 0 (0) | 4 (5) | 0.24 |
| β‐Blocker | 24 (24) | 6 (38) | 18 (21) | 0.16 |
| Diuretics | 70 (69) | 13 (81) | 57 (67) | 0.24 |
| Dose of loop diuretics|| | 19.5±15.8 | 25.3±17.3 | 18.3±15.3 | 0.12 |
| MRA | 41 (41) | 10 (63) | 31 (36) | 0.052 |
| SGLT2 inhibitor | 13 (13) | 4 (25) | 9 (11) | 0.14 |
Data are given as mean±SD, number (percentage), or median (interquartile range). ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BNP, B‐type natriuretic peptide; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; H/CL, heart/contralateral; hs‐cTnT, high‐sensitivity cardiac troponin T; IVSd, interventricular septal thickness in diastole; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; and SGLT2, sodium‐glucose cotransporter 2.
Score calculated by adding 1 point if hs‐cTnT (cutoff value >0.05 ng/mL) and BNP (cutoff value >250 pg/mL) levels increased and eGFR level (cutoff value <45 mL/min/1.73 m2) decreased more than the cutoff value.
Data available in 83 patients.
Data available in 74 patients.
Data available in 67 patients.
Loop diuretic dose equivalent to furosemide.
Table 2 summarizes the serial cardiac biomarker change, echocardiographic and cardiac magnetic resonance imaging parameters, and medication use during the first year after tafamidis administration in all participants. The serum hs‐cTnT level was significantly decreased, estimated glomerular filtration rate significantly declined, and the left atrial diameter tended to be enlarged at 1 year after tafamidis treatment. Meanwhile, no significant difference in the BNP concentration, interventricular septal thickness, LVEF, LV‐GLS, native T1, and ECV values was found. Compared with the baseline, the prescription rate of β‐blocker was decreased, whereas that of diuretics and sodium‐glucose cotransporter 2 inhibitor was increased at 1 year after tafamidis administration. Enrichment of heart failure treatment was performed in 32% of patients during 1 year after tafamidis administration.
Table 2.
Serial Change in Parameters at Baseline and 1 Year After Tafamidis Administration
| Parameter | Baseline | 1 y After tafamidis | P value |
|---|---|---|---|
| hs‐cTnT, ng/mL | 0.049 (0.036–0.072) | 0.044 (0.033–0.068) | <0.01 |
| Ln (hs‐cTnT) | −2.99±0.52 | −3.07±0.54 | <0.01 |
| BNP, pg/mL | 167 (102–267) | 152 (112–252) | 0.98 |
| Ln (BNP) | 5.11±0.71 | 5.10±0.72 | 0.88 |
| eGFR, mL/min per 1.73 m2 | 52.7±13.8 | 49.7±14.2 | <0.01 |
| IVSd, mm* | 15.6±2.3 | 15.5±2.2 | 0.67 |
| LVEF, %* | 50.2±10.3 | 49.8±9.7 | 0.31 |
| LV‐GLS, %† | −8.4±2.9 | −8.5±2.9 | 0.48 |
| LAD, mm‡ | 41.1±6.4 | 42.1±5.9 | 0.06 |
| TRPG, mm Hg‡ | 26.5±8.7 | 26.6±10.1 | 0.99 |
| Native T1, ms§ | 1418±62 | 1425±58 | 0.93 |
| ECV, %§ | 53.6±13.3 | 53.5±10.5 | 0.59 |
| Medication use‖ | |||
| ACEI/ARB | 45 (47) | 36 (38) | 0.25 |
| β–Blocker | 24 (24) | 16 (17) | <0.01 |
| Diuretics | 70 (69) | 69 (72) | <0.01 |
| Dose of loop diuretics¶ | 25.4±14.0 | 26.7±12.0 | 0.44 |
| MRA | 41 (41) | 41 (43) | 0.18 |
| SGLT2 inhibitor | 13 (13) | 20 (21) | <0.01 |
Data are given as mean±SD, number (percentage), or median (interquartile range). P value was calculated by linear mixed‐effect model for normally distributed variables and by Wilcoxon signed‐rank test for nonnormally distributed variables. Categorical data were compared using the McNemar test. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; BNP, B‐type natriuretic peptide; ECV, extracellular volume; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; IVSd, interventricular septal thickness in diastole; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium‐glucose cotransporter 2; and TRPG, tricuspid regurgitation peak gradient.
