Abstract
Background
Data on the incidence of type 2 non–ST‐segment–elevation myocardial infarction (T2MI) in hospitalized patients with COVID‐19 has been limited to single‐center studies. Given that certain characteristics, such as obesity and type 2 diabetes, have been associated with higher mortality in COVID‐19 infections, we aimed to define the incidence of T2MI in a national cohort and identify pre‐hospital patient characteristics associated with T2MI in hospitalized patients with COVID‐19.
Methods and Results
Using the national American Heart Association COVID‐19 Cardiovascular Disease Quality Improvement Registry, we performed a retrospective 4:1 matched (age, sex, race, and body mass index) analysis of controls versus cases with T2MI. We performed (1) conditional multivariable logistic regression to identify predictive pre‐hospital patient characteristics of T2MI for patients hospitalized with COVID‐19 and (2) stratified proportional hazards regression to investigate the association of T2MI with morbidity and mortality. From January 2020 through May 2021, there were 709 (2.2%) out of 32 015 patients with T2MI. Five hundred seventy‐nine cases with T2MI were matched to 2171 controls (mean age 70; 43% female). Known coronary artery disease, heart failure, chronic kidney disease, hypertension, payor source, and presenting heart rate were associated with higher odds of T2MI. Anti‐hyperglycemic medication and anti‐coagulation use before admission were associated with lower odds of T2MI. Those with T2MI had higher morbidity and mortality (hazard ratio, 1.40 [95% CI, 1.13–1.74]; P=0.002).
Conclusions
In hospitalized patients with COVID‐19, those with a T2MI compared with those without had higher morbidity and mortality. Outpatient anti‐hyperglycemic and anti‐coagulation use were the only pre‐admission factors associated with reduced odds of T2MI.
Keywords: anti‐coagulation, anti‐hyperglycemic, COVID‐19, demand ischemia, registry, type 2 NSTEMI
Subject Categories: Myocardial Infarction, Cardiovascular Disease, Anticoagulants, Acute Coronary Syndromes
Nonstandard Abbreviations and Acronyms
- qCSI
Quick COVID‐19 Severity Index
- T2MI
type 2 myocardial infarction
Clinical Perspective.
What Is New?
In hospitalized patients with COVID‐19, having a type 2 myocardial infarction was associated with higher morbidity, including adverse cardiac events, and mortality.
What Are the Clinical Implications?
Prehospital antihyperglycemic or anti‐coagulation use was associated with lower odds of type 2 myocardial infarction in hospitalized patients with COVID‐19.
Non–ST‐segment–elevation myocardial infarction is a subtype of acute coronary syndrome that results from either partial or total occlusion of coronary artery blood flow. 1 The majority of non–ST‐segment–elevation myocardial infarction cases are partial, flow‐limiting occlusions rather than total obstructions. 2 , 3 While type 1 myocardial infarction is characterized primarily by intracoronary atherothrombotic plaque rupture, a type 2 myocardial infarction (T2MI) is one that occurs due to an oxygen supply and demand mismatch in the absence of acute atherothrombosis. 4 T2MI can occur in the setting of fixed atherosclerosis without plaque rupture, alone or in combination with coronary vasospasm, sustained tachyarrhythmias, severe hypertension, severe bradyarrhythmias, respiratory failure, or severe anemia, among other causes. 2 , 4 Comorbid risk factors for patients with T2MI include anemia, sepsis, chronic kidney disease (CKD), heart failure (HF), hypertension, and arrhythmias. 4 , 5 T2MI has been associated with higher mortality when compared with type 1 myocardial infarction and typically occurs in patients who are older, more often female, and with higher prevalence of cardiac and noncardiac comorbidities. 2 , 5
Since the early stages of the COVID‐19 pandemic, a reported cardiovascular complication with associated adverse outcomes of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been myocardial injury, characterized as acute or chronic. 6 Acute myocardial injury is defined as a significant elevation of cardiac troponin above the 99th percentile upper reference limit with a rise and/or fall of cardiac troponin values. 6 Meanwhile, chronic myocardial injury is reflective of stable cardiac troponin levels that are not markedly higher (<20%). 7 Myocardial injury can result from a T2MI during acute viral infections as a result of hypoxia‐related increased cardiometabolic demand. 6 However, other mechanisms of cardiac injury can occur from direct viral‐mediated myocardial damage, systemic inflammation, or electrolyte imbalances. 6 Examples of myocardial injuries that often lead to an elevation of cardiac troponin include myocarditis, stress‐cardiomyopathy, arrhythmias, cardiogenic shock, and cardiac arrest. 6
Known cardiovascular disease (CVD), hypertension, and diabetes are associated with greater mortality in patients infected with SARS‐CoV‐2. 8 A single‐center case series of 187 patients hospitalized with COVID‐19 in Wuhan City, China early in the pandemic reported myocardial injury in 27.8% of patients that was associated with more severe complications of SARS‐CoV‐2 and higher mortality. 9 However, there is currently a paucity of data that specifically evaluate the incidence of T2MI (versus nonischemic myocardial injury) with SARS‐CoV‐2 in diverse cohorts. Furthermore, the prognosis of patients who have been infected with SARS‐CoV‐2 who experience T2MI is not well described. This study aims to define the incidence of T2MI in a national cohort and identify prehospital patient characteristics associated with a diagnosis of T2MI in hospitalized patients with COVID‐19. We will also examine the association of T2MI with morbidity and mortality.