Data available in 100 patients.
Data available in 44 patients.
Data available in 93 patients.
Data available in 10 patients.
Data available in 96 patients.
Loop diuretic dose equivalent to furosemide.
Association Between Serial Change in Biomarkers and Clinical Outcomes
The frequency of increase in hs‐cTnT and BNP levels at 1 year after tafamidis commencement among those with and without composite outcomes was compared (Figure 2). Compared with patients without composite outcomes, more patients with composite outcomes tended to show an increase in BNP level at 1 year after tafamidis administration (69% versus 45%; P=0.07). However, no considerable difference in the frequency of increase in hs‐cTnT concentration between the 2 groups was found (50% versus 31%; P=0.13). Notably, the frequency of an increase in both hs‐cTnT and BNP levels was significantly higher in patients with composite outcomes than those without (44% versus 15%; P=0.01).
Figure 2. Comparison of cardiac biomarker change among patients with and without composite outcomes.
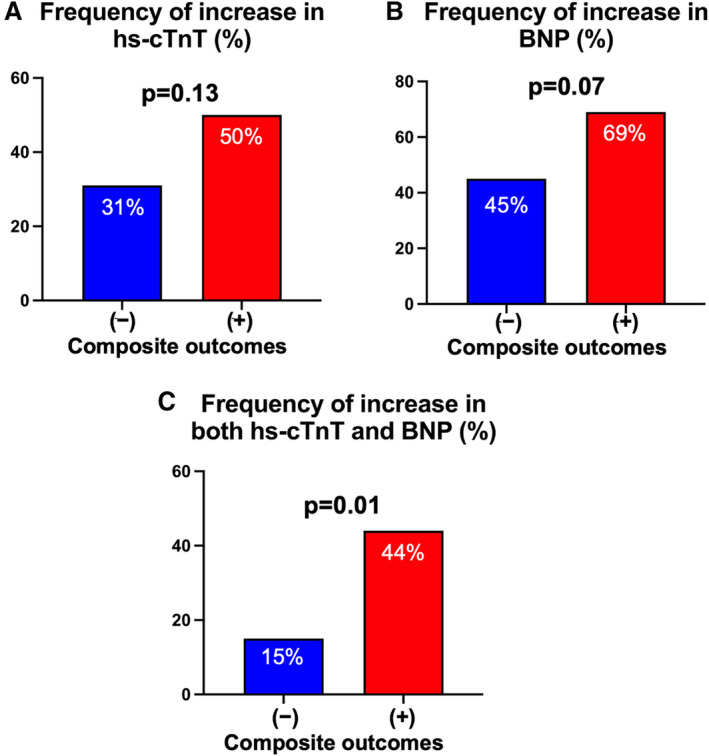
The frequencies of increase in the levels of high‐sensitivity cardiac troponin T (hs‐cTnT) (A), B‐type natriuretic peptide (BNP) (B), and both hs‐cTnT and BNP (C) during the first year after tafamidis administration were compared between the patients with and without composite outcomes during the follow‐up period.
Prognostic Impact of Deterioration in Cardiac Biomarkers From Baseline to 1 Year
Kaplan‐Meier survival analysis compared the composite outcome‐free survival rate between the divided groups depending on the serial change in biomarkers during the follow‐up period. Despite the absence of significant difference in the composite outcome‐free survival rate among patients with an increase and decrease in hs‐cTnT level (log‐rank P=0.11; Figure 3A), the subgroup showing an increase in BNP level exhibited a significantly higher probability of composite outcomes compared than those with decreased BNP level (log‐rank P=0.01; Figure 3B). Notably, the subgroup exhibiting increased hs‐cTnT and BNP levels showed a significantly increased cumulative risk of the appearance of composite outcomes compared with those showing decreased hs‐cTnT and BNP levels (log‐rank P<0.01; Figure 3C).
Figure 3. Kaplan‐Meier survival analysis for composite outcome‐free survival rate during the follow‐up period stratified by cardiac biomarker change during the first year after tafamidis administration.