METHODS
Study Population
This analysis was performed using data from the American Heart Association's (AHA) COVID‐19 Cardiovascular Disease (CVD) Registry powered by the Get With The Guidelines quality improvement program. The Get With The Guidelines programs are provided by the AHA. Anonymized data and materials are available via the AHA's COVID‐19 CVD Registry where IQVIA managed the data collection platform, and the Duke Clinical Research Institute served as the coordinating center. Details regarding the establishment and implementation of this registry have been previously described. 10 The registry included any adult patient hospitalized with an active COVID‐19 infection, confirmed with a reverse transcription polymerase chain reaction test (either before or during the hospitalization), a positive immunoglobulin M antibody test, or a clinical diagnosis using hospital‐specific criteria. This may or may not have been the primary diagnosis for hospitalization. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Medical College of Wisconsin Institutional Review Board Committee approved the study and waived the need for obtaining informed consent because data were collected as part of routine quality‐improvement activities and no direct patient interactions or postdischarge follow‐up occurred. No one received compensation or was offered any incentive for participating in this study.
From January 2020 through May 2021, the registry had ~32 000 admission records from 122 centers across the United States. Included patients were adults hospitalized with active COVID‐19 disease confirmed by reverse transcription polymerase chain reaction test or positive immunoglobulin M antibody test. We excluded patients with a ST‐segment–elevation myocardial infarction or type 1 myocardial infarction event during the index hospitalization. There are >200 data elements that were retrospectively abstracted from the patient medical record using a standardized case report form provided by the AHA. Many of the data points in the case report form, including medical history, outpatient medications, and in‐hospital complications, were reported with checkboxes of either “Yes,” or “No/ND” (ND=not documented). Follow‐up clarification was typically not a mandatory field. For example, when inputting data for lipid‐lowering therapy, “Yes” would be checked but specifically answering whether the patient was on “Ezetimibe,” “PCSK 9 Inhibitor,” “Statin,” or “Other lipid‐lowering med” was not mandatory. Participation in this quality improvement registry was approved or waived for review by each participating hospital's institutional review board.
Inclusion/Exclusion Criteria
All patients aged ≥18 years hospitalized with COVID‐19 within the registry were included in our initial data set. Patients with a type 1 myocardial infarction, ST‐segment–elevation myocardial infarction, and those hospitalized outside the pandemic range (January 1, 2020–May 31, 2021) were removed from the data set. Nineteen patients were excluded from length of stay analyses due to inaccurate entry for time of admission. T2MI was captured in the registry with a checkbox within the “Hospitalization” section in the case report form. Fifty‐seven centers did not have at least 1 reported T2MI, so they were also excluded. There were 702 patients with a reported T2MI.
Study Measures
In this study, we explore T2MI as both an outcome and as an exposure preceding an even more dire outcome. For our primary aim, exposure variables (predictors) of interest for a diagnosis of T2MI (outcome variable) included data from 3 categories: (1) prehospital patient characteristics, (2) comorbidities, and (3) outpatient (before admission) medications. Prehospital patient characteristics included age, sex, race, insurance status, initial labs (hemoglobin A1C, C‐reactive protein, d‐dimer, creatinine, and troponin), smoking status, body mass index (BMI) when available, and admission vitals (blood pressure, heart rate, oxygen saturation). Medical history was entered as a binary indicator in the registry by research personnel based on review of the medical record. Comorbidities considered included hypertension, coronary artery disease (CAD) (defined as prior coronary artery bypass graft surgery, myocardial infarction, or percutaneous coronary intervention), HF, diabetes, pulmonary disease (including chronic obstructive pulmonary disease, asthma, or interstitial lung disease), CKD, and cancer. Outpatient medications included antihypertensives, lipid‐lowering therapy, antiplatelet agents, anticoagulants, antihyperglycemic medications, immunosuppressive medications, chemotherapy, and hydroxychloroquine.
For our secondary aim, the exposure variable was T2MI, and the outcomes of interest included (1) in‐hospital death or discharge to hospice and (2) in‐hospital morbidity. We defined morbidity as the number of days in intensive care unit, length of intubation, new hemodialysis or continuous renal replacement therapy, adverse cardiac events (defined as: cardiac arrest, in‐hospital shock, veno‐arterial or veno‐venous extracorporeal membrane oxygenation), adverse vascular events (defined as: deep vein thrombosis, pulmonary embolus, acute limb ischemia, ischemic or hemorrhagic stroke), clinical bleeding requiring transfusion, and length of stay.
Statistical Analysis
The American Heart Association Precision Medicine Platform (https://precision.heart.org/) was used for data analysis, and IQVIA (Parsippany, New Jersey) serves as the data collection and coordination center. If dates of admission and discharge were outside of the included pandemic range (January 1, 2020–May 31, 2021), length of stay was considered missing. The outcome of T2MI was defined as an event if it occurred before the morbid events listed above (hemodialysis or continuous renal replacement therapy, coagulopathy, ventilation, cardiac arrest, extracorporeal membrane oxygenation (both veno‐arterial and veno‐venous), shock, intracardiac thrombus, ischemic stroke/intracranial hemorrhage, or seizure). If the timing of those morbid events was unknown, it was assumed the T2MI occurred first. Race and ethnicity were categorized into Non‐Hispanic Black, Hispanic, Non‐Hispanic White, Asian, and Other. To ameliorate any concerns over the adjudication of T2MI, we performed a sensitivity analysis using troponin data within the registry. In addition, there were 57 sites reporting no observations of T2MI. Due to concern of misreporting issues, these sites were excluded from analysis.
Since T2MI is rare while controls are abundant, we performed a matched analysis (1 case to 4 control) to increase the power by variance reduction. There was an exact match on age, sex, and race. When BMI data were not missing, the closest BMI was matched; otherwise, the control was selected at random. Control complications were defined by the same length of follow‐up as their matched cases (ie, the controls were followed from the same relative day of the T2MI case). Between cases and controls, the complications were compared using Pearson's χ2 tests for categorical data and Mann–Whitney‐Wilcoxon tests for continuous comparisons. Variables of interest at admission considered for model adjustment were as follows: payment type, smoking history, hypertension, CAD, HF, diabetes, chronic obstructive pulmonary disease, CKD, cancer, antihypertensive, lipid‐lowering therapy, antiplatelet, anticoagulant, antihyperglycemic, corticosteroid, immunosuppressive medications (other than steroids), chemotherapy or biological treatment for cancer, hydroxychloroquine, systolic and diastolic blood pressure, heart rate, temperature, oxygen saturation (%), serum creatinine, hemoglobin A1C (%), C‐reactive protein, and d‐dimer.
The matching variables (age, sex, race, and BMI) were considered descriptively for T2MI cases versus controls. The odds ratio of a case due to covariates was estimated by a matched analysis using conditional logistic regression with forward variable selection to predict T2MI. Similarly, for death (or discharge to hospice), the case/control matches were adjusted via stratified Cox proportional hazard regression (each stratum is a match) that used forward variable selection. Given the large number of variables we analyzed, we built predictive models for our regression, which only included the significant variables to reduce the noise within the data.
To account for the severity of COVID‐19 infection on our outcomes data, we calculated a modified Quick COVID‐19 Severity Index (qCSI) score for both cases and controls. This score has been validated to accurately predict patients who will decompensate with early hospital respiratory failure. 11 The qCSI assigns a score based on the patient's respiratory rate, oxygen saturation, and oxygen flow rate. Given the restriction of the registry data, we modified the last variable in the qCSI where if the patient was on room air, they would be assigned 0 points and any oxygen use would be 4 points. In addition to comparing qCSI, and admission vitals, we also compared the admission chest radiographs for both groups to help account for the severity of COVID‐19.
RESULTS
As of May 31, 2021, there were 32 015 patients in the registry, with 709 having a T2MI. The incidence of T2MI was 2.2%. After performing the 4:1 matched analysis, there were 579 cases with T2MI matched to 2173 controls (130 cases were excluded from the matched analysis since they were unable to be matched).
Unadjusted Analyses
In our unadjusted analysis, we measured the baseline differences between cases and controls. Demographic and baseline characteristics are displayed in the Table. Given that we matched on age, sex, race, and BMI, those variables were not statistically different between the 2 groups. The mean age and BMI of the cohort were 70 years and 29, respectively, with 43% female. The T2MI group had a higher prevalence of hypertension (81% versus 72%, P <0.001), CAD (23% versus 14%, P <0.001), HF (30% versus 17%, P <0.001), and CKD (30% versus 18%, P <0.001). There was no difference between the 2 groups in terms of diabetes, chronic obstructive pulmonary disease, history of cancer, or smoking status. A higher percentage of T2MI (cases) were insured through Medicaid/Title 19 (13% versus 8%). On admission, patients with T2MI had a faster heart rate (95 beats per minute versus 90 beats per minute, P <0.001) but did not differ in terms of systolic or diastolic blood pressure, temperature, or initial oxygen saturation. Abnormal chest radiograph on admittance was recorded in 56% of controls and 76% of cases (only 5% of patients in our study did not have a chest radiograph). This was significant with an odds ratio (OR) 1.341 (P=0.009). In terms of medications before admission, the only differences between the 2 groups were anti‐platelet and anti‐hypertensive use (43% T2MI versus 36% control, P=0.002 and 74% T2MI versus 68% control, P=0.017, respectively). Other classes of medications including lipid‐lowering and anti‐hyperglycemic therapy are shown in the Table. Comparing the modified qCSI, controls had an average score of 3, which equates to a low‐level risk of decompensation. Cases had an average score of 3.5, which was the next risk category, low‐intermediate risk of decompensation. Patients with a T2MI had a more severe course of COVID‐19 (by qCSI) with OR 1.107 (95% CI, 1.062–1.154, P <0.001).
Table .