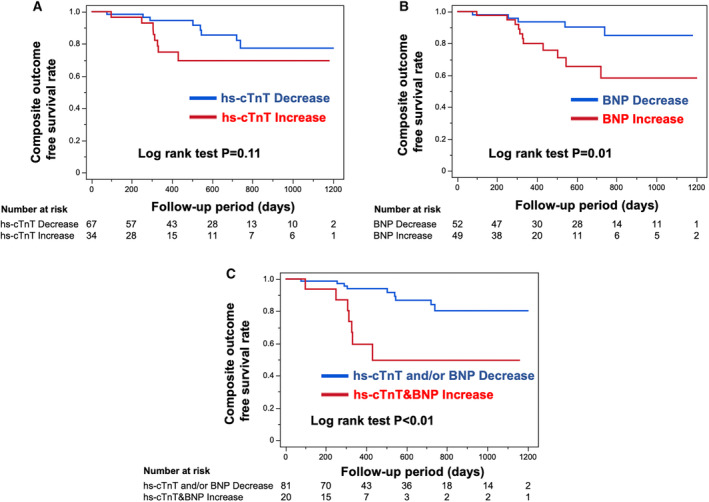
(A and B) Kaplan‐Meier survival analysis for composite outcome‐free survival rate divided by an increase and decrease in the levels of high‐sensitivity cardiac troponin T (hs‐cTnT) (A) and B‐type natriuretic peptide (BNP) (B). (C) Kaplan‐Meier survival analysis for composite outcome‐free survival rate stratified by an increase in the levels of both hs‐cTnT and BNP and decrease in hs‐cTnT and BNP level. The number at risk is shown below each curve.
To investigate the association between the degree of change in cardiac biomarkers and outcomes, the patients were further divided into subgroups depending on the percentage change in cardiac biomarkers (±10%; Figure 4). The hs‐cTnT or BNP increase group tended to have a higher risk of composite outcomes compared with that had by the stable or decrease group, although no significance was identified (Figure 4A and 4B). Importantly, patients who exhibited extensive deterioration in both hs‐cTnT and BNP levels (>10%) had a tendency toward poor prognosis compared with that observed with other patients (Figure 4C).
Figure 4. Kaplan‐Meier survival analysis for composite outcome‐free survival rate during the follow‐up period according to the subgroups.
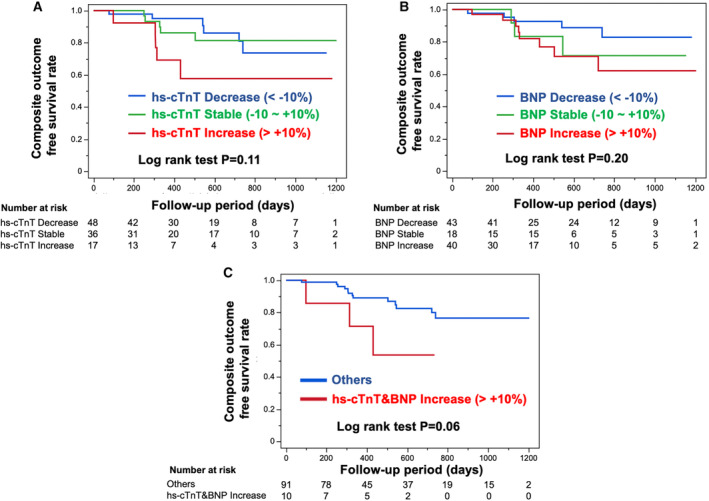
Subgroups include the increase group (percentage change in the high‐sensitivity cardiac troponin T [hs‐cTnT] or B‐type natriuretic peptide [BNP] level >10%), stable group (percentage change in the hs‐cTnT or BNP level between −10% and 10%), and decrease group (percentage change in the hs‐cTnT or BNP <−10%). (A and B) Kaplan‐Meier survival analysis for composite outcome‐free survival rate according to the increase, stable, and decrease groups of hs‐cTnT (A) and BNP (B) levels. (C) Kaplan‐Meier survival analysis for composite outcome‐free survival rate stratified by the subgroup of >10% increase in the percentage change of both hs‐cTnT and BNP levels and others. The number at risk is shown below each curve.