Patient Characteristics of Cases and Controls (Matched on Age, Sex, Race, and Body Mass Index)
| Controls (n=2173) | Type 2 NSTEMI (n=579) | P value | |
|---|---|---|---|
| Age, y | 70±14 | 71±15 | |
| Body mass index | 29±8 | 29±8 | |
| Sex | |||
| Male | 1231 (57%) | 326 (56%) | |
| Female | 942 (43%) | 253 (44%) | |
| Race and ethnicity | |||
| Asian | 89 (4%) | 27 (5%) | |
| Black | 606 (28%) | 164 (28%) | |
| Hispanic | 225 (10%) | 61 (11%) | |
| Other | 136 (6%) | 36 (6%) | |
| White | 1117 (51%) | 291 (50%) | |
| Payment source | 0.011 | ||
| Medicare/Title 18 | 932 (43%) | 228 (39%) | |
| Medicaid/Title 19 | 177 (8%) | 73 (13%) | |
| Private insurance | 387 (18%) | 93 (16%) | |
| VA/CHAMPVA/Tricare/Self‐Pay/Other/Unknown | 137 (6%) | 34 (6%) | |
| More than 1 | 540 (25%) | 151 (26%) | |
| Past medical history | |||
| Cancer | 324 (15%) | 95 (16%) | 0.322 |
| Chronic kidney disease | 386 (18%) | 176 (30%) | <0.001 |
| COPD | 288 (13%) | 95 (16%) | 0.051 |
| Coronary artery disease | 296 (14%) | 135 (23%) | <0.001 |
| Diabetes | 862 (40%) | 244 (42%) | 0.254 |
| Heart failure | 371 (17%) | 173 (30%) | <0.001 |
| Hypertension | 1559 (72%) | 468 (81%) | <0.001 |
| Smoking | 150 (7%) | 47 (8%) | 0.275 |
| Pre‐admission vitals | |||
| Systolic BP pre‐admission | 133±25 | 131±30 | 0.056 |
| Diastolic BP pre‐admission | 75±15 | 75±19 | 0.739 |
| Heart rate pre‐admission | 90±19 | 95±22 | <0.001 |
| Temperature pre‐admission | 99±1.6 | 99±1.8 | 0.556 |
| Oxygen saturation pre‐admission | 94±5 | 94±5.7 | 0.432 |
| Outpatient medications | |||
| Anticoagulation therapy | 358 (17%) | 94 (17%) | 0.937 |
| Antihyperglycemic therapy | 658 (31%) | 160 (30%) | 0.521 |
| Antihypertensive therapy | 1454 (68%) | 400 (74%) | 0.017 |
| Antiplatelet therapy | 788 (36%) | 250 (43%) | 0.002 |
| Immunosuppressive therapy | 287 (13%) | 79 (14%) | 0.641 |
| Lipid‐lowering therapy | 1067 (50%) | 287 (53%) | 0.131 |
Data are displayed as: n (column percent), mean±SD. BP indicates blood pressure; CHAMPVA, Civilian Health and Medical Program of the Department of Veterans Affairs; COPD, chronic obstructive pulmonary disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; and VA, Veterans Affairs.
In patients with a T2MI during the index hospitalization, there was an increase in death or discharge to hospice (hazard ratio [HR], 1.40 [95% CI, 1.13–1.74]; P=0.002) compared with controls. Having a T2MI was associated with higher morbidity, including adverse cardiac events (HR, 3.86 [95% CI, 3.08–4.83; P <0.001]), adverse vascular events (HR, 2.31 [95% CI, 1.70–3.15]; P <0.001), intubation (HR 2.98 [95% CI, 2.39–3.72]; P <0.001), major bleeding requiring transfusion (HR, 2.63 [95% CI, 1.82–3.81]; P <0.001), transfer to the intensive care unit (HR, 2.88 [95% CI, 2.37–3.51]; P <0.001), new hemodialysis or continuous renal replacement therapy (HR, 2.70 [95% CI, 1.81–4.03]; P <0.001), higher length of stay and time in the intensive care unit. These morbidity and mortality outcomes with hazard ratios and 95% CIs are summarized in Figure 1 and Table S1.
Figure 1. Hazard ratios for morbidity and mortality outcomes in COVID‐19 and type 2 NSTEMI.
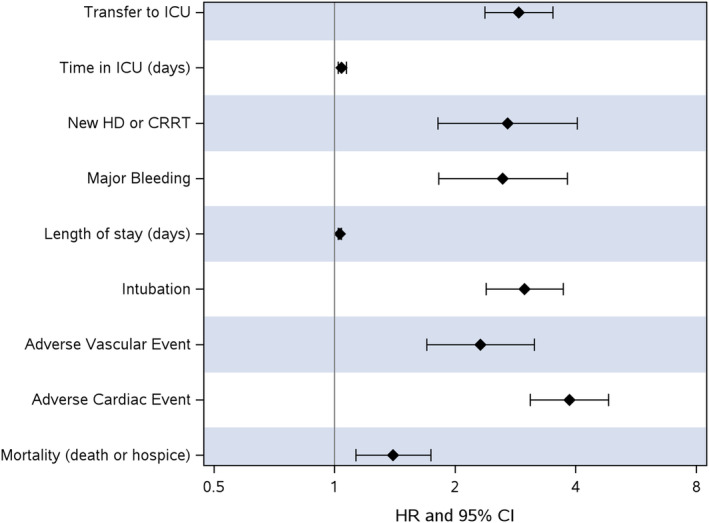
CRRT indicates continuous renal replacement therapy; HD, hemodialysis; HR, hazard ratio; ICU, intensive care unit; NSTEMI, non–ST‐segment–elevation myocardial infarction; and T2MI, type 2 myocardial infarction.
Adjusted Analyses
We performed conditional logistic regression modeling with forward selection in our adjusted analysis. Model selection excluded the following variables due to high missingness: serum creatinine, admission SpO2, and admission labs (hemoglobin A1C, troponin, C‐reactive protein, and d‐dimer). Known CAD (OR, 1.87 [95% CI, 1.43–2.44]; P <0.001), HF (OR, 1.84 [95% CI, 1.43–2.37]; P <0.001), hypertension (OR, 1.41 [95% CI, 1.08–1.84], P=0.012), CKD (OR, 1.84 [95% CI, 1.43–2.36]; P <0.001), and payor source (Medicaid/Title 19 versus private; OR, 2.23 [95% CI, 1.47–3.39]; P <0.001) were strongly associated with higher odds of T2MI (Figure 2 and Table S2). Admission heart rate was also directly associated with higher odds of T2MI (OR, 1.16 [95% CI, 1.10–1.22] per 10 beats; P <0.001). Anti‐hyperglycemic medication use before admission was associated with lower odds of T2MI (OR, 0.76 [95% CI, 0.60–0.95]; P=0.018) as was outpatient anticoagulation use (OR, 0.72 [95% CI, 0.54–0.95]; P=0.022).
Figure 2. Odds ratios for pre‐admission factors associated with type 2 NSTEMI in COVID‐19.
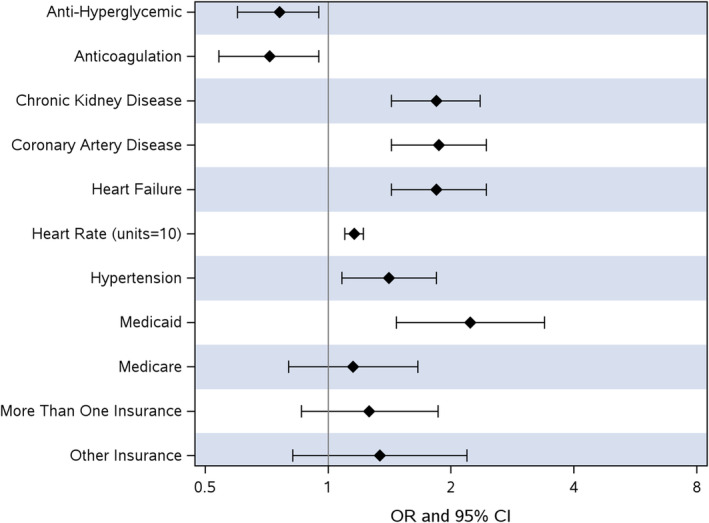
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; and OR, odds ratio.
When comparing patients with T2MI to the matched controls, admission heart rate was directly associated with inpatient mortality or discharge to hospice (HR, 1.16 [95% CI, 1.09–1.23] per 10 beats; P <0.001). Admission systolic blood pressure was inversely associated with mortality (HR, 0.90 [95% CI, 0.86–0.95] per 10 mm Hg; P<0.001). Payor source was associated with mortality (Medicaid versus private; HR, 2.62 [1.29–5.31]; P=0.008 and Medicare versus private; HR, 1.74 [95% CI, 1.03–2.96]; P=0.040); Figure 3 and Table S3. T2MI was associated with mortality (HR, 1.28 [95% CI, 1.004–1.62]; P=0.046).
Figure 3. Hazard ratios for factors associated with inpatient mortality or discharge to hospice.
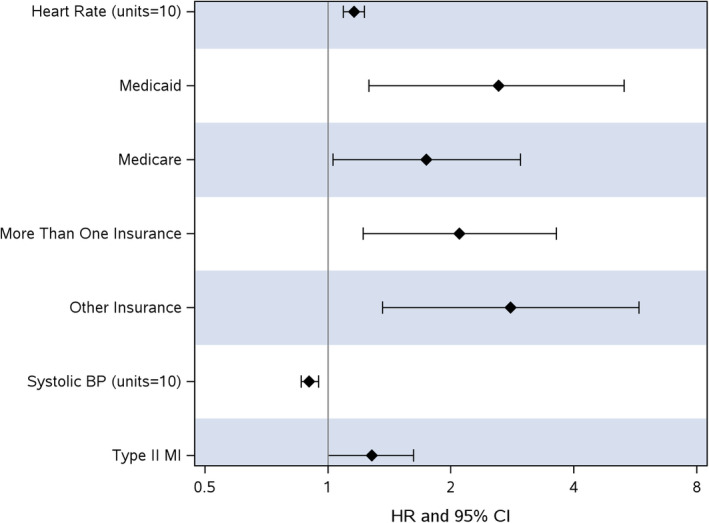
BP indicates blood pressure; HR, hazard ratio; and MI, myocardial infarction.
DISCUSSION
In this national and diverse, multicenter study of patients hospitalized with COVID‐19, the incidence of T2MI was 2.2%. A prior history of CAD, HF, hypertension, CKD, and admission heart rate were associated with higher odds of T2MI during the index hospitalization. T2MI was associated with higher mortality and morbidity, including adverse cardiovascular events, major bleeding, acute kidney injury necessitating hemodialysis or continuous renal replacement therapy, and transfer to the intensive care unit. This may be in the context of more severe COVID‐19 infection, given that cases had higher admission heart rates, higher incidence of abnormal chest radiograph, and higher (modified) COVID‐19 severity scores. This is an expected finding given that T2MI occurs due to myocardial oxygen supply/demand mismatch without acute atherothrombotic plaque disruption, and COVID‐19 primarily manifests as an acute respiratory illness.
Anticoagulation use before admission was associated with lower odds of T2MI. Although not a primary hypothesis of our analysis, anti‐hyperglycemic medication use before admission was associated with reduced odds of T2MI, an interesting finding worthy of more discussion given growing epidemiological data showing potential benefit in other studies of COVID‐19. This observation may have particular relevance given that many studies show higher morbidity and mortality in those with type 2 diabetes and COVID‐19 infection. 12 , 13 , 14
A major strength of our study compared with others is the specific focus on T2MI epidemiology and related outcomes rather than the broader, more common diagnosis of myocardial injury in hospitalized patients with COVID‐19. For example, one of the earliest published case series by Guo et al. during the pandemic was based on 187 patients in Wuhan City, China, demonstrating that 27.8% of those hospitalized patients had myocardial injury. The mortality during hospitalization was 7.6% for patients without underlying CVD and normal troponin T levels, 37.5% for those without underlying CVD but elevated troponin T levels, and 69.4% for those with underlying CVD and elevated troponin T. Patients with underlying CVD were more likely to exhibit elevation of troponin T levels compared with the patients without CVD. 9 The investigators did not differentiate between T2MI nor between acute or chronic myocardial injury.