Cox regression analysis was conducted to evaluate an impact of biomarker changes during the first year after tafamidis administration on composite outcomes (Table 3). In the univariable analysis, increase in both hs‐cTnT and BNP levels was significantly associated with composite outcomes compared with decreased hs‐cTnT and BNP levels (HR, 5.06 [95% CI, 1.84–13.9]; P<0.01). Multivariable analysis revealed that increase in both hs‐cTnT and BNP concentrations was an independent predictor of composite outcomes despite the adjustment for age and sex (multivariable model 1), baseline hs‐cTnT and BNP levels (model 2), LVEF and LV‐GLS levels (model 3), native T1 and ECV values (model 4), and enrichment of heart failure treatment (model 5).
Table 3.
Cox Regression Analysis for Composite Outcomes During the Follow‐up Period in Patients Treated With Tafamidis
| Increase in both hs‐cTnT and BNP (vs decrease in hs‐cTnT, BNP, or both) | HR | 95% CI | P value |
|---|---|---|---|
| Univariable model | 5.06 | 1.84–13.9 | <0.01 |
| Multivariable model 1 (adjusted for age and female sex) | 4.30 | 1.33–13.9 | 0.02 |
| Multivariable model 2 (adjusted for hs‐cTnT and BNP) | 5.11 | 1.54–16.9 | <0.01 |
| Multivariable model 3 (adjusted for LVEF and LV‐GLS) | 6.16 | 1.80–21.0 | <0.01 |
| Multivariable model 4 (adjusted for native T1 and ECV) | 19.7 | 1.83–211 | 0.01 |
| Multivariable model 5 (adjusted for enrichment of HF treatment) | 5.05 | 1.76–14.5 | <0.01 |
Multivariable models were conducted using restricted cubic splines analysis to estimate the nonlinear covariate effects. BNP indicates B‐type natriuretic peptide; ECV, extracellular volume; HF, heart failure; HR, hazard ratio; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; and LV‐GLS, left ventricular global longitudinal strain.
Discussion
This study investigated the relationship between cardiac biomarker change and clinical events during tafamidis treatment. Patients treated with tafamidis with deterioration in both hs‐cTnT and BNP levels at 1 year after tafamidis commencement had a high cumulative incidence of clinical adverse events compared with those with improved hs‐cTnT and BNP, indicating that serial biomarker assessment during tafamidis treatment has a good potential for evaluating the therapeutic effect of tafamidis and predict clinical outcomes.
In clinical practice, appropriate monitoring of ATTRwt‐CM progression and identifying responders to tafamidis are essential because tafamidis is the most expensive cardiovascular drug. Although tafamidis‐mediated improvement in heart/contralateral ratio on bone scintigraphy has been shown in previous studies, 12 , 13 , 14 the clinical significance of improved heart/contralateral ratio on prognosis during tafamidis treatment remains unknown. For cardiac magnetic resonance findings represented by native T1 and ECV values, several studies demonstrated no significant change in these parameters by tafamidis treatment. 3 , 15 Therefore, the utility of monitoring these radiographic parameters during tafamidis therapy remains controversial. Likewise, these modalities are expensive and limited in specialized institutions. Meanwhile, given that evaluating cardiac biomarkers can be easily performed with inexpensive cost irrespective of hospital scales, biomarker measurement may be suitable for serial assessment and could objectively and accurately monitor the therapeutic effect of tafamidis.
The hs‐cTnT is highly specific and sensitive for myocardial damage, and an elevation in its concentration is essential when suspecting ATTRwt‐CM and is strongly associated with the prognosis of ATTRwt‐CM. 3 , 7 , 8 A significant decrease in the hs‐cTnT level at 1 year after administering tafamidis was observed both in our current and previous studies 3 ; however, the precise mechanism of tafamidis‐mediated decline in the hs‐cTnT level necessitates further investigation. It is speculated that tafamidis‐mediated attenuation of progressive amyloid deposition may subsequently ameliorate cardiac injury, and diagnosis as ATTRwt‐CM by specialists may allow further optimal heart failure treatment. However, in this study, a fluctuation in hs‐cTnT level could not solely predict the prognosis in patients treated with tafamidis.