Patients with T2MI and myocardial injury are frequently encountered in clinical practice and are at high risk of both major adverse cardiovascular events and noncardiac death. 15 Differentiating T2MI from nonischemic myocardial injury is important because the underlying mechanisms of troponin release differ. T2MI is an ischemic process, and thus consideration for assessment for underlying obstructive coronary artery disease may be reasonable if not previously done. In contrast, nonischemic myocardial injury may result from many mechanisms including myocardial strain (HF, valvular heart disease, or hypertension) or direct cardiotoxicity (myocarditis, stress cardiomyopathy). 16 The evaluation of nonischemic myocardial injury will often focus on a structural examination of the heart with imaging. Because upwards of 60% of hospitalized patients with COVID‐19 meet the definition for myocardial injury, distinguishing from T2MI with ECG, imaging, or symptoms is increasingly important both for prognostication and to provide insight into the specific mechanisms of cardiac involvement. 17 , 18 , 19
Given what we have learned about the pathophysiology of acute cardiac injury in COVID‐19, our finding of pre‐admission anticoagulation use and lower odds of T2MI can be explained. The hypercoagulable state and systemic endothelial tissue injury due to COVID‐19 infection predisposes to microthrombi formation in blood vessels, including the cardiac capillaries, leading to T2MI. 20 Furthermore, macrovascular thrombosis, such as acute pulmonary embolism leading to respiratory failure and/or increased hemodynamic stress, can be averted with anticoagulation, as can coronary embolism. Patients with COVID‐19 have a higher incidence of venous thromboembolism than other hospitalized patients, and randomized control trial data have now led to guideline recommendations for anticoagulation treatment to prevent both venous and arterial thromboembolic complications in hospitalized COVID‐19 patients. 21 , 22 , 23
The finding of anti‐hyperglycemic medication use before admission being associated with a reduced odds of T2MI deserves more discussion. A large meta‐analysis evaluating in‐hospital mortality outcomes in 3 061 584 patients (from 61 studies rated as having low risk of bias) with type 2 diabetes and COVID‐19 found that metformin, glucagon‐like peptide‐1 receptor agonist, and sodium‐glucose cotransporter‐2 inhibitor use before admission were associated with a lower mortality rate. 24 Furthermore, metformin was associated with better outcomes in a dose–response manner with every 250 mg/d increase associated with a 19.7% lower odds of mortality, strengthening the possibility of causality. 24 , 25 Unfortunately, due to missing data (neither “Yes” nor “No/ND” selected for prior‐to‐admission anti‐hyperglycemic medication use) in the AHA COVID‐19 CVD registry, we were underpowered to analyze by class of anti‐hyperglycemic. However, metformin was the most used non‐insulin, anti‐hyperglycemic agent (16% of cases and 17% of controls) in this registry cohort.
Several mechanisms might explain the benefit of metformin use among patients with COVID‐19 infections. These include reduced release of inflammatory cytokines interleukin‐6 and tumor necrosis factor‐α, both of which are implicated in COVID‐19 pathophysiology. 26 In fact, metformin was originally introduced as an anti‐influenza drug with the side effect of glucose‐lowering, and it has additional pleiotropic effects. Metformin modulates angiotensin‐converting enzyme‐2 through monophosphate‐activated protein kinase, decreases coagulation and thrombosis, and improves endothelial function. 24 Metformin has shown in vitro activity against SARS‐CoV‐2 and other RNA viruses. 27 , 28 The COVID‐OUT: Early Outpatient Treatment for SARS‐COV‐2 infection randomized 1432 patients in a 2‐by‐3 factorial design to test the effectiveness of 3 repurposed drugs (metformin, ivermectin, and fluvoxamine) in preventing SARS‐CoV‐2 infection in nonhospitalized adults enrolled within 3 days after a confirmed diagnosis. None of the 3 medications prevented the occurrence of the primary combined end point of hypoxemia, an emergency department visit, hospitalization, or death associated with COVID‐19. However, there was a reduction in the combined end point components of emergency department visit, hospitalization, and death with metformin with an adjusted OR of 0.58 (95% CI, 0.35–0.94). 27 Because this was a secondary end point, the finding cannot be considered definitive, and more investigation remains to be done.
Diabetes predisposes patients with COVID‐19 to poor outcomes. Epidemiological studies have shown that diabetes increases the risk of hospitalizations, admission to critical care, and mortality caused by COVID‐19. 12 , 29 , 30 , 31 , 32 , 33 , 34 In our analyses, a history of type 2 diabetes was not a significant predictor of T2MI or higher morbidity and mortality. We reconcile our findings with those of others by considering another possible confounding variable, obesity. The co‐existence of obesity and diabetes, also called “diabesity,” is another major pandemic that the world currently faces. 29 Diabesity is characterized by a pro‐inflammatory state, driven by cytokines, such as interleukin‐6 and tumor necrosis factor‐α. These patients are at increased risk of uncontrolled inflammation, which could induce a cytokine storm and contribute to an overall poor prognosis. 30 In our analyses, we matched on BMI (age, sex, and race). Perhaps much of the increased risk in COVID‐19 infection found in other studies of patients with diabetes is largely attributable to the complex pathophysiology of “diabesity,” rather than type 2 diabetes itself. 33 From this same registry, Hendren et al. found that individuals who were obese are at higher risk for mortality and morbidity if hospitalized with COVID‐19, even for young adults. 35 Whether the association of diabetes with poor outcomes is direct, or secondary to comorbidities, needs clarification. Alternatively, diabetes “severity” and/or new‐onset diabetes during the index hospitalization that are not captured in our analyses are alternative possible explanations for the neutral finding. 32
The associations of prior history of CAD, HF, hypertension, CKD, and higher admission heart rate with higher odds of T2MI during the index hospitalization are not unexpected. Exacerbations of these conditions may contribute to both increased myocardial demand (tachyarrhythmia, hypertension, HF) and diminished oxygen delivery to the myocardium (fixed CAD) in the setting of serious infection. 36 The association of payor source with more T2MI (Medicaid/Title 19) and mortality (Medicare/Title 18) likely reflect both the demographics of the vulnerable adult population publicly insured (ages 65 years and older, adults aged 19 to 64 years who are disabled or institutionalized) and social determinants of health, such as income level, food access, housing, transportation, and access to health care. A separate publication from this registry concluded that patients hospitalized with COVID‐19 residing in more socially vulnerable communities experienced higher rates of in‐hospital mortality and morbidity, independent of race, ethnicity, and several clinical factors. 37
Our study is not without limitations. Like many registry studies, our analysis was retrospective and relies on data extracted at each hospital from clinical medical records. Because this is an observational study, we cannot conclude causality. We attempted to account for confounders with both multivariable models and matched analyses, but residual confounding cannot be excluded. Criteria for T2MI were not rigorously defined or applied across sites. Though ECGs were not provided for our analyses, the diagnosis of T2MI was determined by the participating site and would be anticipated to include ECG changes (or imaging evidence of ischemia) according to the Fourth Universal Definition of Myocardial Infarction. 8 It is possible that the capture of T2MI as a dichotomous variable may be subject to both under‐ and over‐ascertainment as a result. To check the reliability of the T2MI diagnosis, we ran a sensitivity analysis on the 579 cases that were captured by the checkbox. The sensitivity analysis confirmed that only 12 patients in the 579 cases did not have an elevated troponin greater than that 99th percentile or a dynamic troponin trending on serial draws, further strengthening our adjudication of T2MI. However, using troponin as a sole indicator for T2MI is inherently limited given the high potential for nonischemic myocardial injury in the hospitalized COVID‐19 population. To ameliorate these concerns, we only included sites recording at least one T2MI diagnosis. Given these limitations, it is unclear how to interpret an incidence of 2.2% for T2MI in patients hospitalized with COVID‐19. We suspect that the actual incidence of T2MI in patients hospitalized with COVID‐19 is likely higher. We did attempt to limit this bias with the case–control matching discussed above, strengthening the conclusions of our study. Vaccination status was not accounted for; however, COVID‐19 vaccinations were not yet available during much of the time analyzed in this study. Due to the observational nature of this study, the findings of antihyperglycemic and anticoagulation medication use before admission being associated with a reduced odds of T2MI (and mortality) can only be classified as hypothesis‐generating here.
CONCLUSIONS
The incidence of T2MI in patients hospitalized with COVID‐19 was 2.2%. The strongest predictors for T2MI in patients hospitalized with COVID‐19 were CKD, CAD, HF, hypertension, and higher heart rate on presentation. Those patients with T2MI during the index hospitalization had higher morbidity and mortality. Outpatient antihyperglycemic medication use may be protective against both T2MI and mortality in hospitalized patients with COVID‐19, and this exploratory finding warrants further investigation in experimental studies, given mechanistic plausibility of some of these agents.
Sources of Funding
This work is funded in part by grant funding from the American Heart Association (AHA) to hospitals participating in the AHA Get With The Guidelines COVID‐19 CVD registry. AHA's suite of Registries is funded by multiple industry sponsors. AHA's COVID‐19 CVD Registry is supported by The Moore Foundation.
Disclosures
None.
Supporting information
Tables S1–S3
This manuscript was sent to Hani Jneid, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032572
For Sources of Funding and Disclosures, see page 9.
References