BNP or NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) is an established biomarker for predicting prognosis in patients with heart failure. 16 , 17 Its utility as a hallmark prognosis predictor is consistent for patients with ATTRwt‐CM. 7 , 8 Law et al recently identified deterioration of NT‐proBNP level in treatment‐naïve patients with ATTRwt‐CM as a powerful predictor of mortality 18 ; however, the prognostic impact of its fluctuation in BNP level during tafamidis treatment remains unknown. This study provided a new insight wherein deterioration of BNP level during the first year after tafamidis predicted worse prognosis in patients treated with tafamidis with ATTRwt‐CM. However, BNP sensitively reflects cardiac overload, which is more susceptible for fluctuations depending on heart failure condition during assessment. Indeed, it was previously demonstrated that hs‐cTnT level is more correlated with the amyloid deposition and radiographic parameters of ATTRwt‐CM rather than BNP level. 19 Considering the finding that a combination assessment of both hs‐cTnT and BNP levels provided substantial prognostic information after tafamidis treatment, it is indicated that the combination of hs‐cTnT, a biomarker reflective of the ATTR‐CM progression, and BNP, a biomarker reflective of the current status of heart failure, may enable higher accuracy of predicting clinical adverse events during tafamidis treatment.
This study suggests the possibility that patients exhibiting a sustained deterioration in cardiac biomarkers despite tafamidis treatment can be identified as “tafamidis nonresponders.” Beside tafamidis, several novel agents, including transthyretin small interfering RNA, 20 , 21 antisense oligonucleotide suppression, 22 and antifibril monoclonal antibodies, 23 , 24 have been investigated for treating ATTRwt‐CM. In the future, where these antiamyloid agents have been approved, identifying “nonresponders to tafamidis” may aid in selecting more potent antiamyloid treatments. Further investigations on serial biomarker changes and their association with clinical outcomes under antiamyloid therapies beside tafamidis are necessitated.
Some limitations should be noted. First, this was a single‐center retrospective study. Second, only patients in whom follow‐up clinical data were obtained at 1 year after tafamidis administration were analyzed. Therefore, the patients who died within 1 year after initiating tafamidis treatment were excluded, which could lead to selection bias. Compared with the ATTR‐ACT trial participants, 1 our patients had better prognosis (survival rate at 30 months after tafamidis administration: our study, 91.1% versus ATTR‐ACT trial, 70.5%) and a lower natriuretic peptide level (our study, median BNP level=167 pg/mL versus ATTR‐ACT trial, median NT‐proBNP level=2996 pg/mL). Third, novel heart failure treatment, including sodium‐glucose cotransporter 2 inhibitors and angiotensin receptor–neprilysin inhibitors, was used in a relatively small number of patients. Fourth, all patients were diagnosed with ATTRwt‐CM, suggesting the need to validate the generalizability of our findings in other types of cardiac amyloidosis. Fifth, serum transthyretin concentration and its change after tafamidis treatment were not evaluated in this study. Last, as this was an observational study, the utility of a log‐rank t‐test may be limited because of the variety of baseline characteristics, although the effect of covariates on the prognostic impact of biomarker changes was evaluated in the multivariable Cox regression analysis.
Conclusions
Deterioration in cardiac biomarkers during the first year after tafamidis administration in ATTRwt‐CM was significantly associated with a worse prognosis. This highlighted the importance of serial assessment of cardiac biomarkers during tafamidis treatment, which may assist in monitoring therapeutic response to tafamidis and identifying “tafamidis nonresponders.”
Sources of Funding
This study was supported by Japan Society for the Promotion of Science Grant‐in‐Aid for Scientific Research (Grant No. 23K19595).