- 1. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;130:2354–2394. doi: 10.1161/CIR.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 2. Arora S, Strassle PD, Qamar A, Wheeler EN, Levine AL, Misenheimer JA, Cavender MA, Stouffer GA, Kaul P. Impact of type 2 myocardial infarction (MI) on hospital‐level MI outcomes: implications for quality and public reporting. J Am Heart Assoc. 2018;7:7. doi: 10.1161/JAHA.118.008661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bansal M. Cardiovascular disease and COVID‐19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen M, Visveswaran G. Defining and managing patients with non‐ST‐elevation myocardial infarction: sorting through type 1 vs other types. Clin Cardiol. 2020;43:242–250. doi: 10.1002/clc.23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta S, Vaidya SR, Arora S, Bahekar A, Devarapally SR. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta‐analysis of observational studies. Cardiovasc Diagn Ther. 2017;7:348–358. doi: 10.21037/cdt.2017.03.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandoval Y, Januzzi JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID‐19: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stein GY, Herscovici G, Korenfeld R, Matetzky S, Gottlieb S, Alon D, Gevrielov‐Yusim N, Iakobishvili Z, Fuchs S. Type‐II myocardial infarction—patient characteristics, management and outcomes. PLoS One. 2014;9:e84285. doi: 10.1371/journal.pone.0084285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; for the universal definition of myocardial infarction EGobotJESoCEACoCAAHAAWHFWTF . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 9. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alger HM, Rutan C, Williams JH, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID‐19 CVD registry powered by get with the guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. doi: 10.1161/CIRCOUTCOMES.120.006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunne C, Lang E. In adults hospitalized with COVID‐19, the quick COVID‐19 severity index predicted 24‐h respiratory decompensation. Ann Intern Med. 2021;174:JC23. doi: 10.7326/ACPJ202102160-023 [DOI] [PubMed] [Google Scholar]
- 12. Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. J Diabetes. 2020;12:851–855. doi: 10.1111/1753-0407.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo L, Shi Z, Zhang Y, Wang C, Do Vale Moreira NC, Zuo H, Hussain A. Comorbid diabetes and the risk of disease severity or death among 8807 COVID‐19 patients in China: a meta‐analysis. Diabetes Res Clin Pract. 2020;166:108346. doi: 10.1016/j.diabres.2020.108346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chapman AR, Shah ASV, Lee KK, Anand A, Francis O, Adamson P, McAllister DA, Strachan FE, Newby DE, Mills NL. Long‐term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandoval Y, Smith SW, Sexter A, Thordsen SE, Bruen CA, Carlson MD, Dodd KW, Driver BE, Hu Y, Jacoby K, et al. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high‐sensitivity cardiac troponin I. Am J Med. 2017;130:1431–1439.e4. doi: 10.1016/j.amjmed.2017.05.049 [DOI] [PubMed] [Google Scholar]
- 17. Bertini M, Ferrari R, Rapezzi C. What happened to electrocardiogram as a screening test to recognize cardiovascular complications in COVID‐19 patients? J Am Coll Cardiol. 2020;76:2799–2800. doi: 10.1016/j.jacc.2020.09.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, Danilov T, Kukar N, Shaban N, Kini A, et al. Characterization of myocardial injury in patients with COVID‐19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siripanthong B, Asatryan B, Hanff TC, Chatha SR, Khanji MY, Ricci F, Muser D, Ferrari VA, Nazarian S, Santangeli P, et al. The pathogenesis and long‐term consequences of COVID‐19 cardiac injury. JACC Basic Transl Sci. 2022;7:294–308. doi: 10.1016/j.jacbts.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumann Kreuziger L, Sholzberg M, Cushman M. Anticoagulation in hospitalized patients with COVID‐19. Blood. 2022;140:809–814. doi: 10.1182/blood.2021014527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bohula EA, Berg DD, Lopes MS, Connors JM, Babar I, Barnett CF, Chaudhry SP, Chopra A, Ginete W, Ieong MH, et al. Anticoagulation and antiplatelet therapy for prevention of venous and arterial thrombotic events in critically ill patients with COVID‐19: COVID‐PACT. Circulation. 2022;146:1344–1356. doi: 10.1161/CIRCULATIONAHA.122.061533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institutes of Health . Coronvirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. 2022. Accessed Mar 17, 2023. https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 24. Nguyen NN, Ho DS, Nguyen HS, Ho DKN, Li HY, Lin CY, Chiu HY, Chen YC. Preadmission use of antidiabetic medications and mortality among patients with COVID‐19 having type 2 diabetes: a meta‐analysis. Metab Clin Exp. 2022;131:155196. doi: 10.1016/j.metabol.2022.155196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kan C, Zhang Y, Han F, Xu Q, Ye T, Hou N, Sun X. Mortality risk of antidiabetic agents for type 2 diabetes with COVID‐19: a systematic review and meta‐analysis. Front Endocrinol (Lausanne). 2021;12:708494. doi: 10.3389/fendo.2021.708494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma S, Ray A, Sadasivam B. Metformin in COVID‐19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bramante CT, Huling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM, Cohen K, Puskarich MA, Belani HK, Proper JL, et al. Randomized trial of metformin, ivermectin, and fluvoxamine for Covid‐19. N Engl J Med. 2022;387:599–610. doi: 10.1056/NEJMoa2201662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, et al. A SARS‐CoV‐2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brunton SA. Diabesity. Clin Diabetes. 2022;40:392–393. doi: 10.2337/cd22-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chee YJ, Tan SK, Yeoh E. Dissecting the interaction between COVID‐19 and diabetes mellitus. J Diabetes Investig. 2020;11:1104–1114. doi: 10.1111/jdi.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia—a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID‐19, hyperglycemia, and new‐onset diabetes. Diabetes Care. 2021;44:2645–2655. doi: 10.2337/dc21-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vas P, Hopkins D, Feher M, Rubino F, B Whyte M. Diabetes, obesity and COVID‐19: a complex interplay. Diabetes Obes Metab. 2020;22:1892–1896. doi: 10.1111/dom.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J, Zhang J, Sun X, Wang L, Xu Y, Zhang Y, Liu X, Dong C. Influence of diabetes mellitus on the severity and fatality of SARS‐CoV‐2 (COVID‐19) infection. Diabetes Obes Metab. 2020;22:1907–1914. doi: 10.1111/dom.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, Rosenblatt A, Walchok J, Omar W, Khera R, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID‐19: results from the American Heart Association COVID‐19 cardiovascular disease registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936 [DOI] [PubMed] [Google Scholar]
- 36. Wereski R, Kimenai DM, Bularga A, Taggart C, Lowe DJ, Mills NL, Chapman AR. Risk factors for type 1 and type 2 myocardial infarction. Eur Heart J. 2022;43:127–135. doi: 10.1093/eurheartj/ehab581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Islam SJ, Malla G, Yeh RW, Quyyumi AA, Kazi DS, Tian W, Song Y, Nayak A, Mehta A, Ko YA, et al. County‐level social vulnerability is associated with in‐hospital death and major adverse cardiovascular events in patients hospitalized with COVID‐19: an analysis of the American Heart Association COVID‐19 cardiovascular disease registry. Circ Cardiovasc Qual Outcomes. 2022;15:e008612. doi: 10.1161/CIRCOUTCOMES.121.008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