Disclosures
Dr Tsujita received significant research grants from AMI Co, Ltd, Bayer Yakuhin, Ltd, Bristol‐Myers K.K., EA Pharma Co, Ltd, and Mochida Pharmaceutical Co, Ltd; scholarship funds from AMI Co, Ltd, Bayer Yakuhin, Ltd, Boehringer Ingelheim Japan, Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Edwards Lifesciences Corporation, Johnson & Johnson K.K., Ono Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, and Takeda Pharmaceutical Co, Ltd; and honoraria from Amgen K.K., Bayer Yakuhin, Ltd, Daiichi Sankyo Co, Ltd, Kowa Pharmaceutical Co Ltd, Novartis Pharma K.K., Otsuka Pharmaceutical Co, Ltd, and Pfizer Japan Inc; and belongs to the endowed departments donated by Abbott Japan Co, Ltd, Boston Scientific Japan K.K., Fides‐one, Inc, GM Medical Co, Ltd, ITI Co, Ltd, Kaneka Medix Co, Ltd, Nipro Corporation, Terumo Co, Ltd, Abbott Medical Co, Ltd, Cardinal Heaith Japan, Fukuda Denshi Co, Ltd, Japan Lifeline Co, Ltd, Medical Appliance Co, Ltd, and Medtoronic Japan Co, Ltd. The remaining authors have no disclosures to report.
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 10.
References
- 1. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 2. Rapezzi C, Elliott P, Damy T, Nativi‐Nicolau J, Berk JL, Velazquez EJ, Boman K, Gundapaneni B, Patterson TA, Schwartz JH, et al. Efficacy of tafamidis in patients with hereditary and wild‐type transthyretin amyloid cardiomyopathy: further analyses from attr‐act. JACC Heart Fail. 2021;9:115–123. doi: 10.1016/j.jchf.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 3. Takashio S, Morioka M, Ishii M, Morikawa K, Hirakawa K, Hanatani S, Oike F, Usuku H, Kidoh M, Oda S, et al. Clinical characteristics, outcome, and therapeutic effect of tafamidis in wild‐type transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2023;10:2319–2329. doi: 10.1002/ehf2.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez‐Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, Treibel TA, Whelan CJ, Knight DS, Kellman P, et al. Native t1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2019;12:810–819. doi: 10.1016/j.jcmg.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 5. Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with attr cardiac amyloidosis. JAMA Cardiol. 2016;1:880–889. doi: 10.1001/jamacardio.2016.2839 [DOI] [PubMed] [Google Scholar]
- 6. Chacko L, Martone R, Bandera F, Lane T, Martinez‐Naharro A, Boldrini M, Rezk T, Whelan C, Quarta C, Rowczenio D, et al. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J. 2020;41:1439–1447. doi: 10.1093/eurheartj/ehz905 [DOI] [PubMed] [Google Scholar]
- 7. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. doi: 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 8. Nakashima N, Takashio S, Morioka M, Nishi M, Yamada T, Hirakawa K, Ishii M, Tabata N, Yamanaga K, Fujisue K, et al. A simple staging system using biomarkers for wild‐type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2022;9:1731–1739. doi: 10.1002/ehf2.13847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kruck F, Bablok W, Besenfelder E, Betzien G, Kaufmann B. Clinical and pharmacological investigations of the new saluretic azosemid. Eur J Clin Pharmacol. 1978;14:153–161. doi: 10.1007/BF02089953 [DOI] [PubMed] [Google Scholar]
- 10. Engblom H, Kanski M, Kopic S, Nordlund D, Xanthis CG, Jablonowski R, Heiberg E, Aletras AH, Carlsson M, Arheden H. Importance of standardizing timing of hematocrit measurement when using cardiovascular magnetic resonance to calculate myocardial extracellular volume (ecv) based on pre‐ and post‐contrast t1 mapping. J Cardiovasc Magn Reson. 2018;20:46. doi: 10.1186/s12968-018-0464-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589 [DOI] [PubMed] [Google Scholar]
- 12. Odouard S, Abulizi M, Kharoubi M, Oghina S, Guendouz S, Zaroui A, Teiger E, Itti E, Damy T, Galat A. Tafamidis decreases cardiac uptake of (99m)tc‐hmdp in transthyretin cardiac amyloidosis. JACC Cardiovasc Imaging. 2022;15:2149–2151. doi: 10.1016/j.jcmg.2022.06.013 [DOI] [PubMed] [Google Scholar]
- 13. Rettl R, Wollenweber T, Duca F, Binder C, Cherouny B, Dachs TM, Camuz Ligios L, Schrutka L, Dalos D, Beitzke D, et al. Monitoring tafamidis treatment with quantitative spect/ct in transthyretin amyloid cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2023;24:1019–1030. doi: 10.1093/ehjci/jead030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papathanasiou M, Kessler L, Bengel FM, Jakstaite AM, Kersting D, Varasteh Z, Luedike P, Carpinteiro A, Herrmann K, Rassaf T, et al. Regression of myocardial (99m)tc‐dpd uptake after tafamidis treatment of cardiac transthyretin amyloidosis. J Nucl Med. 2023;64:1083–1086. doi: 10.2967/jnumed.122.265352 [DOI] [PubMed] [Google Scholar]
- 15. Rettl R, Mann C, Duca F, Dachs TM, Binder C, Ligios LC, Schrutka L, Dalos D, Koschutnik M, Dona C, et al. Tafamidis treatment delays structural and functional changes of the left ventricle in patients with transthyretin amyloid cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022;23:767–780. doi: 10.1093/ehjci/jeab226 [DOI] [PubMed] [Google Scholar]
- 16. Price JF, Thomas AK, Grenier M, Eidem BW, O'Brian Smith E, Denfield SW, Towbin JA, Dreyer WJ. B‐type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation. 2006;114:1063–1069. doi: 10.1161/CIRCULATIONAHA.105.608869 [DOI] [PubMed] [Google Scholar]
- 17. Nishii M, Inomata T, Takehana H, Naruke T, Yanagisawa T, Moriguchi M, Takeda S, Izumi T. Prognostic utility of b‐type natriuretic peptide assessment in stable low‐risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol. 2008;51:2329–2335. doi: 10.1016/j.jacc.2007.11.085 [DOI] [PubMed] [Google Scholar]
- 18. Law S, Petrie A, Chacko L, Cohen OC, Ravichandran S, Gilbertson JA, Rowczenio D, Wechalekar AD, Martinez‐Naharro A, Lachmann HJ, et al. Change in n‐terminal pro‐b‐type natriuretic peptide at 1 year predicts mortality in wild‐type transthyretin amyloid cardiomyopathy. Heart. 2022;108:474–478. doi: 10.1136/heartjnl-2021-319063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morioka M, Takashio S, Nakashima N, Nishi M, Fujiyama A, Hirakawa K, Hanatani S, Usuku H, Yamamoto E, Kidoh M, et al. Correlation between cardiac images, biomarkers, and amyloid load in wild‐type transthyretin amyloid cardiomyopathy. J Am Heart Assoc. 2022;11:e024717. doi: 10.1161/JAHA.121.024717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Habtemariam BA, Karsten V, Attarwala H, Goel V, Melch M, Clausen VA, Garg P, Vaishnaw AK, Sweetser MT, Robbie GJ, et al. Single‐dose pharmacokinetics and pharmacodynamics of transthyretin targeting n‐acetylgalactosamine‐small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther. 2021;109:372–382. doi: 10.1002/cpt.1974 [DOI] [PubMed] [Google Scholar]
- 21. Adams D, Tournev IL, Taylor MS, Coelho T, Plante‐Bordeneuve V, Berk JL, Gonzalez‐Duarte A, Gillmore JD, Low SC, Sekijima Y, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin‐mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30:1–9. doi: 10.1080/13506129.2022.2091985 [DOI] [PubMed] [Google Scholar]
- 22. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: Jacc state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Michalon A, Hagenbuch A, Huy C, Varela E, Combaluzier B, Damy T, Suhr OB, Saraiva MJ, Hock C, Nitsch RM, et al. A human antibody selective for transthyretin amyloid removes cardiac amyloid through phagocytic immune cells. Nat Commun. 2021;12:3142. doi: 10.1038/s41467-021-23274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Galant NJ, Bugyei‐Twum A, Rakhit R, Walsh P, Sharpe S, Arslan PE, Westermark P, Higaki JN, Torres R, Tapia J, et al. Corrigendum: substoichiometric inhibition of transthyretin misfolding by immune‐targeting sparsely populated misfolding intermediates: a potential diagnostic and therapeutic for ttr amyloidoses. Sci Rep. 2016;6:27679. doi: 10.1038/srep27679 [DOI] [PMC free article] [PubMed] [Google Scholar]


